Abstract
Background & Aims
Well established risk factors for intrahepatic cholangiocarcinoma such as biliary tract inflammation and liver flukes are not present in most patients in Western countries. Although cirrhosis and other causes of chronic liver disease have been implicated, their contribution as risk factors for cholangiocarcinoma is unclear and our aims were to analyze these emerging potential risk factors by systematic examination of case-control series from geographically diverse regions.
Methods
We performed a literature review and meta-analysis of case-control studies on intrahepatic cholangiocarcinoma and cirrhosis and related risk factors. Tests of heterogeneity, publication bias and sensitivity analyses were performed and an overall odds ratio and 95% confidence intervals calculated.
Results
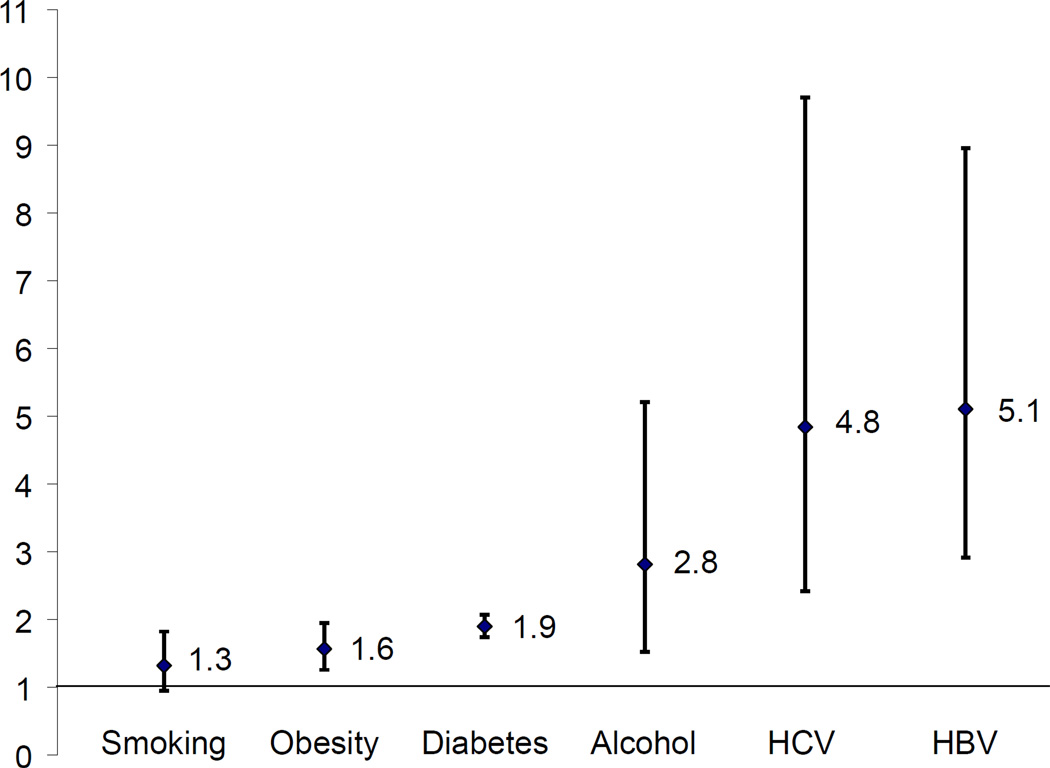
Eleven studies from both high and low prevalence regions were identified. All studies except for those evaluating cirrhosis, diabetes, and obesity exhibited significant heterogeneity. Cirrhosis was associated with a combined OR of 22.92 (95% CI = 18.24 – 28.79). Meta-analysis estimated the overall odds ratio (with 95% confidence intervals) for defined risk factors such as hepatitis B: 5.10 (2.91–8.95), hepatitis C: 4.84 (2.41–9.71), obesity: 1.56 (1.26–1.94), diabetes mellitus type II: 1.89 (1.74–2.07), smoking: 1.31 (0.95–1.82), and alcohol use: 2.81 (1.52–5.21). Sensitivity analysis did not alter the odds ratio for any risk factors except smoking and there was no evidence of publication bias.
Conclusions
Cirrhosis, chronic hepatitis B and C, alcohol use, diabetes, and obesity are major risk factors for intrahepatic cholangiocarcinoma. These data suggest a common pathogenesis of primary intrahepatic epithelial cancers.
Keywords: cholangiocarcinoma, risk factors, biliary neoplasia
Introduction
Cholangiocarcinomas are aggressive malignancies arising from the biliary tract and are challenging to diagnose, prevent or treat [1]. The two major clinical phenotypes of cholangiocarcinoma are intrahepatic and ductal / peri-hilar cancers. These phenotypes differ in their anatomical locations, presentation, natural history, and management. Intrahepatic cholangiocarcinomas (IH-CCA) are similar to hepatocellular cancer (HCC) in their presentation as mass lesions within the liver. Both HCC and IH-CCA are primary epithelial malignancies of the liver and are often classified together as primary liver cancers in epidemiological reports. The incidence of HCC is increasing in the United States and other countries [2, 3]. In contrast to HCC, the true incidence and risk factors for IH-CCA are less well recognized possibly because of the much lower prevalence of these cancers relative to HCC. Recent reports suggest that the incidence of IH-CCA is also increasing [4–9]. Data from the Surveillance, Epidemiology and End Results program, for example, indicated an increase in the age-adjusted annual incidence of IH-CCA in the United States from 0.13 to 0.58 per 100,000 over a twenty-five year period with similar trends observed from many other countries worldwide [7, 8]. However, more recent analyses indicate that these changes may have reflected differences in coding and diagnosis [10–12]. Hilar cholangiocarcinomas, for example, are inconsistently classified and their designation as intra-hepatic cholangiocarcinoma has made accurate determinations of true incidence rates for intrahepatic cancers impossible.
Most patients with IH-CCA do not have any apparent risk factors. Both infectious and non-infectious etiologies have been implicated as risk factors for IH-CCA [13]. Indeed parasitic liver-flukes, primary sclerosing cholangitis, biliary cysts, hepatolithiasis, and toxins are well recognized risk-factors [1, 14–16]. Some of the variations in the incidence of IH-CCA worldwide can be accounted for by differences in the geographic prevalence of causative liver fluke infectious. Other than in regions where liver flukes are endemic such as South East Asia, the majority of patients with IH-CCA do not have any of these risk factors. Salmonella and Helicobacter infections have been implicated in gallbladder cancer but their contribution to IH-CCA is unknown. Recent studies have identified several other conditions that can contribute to an increased risk of IH-CCA such as chronic viral hepatitis B and C, obesity, diabetes, alcohol, and smoking [9, 17–27]. The evaluation of these risk factors has led to conflicting results in some cases. It is unclear to what extent these differences reflect geographic differences, the study design or the population studied. Thus, our objectives were to evaluate these emerging risk factors for IH-CCA by systematically examining the results of reported case-control series from geographically diverse regions. The results of these meta-analyses identify that IH-CCA has many risk factors in common with HCC, and quantitate the overall odds ratios for selected risk factors. These studies support the presence of common mechanisms for the pathogenesis of primary epithelial neoplasia within the liver and lead to exciting hypotheses regarding the etiology and pathogenesis of these cancers. By defining populations at risk for IH-CCA, these observations can form the basis for future efforts at screening or surveillance for these cancers with the goal of decreasing the incidence and mortality from these cancers.
Materials and Methods
Literature search
Studies were identified by searching both the National Library of Medicine’s MEDLINE database using PubMed and by using Google Scholar search. Studies were not limited by language or to any geographic region. The most recent search was performed on August 12, 2011. The search strategy was based on combinations of the key words, “risk”, “smoking”, “diabetes”, “obesity”, “hepatitis” with “cholangiocarcinoma” or “biliary tract cancers”, and restricted to studies performed after 1990 to avoid any possible inconsistencies in diagnostic criteria used. In addition, a manual search of the citations in selected papers was also performed.
Inclusion and exclusion criteria
We included studies that met the following criteria: (a) case-control study design; (b) reported outcomes specifically for cases of IH-CCA; (c) examined individual risk factors using defined criteria; (d) provided enough information to calculate the odds ratio. Studies where the materials and methods were inadequately described, raw data was unavailable, or where cases did not specifically include IH-CCA were excluded. The quality of individual studies was evaluated based on reported study methodology, analyses, and identification of cases and controls. The criteria reviewed included (a) description of the subject selection for both cases and controls, to ensure that there were no obvious biases; (b) methods used to determine presence or absence of risk factor, and (c) approach for analysis of results and their interpretation. Studies were selected for inclusion in our meta-analysis in an unblinded standardized manner by one of the authors. None of the identified studies were excluded from the analysis.
Data extraction
Information was extracted from each included study on authors, journal, inclusion and exclusion criteria, outcome measures, documentation of risk factor, and numbers of individuals with IH-CCA (cases) or without IH-CCA (controls) who either had or did not have the risk factor of interest.
Statistical analysis
Study heterogeneity was assessed using the Cochran’s Q statistic and I2 statistic. A meta-analysis was performed using a random effects model using the DerSimonian and Laird method where there was significant heterogeneity (Q: p<0.01, or I2>60%), or using a fixed effect model and the Mantel-Haenszel weighting algorithm where there was no significant heterogeneity. A pooled odds ratio (OR) and 95% confidence intervals (CI) were calculated for each risk factor. Statistical analyses were performed using Mix 1.7 software (BiostatXL) and Forest plots generated for each risk factor analyzed. Funnel plots were generated to evaluate for potential publication bias.
Results
Study characteristics
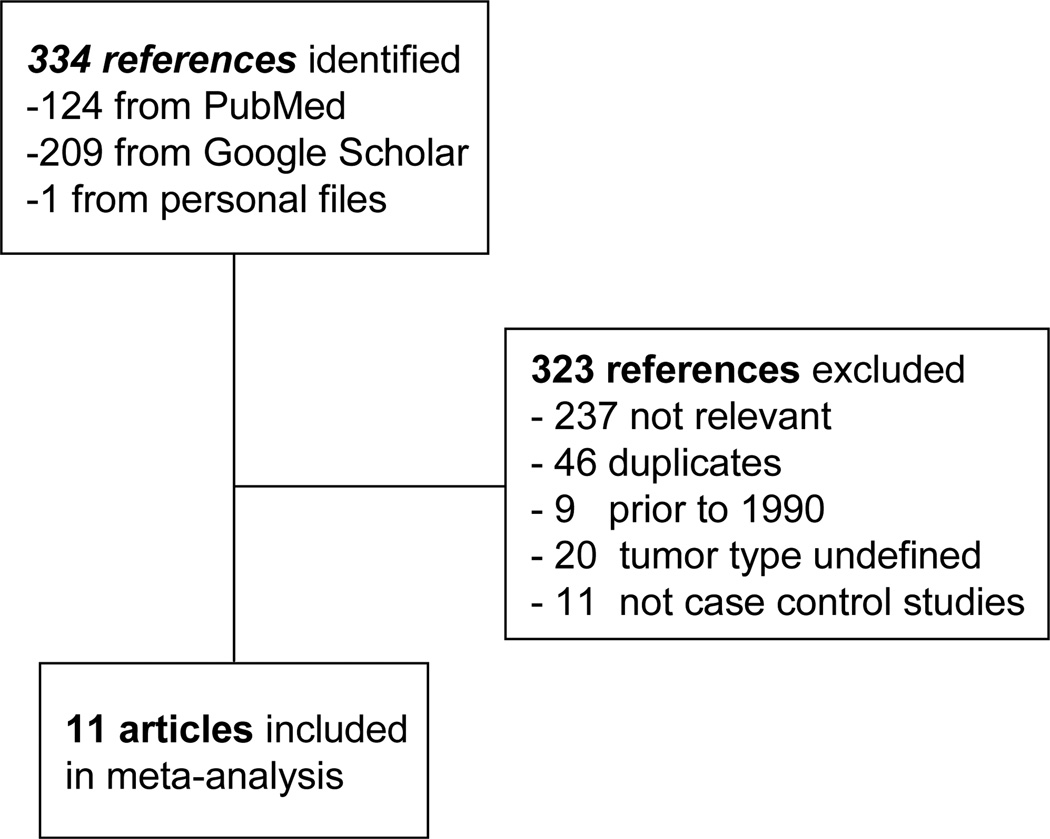
A search of Pubmed and Google Scholar identified a total of 334 citations, of which 323 were excluded as they did not fulfill the selection criteria, were published prior to 1990, or were duplicates (Fig 1). 11 studies qualified for inclusion. The characteristics of the populations studied in these reports are summarized in Table 1. Studies were performed in both regions of high prevalence of hepatobiliary cancers such as Japan (1 study), Korea (1 study) and China (3 studies), as well as regions of lower prevalence such as the United States (4 studies), Italy (1 study), and Denmark (1 study). Age and gender adjustments were reported in all studies. Several of these studies evaluated more than one risk factor. The studies ranged from single-institution studies (7 studies), to population database studies (4 studies). All of the studies except for those evaluating cirrhosis, diabetes mellitus type II, or obesity exhibited significant heterogeneity (Table 2).
Fig. 1. Selection of studies for analysis.
Flow diagram of identification of studies evaluated in this analysis. 11 case-control studies evaluating risk factors of interest for intrahepatic cholangiocarcinoma were identified.
Table 1.
Case-control studies examining selected risk factors for Intrahepatic Cholangiocarcinoma.
| Study | Region | Dates | Cases | Controls | Risk factor | |||
|---|---|---|---|---|---|---|---|---|
| Total | With risk factor |
Total | With risk factor |
|||||
| Hepatitis B | Yamamoto, 2004[23] | Japan | 1991–2002 | 50 | 2 | 205 | 5 | HBsAg+ |
| Shaib, 2005[18] | US | 1993–1999 | 625 | 2 | 90,834 | 182 | HBV | |
| Donato, 2001 [38] | Italy | 1995–2000 | 23 | 3 | 824 | 45 | HBsAg+ | |
| Lee, 2008[17] | Korea | 2000–2004 | 622 | 84 | 2,488 | 125 | HBsAg+ | |
| Zhou, 2008[25] | China | 2004–2006 | 312 | 151 | 438 | 42 | HBsAg+ | |
| Welzel, 2011 [21] | US | 1993–2005 | 743 | 11 | 195,953 | 442 | ICD9 | |
| Zhou, 2010[23] | China | 2003–2006 | 317 | 154 | 634 | 42 | HBsAg+ | |
| Tao, 2010[20] | China | 1998–2008 | 61 | 17 | 380 | 19 | HBsAg+ | |
| Hepatitis C | Yamamoto, 2004[23] | Japan | 1991–2002 | 50 | 18 | 205 | 7 | Anti-HCV+ |
| Shaib, 2007[19] | US | 1992–2002 | 83 | 5 | 236 | 2 | Anti-HCV+ | |
| Shaib, 2005[18] | US | 1993–1999 | 625 | 5 | 90,834 | 161 | HCV/ICD9 | |
| Welzel, 2007[9] | US | 1993–1999 | 535 | 5 | 102,782 | 142 | HCV/ICD9 | |
| Donato, 2001 [38] | Italy | 1995–2000 | 24 | 6 | 824 | 50 | Anti-HCV+ | |
| Lee, 2008[17] | Korea | 2000–2004 | 622 | 12 | 2,488 | 47 | Anti-HCV+ | |
| Zhou, 2008[25] | China | 2004–2006 | 312 | 9 | 438 | 6 | Anti-HCV+ | |
| Welzel, 2011 [21] | US | 1993–2005 | 743 | 20 | 195,953 | 616 | ICD9 | |
| Obesity | Welzel, 2007[] | Denmark | 1978–1991 | 764 | 6 | 3,056 | 12 | ICD9 |
| Welzel, 2007[9] | US | 1993–1999 | 535 | 23 | 102,782 | 3,201 | ICD9 | |
| Welzel, 2011 [21] | US | 1993–2005 | 743 | 59 | 195,953 | 9,983 | ICD9 | |
| Diabetes Mellitus II | Welzel, 2007[22] | Denmark | 1978–1991 | 764 | 15 | 3,056 | 43 | ICD9 |
| Yamamoto, 2004[23] | Japan | 1991–2002 | 50 | 11 | 205 | 24 | Required meds | |
| Shaib, 2007[19] | US | 1992–2002 | 83 | 12 | 236 | 20 | PMHx | |
| Shaib, 2005[18] | US | 1993–1999 | 625 | 165 | 90,834 | 14,201 | ICD9 | |
| Welzel, 2007[9] | US | 1993–1999 | 535 | 177 | 102,782 | 22,764 | ICD9 | |
| Lee, 2008[17] | Korea | 2000–2004 | 622 | 96 | 2,488 | 139 | PMHx | |
| Zhou, 2008[25] | China | 2004–2006 | 312 | 13 | 438 | 11 | ICD9 | |
| Welzel, 2011[21] | US | 1993–2005 | 743 | 299 | 195,953 | 52,691 | ICD9 | |
| Tao, 2010[20] | China | 1998–2008 | 61 | 3 | 380 | 36 | Chart review | |
| Smoking | Yamamoto, 2004[23] | Japan | 1991–2002 | 50 | 17 | 205 | 90 | Any |
| Shaib, 2007[19] | US | 1992–2002 | 83 | 20 | 236 | 37 | ≥25 pack/year | |
| Shaib, 2005[18] | US | 1993–1999 | 625 | 24 | 90,834 | 1,927 | ICD9 | |
| Welzel, 2007[9] | US | 1993–1999 | 535 | 12 | 102,782 | 1,212 | ICD9 | |
| Lee, 2008[17] | Korea | 2000–2004 | 622 | 293 | 2,488 | 1,135 | Any | |
| Zhou, 2008[25] | China | 2004–2006 | 312 | 43 | 438 | 67 | ≥4d/wk for ≥6m | |
| Welzel, 2011 [21] | US | 1993–2005 | 743 | 78 | 195,953 | 9,647 | ICD9 | |
| Tao, 2010[20] | China | 1998–2008 | 61 | 18 | 380 | 122 | Any | |
| Alcohol | Yamamoto, 2004[23] | Japan | 1991–2002 | 50 | 1 | 205 | 11 | >5gosake/day > 10y |
| Shaib, 2007[19] | US | 1992–2002 | 83 | 18 | 236 | 9 | ≥ 80g/day | |
| Shaib, 2005[18] | US | 1993–1999 | 625 | 14 | 90,834 | 282 | EtOH liver disease | |
| Welzel, 2007[9] | US | 1993–1999 | 535 | 5 | 102,782 | 310 | EtOH liver disease | |
| Donato, 2001 [38] | Italy | 1995–2000 | 26 | 6 | 824 | 271 | ≥ 80g/day | |
| Lee, 2008[17] | Korea | 2000–2004 | 622 | 112 | 2,488 | 78 | ≥ 80g/day | |
| Zhou, 2008[25] | China | 2004–2006 | 312 | 39 | 438 | 41 | ≥ 1 d/wk for >=6m | |
| Welzel, 2011 [21] | US | 1993–2005 | 743 | 21 | 195,953 | 832 | EtOH liver disease | |
| Zhou, 2010[24] | China | 2003–2006 | 317 | 6 | 634 | 2 | EtOH liver disease | |
| Tao, 2010[20] | China | 1998–2008 | 61 | 15 | 380 | 85 | Any | |
HBsAg, Hepatitis B surface antigen, Anti-HBc, hepatitis B core antibody, HBV, hepatitis B, ICD, International classification of disease, anti-HCV, hepatitis C antibodies, PMHx, past medical history, EtOH, alcohol
Table 2.
Measures of the degree of heterogeneity between studies analyzed for each risk factor.
| Risk factor | z | p | Q | p | H | 95% CI | I2 | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Alcohol | 12.6 | <0.0001 | 90.3 | <0.0001 | 3.2 | 2.5 – 4.1 | 90.0% | 83.8–93.9% |
| HBV | 20.6 | <0.0001 | 51.2 | <0.0001 | 2.7 | 2.0 – 3.6 | 86.3% | 75.1–92.5% |
| HCV | 9.1 | <0.0001 | 42.7 | <0.0001 | 2.5 | 1.8 – 3.4 | 83.6% | 69.3–91.3% |
| Smoking | 3.9 | <0.0001 | 41.4 | <0.0001 | 2.4 | 1.8 – 3.3 | 83.1% | 68.2–91.0% |
| DM | 14.3 | <0.0001 | 18.9 | 0.015 | 1.5 | 1.1 – 2.2 | 57.8% | 11.4–79.9% |
| Obesity | 4.0 | <0.0001 | 0.6 | 0.754 | 1.0 | 1.0 – 3.1 | 0.0% | 0–89.6% |
Significant heterogeneity was observed in studies evaluating hepatitis B, hepatitis C, alcohol or smoking using either Q or I2 statistics. HBV, Hepatitis B, HCV, Hepatitis C; DM, Diabetes mellitus, type II; CI, confidence intervals
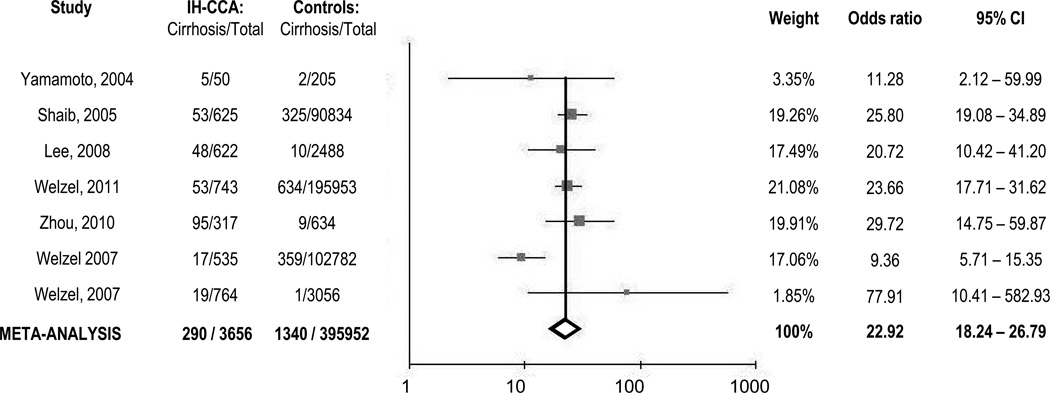
Cirrhosis
An increased predisposition for IH-CCA in patients with cirrhosis has been recognized for many years [28–32]. We performed a meta-analysis of results from seven case-control studies that provided data on cirrhosis in a total study population of 399,608 patients with or without IH-CCA. The diagnosis of cirrhosis was made on the basis of clinical criteria, imaging, biopsy, or International Classification of Diseases (ICD)-9 coding. Three of the studies specifically examined non-specific cirrhosis and excluded chronic viral or alcohol related cirrhosis. Three studies were performed in the United States with one study from each of the following: Japan, Italy, Denmark, and China. A moderate degree of heterogeneity was noted (I2=62.4%), and thus the overall odds ratio (OR) was estimated using a random effects model. Cirrhosis was associated with an overall OR of 22.92 (95% CI = 18.24 – 28.79) for IH-CCA.
This analysis confirms that cirrhosis is a major risk factor for IH-CCA. In order to determine the potential risk associated with specific underlying causes of chronic liver disease, we next examined the risk associated with known individual risk factors for cirrhosis.
Hepatitis B
Chronic infection with hepatitis B virus (HBV) has been evaluated as a possible risk factor for IH-CCA in several studies. Nine case control studies investigating hepatitis B as a risk factor were selected for meta-analysis. Of these, we excluded one study where hepatitis B infection was identified solely on the basis of a positive Hepatitis B core antibody. In the other studies, hepatitis B infection was defined by presence of HBsAg (6 studies), HBV DNA in serum (1 study), or by ICD9 codes 070.22, 070.23, 070.32, 070.33, V02.61 (1 study). Study data collection ranged from 1991 through 2008. These studies encompassed a total study population of 294,828 patients. Three of the nine studies did not indicate any increased risk of IH-CCA with hepatitis B, with an OR close to unity. These three studies were geographically different (US, Italy, Japan) with all three studies concluding prior to 2002. However, the meta-analysis indicated that presence of hepatitis B virus was associated with a combined OR of 5.54, with 95% confidence intervals of 3.19 – 9.63 for IH-CCA. Five studies analyzed were performed in high prevalence regions in Eastern nations such as Japan, Korea, and China whereas three studies were from Western nations with a low-to-intermediate prevalence regions such as the United States and Italy. A separate analysis of these two groups did not reveal any significant difference between the two regions (Table 3).
Table 3.
Chronic viral hepatitis as a risk factor for IH-CCA
| Risk factor | Region | Number of studies |
Number of participants |
OR | 95% CI |
|---|---|---|---|---|---|
| HBV | East | 5 | 5507 | 6.04 | 2.90 – 12.57 |
| HBV | West | 3 | 289002 | 3.97 | 2.38 – 6.62 |
| HCV | East | 3 | 4115 | 3.20 | 0.60 – 16.91 |
| HCV | West | 5 | 392639 | 6.91 | 4.87 – 9.81 |
The odds ratio and 95% confidence intervals for chronic viral hepatitis B or C were analyzed from studies from Eastern nations (Japan, China, Korea) or for studies from Western nations (U.S. and Italy). HBV, Hepatitis B, HCV, Hepatitis C; OR, Odds Ratio; CI, confidence intervals
Hepatitis C
Some studies have identified chronic hepatitis C virus (HCV) infection as a risk factor for IH-CCA whereas others have not. We identified eight case-control studies that evaluated HCV infection as a risk factor for ICC. HCV was defined by presence of serum anti-HCV+ in 5 studies, or HCV-RNA positivity in serum, or by ICD9 codes 070.41, 070.44, 070.51, 070.54, V02.62 in the rest of the studies. Four of the studies were performed in the United States, with one from each of Japan, Italy, Korea, and China. These studies incorporated a total case and control population of 396,754 individuals, with study data collection occurring from 1991 through 2006. The meta-analysis indicated that the presence of Hepatitis C virus was associated with an overall OR of 4.84, with a 95% confidence interval of 2.41–9.71. However, in two studies, both performed in Asia, there was no difference noted [17, 25], When studies from Eastern nations (Japan, Korea and China) were separately analyzed from studies from Western nations (United States and Italy), the OR was significantly different from unity only for the latter (Table 3).
Obesity
There is limited information regarding the role of obesity as a risk factor for IH-CCA. We identified only three studies, two from the United States, and one from Denmark, in which obesity was defined by ICD-9 coding, with a total study population of 304,134 patients. As expected, there was no significant heterogeneity (Q=0.5646, p=0.75; I2=0%). All three studies reported an increased risk of IH-CCA with obesity. Using a fixed effects estimate, combined analysis revealed an overall odds ratio of 1.56 with 95% confidence intervals ranging from 1.26 to 1.94. These observations are consistent with several other recent reports indicating an increased risk of many cancers with obesity.
Diabetes Mellitus Type II
Data for analysis of diabetes mellitus II as a risk factor for IH-CCA were obtained from nine case control studies. The diagnosis was based on ICD-9 coding (250.x0, 250.x2) in 5 studies, or based on chart review identifying a history of diabetes or need for diabetic medications in 4 studies. Four of the studies were performed in the United States, with one study from each of Denmark, Japan, China, and Korea. All studies adjusted for age and sex. Study data collection ranged from 1991 through 2008. Five of the studies did not indicate any increased risk of diabetes. However, using a fixed effects analysis, meta-analysis of the total study population of 400,167 patients showed that diabetes was associated with an overall OR of 1.89 with 95% confidence intervals of 1.74–2.07 for IH-CCA.
Smoking
Tobacco use has also been investigated as a possible risk factor for IH-CCA. Eight studies with a total study population of 396,347 provided case-control information for the risk of smoking. Tobacco use was based on ICD-9 coding (3 studies), or history of use (5 studies). Given the heterogeneity between studies, a random effects method was used for analysis. An overall odds ratio of 1.31 with 95% confidence intervals of 0.95 to 1.82 was estimated. Thus, the association between smoking and risk of IH-CCA is not as defined compared to the other risk factors analyzed, and the increased risk, if any, is much lower. The relationship between smoking and IH-CCA will need to be further defined.
Alcohol
Ten case control studies investigating alcohol as a risk factor for ICC were selected for meta-analysis. Alcohol exposure was defined as greater than 80g per day of alcohol (3 studies), the presence of alcoholic liver disease (4 studies), or based on a defined threshold for alcohol use for at least 6 months (3 studies). Studies were performed in the United States (4 studies), Japan, Italy, Korea, (1 from each), and China (3 studies). Study data collection ranged from 1991 through 2008. In a total study population of 398,048 patients, alcohol use was associated with an overall OR of 2.81 (95% CI = 1.52–5.21) for IH-CCA.
Publication bias and Sensitivity analysis
Funnel plots were generated to examine for publication bias, but did not identify any possible bias in the studies (Supplementary figure 1). Because of the differences in study population, we performed a sensitivity analysis to identify the impact of exclusion of population based studies, or of studies with the greatest weighting on the overall results of the meta-analysis. A sensitivity analysis was not performed for studies evaluating obesity because of the small number of studies. With the exception of smoking, there was no significant difference noted in the overall odds ratio for cirrhosis, or for chronic HBV, HCV, alcohol use, tobacco use or diabetes, indicating that the results of the meta-analyses for these factors were not influenced by individual studies or by specific types of studies.
Discussion
While the predisposition of biliary tract inflammation to carcinogenesis is recognized in the biliary tract, many patients with IH-CCA do not have readily recognizable biliary tract inflammation or infection. Traditional infectious risk factors for IH-CCA include the liver flukes Clonorchis sinensis and Opisthorchis viverrini, both of which are now recognized as group 1 carcinogens and causes of cholangiocarcinoma by the International Agency for Research in Cancer of the World Health Organization [33]. Intrahepatic ductal inflammation associated with hepatolithiasis and hepatic schistosomiasis can also predispose to tumor formation. However the prevalence of these conditions is highly restricted to certain geographic regions. The increasing recognition of IH-CCA in many other regions of the world highlights the critical importance of defining the risk factors for these cancers.
These meta-analyses of case-control series confirm that liver cirrhosis is associated with a dramatically increased risk of IH-CCA. Moreover, they identify an increased risk of IH-CCA in individuals who have chronic viral hepatitis B or C infection, type II diabetes mellitus or obesity and those who use alcohol. These are all established risk factors for HCC. Similar to HCC, IH-CCA presents most often as an intrahepatic mass lesion. Although IH-CCA and HCC may have different characteristics on imaging studies, histological examination may be necessary to distinguish between these two cancers. However, biopsies are not routinely obtained raising the possibility that some instances of IH-CCA could be misdiagnosed as HCC and misrepresented as such in epidemiological studies. Although HBV and HCV are well-established risk factors for HCC, the increased risk of IH-CCA with these conditions has not been widely recognized. Indeed, the variable conclusions reported for risk factors such as HBV and HCV have resulted in uncertainty about their involvement in IH-CCA. The results of the current meta-analyses should allow us to now focus on understanding the contribution of these risk factors to the pathogenesis of these cancers. Our analysis raises the possibility of geographic differences in the risk of IH-CCA in patients with HCV but is limited by the small number of studies and participants, and additional studies from regions in the East are necessary.
Although the evidence for the relationship between cigarette smoking and HCC is supported by several epidemiological studies [34], our analysis did not provide sufficient evidence of an increased risk for IH-CCA. The reason for this is most likely related to the limited number of studies that have examined this risk factor. The estimated overall odds ratios for smoking as a risk factor were sensitive to exclusion of individual studies and to the analytic models used. Thus, additional studies will be required to establish the absence or presence of smoking as a risk factor and to determine the precise contribution of cigarette smoking on the risk of IH-CCA.
The inclusion of different types of studies ranging from population based to single center studies, along with the sample sizes and geographical diversity are key strengths of the analyses. We did not geographically restrict inclusion of studies in our analyses, but did perform separate analyses for risk in Eastern and Western nations for chronic viral hepatitis B and C. The other risk factors examined are less restricted geographically. There are some limitations of this study. Although criteria for identifying non-viral risk factors such as alcohol use and smoking were appropriately chosen, they varied between studies. Future studies could examine the relationship between duration of exposure to these risk factors and the risk of IH-CCA. There were limited studies available from regions with high endemicity for chronic viral hepatitis. The precise contribution of the risk of these factors on liver-fluke induced cholangiocarcinoma will be important to determine in order to establish whether these constitute an entirely distinct type of IH-CCA. The relationship of these risk factors to extrahepatic, ductal cholangiocarcinoma was not examined. These cancers differ from IH-CCA in their clinical presentation and natural history. These important questions warrant evaluation in future studies.
The recognition of the similarity of risk factors provides new insights into the pathogenesis of these primary epithelial malignancies by suggesting a common pathogenesis for hepatobiliary neoplasia. Recent hypotheses regarding the contribution of intrahepatic cancer progenitor cells to the pathogenesis of both HCC and IH-CCA offer biological plausibility to these observations. According to these hypotheses, stem cell niches residing in the canals of Hering or within the peribiliary glands of the bile ducts can be activated to differentiate while undergoing oncogenic stimulation in the context of chronic hepatic injury. The extent of differentiation eventually reflects the phenotypic nature of the resulting lesion, such as HCC, IH-CCA, or an intermediate phenotype. This postulate is supported by recent studies that identify HCC, IH-CCA or mixed phenotype tumors encompassing this spectrum as well as the identification of overlapping signature genes between HCC and IH-CCA, and of cholangiocarcinoma-like gene expression signatures in some hepatocellular cancers [35–37]. By predicting that these cancers arise from a single precursor population, this hypothesis explains the commonality of risk factors. These risk factors can contribute to the milieu of chronic hepatic injury underlying oncogenic transformation which can result in HCC, IH-CCA or intermediate cancer phenotypes.
Supplementary Material
Funnel plot analysis to detect publication bias evaluating the association between intrahepatic cholangiocarcinoma and the following risk factors; (A) Hepatitis B, (B) Hepatitis C, (C) Alcohol use, (D) Smoking, (E) Diabetes Mellitus type II and (F) Obesity. Each spot represents a separate case-control study evaluating that risk factor for intrahepatic cholangiocarcinoma. Horizontal axis indicates the Log odds ratio, vertical axis indicates the inverse standard error.
Fig. 2. Cirrhosis as a risk factor for Intrahepatic Cholangiocarcinoma.
A forest plot of case control studies for the association of cirrhosis with intrahepatic cholangiocarcinoma using a fixed effects analysis. The horizontal axis indicates the odds ratio on a log scale. Horizontal lines, 95% CI of point estimates indicated as solid squares, the size of which reflects the percent weight accorded the study in the analysis. The vertical axis indicates individual studies and line of null effect. Vertical solid line indicates the pooled estimate.
Fig. 3. Meta-analyses of case-control studies.
A forest plot of case control studies for the association of selected risk factors with intrahepatic cholangiocarcinoma was generated using a random effects analysis for (A) Hepatitis B, (B) Hepatitis C, (C) Alcohol use, (D) Smoking; or using a fixed effects analysis for (E) Diabetes Mellitus type II and (F) Obesity. The horizontal axis indicates the odds ratio (log scale). Horizontal lines, 95% CI of point estimates indicated as solid squares, the size of which reflects the percent weight accorded the study. The vertical axis indicates individual studies and line of null effect.
Fig. 4. Risk factors for Intrahepatic Cholangiocarcinoma.
Overall odds ratio (vertical axis) with 95% confidence intervals for intrahepatic cholangiocarcinoma are shown for selected risk factors based on meta-analysis of case-control studies. HBV, Hepatitis B; HCV, Hepatitis C.
Acknowledgments
Financial support: Supported in part by NIH grant DK 069370 (TP).
Abbreviations
- IH-CCA
Intrahepatic cholangiocarcinoma
- HCC
hepatocellular carcinoma
- OR
Odds Ratio
- CI
confidence intervals
- ICD
International classification of disease
- HBV
hepatitis B virus
- HCV
hepatitis C virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: There are no conflicts of interest to disclose
References
- 1.Patel T. Cholangiocarcinoma--controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8:189–200. doi: 10.1038/nrgastro.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 4.Chang KY, Chang JY, Yen Y. Increasing incidence of intrahepatic cholangiocarcinoma and its relationship to chronic viral hepatitis. J Natl Compr Canc Netw. 2009;7:423–427. doi: 10.6004/jnccn.2009.0030. [DOI] [PubMed] [Google Scholar]
- 5.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Mouzas IA, Dimoulios P, Vlachonikolis IG, Skordilis P, Zoras O, Kouroumalis E. Increasing incidence of cholangiocarcinoma in Crete 1992–2000. Anticancer Res. 2002;22:3637–3641. [PubMed] [Google Scholar]
- 7.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 8.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5:1221–1228. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welzel TM, McGlynn KA, Hsing AW, O'Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006 Jun 21;98(12):873–875. doi: 10.1093/jnci/djj234. [DOI] [PubMed] [Google Scholar]
- 11.Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang Y, et al. Comparison of incidence of intrahepatic and extrahepatic cholangiocarcinoma--focus on East and South-Eastern Asia. Asian Pac J Cancer Prev. 2010;11(5):1159–1166. [PubMed] [Google Scholar]
- 12.Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, et al. Rising trends in cholangiocarcinoma: Is the ICD classification system misleading us? J Hepatol. 2011 Dec 13; doi: 10.1016/j.jhep.2011.11.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–184. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, et al. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer science. 2010;101:579–585. doi: 10.1111/j.1349-7006.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broome U, Lofberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995;22:1404–1408. doi: 10.1002/hep.1840220511. [DOI] [PubMed] [Google Scholar]
- 16.Su CH, Shyr YM, Lui WY, P'Eng FK. Hepatolithiasis associated with cholangiocarcinoma. The British journal of surgery. 1997;84:969–973. doi: 10.1002/bjs.1800840717. [DOI] [PubMed] [Google Scholar]
- 17.Lee TY, Lee SS, Jung SW, Jeon SH, Yun SC, Oh HC, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. The American journal of gastroenterology. 2008;103:1716–1720. doi: 10.1111/j.1572-0241.2008.01796.x. [DOI] [PubMed] [Google Scholar]
- 18.Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620–626. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 19.Shaib YH, El-Serag HB, Nooka AK, Thomas M, Brown TD, Patt YZ, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. The American journal of gastroenterology. 2007;102:1016–1021. doi: 10.1111/j.1572-0241.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 20.Tao LY, He XD, Qu Q, Cai L, Liu W, Zhou L, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case-control study in China. Liver international : official journal of the International Association for the Study of the Liver. 2010;30:215–221. doi: 10.1111/j.1478-3231.2009.02149.x. [DOI] [PubMed] [Google Scholar]
- 21.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welzel TM, Mellemkjaer L, Gloria G, Sakoda LC, Hsing AW, El Ghormli L, et al. Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. International journal of cancer Journal international du cancer. 2007;120:638–641. doi: 10.1002/ijc.22283. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto S, Kubo S, Hai S, Uenishi T, Yamamoto T, Shuto T, et al. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer science. 2004;95:592–595. doi: 10.1111/j.1349-7006.2004.tb02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou H, Wang H, Zhou D, Wang Q, Zou S, Tu Q, et al. Hepatitis B virus-associated intrahepatic cholangiocarcinoma and hepatocellular carcinoma may hold common disease process for carcinogenesis. European journal of cancer. 2010;46:1056–1061. doi: 10.1016/j.ejca.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhou YM, Yin ZF, Yang JM, Li B, Shao WY, Xu F, et al. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World journal of gastroenterology : WJG. 2008;14:632–635. doi: 10.3748/wjg.14.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu TH, Yuan RH, Chen YL, Yang WC, Hsu HC, Jeng YM. Viral hepatitis is associated with intrahepatic cholangiocarcinoma with cholangiolar differentiation and N-cadherin expression. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24:810–819. doi: 10.1038/modpathol.2011.41. [DOI] [PubMed] [Google Scholar]
- 27.Yin F, Chen B. Detection of hepatitis C virus RNA sequences in hepatic portal cholangiocarcinoma tissue by reverse transcription polymerase chain reaction. Chinese medical journal. 1998;111:1068–1070. [PubMed] [Google Scholar]
- 28.Kobayashi M, Ikeda K, Saitoh S, Suzuki F, Tsubota A, Suzuki Y, et al. Incidence of primary cholangiocellular carcinoma of the liver in japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000;88:2471–2477. doi: 10.1002/1097-0142(20000601)88:11<2471::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Altaee MY, Johnson PJ, Farrant JM, Williams R. Etiologic and clinical characteristics of peripheral and hilar cholangiocarcinoma. Cancer. 1991;68:2051–2055. doi: 10.1002/1097-0142(19911101)68:9<2051::aid-cncr2820680934>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 30.Kuper H, Ye W, Broome U, Romelsjo A, Mucci LA, Ekbom A, et al. The risk of liver and bile duct cancer in patients with chronic viral hepatitis, alcoholism, or cirrhosis. Hepatology. 2001;34:714–718. doi: 10.1053/jhep.2001.28233. [DOI] [PubMed] [Google Scholar]
- 31.Sorensen HT, Friis S, Olsen JH, Thulstrup AM, Mellemkjaer L, Linet M, et al. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998;28:921–925. doi: 10.1002/hep.510280404. [DOI] [PubMed] [Google Scholar]
- 32.Shin HR, Lee CU, Park HJ, Seol SY, Chung JM, Choi HC, et al. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol. 1996;25:933–940. doi: 10.1093/ije/25.5.933. [DOI] [PubMed] [Google Scholar]
- 33.Part B: Biological Agents. Lyon, France: World Health Organization International Agency for Research on Cancer; 2009. Evaluation of Carcinogenic Risks to Humans: A review of human carcinogens. [Google Scholar]
- 34.Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. 2009;38:1497–1511. doi: 10.1093/ije/dyp280. [DOI] [PubMed] [Google Scholar]
- 35.Chen F, Li S, Castranova V. Overlapping signature genes between hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Eur J Gastroenterol Hepatol. 2009;21:1320–1321. doi: 10.1097/MEG.0b013e32832b2123. [DOI] [PubMed] [Google Scholar]
- 36.Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544–1556. doi: 10.1002/hep.22238. [DOI] [PubMed] [Google Scholar]
- 347.Woo HG, Lee JH, Yoon JH, Kim CY, Lee HS, Jang JJ, et al. Identification of a Cholangiocarcinoma-Like Gene Expression Trait in Hepatocellular Carcinoma. Cancer Res. 2010;70:3034–3041. doi: 10.1158/0008-5472.CAN-09-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donato F, Gelatti U, Tagger A, Favret M, Ribero ML, Callea F, et al. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer causes & control : CCC. 2001;12:959–964. doi: 10.1023/a:1013747228572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plot analysis to detect publication bias evaluating the association between intrahepatic cholangiocarcinoma and the following risk factors; (A) Hepatitis B, (B) Hepatitis C, (C) Alcohol use, (D) Smoking, (E) Diabetes Mellitus type II and (F) Obesity. Each spot represents a separate case-control study evaluating that risk factor for intrahepatic cholangiocarcinoma. Horizontal axis indicates the Log odds ratio, vertical axis indicates the inverse standard error.