Abstract
We previously reported an “athlete’s paradox” in which endurance-trained athletes, who possess a high oxidative capacity and enhanced insulin sensitivity, also have higher intramyocellular lipid (IMCL) content. The purpose of this study was to determine whether moderate exercise training would increase IMCL, oxidative capacity of muscle, and insulin sensitivity in previously sedentary overweight to obese, insulin-resistant, older subjects. Twenty-five older (66.4 ± 0.8 yr) obese (BMI = 30.3 ± 0.7 kg/m2) men (n = 9) and women (n = 16) completed a 16-wk moderate but progressive exercise training program. Body weight and fat mass modestly but significantly (P < 0.01) decreased. Insulin sensitivity, measured using the euglycemic hyperinsulinemic clamp, was increased (21%, P = 0.02), with modest improvements (7%, P = 0.04) in aerobic fitness (V̇O2peak). Histochemical analyses of IMCL (Oil Red O staining), oxidative capacity [succinate dehydrogenase activity (SDH)], glycogen content, capillary density, and fiber type were performed on skeletal muscle biopsies. Exercise training increased IMCL by 21%. In contrast, diacylglycerol and ceramide, measured by mass spectroscopy, were decreased (n = 13; −29% and −24%, respectively, P < 0.05) with exercise training. SDH (19%), glycogen content (15%), capillary density (7%), and the percentage of type I slow oxidative fibers (from 50.8 to 55.7%), all P ≤ 0.05, were increased after exercise. In summary, these results extend the athlete’s paradox by demonstrating that chronic exercise in overweight to obese older adults improves insulin sensitivity in conjunction with favorable alterations in lipid partitioning and an enhanced oxidative capacity within muscle. Therefore, several key deleterious effects of aging and/or obesity on the metabolic profile of skeletal muscle can be reversed with only moderate increases in physical activity.
Keywords: insulin sensitivity, aging, diacylglycerol, ceramide
Several studies have demonstrated strong associations between high intramyocellular lipid (IMCL) content and skeletal muscle insulin resistance in obesity (25, 44), aging (11, 42, 45, 52), and type 2 diabetes (T2DM) (31, 36, 58). Yet, despite these numerous observations, we (23) described an “athlete’s paradox” that has since been confirmed by others (54, 58) in which highly insulin-sensitive, endurance-trained athletes have IMCL content similar to that observed in insulin-resistant obese and T2DM subjects. We (46) later reported that the exercise training-induced increase in IMCL was not limited to young, lean, highly trained athletes; in a group of older (~67 yr), nonobese subjects, moderate aerobic exercise training increased IMCL content concomitant with improved oxidative capacity and overall fitness. Unfortunately, insulin sensitivity was not directly assessed in these subjects. Therefore, it is not clear whether exercise training enhances insulin sensitivity in conjunction with increases in IMCL in previously sedentary, overweight to obese, insulin-resistant subjects.
Several studies have suggested that aging and obesity are similarly associated with insulin resistance (7, 12, 19, 22, 35), poor oxidative capacity (35, 37, 59), and a reduced capacity for substrate delivery, i.e., capillary number (21). However, it is not clear to what extent these negative attributes are caused by physical inactivity in aging and obesity. In young sedentary subjects, exercise can clearly induce several changes in skeletal muscle that reflect an overall increase in metabolic flexibility, including increased insulin sensitivity (29), oxidative enzyme capacity (26, 39), and capillary density (40), as well as an increase in glycogen storage (27, 43). It is uncertain whether similar adaptations can occur in older, obese, insulin-resistant subjects. Some studies have suggested that exercise improves the oxidative capacity of muscle but does not improve insulin sensitivity older men and women (51). Others have reported improvements in insulin sensitivity (9, 15, 17) and enhanced oxidative capacity in older adults (51), although most of these studies have employed rigorous exercise regimes in nonobese, normal-weight subjects. We sought to test the hypothesis that moderate exercise would increase IMCL in conjunction with improvements in insulin sensitivity in older, overweight to obese, insulin-resistant adults and that these positive adaptations would be associated with an enhanced metabolic profile within skeletal muscle.
METHODS
Study Population
Men and women aged 60–75 yr were recruited though print advertisements in the Pittsburgh, PA, area. Eligibility for inclusion included volunteers who were sedentary by self-report (exercise ≤ 1 day/wk), weight stable (<3 kg weight loss or gain in the previous 6 mo), overweight to moderately obese [body mass index (BMI) 25.0–35.0 kg/m2], and nonsmokers. Volunteers who passed the initial phone screen were further evaluated at the Clinical Translational Research Center. Uncontrolled hypertension (blood pressure >150 mmHg systolic and >95 mmHg diastolic), anemia (Hct <34%), elevated liver enzymes (25% above normal), proteinuria, or hypothyroidism (sensitive TSH >8 mIU/l) were considered exclusion criteria, as well as chronic medications known to adversely affect glucose homeostasis. Furthermore, if EKG abnormalities (i.e., tachycardia, uncontrolled arrhythmias, unstable ischemia) were observed during rest or the graded exercise test, the subject was referred to their primary care physician for further evaluation.
Following the medical screen, volunteers completed a 2-h, 75-g oral glucose tolerance test (OGTT) to determine glucose tolerance status. Volunteers with impaired fasting glucose (≥ 100 mg/dl), impaired glucose tolerance (2-h OGTT glycemia >140 mg/dl, but <200 mg/dl), and normal glucose tolerance (2-h OGTT glycemia <140 mg/dl) were enrolled. All subjects gave written consent to the protocol, which was approved by the University of Pittsburgh’s Institutional Review Board.
Exercise Intervention
Subjects were progressed to 4–5 days/wk, 45 min/session (~180 min/wk), of moderate-intensity [75% of heart rate maximum, determined by heart rate or perceived exertion (46)] supervised exercise (mostly walking and stationary cycling) for 16 wk. After the intervention, subjects were instructed on methods to maintain regular physical activity and encouraged to meet with the registered dietitian for nutrition counseling.
Insulin Sensitivity
Rates of insulin-stimulated glucose disposal, considered the gold standard in vivo measurement of insulin resistance (13), were assessed before and after the intervention using a hyperinsulinemic euglycemic clamp (24, 48). Briefly, a continuous infusion of insulin (Humulin; Eli Lilly) was given at a rate of 40 mU · m2 · min−1 for 4 h and euglycemia (target = 90 mg/dl) maintained using an adjustable infusion of 20% dextrose. Previous studies have demonstrated a near-complete suppression of hepatic glucose output at this rate in subjects with this range of blood glucose values (55). Thus, the glucose infusion rate was assumed to represent a measurement of skeletal muscle insulin-stimulated glucose disposal (2, 5). The glucose clamp was performed 48 h following the last exercise session to avoid the potentially confounding effect of acute exercise on insulin sensitivity (14).
Body Composition and Peak Aerobic Capacity
Total body fat and lean mass were assessed using dual-energy X-ray absorptiometry (GE Lunar Prodigy and Encore 2005 software v9.30). As previously described (46), a peak aerobic capacity (V̇O2peak) test was employed to determine both changes in physical fitness and the appropriate exercise intensity. Briefly, subjects performed a standard graded exercise test on a cycle ergometer until volitional exhaustion or one of the established criteria for V̇O2peak was reached (1). Heart rate, blood pressure, and EKG were recorded prior to, during, and immediately following this test.
Tissue Analysis
Percutaneous biopsy samples were obtained in the fasted state on the mornings of the glucose clamp as described previously (18, 46). Following the excision, samples were cleared of any visible adipocytes with a standard dissecting microscope and blotted dry. Portions of the tissue sample were snap-frozen in liquid nitrogen for biochemical analysis of lipid metabolites. Briefly, liquid nitrogen-frozen samples (~30 mg) were homogenized in ice-cold buffer (250 mM sucrose, 25 mM KCl, 50 mM Tris, and 0.5 mM EDTA, pH 7.4). Total diacylglycerol and ceramide content were measured by high-performance liquid chromatography-tandem mass spectrometry as previously described in detail (4).
Samples used for histochemistry were mounted on a small piece of cork with mounting medium, placed into isopentane cooled with liquid nitrogen for 2–3 min, and then placed into liquid nitrogen. All samples were stored at −70°C until analysis. Histochemical analyses were performed on serial sections using methods previously used in our laboratory (30, 31, 46). Samples from pre- and postintervention were sectioned (10 μm) on a cryostat (Cryotome E; Shandon Scientific) at −20°C and placed on individual precleaned glass slides. Slides representing four to five subjects were analyzed together to minimize staining bias. Each analysis included data from 150–300 fibers, and intra-assay variability was <5%. Images were visualized using a Leica microscope (Leica DM 4000B; Leica Microsystems), digitally captured (Retiga 2000R camera; Q Imaging), and analyzed using specialized software (Northern Eclipse, v6.0; Empix Imaging). For analysis of intensity of staining (see Intramyocellular lipid content, Mitochondria activity, and Glycogen content below), four to five images from both pre- and postintervention sections were captured in 16-bit grayscale and averaged.
Intramyocellular lipid content
Triglyceride content was determined using Oil Red O staining as described previously (30).
Mitochondria activity
Succinate dehydrogenase activity was measured using histochemical methods as described previously (46).
Glycogen content
Skeletal muscle glycogen content was assessed using a standard Shiffs reagent protocol (31).
Capillary density
Capillary density was determined using modified methods of Frisbee (20). Briefly, samples were allowed to air dry for 15 min and then fixed for 1 h in 0.25% formaldehyde. Sections were incubated for 2 h with lectin (25 μg/ml) and rinsed, and coverslips were applied. Capillaries were visualized using a tetramethylrhodamine isothiocyanate (TRITC) excitation filter. Capillary density was calculated as the total number of capillaries per total muscle area.
Fiber type analysis
The determination of type I slow oxidative and type II fast glycolytic skeletal muscle fiber types was determined using immnuohistochenistry. Briefly, antibodies specific for type I and type IIa fibers (Santa Cruz Biotechnology, Santa Cruz, CA) were applied using the manufacturer’s recommendations. Signals for specific fibers were recorded using a flourescein isothiocyanate excitation filter (type I) and a TRITC excitation filter (type IIa). Type IIx fibers were assumed to be those that did not fluoresce with either filter. Approximately 100–300 total fibers were manually counted, and relative fiber type percentage was determined.
Statistical Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS for Mac, v11). To address the effects of intervention on insulin sensitivity and skeletal muscle parameters, a paired t-test was applied to all data. Pearson’s correlation analysis addressed the relation between changes in insulin sensitivity and tissue measures. The unequal numbers of males and females precluded a sex difference analysis owing to a lack of statistical power. Statistical significance was assumed a priori at P < 0.05.
RESULTS
Study Subjects, Insulin Sensitivity, and Aerobic Fitness
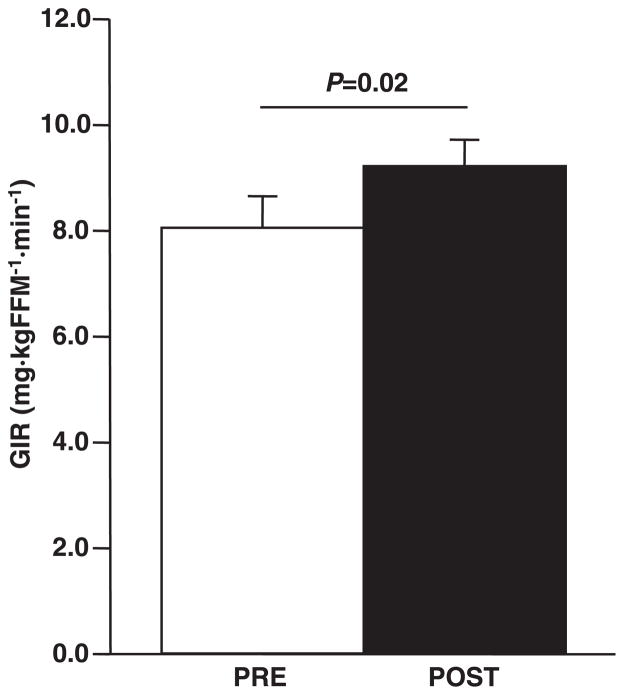
Thirty previously sedentary overweight to obese (BMI 30.3 ± 0.7 kg/m2) older (66.4 ± 0.8 yr) men (n = 10) and women (n = 20) were enrolled. Five subjects did not finish the intervention (dropout rate 16%), four due to time commitment conflicts and one to a newly diagnosed oncological disease. Only the 25 participants (men, n = 9; women, n = 16) that completed the study were included in the analysis. The subject compliance rate was 87.1 ± 19.4% (means ± SD, mean weekly sessions completed/recommended weekly sessions) for the exercise program, as evidenced by achieving the recommended number of sessions per week (3.5 ± 0.8, means ± SD). Moreover, additional data were collected to assess appropriate training intensities. Subjects expended an average of 833.3 ± 78.6 kcal/wk and 233.7 ± 18.8 kcal/session. The exercise intensity was, on average, 70.4 ± 2.3% of V̇O2peak. Body weight and fat mass were modestly, but significantly (P < 0.01), decreased (Table 1). Fat-free mass was unchanged by the aerobic training protocol. There was a fairly robust improvement (21%, P = 0.02) in insulin sensitivity with intervention (Fig. 1). When adjusted for changes in body weight and composition, the improvements in insulin sensitivity remained. Moderate aerobic training induced a modest improvement (7%, P = 0.04) in aerobic fitness (Table 1).
Table 1.
Subject characteristics and response to intervention
| Pre | Post | |
|---|---|---|
| n (Male/female) | 25 (9/16) | |
| Body weight, kg | 83.9±2.0 | 82.6±1.9* |
| BMI, kg/m2 | 30.3±0.7 | 29.9±0.7* |
| Fat mass, kg | 35.5±1.5 | 33.8±1.5* |
| FFM, kg | 45.2±1.7 | 45.7±1.7 |
| V̇O2peak, ml · kg FFM−1 · min−1 | 34.4±6.7 | 36.7±1.4* |
Values are means ± SE. Pre, preintervention; Post, postintervention; BMI, body mass index; FFM, fat-free mass; V̇O2peak, peak aerobic capacity.
P < 0.05, significant Pre vs. Post differences.
Fig. 1.
Effect of exercise on insulin sensitivity. Insulin sensitivity was determined using a hyperinsulinemic euglycemic clamp prior to and subsequent to exercise training, as described in METHODS; n = 25. Glucose infusion rate (GIR) is expressed relative to fat-free mass (FFM). Statistical significance is indicated. Data are means ± SE. PRE, preintervention; POST, postintervention.
Skeletal Muscle Tissue Analysis
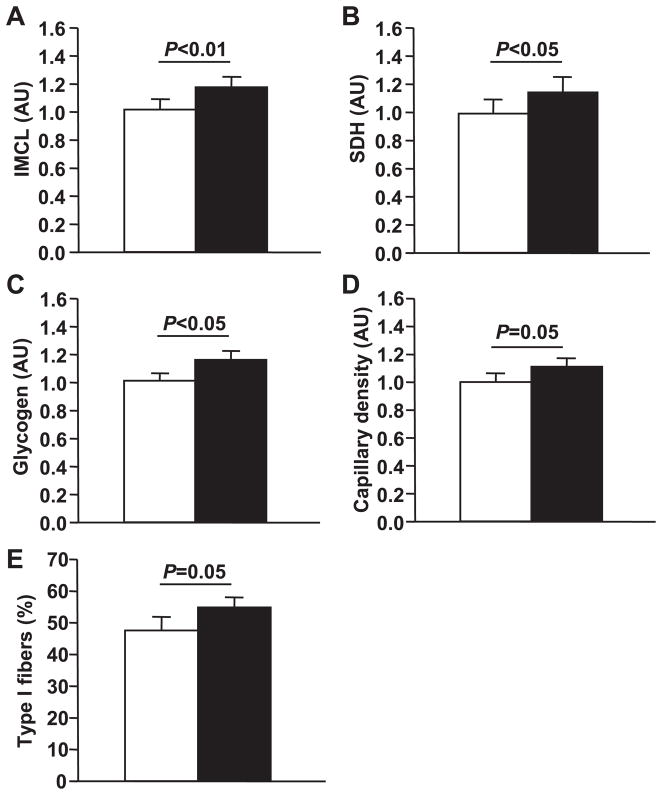
At baseline, no marker of skeletal muscle substrate availability or utilization was associated with insulin sensitivity. Additionally, baseline total body weight, BMI, and fat mass were unrelated to insulin sensitivity. Figure 2 demonstrates the changes in skeletal muscle substrate storage and capacity for oxidation following exercise training. Moderate aerobic exercise training significantly increased total IMCL content (21%, P < 0.01), as measured by Oil Red O staining. Oxidative capacity, as measured by succinate dehydrogenase activity, was significantly increased (22%, P < 0.05) with exercise training. Glycogen was significantly increased (16%, P < 0.05). Capillary density increased by 7%, and the percentage of type I slow oxidative fibers increased (11%, both P = 0.05). However, none of the changes observed in IMCL or glycogen content, i.e., skeletal muscle substrate availability, or in capillary density or oxidative capacity predicted the improvements in insulin sensitivity. Moreover, none of the adaptations in skeletal muscle metabolism predicted the improvements in aerobic fitness.
Fig. 2.
Effects of exercise on substrate availability and capacity for oxidation. Histochemical analyses were performed on skeletal muscle biopsy samples obtained as described in METHODS; n = 25. A: intramyocellular lipid (IMCL) content was measured by Oil Red O staining. B: oxidative capacity was measured by succinate dehydrogenase (SDH) staining. C: glycogen content was measured by a Schiff’s reagent protocol. D: capillary density. E: percentage of type I slow oxidative fibers. Statistical significance is indicated. Data are means ± SE. AU, arbitrary units.
Skeletal muscle diacylglycerol and ceramide content were measured in a subset (n = 13) of men (n = 4) and women (n = 9) who completed the protocol due to limited sample size of the biopsies in some subjects. Their baseline characteristics and responses to intervention were similar to those for the large subject population (data not shown). Both total diacylglycerol (P = 0.03) and ceramide (P = 0.01) were decreased with exercise training (Fig. 3). The decrease in ceramide content was associated with the improvement in insulin sensitivity (r2 = 0.46, P < 0.01). The decrease in diacylglycerol, however, was not associated with improvements in insulin sensitivity. In addition, changes in neither diacylglycerol nor ceramide were related to alterations in skeletal muscle substrate storage or capacity for oxidation.
Fig. 3.
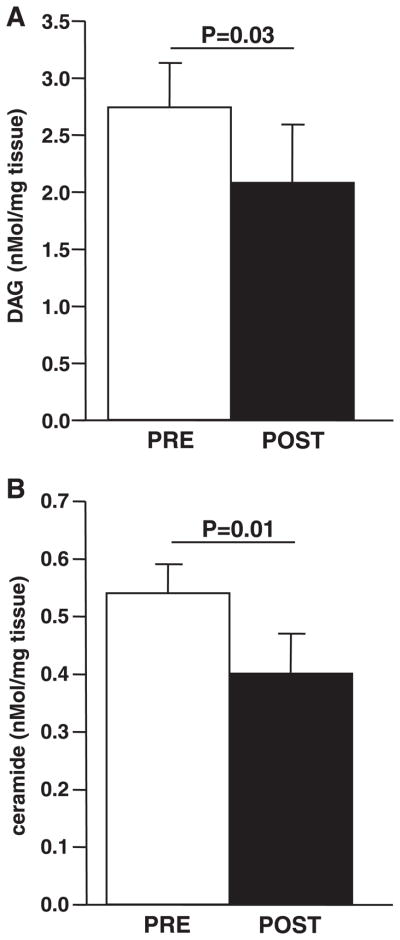
Effects of exercise on diacylglycerol (DAG) and ceramide content. DAG (A) and ceramide (B) content were assessed from skeletal muscle biopsy samples as described in METHODS; n = 13. Statistical significance is indicated. Data are means ± SE.
DISCUSSION
Increased skeletal muscle IMCL lipid content has been associated with insulin resistance in human obesity (25, 44), aging (11, 42, 52), and T2DM (31, 36, 58). However, we (23) have previously demonstrated the athlete’s paradox in which endurance-trained athletes have IMCL content similar to that observed in insulin-resistant subjects. Moreover, there is evidence that physical inactivity has adverse effects on skeletal muscle metabolism, manifesting in poor oxidative capacity (32, 38), lower glycogen storage (10), and decreased capillary density (61). Therefore, we used a progressive, moderate-intensity, aerobic training protocol to test the hypothesis that exercise training would induce positive adaptations in skeletal muscle substrate availability and utilization that would include increased IMCL content and enhanced insulin action.
The primary finding in this study was that just moderate increases in physical activity were sufficient to increase intramuscular triglycerides and decrease both diacylglycerol and ceramide content. These apparently favorable alterations in lipid partitioning within skeletal muscle were observed in conjunction with improved insulin sensitivity in these previously sedentary, insulin-resistant subjects. In addition, this moderate exercise program induced other positive adaptations in skeletal muscle, including enhanced glycogen storage, oxidative capacity, and capillary density in previously sedentary older adults. Therefore, many deleterious characteristics of skeletal muscle attributed to physical inactivity observed in aging and/or obesity can be largely restored with moderate exercise.
Insulin resistance is thought to be a key unifying feature of a myriad of conditions, including obesity and cardiovascular disease, and has been demonstrated to precede the development of type 2 diabetes mellitus. Although exercise training clearly improves insulin sensitivity in younger (6) and middle-aged adults (16), the same may not be true for older populations (51). Here we demonstrate that, in a group of primarily obese, previously sedentary older adults, moderate aerobic training induced a robust increase in insulin sensitivity irrespective of baseline metabolic status. It is important to note that, although our subjects lost a slight amount of fat mass, these decreases are not nearly as robust as those observed with diet-induced weight loss (24). Moreover, when adjusted for weight loss, improvements in insulin sensitivity were still present. Therefore, the improvement in insulin sensitivity was due primarily to the increase in physical activity.
Physical inactivity is a key factor in the development of obesity (37), osteoporosis (3), and sarcopenia (28) observed in aging. Thus, the addition of regular exercise is often prescribed to ameliorate these conditions and other chronic diseases. Yet despite our understanding of the overwhelming benefits of exercise, controversy still remains regarding the appropriate quantity of exercise required for metabolic improvement and in cardiorespiratory fitness. In agreement with our previous observation in older adults (46), relatively moderate-intensity aerobic training modestly improved fitness in this population. Moreover, these improvements are similar to those observed in younger adults with a similar exercise program (54).
The role of muscle triglycerides in insulin resistance is currently a topic of great interest. Higher amounts of IMCL as muscle triglycerides, although clearly associated with insulin resistance observed in obesity and type 2 diabetes, are also paradoxically observed in endurance-trained athletes. This study is the first to directly extend a paradoxical increase in both IMCL and insulin sensitivity to obese older adults with insulin resistance. In accord with these observations, we have previously observed an increase (~12%) in IMCL following exercise training in normal, glucose-tolerant, nonobese older adults (46). Similar results have been observed in lean, young adults (54) and following acute exercise (50). Importantly, we did not observe any relation between IMCL content and insulin sensitivity at baseline in this cohort, possibly owing to the relatively homogeneous population investigated (23). Thus, IMCL as triglycerides per se may not confer insulin resistance, but rather, the increases in IMCL content provide substrate for energy metabolism in the exercise-trained state (47, 57). These findings are supported by the mounting evidence, mostly in animal and cell culture models, that other lipid metabolites, such as diacylglycerol and/or ceramide, may be more directly linked to the development of insulin resistance (53, 56, 60).
Another important finding in our study was that both diacylglycerol and ceramide content are decreased with exercise training in conjunction with improved skeletal muscle insulin sensitivity. These results are in accordance with those of Bruce et al. (6), who found that exercise decreased these lipid metabolites along with enhanced glucose tolerance. However, although insulin sensitivity was not directly measured in their study, markers of enhanced fatty acid oxidation were observed. Thus, the decrease in lipid metabolite concentration following exercise may be related to an elevated oxidative capacity of muscle that may relate to improvements in insulin sensitivity. However, to date there is no direct evidence to support this mechanism. Although the baseline values of lipid metabolites in the current study were unrelated to insulin sensitivity, the alterations in skeletal muscle ceramide content did predict improvements in insulin-stimulated glucose uptake. However, these correlation data should be interpreted with caution given the homogeneous population and small sample size.
It has been reported (35) that obesity results in decreased postabsorptive rates of fatty acid metabolism despite similar rates of fatty acid uptake compared with lean controls. Moreover, rates of fatty acid oxidation are decreased with aging (51). Therefore, both aging and obesity contribute to a profile of metabolic inflexibility resulting in a greater reliance on skeletal muscle glycogen content for energy production. An important finding of the current study, in agreement with a previous report (33), was that moderate exercise training increased glycogen content, probably due in part to an increased insulin sensitivity.
Mitochondrial defects have been observed in both obesity (32) and aging (34, 59) concomitant with impaired rates of substrate oxidation during rest and exercise. However, aerobic exercise increases the capacity of muscle for oxidation in nonobese young (8, 54) and older (46) individuals. Here we demonstrate that the markers of oxidative capacity of muscle from overweight to obese, mildly insulin-resistant older adults are increased following exercise training. Our previous finding of increased mitochondrial electron transport chain activity following exercise training in leaner, older adults (41) supports these data. Moreover, the percentage of oxidative fibers was increased, in agreement with previous reports (46, 49). It is still not clear, however, whether or not changes in fiber type or enhanced oxidative capacity cause alterations in muscle lipid levels or improved insulin sensitivity. Further studies, most likely to be conducted using animal models, should be performed to determine whether these contrasting changes in muscle triglyceride vs. diacylglycerol and ceramide are consistent in high-oxidative and low-oxidative muscles. Taken together, the current data reflect a positive adaptation for increased skeletal muscle oxidative capacity with just moderate-intensity exercise.
The capacity for substrate or nutrient delivery was also increased, as evidenced by an increase in capillary density following exercise training. Decreases in microvessel density have been reported in human (21) and animal (20) models of insulin resistance. This deleterious effect of nutrient oversupply and physical inactivity may indeed exacerbate the defects in oxidative capacity by limiting the flux of substrates into and out of the cell. Thus, increasing capillary density per se may partially ameliorate perturbations in glucose and fatty acid homeostasis.
In summary, enhanced insulin action due to moderate increases in physical activity in older, overweight to obese, insulin-resistant adults is accompanied by a repartitioning of skeletal muscle lipid content demonstrated by increases in intramyocellular triglyceride but decreases in both diacylglycerol and ceramide. These exercise-induced changes were found in the context of an overall improvement in metabolic profile evidenced by increased oxidative enzyme activity, a shift toward more oxidative fiber type composition, and enhanced substrate delivery and storage capacity of muscle. This study suggests that physical inactivity plays a primary role in the development of insulin resistance in obesity and aging, perhaps through altered lipid partitioning within skeletal muscle and aberrant skeletal muscle metabolic profiles. Moreover, these data provide further support for the recommendation of lifestyle interventions, specifically aerobic exercise, for the treatment and prevention of insulin resistance.
Acknowledgments
We thank the volunteers for their participation in this study. We appreciate the time, dedication, and effort of the intervention staff (Krista Clark, Chuck Fiedler, George Grove, and Steve Anthony) and Clinical Translational Research Center nurses.
GRANTS
This work was funded by American Diabetes Association Clinical Research Award and R01-AG-20128 (B. H. Goodpaster) and the Obesity and Nutrition Research (1P30DK46204) and Clinical Translational Research (5 M01RR00056) Centers.
References
- 1.American College of Sports Medicine. . ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 2.Bachmann OP, Dahl DB, Brechtel K, Machann J, Haap M, Maier T, Loviscach M, Stumvoll M, Claussen CD, Schick F, Haring HU, Jacob S. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes. 2001;50:2579–2584. doi: 10.2337/diabetes.50.11.2579. [DOI] [PubMed] [Google Scholar]
- 3.Bakhireva LN, Barrett-Connor E, Kritz-Silverstein D, Morton DJ. Modifiable predictors of bone loss in older men: a prospective study. Am J Prev Med. 2004;26:436–442. doi: 10.1016/j.amepre.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612–1617. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- 6.Bruce CR, Thrush AB, Mertz VA, Bezaire V, Chabowski A, Heigen-hauser GJ, Dyck DJ. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab. 2006;291:E99–E107. doi: 10.1152/ajpendo.00587.2005. [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Bergman RN, Pacini G, Porte D., Jr Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta-cell function. J Clin Endocrinol Metab. 1985;60:13–20. doi: 10.1210/jcem-60-1-13. [DOI] [PubMed] [Google Scholar]
- 8.Coggan AR, Habash DL, Mendenhall LA, Swanson SC, Kien CL. Isotopic estimation of CO2 production during exercise before and after endurance training. J Appl Physiol. 1993;75:70–75. doi: 10.1152/jappl.1993.75.1.70. [DOI] [PubMed] [Google Scholar]
- 9.Coker RH, Hays NP, Williams RH, Brown AD, Freeling SA, Kortebein PM, Sullivan DH, Starling RD, Evans WJ. Exercise-induced changes in insulin action and glycogen metabolism in elderly adults. Med Sci Sports Exerc. 2006;38:433–438. doi: 10.1249/01.mss.0000191417.48710.11. [DOI] [PubMed] [Google Scholar]
- 10.Costill DL, Fink WJ, Hargreaves M, King DS, Thomas R, Fielding R. Metabolic characteristics of skeletal muscle during detraining from competitive swimming. Med Sci Sports Exerc. 1985;17:339–343. [PubMed] [Google Scholar]
- 11.Cree MG, Newcomer BR, Katsanos CS, Sheffield-Moore M, Chinkes D, Aarsland A, Urban R, Wolfe RR. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89:3864–3871. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- 12.Davis SN, Monti L, Piatti PM, Moller N, Ng L, Coppack S, May M, Brown MD, Orskov H, Alberti KG. Estimates of insulin action in normal, obese and NIDDM man: comparison of insulin and glucose infusion test, CIGMA, minimal model and glucose clamp techniques. Diabetes Res. 1993;23:1–18. [PubMed] [Google Scholar]
- 13.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 14.Dela F, Mikines KJ, Larsen JJ, Galbo H. Glucose clearance in aged trained skeletal muscle during maximal insulin with superimposed exercise. J Appl Physiol. 1999;87:2059–2067. doi: 10.1152/jappl.1999.87.6.2059. [DOI] [PubMed] [Google Scholar]
- 15.DiPietro L, Dziura J, Yeckel CW, Neufer PD. Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J Appl Physiol. 2006;100:142–149. doi: 10.1152/japplphysiol.00474.2005. [DOI] [PubMed] [Google Scholar]
- 16.Duncan GE, Perri MG, Theriaque DW, Hutson AD, Eckel RH, Stacpoole PW. Exercise training, without weight loss, increases insulin sensitivity and postheparin plasma lipase activity in previously sedentary adults. Diabetes Care. 2003;26:557–562. doi: 10.2337/diacare.26.3.557. [DOI] [PubMed] [Google Scholar]
- 17.Evans EM, Racette SB, Peterson LR, Villareal DT, Greiwe JS, Holloszy JO. Aerobic power and insulin action improve in response to endurance exercise training in healthy 77–87 yr olds. J Appl Physiol. 2005;98:40–45. doi: 10.1152/japplphysiol.00928.2004. [DOI] [PubMed] [Google Scholar]
- 18.Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc. 1982;14:101–102. [PubMed] [Google Scholar]
- 19.Ferrannini E, Vichi S, Beck-Nielsen H, Laakso M, Paolisso G, Smith U. Insulin action and age. European Group for the Study of Insulin Resistance (EGIR) Diabetes. 1996;45:947–953. doi: 10.2337/diab.45.7.947. [DOI] [PubMed] [Google Scholar]
- 20.Frisbee JC. Hypertension-independent microvascular rarefaction in the obese Zucker rat model of the metabolic syndrome. Microcirculation. 2005;12:383–392. doi: 10.1080/10739680590960241. [DOI] [PubMed] [Google Scholar]
- 21.Gavin TP, Stallings HW, 3rd, Zwetsloot KA, Westerkamp LM, Ryan NA, Moore RA, Pofahl WE, Hickner RC. Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J Appl Physiol. 2005;98:315–321. doi: 10.1152/japplphysiol.00353.2004. [DOI] [PubMed] [Google Scholar]
- 22.Golay A, Munger R, Assimacopoulos-Jeannet F, Bobbioni-Harsch E, Habicht F, Felber JP. Progressive defect of insulin action on glycogen synthase in obesity and diabetes. Metabolism. 2002;51:549–553. doi: 10.1053/meta.2002.31972. [DOI] [PubMed] [Google Scholar]
- 23.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 24.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 25.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49:467–472. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 26.Green HJ, Jones S, Ball-Burnett M, Farrance B, Ranney D. Adaptations in muscle metabolism to prolonged voluntary exercise and training. J Appl Physiol. 1995;78:138–145. doi: 10.1152/jappl.1995.78.1.138. [DOI] [PubMed] [Google Scholar]
- 27.Green HJ, Jones S, Ball-Burnett ME, Smith D, Livesey J, Farrance BW. Early muscular and metabolic adaptations to prolonged exercise training in humans. J Appl Physiol. 1991;70:2032–2038. doi: 10.1152/jappl.1991.70.5.2032. [DOI] [PubMed] [Google Scholar]
- 28.Greenlund LJ, Nair KS. Sarcopenia— consequences, mechanisms, and potential therapies. Mech Ageing Dev. 2003;124:287–299. doi: 10.1016/s0047-6374(02)00196-3. [DOI] [PubMed] [Google Scholar]
- 29.Hasbum B, Real JT, Sanchez C, Priego MA, Diaz J, Viguer A, Basanta M, Martinez-Valls J, Marin J, Carmena R, Ascaso JF. Effects of a controlled program of moderate physical exercise on insulin sensitivity in nonobese, nondiabetic subjects. Clin J Sport Med. 2006;16:46–50. doi: 10.1097/01.jsm.0000180021.67759.16. [DOI] [PubMed] [Google Scholar]
- 30.He J, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on muscle lipid content and droplet size. Obes Res. 2004;12:761–769. doi: 10.1038/oby.2004.92. [DOI] [PubMed] [Google Scholar]
- 31.He J, Kelley DE. Muscle glycogen content in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2004;287:E1002–E1007. doi: 10.1152/ajpendo.00015.2004. [DOI] [PubMed] [Google Scholar]
- 32.Heilbronn LK, Gan SK, Turner N, Campbell LV, Chisholm DJ. Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects. J Clin Endocrinol Metab. 2007;92:1467–1473. doi: 10.1210/jc.2006-2210. [DOI] [PubMed] [Google Scholar]
- 33.Hughes VA, Fiatarone MA, Fielding RA, Kahn BB, Ferrara CM, Shepherd P, Fisher EC, Wolfe RR, Elahi D, Evans WJ. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 1993;264:E855–E862. doi: 10.1152/ajpendo.1993.264.6.E855. [DOI] [PubMed] [Google Scholar]
- 34.Karakelides H, Nair KS. Sarcopenia of aging and its metabolic impact. Curr Top Dev Biol. 2005;68:123–148. doi: 10.1016/S0070-2153(05)68005-2. [DOI] [PubMed] [Google Scholar]
- 35.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 36.Kelley DE, Goodpaster BH, Storlien L. Muscle triglyceride and insulin resistance. Annu Rev Nutr. 2002;22:325–346. doi: 10.1146/annurev.nutr.22.010402.102912. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy RL, Chokkalingham K, Srinivasan R. Obesity in the elderly: who should we be treating, and why, and how? Curr Opin Clin Nutr Metab Care. 2004;7:3–9. doi: 10.1097/00075197-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039–E1044. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- 39.Linossier MT, Dormois D, Perier C, Frey J, Geyssant A, Denis C. Enzyme adaptations of human skeletal muscle during bicycle short-sprint training and detraining. Acta Physiol Scand. 1997;161:439–445. doi: 10.1046/j.1365-201X.1997.00244.x. [DOI] [PubMed] [Google Scholar]
- 40.McCall GE, Byrnes WC, Dickinson A, Pattany PM, Fleck SJ. Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol. 1996;81:2004–2012. doi: 10.1152/jappl.1996.81.5.2004. [DOI] [PubMed] [Google Scholar]
- 41.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61:534–540. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakagawa Y, Hattori M, Harada K, Shirase R, Bando M, Okano G. Age-related changes in intramyocellular lipid in humans by in vivo H-MR spectroscopy. Gerontology. 2007;53:218–223. doi: 10.1159/000100869. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen JN, Wojtaszewski JF. Regulation of glycogen synthase activity and phosphorylation by exercise. Proc Nutr Soc. 2004;63:233–237. doi: 10.1079/PNS2004348. [DOI] [PubMed] [Google Scholar]
- 44.Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 45.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pruchnic R, Katsiaras A, He J, Kelley DE, Winters C, Goodpaster BH. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am J Physiol Endocrinol Metab. 2004;287:E857–E862. doi: 10.1152/ajpendo.00459.2003. [DOI] [PubMed] [Google Scholar]
- 47.Rico-Sanz J, Moosavi M, Thomas EL, McCarthy J, Coutts GA, Saeed N, Bell JD. In vivo evaluation of the effects of continuous exercise on skeletal muscle triglycerides in trained humans. Lipids. 2000;35:1313–1318. doi: 10.1007/s11745-000-0647-2. [DOI] [PubMed] [Google Scholar]
- 48.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 49.Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, Deriaz O. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes. 2003;52:2874–2881. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- 50.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest. 2007;117:1690–1698. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52:1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- 52.St-Onge MP. Relationship between body composition changes and changes in physical function and metabolic risk factors in aging. Curr Opin Clin Nutr Metab Care. 2005;8:523–528. [PubMed] [Google Scholar]
- 53.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1271–R1278. doi: 10.1152/ajpregu.00472.2006. [DOI] [PubMed] [Google Scholar]
- 55.Thomaseth K, Pavan A, Berria R, Glass L, Defronzo R, Gastaldelli A. Model-based assessment of insulin sensitivity of glucose disposal and endogenous glucose production from double-tracer oral glucose tolerance test. Comput Methods Programs Biomed. 2007;89:132–140. doi: 10.1016/j.cmpb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Turinsky J, O’Sullivan DM, Bayly BP. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem. 1990;265:16880–16885. [PubMed] [Google Scholar]
- 57.van Loon LJ. Use of intramuscular triacylglycerol as a substrate source during exercise in humans. J Appl Physiol. 2004;97:1170–1187. doi: 10.1152/japplphysiol.00368.2004. [DOI] [PubMed] [Google Scholar]
- 58.van Loon LJ, Koopman R, Manders R, van der Weegen W, van Kranenburg GP, Keizer HA. Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. Am J Physiol Endocrinol Metab. 2004;287:E558–E565. doi: 10.1152/ajpendo.00464.2003. [DOI] [PubMed] [Google Scholar]
- 59.Van Remmen H, Richardson A. Oxidative damage to mitochondria and aging. Exp Gerontol. 2001;36:957–968. doi: 10.1016/s0531-5565(01)00093-6. [DOI] [PubMed] [Google Scholar]
- 60.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 61.Zoladz JA, Semik D, Zawadowska B, Majerczak J, Karasinski J, Kolodziejski L, Duda K, Kilarski WM. Capillary density and capillary-to-fibre ratio in vastus lateralis muscle of untrained and trained men. Folia Histochem Cytobiol. 2005;43:11–17. [PubMed] [Google Scholar]