Abstract
Carotene-15,15'-monooxygenase (CMO-I) cleaves β-carotene to form vitamin A while carotene-9’,10’-monooxygenase (CMO-II) preferentially cleaves non-provitamin A carotenoids. Recent reports indicate that beta-carotene metabolites regulate dietary lipid uptake while lycopene regulates peroxisome-proliferated activator receptor (PPAR) expression. To determine the physiologic consequences of carotenoids and their interactions with CMO-I and CMO-II, we characterized mammalian carotenoid metabolism, metabolic perturbations and lipid metabolism in female CMO-I−/− and CMO-II−/− mice fed lycopene or tomato-containing diets for 30 days. We hypothesized that there would be significant interactions between diet and genotype on carotenoid accumulation and lipid parameters. CMO-I−/− mice had higher levels of leptin, insulin and hepatic lipidosis, but lower levels of serum cholesterol. CMO-II−/− mice had increased tissue lycopene and phytofluene accumulation, reduced IGF-1 levels and cholesterol levels, but elevated liver lipids and cholesterol compared with WT mice. The diets did not modulate these genotypic perturbations, but lycopene and tomato powder did significantly decrease serum insulin-like growth factor-I. Tomato powder also reduced hepatic PPAR expression, independent of genotype. These data point to the pleiotropic actions of CMO-I and CMO-II supporting a strong role of these proteins in regulating tissue carotenoid accumulation and the lipid metabolic phenotype, as well as tomato carotenoid-independent regulation of lipid metabolism.
Keywords: Lycopene, phytoene, tomato, lipids, cholesterol, CMO-I, CMO-II, mice
1. Introduction
The most predominant carotenoids found in human serum are β-carotene, α-carotene, β-cryptoxanthin, lycopene, lutein and zeaxanthin [1]. Carotenoids are derived from a C40 tetraterpene structure and are commonly produced in the photosynthetic and reproductive tissues of plants and algae. They can be grouped either as hydrocarbon carotenes or their oxygenated derivatives, the xanthophylls [2]. Consumption of provitamin A carotenoids (β-carotene, α-carotene, and β-cryptoxanthin) helps to alleviate vitamin A deficiency symptoms which can progress to blindness, impaired immune function, increased mortality and numerous other negative health outcomes [2,3,4]. Provitamin A and non-provitamin A carotenoids (e.g. lycopene, lutein and zeaxanthin) may also have health promoting activities and have been shown to influence the risk and progression of cancers of the lung, breast, prostate, cervix and ovary [3].
There are two primary mammalian carotenoid cleavage enzymes. Carotene-15,15’-monooxygenase (CMO-I) was identified as a cytoplasmic enzyme and is expressed in many tissues including, but not limited to the liver, kidney, prostate, testis, ovary, colon and skeletal muscle [5]. Carotene-9’,10’-monooxygenase (CMO-II) is likely responsible for the asymmetric cleavage of carotenoids (including β-carotene and lycopene [6,7]) whereas CMO-I cleaves carotenoids at the central carbon double bond and generates two molecules of retinal from one molecule of β-carotene [7]. The optimal pH for CMO-II activity differs from CMO-I (8.5 vs. 7.7) and CMO-II is often, though not exclusively, expressed in tissues lacking CMO-I (spleen, brain, lung and heart [5,7]), which suggests that these enzymes perform different functions [6]. Moreover, CMO-II was identified as a mitochondrial enzyme and is thought to protect against oxidative damage caused by excess carotenoid accumulation [8]. The carotenoid metabolites produced by CMO-I and CMO-II appear to be biologically active and important for optimal nutrition and human health [9–12].
A crosstalk between lipid homeostatic and carotenoid metabolic pathways has recently emerged. Previously, it was shown that ablation of CMO-I induced the expression of hepatic fatty acid oxidase (ACOX1) which catalyzes the first step of beta-oxidation [13]. Loss of ACOX1 has been shown to induce the expression of peroxisomal proliferator-activated receptors (PPARs) [14]. PPARs are nuclear receptors that heterodimerize with other nuclear receptors to alter gene transcription, notably that of a number of lipid metabolic genes [15]. Additionally, a PPAR response element (PPRE) exists in the promoter region of the CMO-I gene, suggesting that its activity is likely regulated by PPARs [16]. Lycopene feeding in rats decreases mRNA expression of both CMO-I and PPARγ [17], the latter of which is known to heterodimerize with retinoid-X-receptor (RXR). The primary ligand for RXRs (9-cis-retinoic acid) is a metabolite of retinal, which is itself the primary cleavage product of CMO-I, suggesting that CMO-I status may affect transport, metabolism and adipocyte uptake of fatty acids [18]. Additionally, CMO-I is necessary for the regulation of scavenger receptor B type 1 (SR-B1) by β-carotene-derived retinoic acid - consequently, a lack of CMO-I increases intestinal absorption of cholesterol and other lipids [19]. Not only are the central cleavage products of carotenoids bioactive, but also several eccentric cleavage products of β-carotene were recently shown to bind and antagonize the retinoic acid receptors (α,β,γ) [20,21]. Lastly, the expression of CMO-I or serum β-carotene levels are inversely associated with diabetes and metabolic syndrome [22,23]. Based on these reports, it is necessary to determine if lycopene, along with other commonly consumed tomato carotenoids, has physiologically relevant interactions with CMO-I and CMO-II, and whether these interactions impact lipid metabolism, homeostasis and signaling pathways.
We hypothesized that diet (tomato powder and lycopene), genotype (CMO-I−/− and CMO-II−/− mice), and diet by genotype interactions would alter tissue carotenoid concentrations, tissue lipids and metabolism, and circulating peptide hormones and adipokines. Therefore, we evaluated tomato carotenoid (lycopene, phytoene and phytofluene) accumulation and assessed the impact of carotenoid cleavage enzyme expression (CMO-I and CMO-II) and the presumable production of carotenoid metabolites on serum and tissue lipids and lipid metabolism.
2. Methods and Materials
2.1 Study Design
All animal handling procedures were approved by the University of Illinois Institutional Animal Care and Use Committee. The generation of CMO-I−/− (B6;129S6-Bcmo1tm1Dnp) and CMO-II−/− (B6;129S6-Bcdo2tm1Dnp) mice has been previously discussed [8,13]. Mice in this study were bred at the University of Illinois and genomic DNA was used to confirm genotype, as described previously [24]. Wild-type (WT) C57BL/6 × 129/SvJ F1 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and were housed at the University of Illinois and fed rodent chow (Harlan Teklad 8604 unpurified diet) until 7 months of age. Seven month-old female WT, CMO-I−/− and CMO-II−/− mice were acclimated to a powdered AIN-93G basal diet for one week prior to randomization onto one of four experimental diets (n = 10 / group): AIN-93G control, 10% tomato powder, placebo beadlet-supplemented control, or lycopene beadlet-supplemented. All diets were provided ad libitum for 30 days. The compositions of the diets used in this work are described in Table 1, similar to a previous study [24]. The vitamin A level in the diets was slightly reduced to 1,500 IU/kg diet to ensure adequate carotenoid absorption without resulting in vitamin A deficiency [19]. As determined by high-performance liquid chromatography (HPLC) analysis, the tomato powder diet provided 181 ± 42 nmol lycopene, 10.1 nmol phytoene, 2.6 nmol phytofluene and 0.8 nmol β-carotene per gram diet from drum dried tomato powder (FutureCeuticals, Momence, IL) and the lycopene diet provided 152 ± 7 nmol lycopene/g diet from water soluble beadlets (DSM, Basel, Switzerland). Fresh diet was provided every 48 hours and new diets were made monthly and stored in the dark at 4 °C. Mice were weighed every other day and at the conclusion of the study mice were fasted 4 hours prior to euthanization by CO2 asphyxiation and cardiac exsanguination. Tissues were either snap frozen in liquid nitrogen and stored at −80 °C or fixed in formalin prior to paraffin embedding.
Table 1.
Diet Composition
| Ingredient | AIN | Tomato1 | Placebo2 | Lycopene3 |
|---|---|---|---|---|
|
g/kg diet |
||||
| Cornstarch | 398 | 331 | 398 | 398 |
| Casein | 200 | 188 | 200 | 200 |
| Maltodextrin | 102 | 132 | 102 | 102 |
| Sucrose | 100 | 100 | 100 | 100 |
| Nonnutritive cellulose | 50 | 34 | 50 | 50 |
| Mineral mix4 | 35 | 35 | 35 | 35 |
| Vitamin mix5 | 10 | 10 | 10 | 10 |
| L-Cystine | 3 | 3 | 3 | 3 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 |
| Soybean oil | 100 | 65 | 100 | 100 |
| TP | 0 | 100 | 0 | 0 |
| 10% LYC | 0 | 0 | 0 | 0.1 |
| PB | 0 | 0 | 0.1 | 0 |
As measured by HPLC; the tomato powder (FutureCeuticals, Momence, IL) diet contained 181 ± 42 nmol lycopene, 10.1 nmol phytoene, 2.6 nmol phytofluene and 0.8 nmol β-carotene per gram of diet.
As measured by HPLC; the placebo beadlet (DSM) diet provided 0 mg carotenoids/g diet.
As measured by HPLC; the lycopene-beadlet (10% DSM) diet provided 152 ± 7 nmol lycopene per gram of diet.
AIN93M-MX formulation.
AIN93-VX formulation with 1.57 mol retinyl palmitate/g diet.
2.2 Carotenoid Analysis
All carotenoid samples were extracted and analyzed under yellow lights and on ice using the procedure previously described [25,26]. Briefly, ethanol containing 0.01% butylated hydroxytoluene and potassium hydroxide was added to minced liver or adipose tissue followed by saponification in a shaking water bath at 60 °C for 30 minutes. Carotenoids were extracted three times with 6 mL of hexane, dried under reduced pressure (SpeedVac concentrator, model AES1010, Savant, Milford, MA) and stored with an argon gas atmosphere to prevent degradation. For analysis, sample extracts were reconstituted in methyl tert-butyl ether, separated on a reverse-phase C30 HPLC column (4.6 × 150 mm, 3µm; YMC, Wilmington, NC) maintained at 18 °C and carotenoids were detected by a photodiode array detector (model 2996, Waters, Milford, MA). A phase gradient method was used for carotenoid separation has been previously described [27]. Standards for individual carotenoids (lycopene, DSM; phytoene and phytofluene, BASF (Ludwigshafen, Germany); β-carotene, (Sigma-Aldrich St. Louis, MO) were used for identification by comparison of elution times and absorption spectra, and quantitation by an external standard curve method.
2.3 Serum hormones and growth factors
Fasting blood glucose was measured directly using a Bayer Contour Glucometer (Bayer, Tarrytown, New York). Fasting serum insulin, leptin and adiponectin concentrations (n ≥ 3 per group) were measured using a Luminex-based LINCOplex bead array assay (Millipore, Billerica, MA) on a BioRad BioPlex multianalyte detection system (BioRad, Inc., Hercules, CA). Fasting serum insulin-like growth factor (IGF)-1 (n ≥ 3 per group) was measured using an ELISA (R&D Systems, Minneapolis, MN) on a Synergy 2 plate reader (BioTek, Winooski, VT).
2.4 Hepatic Lipids
Total liver lipids were extracted using a modification of the Folch method [28,29]. Briefly, the liver sample (~0.3 g), n = 10 per group, was homogenized in chloroform: methanol (1:1) (Fisher Scientific, Pittsburg, PA.) and filtered by gravity. The mixture was washed with 0.29% sodium chloride solution and then centrifuged at 183 x g for 5 min at 25 °C, after which chloroform was added to further separate lipids. These steps were repeated 2 more times before samples were dried in a desiccator under reduced pressure for at least 48 hours and then weighed to determine total lipids.
2.5 Hepatic Pathology
Livers (n = 8 randomly selected per group) were fixed in 10% formalin, transferred to ethanol after 24 hours and processed into paraffin blocks within a week of study completion. The embedded tissues were cut into 4-µm slices, mounted on slides and dried in a 60 °C oven. Slides were then stained using a standard hematoxylin and eosin (H&E) staining procedure in the Veterinary Medicine Pathology Lab at the University of Illinois at Urbana-Champaign. The slides were analyzed by a licensed veterinary pathologist from the Department of Pathobiology at the College of Veterinary Medicine at the University of Illinois-Urbana/Champaign.
2.6 Serum and Hepatic Cholesterol
Serum and hepatic total cholesterol (n = 10 per group) were analyzed by enzymatic colorimetric assay (Wako Chemicals USA, Richmond, VA) according to the manufacturer’s instructions. For hepatic cholesterol analysis, 1 mL of 10% Triton X-100 (Sigma-Aldrich) in isopropanol (Fisher Scientific) was added to the liver lipid extracts and vortexed briefly before enzyme addition. Samples were quantitatively analyzed on a µQuant plate reader (BioTek U.S., Winooski, VT) at 600 nm.
2.7 mRNA Expression
Total RNA was isolated (n = 6 randomly selected per group) by the TRIzol (Invitrogen, Carlsbad, CA) method as previously described [24]. Concentration and quality of mRNA was confirmed by gel electrophoresis and spectrophotometry (Nanodrop, Thermo Scientific, Wilmington, DE). Complementary DNA was synthesized using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). mRNA expression was measured by qRT-PCR using TaqMan Primer Probes (Austin, TX) and compared with beta-actin using the 2−ΔΔCt method. Reactions were monitored by a ViiA 7 thermocycler (Applied Biosystems, Carlsbad, CA).
2.8 Statistical analyses
Power analyses were conducted on the primary outcomes with an alpha of 0.05 and a power of 80% to determine the appropriate sample size. Carotenoid accumulation in response to a tomato powder diet was analyzed by one-way ANOVA followed by Tukey’s post hoc analysis. All other data was analyzed by 2-way ANOVA followed by Tukey’s post hoc analysis using SAS 9.3 (Cary, NC). When assumptions for ANOVA were violated, data were transformed by natural log. Results were expressed as means ± SD. Statistical significance was considered to be achieved when P ≤ 0.05.
3. Results
3.1 Differential accumulation of carotenoids in CMO-I−/− mice and CMO-II−/− mice
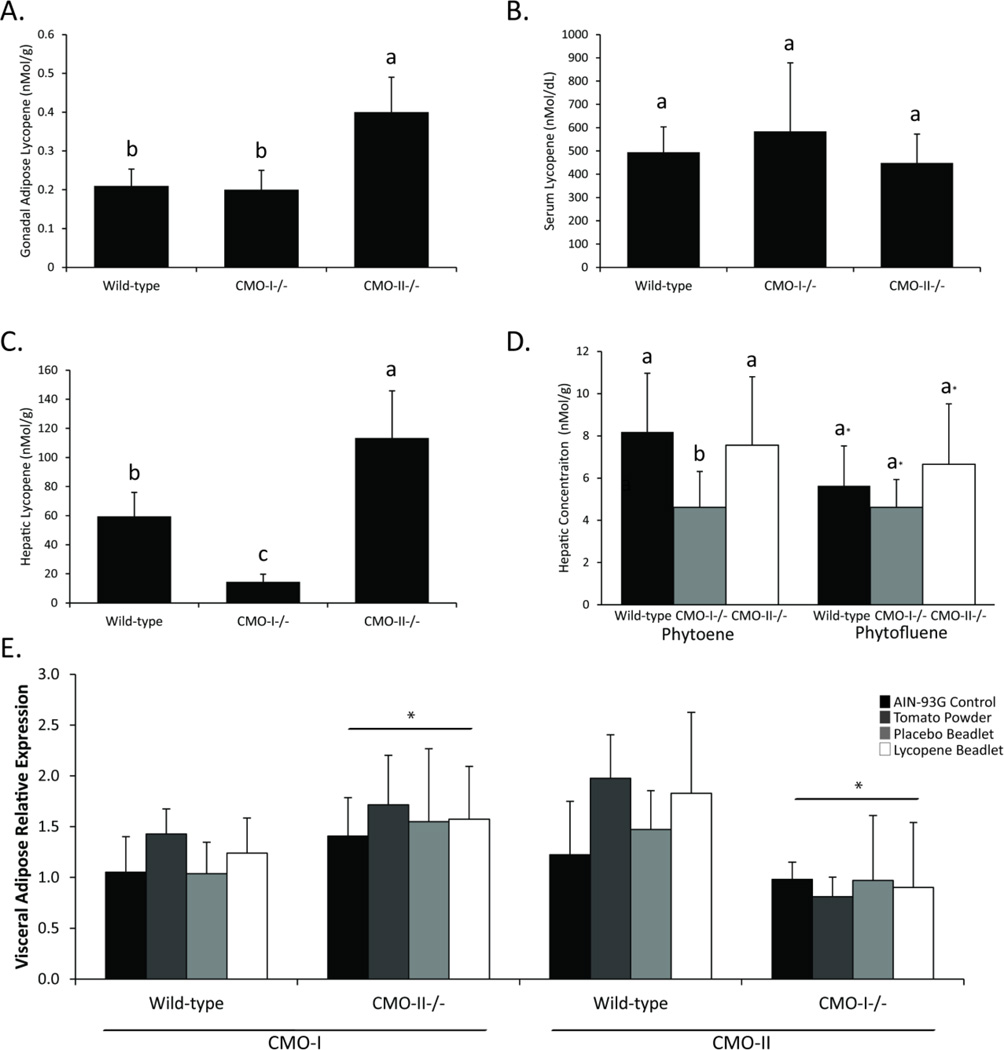
To confirm previous observations from male mice [24], we analyzed lycopene accumulation in female mice fed a 10% tomato powder diet for 1 month. Within the perigonadal adipose tissue, CMO-II−/− mice accumulated significantly more lycopene (0.4 ± 0.08 nmol/g) relative to WT mice (0.21 ± 0.04 nmol/g; P = 0.03; Figure 1A). Moreover, CMO-II−/− mice also accumulated significantly more hepatic lycopene (113 ± 32.4 nmol/g) while CMO-I−/− mice accumulated significantly less total hepatic lycopene (14.4 ± 5.31 nmol/g) relative to WT mice (59.5 ± 16.4 nmol/g; P < 0.01; Figure 1C). Circulating serum lycopene was not altered by genotype (Figure 1B).
Figure 1.
Lycopene accumulation in A.) gonadal adipose, B.) serum, C.) liver and accumulation of phytoene and phytofluene in D.) liver tissue of WT, CMO-I−/− and CMO-II−/− mice in response to diets containing 10% dried tomato powder. Different letters indicate statistical differences as determined by one-way analysis of variance (ANOVA) with P < 0.05 considered significant. Data are presented as means ± SD, n = 10 per group. The asterisk in the phytofluene data indicates that ANOVA compared phytofluene accumulation separate from phytoene hepatic accumulation. E.) Relative mRNA expression of CMO-I (left) and CMO-II (right) in visceral gonadal adipose tissue. Asterisks in the relative expression figure indicate a significant effect of genotype as determined by two-way ANOVA.
Because the tomato powder diet contained small amounts of other carotenoids, including phytoene, phytofluene and β-carotene, serum and tissue levels of these carotenoids were also measured. For the first time, we report that hepatic accumulation of the lycopene precursor, phytoene was significantly lower in CMO-I−/− mice relative to WT mice (P < 0.05; Figure 1D). Accumulation of phytoene and phytofluene in serum and adipose tissue was below detectable limits or not present most likely due to the very low concentrations in the tomato powder. (data not shown).
3.2 Genetic ablation of CMO-I or CMO-II alters the expression of carotenoid oxygenases in visceral adipose
CMO-I and CMO-II mRNA were readily detectable in visceral adipose tissue of wild-type mice (Figure 1E). Additionally, CMO-II expression was significantly greater (P < 0.01) in CMO-I−/− mice while CMO-I expression was significantly reduced (P < 0.01) in CMO-II−/− mice relative to wild-type mice. The tomato powder and lycopene diets did not significantly impact the expression of CMO-I or CMO-II in WT, CMO-I−/− or CMO-II−/− mice.
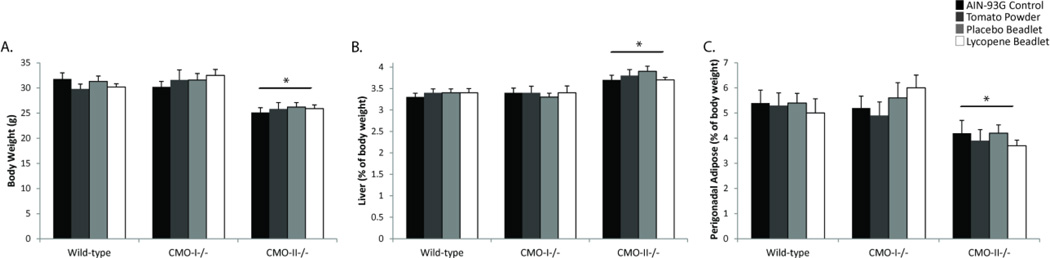
3.3 CMO-II−/− mice have altered body and organ weights
As an indicator of obesity and metabolic alterations, mice were weighed throughout the study and organ weights were recorded at euthanization. CMO-II−/− mice were significantly smaller than WT mice and CMO-I−/− mice (P <0.01; Figure 2A), and visceral gonadal adipose weights, as a percentage of total body weight, were smaller in CMO-II−/− mice relative to WT mice (P < 0.02; Figure 1C). In contrast, CMO-II−/− mice had significantly larger livers, as a percentage of total body weight, relative to WT mice (P <0.01; Figure 1B). Consumption of tomato or lycopene diets did not affect body, visceral adipose tissue or liver weights relative to control fed mice. Additionally, the diets and genotype did not alter other organ weights (kidney, spleen or retroperitoneal adipose), as a percentage of total body weight. Total body fat and lean body mass were not measured in this study.
Figure 2.
The impact of genotype (WT, CMO-I−/− and CMO-II−/−) and diet (AIN-93G control, 10% tomato powder, placebo or lycopene) on A.) body weight, B.) liver weight as a percentage of body weight and C.) perigonadal weight as a percentage of body weight. Data were analyzed by two-way ANOVA followed by Tukey’s post hoc analysis and P < 0.05 was considered statistically significant. Data are presented as means ± SD, n = 10 per group. Asterisks indicate a significant effect of genotype. No significant effects of diet or interactions of diet and genotype were identified in these analyses.
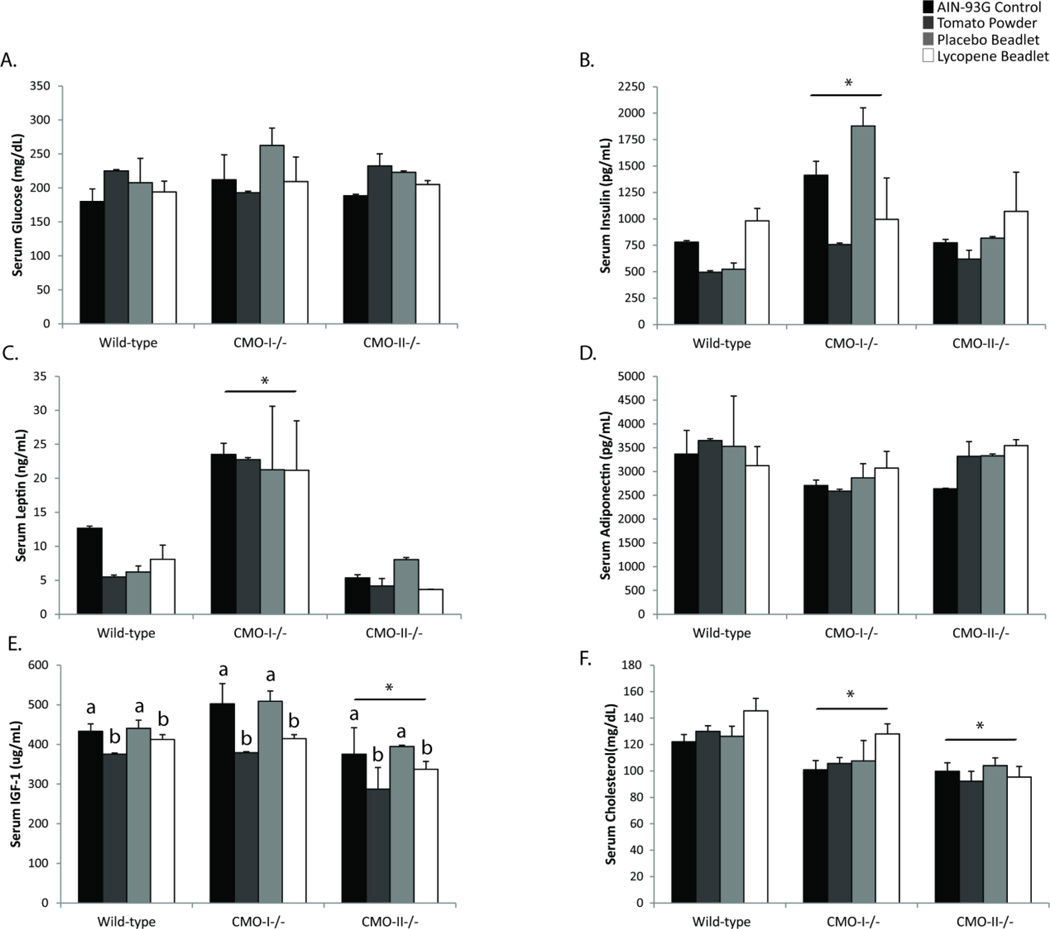
3.4 Serum hormones, growth factors and cholesterol are altered by carotenoid consumption and expression of CMO-I and CMO-II
To investigate the physiological impacts of dietary carotenoids and carotenoid metabolizing enzyme genotypes on serum indicators of metabolic perturbations, numerous assays were performed. CMO-I−/− mice have significantly elevated levels of fasting insulin (P = 0.03) and leptin, (P = 0.04) but reduced levels of fasting serum cholesterol (P < 0.01) relative to WT mice (Figure 3B,C,F). CMO-II−/− mice also had reduced serum cholesterol (P < 0.01) and IGF-1 (P < 0.01) levels relative to WT mice (Figure 3E,F). There were no significant effects of CMO-I or CMO-II expression on fasting serum glucose or adiponectin (Figure 3A,D).
Figure 3.
Serum hormone, adipokine, growth factor and cholesterol levels in WT, CMO-I−/− and CMO-II−/− mice in response to a diet containing 10% tomato powder or lycopene. Data were analyzed by two-way ANOVA followed by Tukey’s post hoc analysis with P < 0.05 considered statistically significant. Data are presented as means ± SD, n ≥ 3/group. Asterisks indicate a significant effect of genotype, while different letters indicate an effect of diet within genotype. No significant interactions between diet and genotype were identified in these analyses.
Consumption of the tomato powder diet or lycopene diet did not affect serum glucose, insulin, leptin, adiponectin or cholesterol levels. In contrast, both the tomato powder and lycopene beadlet diets significantly reduced serum levels of IGF-1 (P = 0.04; Figure 3E), independently of genotype.
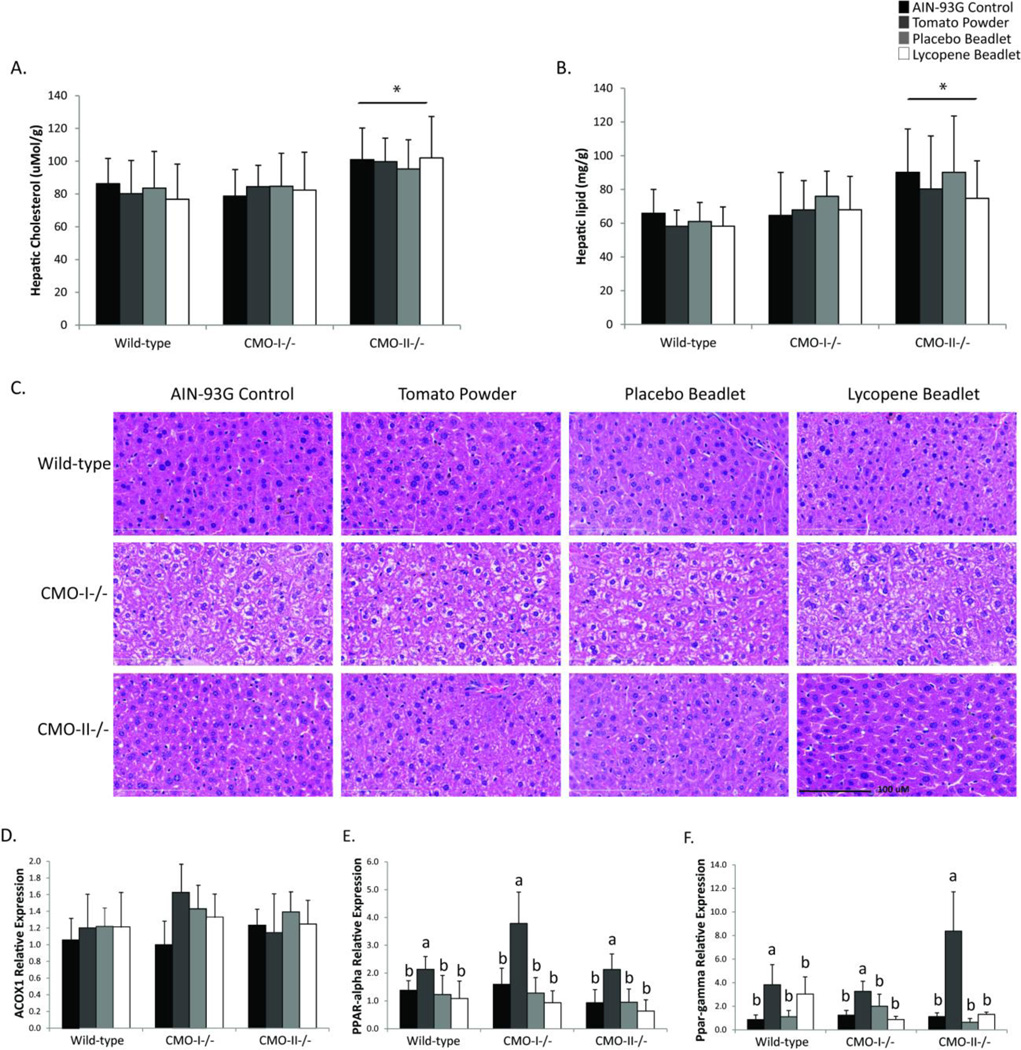
3.5 Hepatic perturbations in CMO-I−/−and CMO-II−/− mice fed tomato powder or lycopene diets
Due to previous reports of hepatic lipid changes in CMO-I−/− and CMO-II−/− male mice [8,13], we investigated the effects of genotype and diet on hepatic lipid accumulation parameters in female mice (Figure 4). CMO-II−/− mice accumulated significantly more hepatic cholesterol (P = 0.03; Figure 4A) and total lipids (P = 0.04; Figure 4B) compared with WT mice. Diet did not significantly alter either of these parameters. Veterinary pathological assessment indicated hepatic lipidosis was evident in 25% of CMO-I−/−mice (Figure 4C), but not in livers from CMO-II −/−or WT mice. Carotenoid-containing diets did not alter the incidence of hepatic lipidosis in any of the mouse models. Lastly, we investigated the impact of CMO-I and CMO-II expression and carotenoid consumption on the expression of fatty acid oxidase I (ACOX1), the enzyme catalyzing the first step of beta-oxidation, and key lipid-related transcription factors, PPAR-α and PPAR-γ (Figure 4D, E). Although genotype did not impact the expression of these three genes, expression of hepatic PPAR-a and PPAR-γwas significantly increased by the tomato powder diet (P = 0.01, P < 0.01, respectively) relative to the control diets in all genotypes. Diet did not significantly impact ACOX1 mRNA expression.
Figure 4.
Hepatic lipid perturbations in response to genotype (WT, CMO-I−/− and CMO-II−/−) and diet (AIN-93G control, 10% tomato powder, placebo or lycopene). Hepatic A.) cholesterol, B.) lipid content, C.) pathology, D.) ACOX1 gene expression E.) PPAR-α gene expression, and F.) PPAR-γ gene expression. Quantitative data were analyzed by two-way ANOVA followed by Tukey’s post hoc analysis with P < 0.05 considered statistically significant. Data are presented as means ± SD, n = 8–10 per group. Asterisks indicate a significant effect of genotype, while different letters indicate an effect of diet within genotype. No significant interactions between diet and genotype were identified in these analyses.
3.6 Inflammatory gene expression in adipose tissue is not altered by diet or genotype
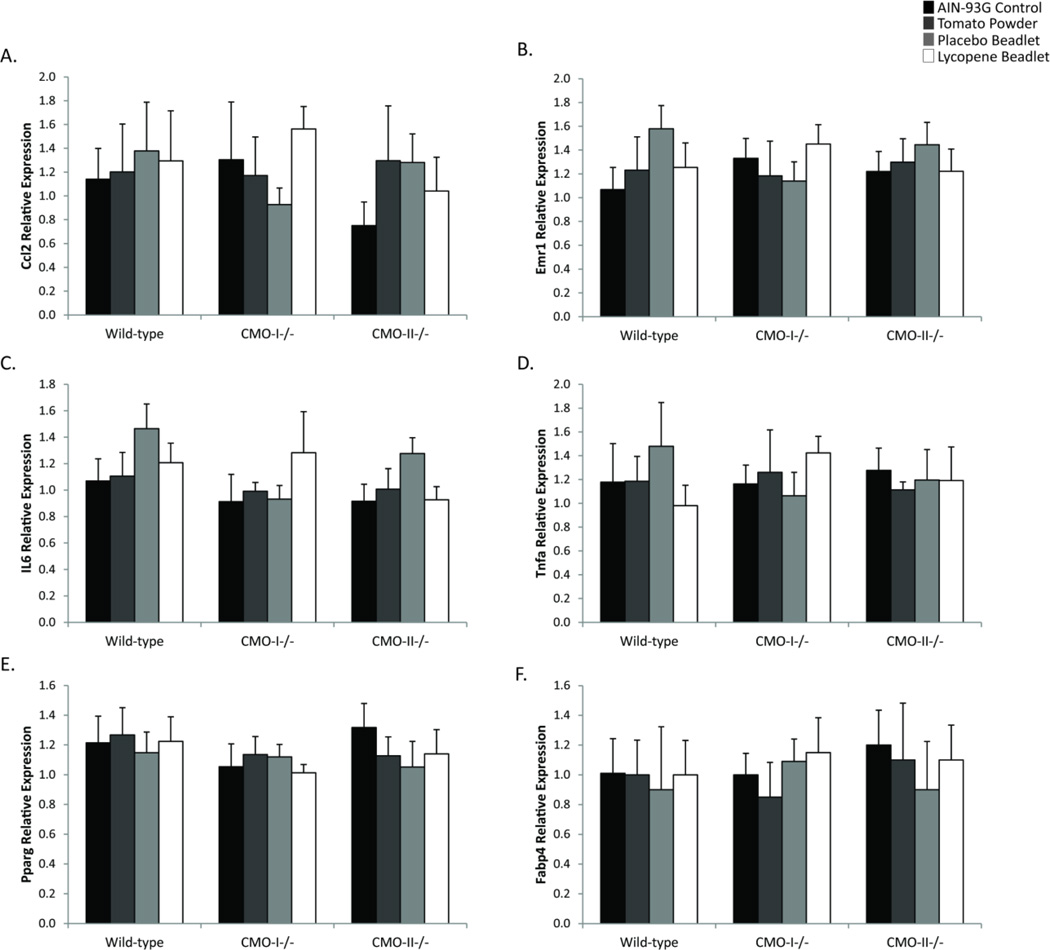
Despite metabolic changes in lipid metabolism observed in serum and liver tissue in response to consumption of tomato carotenoids or expression of CMO-I and CMO-II, the expression of inflammatory genes (Ccl2, Emr1, Tnfa or Il6) and PPAR-γ or downstream target FABP4 was not significantly altered in perigonadal adipose tissue from CMO-I−/−, CMO-II−/− or WT mice (Figure 5).
Figure 5.
Effects of genotype (WT, CMO-I−/− and CMO-II−/− ) and diet (AIN-93G control, 10% tomato powder, placebo or lycopene) on genes indicative of visceral adipose inflammation and lipid metabolism. Relative expression of A.) Ccl2 (macrophage chemotactic protein-1), B.) Emr1 (a macrophage marker, F4/80), C.) IL-6, D.) TNF-a, E.) PPARγ and F.) FABP-4 was analyzed by two-way ANOVA followed by Tukey’s post hoc analysis with P < 0.05 considered statistically significant. Data are presented as means ± SD, n = 6 per group. No significant effects of genotype, diet or interaction were identified in these analyses.
4. Discussion
CMO-I is known to produce vitamin A from some carotenes by central cleavage [4], while CMO-II asymmetrically cleaves many carotenoids to produce metabolites, some of which are bioactive [7,12,20,30]. However, recent evidence suggests CMO-I and CMO-II may have other functions that extend beyond carotenoid metabolism [13,16,18,19]. Our findings support the following hypotheses which were to further characterize carotenoid metabolism in female mice fed a tomato powder or lycopene-supplemented diets, to identify any physiologic consequences of cross-talk between expression of the CMO enzymes and lipid metabolism, and to test if feeding tomato powder or lycopene could mitigate changes in metabolism or lipid homeostasis.
Tomato carotenoid accumulation was investigated in CMO-I−/− and CMO-II−/− mice and in agreement with previous findings in male CMO-II−/− mice [24], female CMO-II−/− mice preferentially accumulate lycopene in perigonadal adipose and liver tissue, again suggesting that lycopene is either a substrate for CMO-II activity or CMO-II inhibits lycopene uptake and accumulation at the tissue level. Moreover, CMO-I−/− mice accumulated significantly less lycopene in liver tissue, potentially due to increased CMO-II expression and therefore cleavage activity; this effect has been previously shown in other tissues [24]. We report that accumulation of the colorless carotenoid precursor to lycopene, phytoene, is also significantly reduced in liver tissue of CMO-I−/− mice, possibly due to alterations in uptake, metabolism or storage of phytoene in this mouse model. Therefore, the potential differential production of phytoene metabolites between these mouse models may explain some of the differential effects between lycopene and tomato powder (PPAR-α and PPAR- γ). Consumption of the lycopene beadlet diet produced different metabolic changes relative to the tomato powder diet suggesting that other components of the tomato powder – such as phytoene – may be bioactive. Together, these data offer insight into additional functions of carotenoid oxygenases and may point to the production of unidentified and potentially bioactive phytoene or lycopene metabolites.
The lycopene and tomato powder diets both reduced fasting serum IGF-1 levels in all three models, suggesting that the effect can be attributed to intact lycopene, rather than other components of tomato powder or carotenoid metabolites of CMO-I or CMO-II cleavage. This finding is particularly intriguing because IGF-1 is esponsible for signaling growth and proliferation through the mammalian target of rapamycin (mTOR) pathway, and controls cardiac growth, promotes myocardial contractility and alters growth of the vascular system. Together these pathways influence risk for cancers and cardiovascular disease [12,31]. The positive relationship between serum IGF-1 levels and prostate cancer risk [12] and IGF-1 levels and chronic heart failure have been demonstrated [31]. The tomato powder diet also caused up regulation of PPAR-α and PPAR-γ expression, independent of genotype. Known endogenous ligands of PPARs include free fatty acids and eicosanoids, and more specifically PPAR-γ is activated by PGJ2 (a prostaglandin) and PPAR-α is activated by a subset of leukotrienes. PPAR agonists [32] are used for the control of serum lipids (PPAR-α) and regulation of blood glucose (PPAR-γ) therefore consuming tomatoes may be a dietary approach to assist in the control of risk factors for diabetes, metabolic syndrome and coronary diseases.
Interactions between lipid and carotenoid metabolic pathways are plausible for several reasons. Firstly, PPAR-γ and RXR heterodimerize and binds with the peroxisome proliferators response element (PPRE) in the promoter region of CMO-I to regulate its expression [16]. Secondly, CMO-I−/− mice display increased expression of SR-B1, and accumulation of hepatic lipids, develop hepatic steatosis and have elevated levels of circulating unesterified free fatty acids [13,19]. Thirdly, hepatic lipid droplet formation is elevated in CMO-II−/− mice [8]. Moreover, relative to WT mice, CMO-I−/− mice have elevated levels of serum insulin and leptin, and increased incidence of hepatic lipidosis; the latter of which may be attributed to elevated intestinal lipid uptake through SR-B1 [19]. Additionally, it is possible that serum cholesterol was reduced in CMO-I−/− mice (relative to WT mice) due to elevated levels of SR-B1 shuttling cholesterol esters from plasma lipoproteins to the liver for incorporation into bile. CMO-II−/−mice also showed metabolic perturbations including reduced measures of visceral adiposity, serum IGF-1 and serum cholesterol levels, as well as enlarged livers with elevated accumulation of lipids and cholesterol. This may suggest a preferential storage of lipids and cholesterol in liver tissue rather than in perigonadal adipose tissue in these mice. Decreased serum IGF-1 levels may be due to reduced adiposity in these mice [33].
Single nucleotide polymorphisms (SNPs) appear frequently (up to 42% minor allele frequency) in the human CMO-I gene [34–36] and can significantly impact its activity [35,36]. Although studies have not focused on identifying SNPs in the human CMO-II gene, one small study reports specific mutant alleles in the CMO-II gene [39]. Furthermore, evidence for the existence of SNPs in the CMO-II gene is evident in animal models [40–42]. Therefore, data from murine genetic knock-out models and clinical studies [39,43] indicate that it is plausible that individuals with functional alterations to the CMO-I or CMO-II gene may be genetically predisposed to perturbations in lipid metabolism.
Despite what appears to be an interaction between lipid and carotenoid metabolism[9,16,44–49], consumption of the tomato carotenoid-containing diets in this study had little impact on lipid or inflammatory outcomes, and did not appear to influence the gene expression changes found in CMO-I−/− and CMO-II−/− mice. Due to the heterogeneity among previous studies, including the selection of animal models, duration of feeding, specific carotenoid of interest and the concentration of other carotenoids and vitamin A within the diets, it is difficult to determine the mitigating factors whereby dietary carotenoids impact lipid metabolic parameters. However, based upon our data, it is possible that metabolites of tomato carotenoids in these models had little to no impact on the metabolic dysregulation in these mice.
Our study has some limitations including the inability to measure proposed tomato carotenoid metabolites in serum and tissue. Additionally, CMO-I−/− and CMO-II−/− mice were maintained on a mixed C57BL/6 and 129svJ background so there is the potential for differences in genetic backgrounds between the mouse strains. Overall, we conclude that CMO-I and CMO-II appear to have pleiotropic roles in lipid metabolic homeostasis. We [24,29] and others [6–8,13] have established that tomato carotenoids are likely substrates of these enzymes. Additionally, the expression of CMO-I and CMO-II alter serum, adipose, and hepatic lipid products. Overall the impact of tomato powder and lycopene on the phenotypic alterations in lipid metabolism were modest. However, significant alterations in PPAR expression by tomato powder and serum IGF-1 levels by tomato powder and lycopene were independent of CMO expression and are worthy of further investigation.
Acknowledgment
We acknowledge the Institute for Genomic Biology animal facility staff and veterinarians. Additionally, we thank Matt Wallig for the hepatic histological analysis. We thank Josh Smith and Caroline Bauer for their assistance with wet lab analyses and Nancy E. Moran for her assistance in editing the manuscript. NAF was supported by an American Institute for Cancer Research Postdoctoral Fellowship.
List of Abbreviations
- PPAR
peroxisome-proliferator activator receptor
- CMO-I
carotene-15,15’-monooxygenase
- CMO-II
carotene-9’,10’-monooxygenase
- IGF-1
insulin-like growth factor 1
- BHT
butylated hydroxytoluene
- FABP4
fatty acid binding protein 4
- RXR
retinoid-x-receptor
- PPRE
peroxisome-proliferator response element
- SNP
single nucleotide polymorphism
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to report.
References
- 1.Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100:637–640. doi: 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- 2.Britton G, Liaaen-Jensen S, Pfander H. Carotenoids. 1995;1A:328. [Google Scholar]
- 3.Goodman DS, Huang HS, Shiratori T. Mechanism of the biosynthesis of vitamin A from beta-carotene. J Biol Chem. 1966;241:1929–1932. [PubMed] [Google Scholar]
- 4.Britton G, Liaaen-Jensen S, Pfander H. Carotenoids. 2009;5:328. [Google Scholar]
- 5.Lindqvist A, Andersson S. Biochemical properties of purified recombinant human beta-carotene 15,15'-monooxygenase. J Biol Chem. 2002;277:23942–2398. doi: 10.1074/jbc.M202756200. [DOI] [PubMed] [Google Scholar]
- 6.Hu K, Liu C, Ernst H, Krinsky N, Russell R, Wang X. The biochemical characterization of ferret carotene-9',10'-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem. 2006;281:19327–19338. doi: 10.1074/jbc.M512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–14116. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- 8.Amengual J, Lobo G, Golczak M, Li HNM, Klimova T, Hoppel C, Wyss A, Palczewski K, von Lintig J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011;25:948–959. doi: 10.1096/fj.10-173906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang XD. Lycopene metabolism and its biological significance. Am J Clin Nutr. 96. 2012:1214S–1222S. doi: 10.3945/ajcn.111.032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lietz G, Oxley A, Boesch Saadatmandi C, Kobayashi D. Importance of β,β-carotene 15,15'-monooxygenase 1 (BCMO1) and β,β-carotene 9',10'-dioxygenase 2 (BCDO2) in nutrition and health. Mol Nutr Food Res. 2012;56:241–250. doi: 10.1002/mnfr.201100387. [DOI] [PubMed] [Google Scholar]
- 11.Sharoni Y, Linnewiel-Hermoni K, Khanin M, Salman H, Veprik A, Danilenko M, Levy J. Carotenoids and apocarotenoids in cellular signaling related to cancer: a review. Mol Nutr Food Res. 2012;56:259–356. doi: 10.1002/mnfr.201100311. [DOI] [PubMed] [Google Scholar]
- 12.Erdman JW, Jr, Ford NA, Lindshield BL. Are the health attributes of lycopene related to its antioxidant function? Arch Biochem Biophys. 2009;483:229–235. doi: 10.1016/j.abb.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem. 2007;282:33553–33561. doi: 10.1074/jbc.M706763200. [DOI] [PubMed] [Google Scholar]
- 14.Poulsen LlC, Siersbk M, Mandrup S. PPARs: Fatty acid sensors controlling metabolism. Sem in Cell Dev Bio. 2012;23:631–639. doi: 10.1016/j.semcdb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Jia Y, Fu T, Viswakarma N, Bai L, Rao MS, Zhu Y, Borensztajn J, Reddy JK. Sustained activation of PPARalpha by endogenous ligands increases hepatic fatty acid oxidation and prevents obesity in ob/ob mice. FASEB J. 2012;26:628–623. doi: 10.1096/fj.11-194019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulanger A, McLemore P, Copeland N, Gilbert D, Jenkins N, Yu S, Gentleman S, Redmond TM. Identification of beta-carotene 15, 15'-monooxygenase as a peroxisome proliferator-activated receptor target gene. FASEB. 2003;17:1304–1306. doi: 10.1096/fj.02-0690fje. [DOI] [PubMed] [Google Scholar]
- 17.Zaripheh S, Nara T, Nakamura M, Erdman JW., Jr Dietary lycopene downregulates carotenoid 15,15'-monooxygenase and PPAR-gamma in selected rat tissues. J Nutr. 2006;136:932–938. doi: 10.1093/jn/136.4.932. [DOI] [PubMed] [Google Scholar]
- 18.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Devel. 1994;8:1224–1223. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 19.Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J. 2010;24:1656–1656. doi: 10.1096/fj.09-150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eroglu A, Hruszkewycz DP, dela Sena C, Narayanasamy S, Riedl KM, Kopec RE, Schwartz SJ, Curley RW, Jr, Harrison EH. Naturally occurring eccentric cleavage products of provitamin A beta-carotene function as antagonists of retinoic acid receptors. J Biol Chem. 2012;287:15886–15895. doi: 10.1074/jbc.M111.325142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eroglu A, Hruszkewycz DP, Curley RW, Jr, Harrison EH. The eccentric cleavage product of beta-carotene, beta-apo-13-carotenone, functions as an antagonist of RXRalpha. Arch Biochem Biophys. 2010;504:11–16. doi: 10.1016/j.abb.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takitani K, Miyazaki H, Takaya R, Tamai H. Expression of beta-carotene 15,15'-monooxygenase gene and retinol status in type 2 diabetic Goto-Kakizaki rats. Biofactors. 2008;33:77–83. doi: 10.1002/biof.5520330107. [DOI] [PubMed] [Google Scholar]
- 23.Coyne T, Ibiebele TI, Baade PD, McClintock CS, Shaw JE. Metabolic syndrome and serum carotenoids: findings of a cross-sectional study in Queensland, Australia. Br J Nutr. 2009;102:1668–1677. doi: 10.1017/S000711450999081X. [DOI] [PubMed] [Google Scholar]
- 24.Ford N, Clinton S, von Lintig J, Wyss A, Erdman JW., Jr Loss of carotene-9',10'-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice. J Nutr. 2010;140:2134–2138. doi: 10.3945/jn.110.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boileau TW, Clinton SK, Zaripheh S, Monaco MH, Donovan SM, Erdman JW. Testosterone and food restriction modulate hepatic lycopene isomer concentrations in male F344 rats. J Nutr. 2001;131:1746–1752. doi: 10.1093/jn/131.6.1746. [DOI] [PubMed] [Google Scholar]
- 26.Campbell J, Engelmann N, Lila M, Erdman JW., Jr Phytoene, Phytofluene, and Lycopene from Tomato Powder Differentially Accumulate in Tissues of Male Fisher 344 Rats. Nutr Res. 2007;27:794–801. doi: 10.1016/j.nutres.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeum KJ, Booth SL, Sadowski JA, Liu C, Tang G, Krinsky NI, Russell RM. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr. 1996;64:594–602. doi: 10.1093/ajcn/64.4.594. [DOI] [PubMed] [Google Scholar]
- 28.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 29.Lindshield B, King J, Wyss A, Goralczyk R, Lu C, Ford N, Erdman JW., Jr Lycopene biodistribution is altered in 15,15'-carotenoid monooxygenase knockout mice. J Nutr. 2008;138:2367–2371. doi: 10.3945/jn.108.099663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrison EH, dela Sena C, Eroglu A, Fleshman MK. The formation, occurrence, and function of beta-apocarotenoids: beta-carotene metabolites that may modulate nuclear receptor signaling. Am J Clin Nutr. 2012;96:1189S–1192S. doi: 10.3945/ajcn.112.034843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andreassen M, Raymond I, Kistorp C, Hildebrandt Per, Faber J, Østergaard Kristensen L. IGF1 as predictor of all-cause mortality and cardiovascular disease in an elderly population. Euro J Endo. 2009;160:25–31. doi: 10.1530/EJE-08-0452. [DOI] [PubMed] [Google Scholar]
- 32.Ernest A, Adem A, Hasan MY, Tekes K, Kalasz H. Medicinal Chemistry and Actions of Dual and Pan PPAR Modulators. Open Med Chem J. 2011;5:93–98. doi: 10.2174/1874104501105010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hursting SD, Hursting MJ. Growth signals, inflammation, and vascular perturbations: mechanistic links between obesity, metabolic syndrome, and cancer. Arterioscler Thromb Vasc Biol. 2012;32:1766–1770. doi: 10.1161/ATVBAHA.111.241927. [DOI] [PubMed] [Google Scholar]
- 34.Ferrucci L, Perry JR, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, Fried LP, Albanes D, Corsi AM, Cherubini A, Guralnik J, Bandinelli S, Singleton A, Virtamo J, Walston J, Semba RD, Frayling TM. Common variation in the beta- carotene 15,15'-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. 2009;84:123–123. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindqvist A, Sharvill J, Sharvill D, Andersson S. Loss-of-function mutation in carotenoid 15,15'-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J Nutr. 2007;137:2346–2350. doi: 10.1093/jn/137.11.2346. [DOI] [PubMed] [Google Scholar]
- 36.Leung WC, Hessel S, Mplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding beta-carotene 5,15'-monoxygenase alter beta-carotene metabolism in female volunteers. FASEB J. 2009;23:1041–1053. doi: 10.1096/fj.08-121962. [DOI] [PubMed] [Google Scholar]
- 37.Lietz G, Oxley A, Leung W, Hesketh J. Single nucleotide polymorphisms upstream from the beta-carotene 15,15'-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J Nutr. 2012;142:161S–165S. doi: 10.3945/jn.111.140756. [DOI] [PubMed] [Google Scholar]
- 38.Tourniaire F, Gouranton E, von Lintig J, Keijer J, Bonet ML, Amengual J, Lietz G, Landrier JF. beta-Carotene conversion products and their effects on adipose tissue. Genes Nutr. 2009;4:179–187. doi: 10.1007/s12263-009-0128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tourniaire F, Minihane AM, Hesketh J, Lietz G. Do single nucleotide polymorphisms in β- carotene dioxygenase-2 ( BCDO2) gene affect the postprandial response? Proc of the Nutr Soc. 2008:67. [Google Scholar]
- 40.Eriksson J, Larson G, Gunnarsson U, Bed'hom B, Tixier-Boichard M, Stromstedt L, Wright D, Jungerius A, Vereijken A, Randi E, Jensen P, Andersson L. Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet. 2008;4:e1000010. doi: 10.1371/journal.pgen.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vage DI, Boman IA. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries) BMC Genet. 2010;11 doi: 10.1186/1471-2156-11-10. 10,2156-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berry SD, Davis SR, Beattie EM, Thomas NL, Burrett AK, Ward HE, Stanfield AM, Biswas M, Ankersmit-Udy AE, Oxley PE, Barnett JL, Pearson JF, van der Does Y, Macgibbon AH, Spelman RJ, Lehnert K, Snell RG. Mutation in bovine beta-carotene oxygenase 2 affects milk color. Genetics. 2009;182:923–926. doi: 10.1534/genetics.109.101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He M, Cornelis MC, Kraft P, van Dam RM, Sun Q, Laurie CC, Mirel DB, Chasman DI, Ridker PM, Hunter DJ, Hu FB, Qi L. Genome-wide association study identifies variants at the IL18-BCO2 locus associated with interleukin-18 levels. Arterioscler Thromb Vasc Biol. 2010;30:885–890. doi: 10.1161/ATVBAHA.109.199422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amengual J, Gouranton E, van Helden YGJ, Hessel S, Ribot J, Kramer E, Kiec Wilk B, Razny U, Lietz G, Wyss A, Dembinska Kiec A, Palou A, Keijer J, Landrier J, Bonet ML, von Lintig J. Beta-carotene reduces body adiposity of mice via BCMO1. PLoS ONE. 2011;6:e20644. doi: 10.1371/journal.pone.0020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villaa-Chaves G, Pereira S, Saboya C, Ramalho A. Non-alcoholic fatty liver disease and its relationship with the nutritional status of vitamin A in individuals with class III obesity. Obesity Surg. 2008;18:378–385. doi: 10.1007/s11695-007-9361-2. [DOI] [PubMed] [Google Scholar]
- 46.Fuhrman B, Elis A, Aviram M. Hypocholesterolemic effect of lycopene and beta-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophages. Biochem Biophys Res Commun. 1997;233:658–662. doi: 10.1006/bbrc.1997.6520. [DOI] [PubMed] [Google Scholar]
- 47.Sluijs I, Beulens JWJ, Grobbee D, van der Schouw YT. Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. J Nutr. 2009;139:987–992. doi: 10.3945/jn.108.101451. [DOI] [PubMed] [Google Scholar]
- 48.Lobo GP, Amengual J, Li HN, Golczak M, Bonet ML, Palczewski K, von Lintig J. Beta,beta-carotene decreases peroxisome proliferator receptor gamma activity and reduces lipid storage capacity of adipocytes in a beta,beta-carotene oxygenase 1-dependent manner. J Biol Chem. 2010;285:27891–27899. doi: 10.1074/jbc.M110.132571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Helden YG, Godschalk RW, Swarts HJ, Hollman PC, van Schooten FJ, Keijer J. Beta-carotene affects gene expression in lungs of male and female Bcmo1 (−/−) mice in opposite directions. Cell Mol Life Sci. 2011;68:489–504. doi: 10.1007/s00018-010-0461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]