Abstract
The comet analysis of DNA strand break levels in tissues and cells has become a common method of screening for genotoxicity. The large majority of published studies have used fresh tissues and cells processed immediately after collection. However, we have used frozen tissues and cells for more than 10 years, and we believe that freezing samples improve efficiency of the method. We compared DNA strand break levels measured in fresh and frozen bronchoalveolar cells, and lung and liver tissues from mice exposed to the known mutagen methyl methanesulphonate (0, 25, 75, 112.5mg/kg). We used a high-throughput comet protocol with fully automated scoring of DNA strand break levels. The overall results from fresh and frozen samples were in agreement [R 2 = 0.93 for %DNA in tail (%TDNA) and R 2 = 0.78 for tail length (TL)]. A slightly increased %TDNA was observed in lung and liver tissue from vehicle controls; and TL was slightly reduced in bronchoalveolar lavage cells from the high-dose group. In our comet protocol, a small block of tissue designated for comet analysis is frozen immediately at tissue collection and kept deep frozen until rapidly homogenised and embedded in agarose. To demonstrate the feasibility of long-term freezing of samples, we analysed the day-to-day variation of our internal historical negative and positive comet assay controls collected over a 10-year period (1128 observations, 11 batches of frozen untreated and H2O2-treated A549 lung epithelial cells). The H2O2 treatment explained most of the variation 57–77% and the day-to-day variation was only 2–12%. The presented protocol allows analysis of samples collected over longer time span, at different locations, with reduced variation by reducing number of electrophoreses and is suitable for both toxicological and epidemiological studies. The use of frozen tissues; however, requires great care during preparation before analysis, with handling as a major risk factor.
Introduction
The alkaline comet assay is a method to assess DNA strand break levels in eukaryotic cells. The comet assay is based on a relatively simple protocol. Damaged DNA is drawn from the nuclei during electrophoresis to form comet-like images that are quantified by fluorescent microscopy. Data are reported as measure of DNA in tail. The protocol is optimised and internationally validated to achieve standardised and reliable results (1–17). The method is often used in screening for genotoxicity of tissue samples from humans and animals. The increased number of analyses calls for method optimisation and efficiency regarding both the number of samples run per electrophoresis and a more efficient scoring methods. The comet assay has relied on manual scoring of 50–150 nuclei per sample and is thus very time and labour consuming. A high-throughput method can lower the cost and increase the overall capacity, versatility and robustness of the comet assay (4,18). Traditionally, tissue for analysis in the comet assay is prepared from a small fresh tissue sample, which is processed immediately after collection. Frozen tissues and cells have not been considered usable by some (6) but usable by others (2,7–16,19). We have analysed frozen tissues and cells using the comet assay for more than 10 years. We have analysed tissues such as blood, bronchoalveolar lavage (BAL) cells, lung, liver and colon, but also spleen, kidneys (Jacobsen, not published; 20–33).
Here, we present an optimised protocol for a high-throughput comet procedure and compare DNA strand break levels induced by methyl methanesulphonate (MMS) treatment using the comet assay on fresh and frozen tissue. Furthermore, we present data on untreated and H2O2-treated A549 lung epithelial cells that we have used as negative and positive historical internal quality controls for the comet assay. The batches of frozen A549 cells are used to assess the day-to-day variation of the comet assay and to assess the effect of prolonged freezing. The batches were frozen for 1 year or longer and cover over 10-year period.
Materials and Methods
The tissues were collected from animals housed as described (33,34). Animal studies complied with the EC Directive 86/609/EEC and the Danish law regulating experiments on animals (The Danish Ministry of Justice, Animal Experiments Inspectorate, Permission 2006/561–1123).
Effect of tissue sampling techniques on DNA strand breaks
We examined different procedures of liver tissue freezing and preparation for comet analysis. Five female mice (9–10 weeks old, average weight 21.3g, C57BL/6J; Taconic Europe, Ejby, Denmark) were anaesthetised with 3% isoflurane and intratracheally instilled with 10% BAL fluid in sterile saline four times every third day. The mice were killed 3 days after instillation as described (33). Fresh tissues were not used in this experiment for logistical reasons.
Tissue preparation.
Liver samples were prepared using four different techniques. Method 1: small samples of liver tissue (~20–40mg, 3×3 × 3mm) were cut directly at necropsy, placed in NUNC cryotubes assigned for comet analysis and snap frozen in liquid N2. Samples were promptly placed on dry ice, stored at −80°C and kept deep frozen until comet cell treatment. During slide preparation, NUNC cryotubes were placed on dry ice. Tissues were processed one sample at a time. A drop of Merchant’s medium was placed on the tissue to create a protective ice cap. A stainless steel cylindrical sieve was immersed in 1.5ml ice-cold Merchant’s medium. The deep frozen tissue was transferred into the cylindrical sieve using cold tweezers. Tissues were homogenised by pressing through sieves by moving the plunger up and down several times, to gain a cell suspension. An aliquot of the cell suspension was immediately mixed with agarose and placed on the slide. The volume of Merchant’s medium can be adjusted for different tissues and different tissue cube sizes, to gain an optimal cell concentration; method 2: larger samples of liver tissue were collected at necropsy, snap frozen in NUNC cryotubes in liquid N2 and stored at −80°C. Samples were cut (same size as above) on a deep cold metal plate to minimise thawing, placed in frozen Eppendorf tubes, stored at −80°C until comet cell treatment and processed as described above; method 3: larger samples of liver tissue were collected at necropsy, snap frozen in NUNC cryotubes in liquid N2 and frozen at −80°C. Samples were crushed in a porcelain mortar in liquid N2, fragments (size as above) were placed in frozen Eppendorf tubes, stored at −80°C until comet cell treatment and processed as described above; reference treatment: small samples of liver tissue prepared as in method 3 were thawed at room temperature during preparation, placed in cylindrical sieves immersed in 1.5ml ice-cold Merchant’s medium and processed as described above.
Comparison of DNA strand break levels in fresh and frozen tissues and cells
We examined the difference in DNA strand break levels in fresh and frozen tissues. Twenty-four female mice (8-week-old, average weight 18.4g, C57BL/6J; Taconic Europe) were injected intraperitoneally (IP) 20ml/kg mouse with sterile saline or 25, 75, 112.5mg/kg of MMS (Sigma Aldrich 129925) in saline. The concentrations were chosen to give response from marginal to severe, based on a small pilot study. One day after IP mice were anaesthetised with a mixture of Hypnorm-Dormicum and killed by total bleeding by cutting the groin artery.
Tissue preparation.
BAL fluid was collected by flushing mouse lungs twice with 0.9% sterile saline through the trachea with volumes corresponding to 1ml/25g mouse. The BAL was immediately placed on ice until the BAL fluid and cells were separated by centrifugation at 4°C and 400g for 10min. The BAL cells were resuspended in 100 μl HAM’s F12 medium [with 1% penicillin-streptomycin, 1% l-glutamine and 10% foetal bovine serum (FBS)]. Forty microlitres of the cell suspension was mixed with 60 μl freezing medium [HAM’s F12 medium with 1% penicillin-streptomycin, 1% l-glutamine, 10% FBS and 10% dimethyl sulphoxide (DMSO)] and: (i) immediately carried to laboratory to be embedded in agarose (fresh) or (ii) stored at −80°C for later comet analysis (frozen II).
Lung and liver were dissected, small samples from the same area were cut (~20–40mg, 3×3×3mm) and: (i) placed in NUNC cryotubes containing Merchant’s medium, immediately processed and embedded in agarose (fresh), (ii) placed in NUNC cryotubes, snap frozen in liquid N2 and stored at −80°C until comet analysis (frozen I) or (iii) placed in NUNC cryotubes, left for 15min on the necropsy table at room temperature and then snap frozen in liquid N2 and stored at −80°C until comet analysis (frozen II).
A549 cells as a historical internal controls
A549 cells were used as an historical internal negative control and H2O2-exposed A549 cells as a positive control, to monitor the day-to-day variation of the comet assay. A549 cells grown for two passages in HAM’s F12 medium (with 1% penicillin-streptomycin, 1% l-glutamine and 10% FBS) were exposed to phosphate-buffered saline (PBS) (negative control), 15, 30 or 60 μM H2O2 (positive control) for 30min at 4°C. Cells were washed in PBS and resuspended in HAM’S F12 medium (10% DMSO, 1% penicillin-streptomycin, 1% l-glutamine and 10% FBS), frozen and stored at −80°C in aliquots of 100 μl with 1×104 cells for the semi-automated system and 25 μl with 1×105 cells for the fully automated system. Each batch was used for ~1 year.
Comet analysis
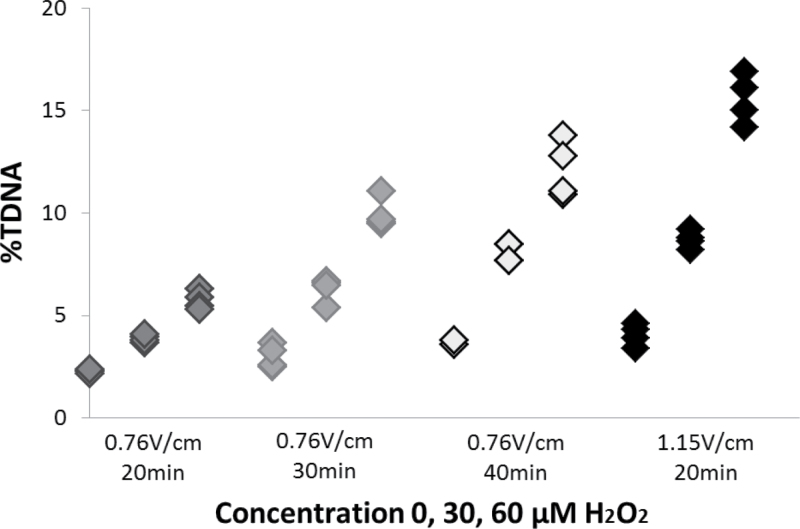
The comet analysis was based on a previously published protocol (24). The method was modified during validation of the fully automated scoring system based on available recommendations (2). Results are presented in Figure 1; and the differences between the protocols are presented in Table I.
Fig. 1.
Effect of electrophoresis strength (voltage and time) on %TDNA of untreated and H2O2-treated (30 and 60 μM) A549 cells. Results were generated by fully automated scoring.
Table I.
Protocols for sample preparation for the semi-automated system and fully automated system
| Protocol differences | Protocol for semi-automated system | Protocol for fully automated system |
|---|---|---|
| Agarose concentration | 0.6% (final) | 0.7% (final) |
| Slides | GelBond® film (Lonza Rockland Inc., Rockland, Maine, USA) (10 μl per well for 40-well format) | TrevigenCometSlides™ (Trevigen®, Gaithersburg, Maryland, USA) (30 μl per well for 20-well slide) |
| Alkaline treatment | 40 min | 40 min |
| Electrophoresis | 25V (0.76V/cm), 292–296 mA, 20 min | 38V (1.15V/cm), 292–296 mA, 25–30 min |
| Neutralisation | 2×5 min | 2×5 min |
| Fixing | 1.5h in 96% ethanol | 5min in 96% ethanol, 15min at 45°C |
| Staining | 20ml bath TE buffered SYBR Gold [Molecular probes®, Eugene, Oregon, USA (purchased from Sigma-Aldrich); 1:10 000] for 10min, wet gel placed on glass slide and cover slip applied | 20ml bath TE buffered SYBR® Green (Lonza Bioscience, Fisher Scientific, Slangerup, Denmark) for 15min, dried 10min at 37°C, ultraviolet filter and cover slip applied |
| Scoring system | KOMET 3.9 and 6 Scored within 2 days |
IMSTAR PathFinder™ Scored same day |
| Comets scored | 50 cells/well | All cells recognised 111–3414/well |
BAL cell suspensions and A549 cells in freezing medium were thawed quickly, one to five samples at a time at 37°C, and embedded rapidly in agarose (low melting point agarose in PBS buffer) with single or multi-pipette onto gels/slides. Organs, fresh or deep frozen were pressed through a stainless steel cylindrical sieve (diameter 0.5cm, mesh size 0.4mm) into 1.5ml ice-cold Merchant’s medium (0.14M NaCl, 1.47mM KH2PO4, 2.7mM KCl, 8.1mM Na2HPO4, 10mM Na2EDTA, pH 7.4) (35). Aliquots of cell suspensions were then rapidly embedded in agarose. The method is described in detail under tissue sampling techniques, method 1. When all the samples were placed on the slides, slides were immersed into 4°C cold lysing solution (2.5M NaCl, 10mM Tris, 100mM Na2EDTA, 1% sodium sarcosinate, 10% DMSO, 1% Triton X-100, pH 10) and kept overnight at 4°C. Slides were rinsed in cold electrophoresis buffer, alkaline treated in ice-cold electrophoresis buffer directly in electrophoresis chamber (0.3M NaOH, 1mM Na2EDTA, pH 13.2) placed on ice and subjected to electrophoresis (pH >13) with 4°C cold circulating buffer. Slides were rinsed in neutralisation buffer and neutralised (0.4M Tris, pH 7.5), the gels/slides were fixed (95% ethanol), stained [SYBR® Gold or SYBR® Green in TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 7.6), pH 7.5 or 8.0, respectively] and comets were scored. DNA damage was quantified as %DNA in tail (%TDNA) and tail length (TL), mean values for all cells counted per well/sample. Electrophoresis efficiency was evaluated by including unexposed and H2O2-exposed A549 cells from a single batch exposure, as negative and positive controls, respectively. The samples were scored by semi-automated system KOMET 3.9 (pilot study with sampling techniques and batches 1–9 for internal A549 cell controls), by semi-automated system KOMET 6 (validation of PathFinder™ system from IMSTAR) and by fully automated PathFinder™ system from IMSTAR (MMS study and batches 10 and 11 for internal A549 cell controls). As a quality control, random sections of all samples analysed by the fully automated PathFinder™ system from IMSTAR were manually viewed to confirm that cells and not artefacts were scored, to assure the validity of the analysis.
Statistics
Effect of tissue sampling techniques on DNA strand breaks.
The overall differences between the levels of %TDNA for different treatments (method 1, 2, 3 and 4) were analysed by non-parametric one-way analysis of variance (Kruskal–Wallis), followed by Tukey’s type multiple comparison test. Analysis was performed in SAS version 9.2.
Comparison of DNA strand break levels in fresh and frozen tissues and cells.
The differences between the levels of %TDNA and TL were analysed by analysis of variance with concentration (0, 25, 75, 112.5mg/kg) and treatment (fresh, frozen I and frozen II) as factors. Analysis was performed in SYSTAT Software package 9.
A549 cells as a historical internal controls.
The DNA strand break levels (%TDNA and TL) differences between untreated and H2O2-treated A549 cells were registered for a period of more than 10 years and evaluated by analysis of variance with batch (1–11), date of electrophoresis, exposure (0, 15, 30 or 60 μm H2O2) and scoring method (semi-automated or fully automated) as factors. Date of electrophoresis was nested under batch and the whole analysis was stratified by scoring method; semi-automated (n = 480) vs. fully automated (n = 648). Proc GLM in SAS version 9.3 was used to implement the analyses. Only main effects were considered.
Results
Effect of tissue sampling techniques on DNA strand breaks
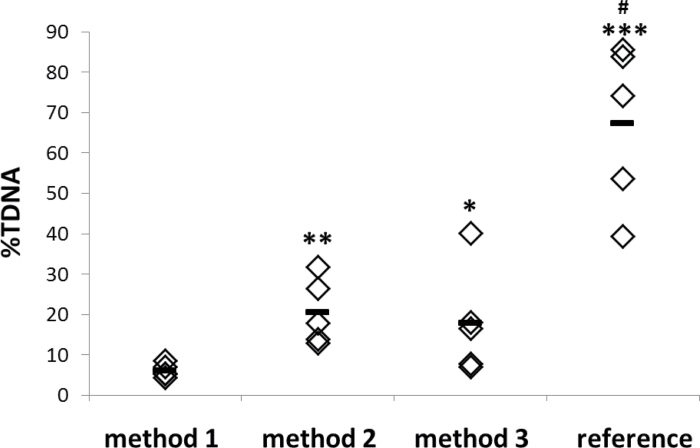
The sample preparation of frozen tissue affected the DNA strand break levels (P = 0.002) (Figure 2). Cutting a small tissue sample at necropsy and storing it deep frozen until comet analysis (method 1) resulted in low DNA strand break levels, with limited variation (%TDNA 6.15±1.62 SD). Cutting a frozen whole tissue on a deep cold metal plate (method 2) induced 3.7-fold higher DNA strand break levels compared with method 1 (%TDNA 20.58±8.17; P < 0.01). Crushing whole tissue in liquid N2 in porcelain mortar (method 3) resulted in 3.4-fold higher DNA strand break levels compared with method 1 (%TDNA 17.92±13.35; P < 0.05). Thawed tissue had significantly higher DNA strand break levels compared with all the other methods (%TDNA 67.28±20.14, P < 0.01), indicating the importance of preventing unintentional thawing of the samples. Thus, cutting tissues prior to freezing (method 1) yielded the lowest background level of DNA strand breaks, followed by mortaring frozen tissue in liquid N2 (method 3) or cutting the frozen tissue on a deep frozen metal plate (method 2).
Fig. 2.
DNA strand break levels (%TDNA) for tissues collected using different sampling techniques. Method 1: liver tissue designated for comet analysis was cut directly at necropsy, snap frozen in liquid N2 and stored in −80°C until comet analysis; method 2: larger samples of liver tissue were collected at necropsy, snap frozen in liquid N2, frozen at −80°C, samples were cut on a deep cold metal and stored in −80°C until comet analysis; method 3: larger samples of liver tissue were collected at necropsy, snap frozen in liquid N2, stored at −80°C, samples were crushed in a porcelain mortar with liquid N2 and stored in −80°C until comet analysis; reference treatment: small samples of liver tissue prepared by crushing in N2 were thawed at room temperature during preparation. *P < 0.05 different from method 1; **P < 0.01 different from method 1; ***P < 0.001 different from method 1; # P < 0.01 different from methods 1, 2 and 3.
Comparison of DNA strand break levels in fresh and frozen tissues and cells
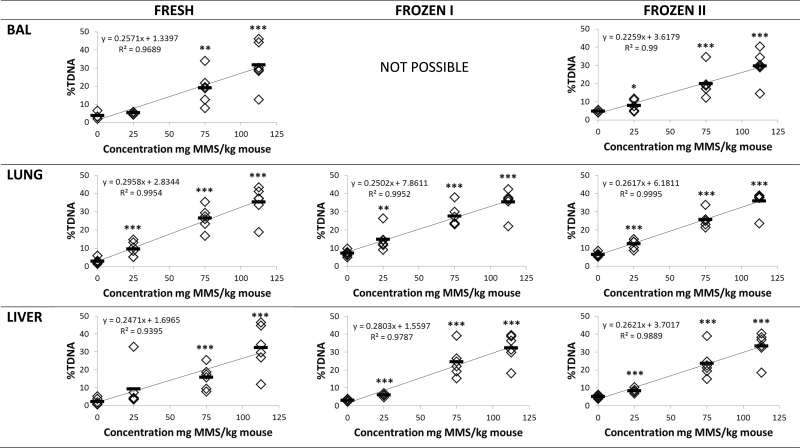
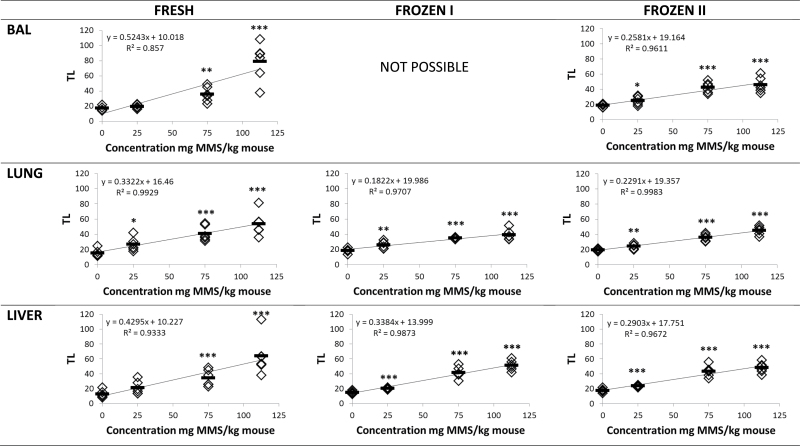
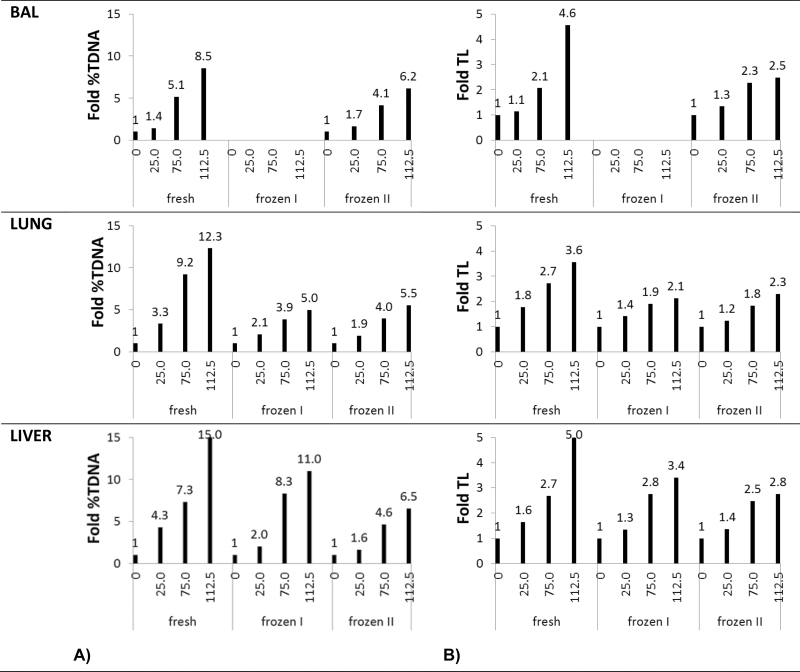
Tissues from MMS-treated mice were analysed as fresh (fresh), frozen immediately after dissection (frozen I) or frozen with 15-min delay (frozen II). MMS treatment (25, 75, 112.5mg/kg) induced DNA stand breaks measured as %TDNA and TL in a dose-dependent manner in BAL cells, lung and liver (P < 0.001) (Figures 3 and 4). For all preparation methods, correlation coefficients were very high, thus, R 2 values were between 0.857 and 0.995 for both %DNA and TL. When the three different tissue preparation methods were compared, no overall statistically significant difference was observed in %TDNA and TL (P > 0.1). To thoroughly assess the data set, the results were also analysed grouped by exposure levels (controls, 25, 75 and 112.5mg/kg MMS). Overall, there was no difference between the differently treated samples in the same exposure level (P > 0.1) except that %TDNA in frozen control lung and liver tissue was significantly increased (P < 0.01); the TL in the BAL cells from mice exposed to 112.5mg/kg MMS was significantly reduced (P = 0.01; Table II).
Fig. 3.
DNA strand break levels (%TDNA) in BAL, lung and liver tissues from mice injected IP 20ml/kg with saline or 25, 75, 112.5mg/kg MMS. Samples were either analysed fresh, frozen immediately by snap freezing in liquid N2 (frozen I) and snap frozen in liquid N2 after a 15-min delay on the necropsy table at room temperature (frozen II). Frozen samples were stored at −80°C until comet analysis. *P < 0.05, **P < 0.01, ***P < 0.001 different from saline IP injected control mice.
Fig. 4.
DNA strand break levels (TL) in BAL, lung and liver tissues from mice injected IP 20ml/kg with saline or 25, 75, 112.5mg/kg MMS. Samples were either analysed fresh, frozen immediately by snap freezing in liquid N2 (frozen I) and snap frozen in liquid N2 after a 15-min delay on the necropsy table at room temperature (frozen II). Frozen samples were stored at −80°C until comet analysis. *P < 0.05, **P < 0.01, ***P < 0.001 different from saline IP injected control mice.
Table II.
DNA strand break levels (%TDNA, TL) in fresh or frozen BAL cell, lung and liver tissues from untreated mice and mice treated to 112.5 mg/kg of MMS
| %DNA | TL | ||||||
|---|---|---|---|---|---|---|---|
| Fresh | Frozen I | Frozen II | Fresh | Frozen I | Frozen II | ||
| Controls | BAL | 3.7 | — | 4.8 (1.3-fold) | 17.3 | — | 18.5 (1.1-fold) |
| Lung | 2.9 | 7.1 (2.5-fold)** | 6.5 (2.3-fold)** | 15.1 | 18.4 (1.2-fold) | 19.8 (1.3-fold) | |
| Liver | 2.2 | 3.0 (1.4-fold) | 5.1 (2.4-fold)* | 12.8 | 15.0 (1.2-fold) | 17.5 (1.3-fold) | |
| High dose | BAL | 31.8 | — | 29.7 (0.9-fold) | 78.9 | — | 45.9 (0.6-fold)* |
| Lung | 35.3 | 35.3 (1.0-fold) | 35.8 (1.0-fold) | 53.7 | 39.3 (0.7-fold) | 45.4 (0.9-fold) | |
| Liver | 32.2 | 32.4 (1.0-fold) | 33.4 (1.0-fold) | 63.9 | 51.2 (0.8-fold) | 48.1 (0.8-fold) | |
The observed fold difference compared with fresh tissue is presented in brackets.
*P < 0.05, **P < 0.01 different from fresh tissue.
The observed fold increase over control in both %TDNA and TL was smaller when using frozen tissue, compared with using fresh tissue (Figure 5). The main reason for the lowered fold induction was slightly higher background levels of %TDNA in controls when using frozen tissue (Table II).
Fig. 5.
Fold increase over control for DNA strand break levels (A for %TDNA, B for TL) in BAL, lung and liver tissues from mice injected IP with 20ml/kg with saline or 25, 75, 112.5mg/kg MMS. Samples were either analysed as fresh, frozen immediately by snap freezing in liquid N2 (frozen I) and snap frozen in liquid N2 after 15-min delay on the necropsy table at room temperature (frozen II). Frozen samples were stored at −80°C until comet analysis. The lowered fold induction in frozen tissues compared with fresh tissues is caused by the slightly increased DNA strand break levels in the controls (presented in Table II).
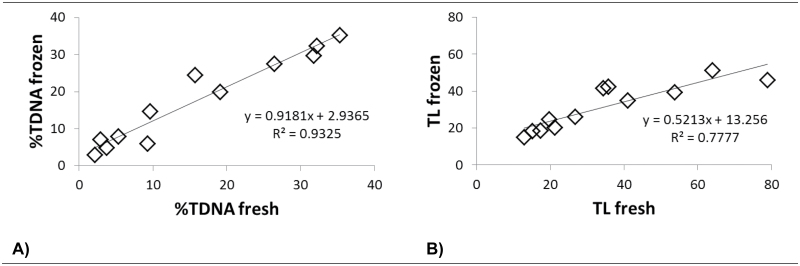
Overall, the correlation between DNA strand break levels in fresh and frozen tissues was R 2 = 0.93 for %TDNA and R 2 = 0.78 for TL, for samples fresh vs. BAL frozen II, lung and liver frozen I (Figure 6).
Fig. 6.
Correlation between DNA strand break levels (A for %TDNA, B for TL) in fresh and frozen tissues (BAL frozen II; lung and liver frozen I).
A549 cells as a historical internal controls
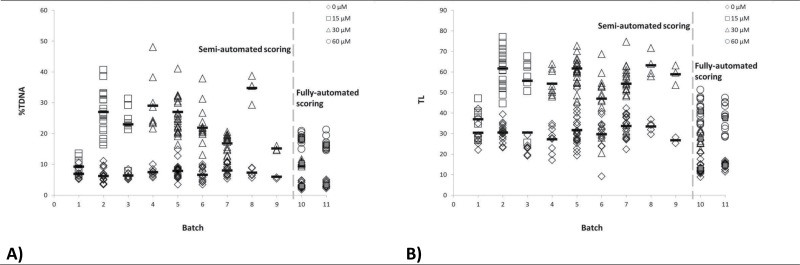
Untreated and H2O2-treated A549 cells were used as negative and positive historical controls for the electrophoresis performed over a 10-year period, with each A549 batch lasting ~1 year. Here, we use the historical data to assess the day-to-day variation of the comet assay and at the same time to assess the effect of prolonged storage at −80°C on DNA strand break levels (Figure 7A and B). The first nine batches of frozen A549 cells were scored using semi-automated comet scoring system, whereas batches 10 and 11 were scored by fully automated scoring system. Other experimental procedures including electrophoresis time and voltage, and staining procedure also differed between the two scoring methods (Table I). In the semi-automated scoring, each observation consisted of 50 cells scored per well on two separate slides (100 in total), run in the same electrophoresis. In the fully automated scoring, one observation consists of the mean of ~700 cells in 2 of 20 wells on each slide, run in the same electrophoresis (minimum eight observations per control per electrophoresis).
Fig. 7.
DNA strand break levels (electrophoresis average; A for %TDNA, B for TL) for untreated and H2O2-treated A549 cells used as negative and positive historical controls for the electrophoresis performed over a 10-year period. A549 cells were exposed to concentrations 15, 30 or 60 μM H2O2. Results were generated by a semi-automated scoring (one observation = 50 cells/well on two slides) or a fully automated scoring (one observation = ~700 cells/well on eight slides). The day-to-day variation was 9–12% in the semi-automated system and 2–6% in the fully automated system.
The 1128 observations (480 observations in batches 1–9 and 648 observations in batches 10 and 11) were analysed by a simple statistical model with %TDNA and TL as dependent variable and batch (n = 11), date of electrophoresis (n = 142) and exposure (0, 15, 30 or 60 μm H2O2) as explanatory variables stratified by scoring method (semi-automated or fully automated). Of the variation, 75–79% was explained by these three variables, for %TDNA, and TL for both scoring systems. ‘Exposure (0, 15, 30 or 60 μm H2O2)’ explained 57–79% of the variation, for %TDNA and TL, for both scoring systems (P < 0.0001). ‘Batch’ explained 9 and 5% of the variation in %TDNA and TL, respectively, for the semi-automated system (P < 0.0001); and 0.2% of %TDNA variation for the fully automated system (P < 0.003), where TL was not significantly different between batches. The ‘date of electrophoresis’ (the day-to-day variation) explained 9 and 12% of %TDNA and TL variation for the semi-automated system (P = 0.02 and P < 0.001, respectively); and 2 and 6% of %TDNA and TL variation for the fully automated system (P < 0.001). The fully automated system was validated by comparison of results with the semi-automated system KOMET 6 (Figure 8). Thus, the day-to-day variation of the comet assay was 9–12% for the semi-automated system and 2–6% for the fully automated system. Data from the fully automated system represent A549 cells that had been frozen for up to 1 year and more. The low day-to-day variation for the fully automated system therefore indicates that prolonged storage at −80°C has little impact on DNA strand break levels in A549 cells.
Fig. 8.
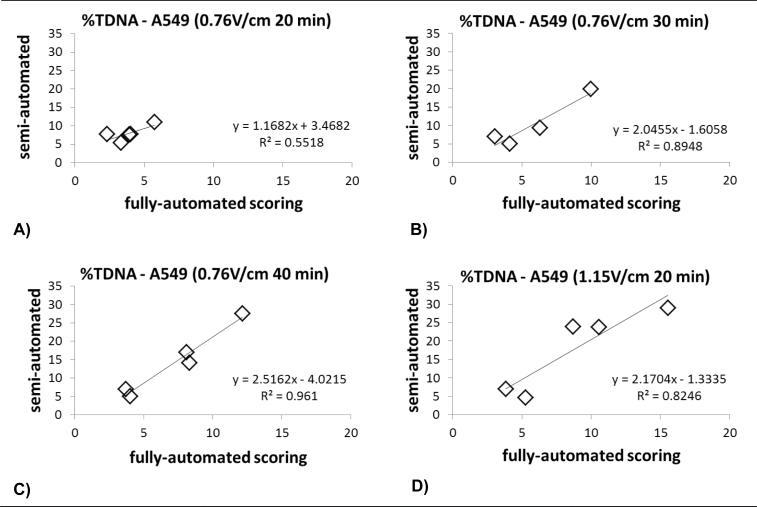
Correlation between results scored on semi-automated and fully automated scoring system for %TDNA of untreated and H2O2-treated (30 and 60 μM) A549 cells. A549 cell were run on electrophoresis with increasing electrophoresis time (A–C) and voltage (D). The best correlation between the two systems was at electrophoresis 1.15V/cm for 20min. After further validation, the recommended electrophoresis time is 25–30min.
Discussion
Here, we have compared the use of fresh and frozen tissues and cells in a high-throughput comet assay with fully automatic scoring. We have confirmed that DNA strand breaks can be detected in both fresh and frozen BAL cells, and lung and liver tissue from MMS-treated mice. Additionally, we have assessed different sampling techniques of frozen tissues for comet analysis and suggest that immediate freezing of a small piece of tissue designated for comet analysis is the preferable method. This procedure generated the least variation, probably due to the limited handling of the deep frozen samples. If this step is not possible, the alternative procedure is to crush a larger frozen tissue section using a mortar in liquid N2; however, a higher variation has to be anticipated. We also present day-to-day variation of frozen untreated and H2O2-treated A549 cells based on data collected over a 10-year period, to demonstrate the limited day-to-day variation and thus the feasibility of this approach.
Work with frozen tissues requires great care. On the one hand, we show that a 15-min delay prior to freezing does not affect the DNA damage levels significantly (Figures 3 and 4). On the other hand, thawing damages the DNA and increases DNA strand break levels in the tested tissue (Figure 2). Cells frozen with DMSO (blood cells or cell lines) have previously been used for comet assay validation (2,7–15). Effect of freezing tissues as cell suspensions frozen in 10% DMSO was investigated using rats exposed by repeated oral gavage to ethyl methanesulphonate (16). DNA strand break levels in fresh and frozen blood, liver and stomach samples were similar at all exposure levels collected 3h after exposure, even in tissues that had undergone transportation. Colon samples were reported to be more sensitive and were affected by transportation. In our experience (22,28,32), colon was equally difficult to work with as other tissues, with relatively low background levels. Whole blood samples frozen in small volume without prior treatment have been shown to give reliable comet data (19).
Here, we present a similar method for freezing tissue. We froze a small sample of tissue at −80°C and kept it deep frozen until homogenisation in Merchant’s medium. When comparing DNA strand break levels in tissues from MMS-treated mice, fresh and frozen BAL, lung and liver samples resulted in virtually similar levels. The only difference was a slightly increased %TDNA in the background level for the frozen tissues and a slightly reduced TL in high doses in the frozen cells. The slightly increased background may be suspected to affect sensitivity of the assay. However, despite the slight reduction in the fold change over control in frozen tissues presented in Figure 5, the DNA stand break levels at the lowest MMS dose were statistically significantly increased. Thus, even small increases of ~20% in DNA strand break levels could be detected in the frozen tissues. The used semi-automated system and fully automated system automatically identify the cells that are scored. However, neither system will recognise highly damaged nor apoptotic cells, also called hedgehogs. Consequently, hedgehogs are not quantified and highly damaged samples will appear as having very low numbers of scored cells when using automated scoring. As a quality measure, we routinely manually screen all samples and pay special attention to samples with unexpected low numbers of scored cells. However, in our experience, the number of hedgehogs is very small and does not seem to change with exposure except in positive controls when high doses are used.
There are a few critical steps during tissue preparation and handling, where frozen tissues can be thawed and consequently damaged, including tissue sorting and transport. It is not always possible to sort samples frozen in liquid N2 directly during collection. Great care during sorting is necessary to assure that samples are not warmed up during this process, especially since tissue samples dedicated for comet analysis are very small and therefore thaw easily. We usually place the samples frozen in liquid N2 on dry ice and quickly place them unsorted at −80°C. Then, we sort small portions at a time. For transport, samples have to be packed in plenty of dry ice, to maintain the frozen conditions. Samples are also stored on dry ice before analysis and they are homogenised into Merchant’s medium while still frozen.
We have used batches of frozen historical internal negative and positive controls in every comet analysis for more than 10 years. We prepared batches of cultured A549 epithelial lung cells unexposed and exposed to H2O2. Batches were prepared about once a year and frozen in small aliquots in HAM’s F12 medium with 10% DMSO. Controls are included in all electrophoreses, as a positive control of the successful electrophoresis and to control the day-to-day variation. We demonstrate that the comet assay has run consistently for over 10 years in our laboratory with a day-to-day variation of 9–12% in semi-automated system and 2–6% in fully automated system. For the fully automated system, the day-to-day variation was assessed for an 18-month period (including two different A549 batches) and thus the observed 2–6% day-to-day variation indicates that prolonged freezing had little impact on DNA strand break levels. It is possible to freeze, thaw and run the cells in comet assay with consistent results. The observed variation between batches of 0.2–9% may be partly explained by the deterioration of H2O2 over time, it may also be influenced by the harvesting process and freezing procedure. We strongly recommend using frozen cells, untreated and damaged (with H2O2, radiation or other), which represent the dynamic range of damage for the target exposure as quality controls in comet assay experiments.
We have performed comet assay analysis using different high-throughput protocols. First, we used a protocol similar as described in references (4,18). Forty samples were applied on GelBond® film and four gels were electrophoresed simultaneously. To expedite the scoring process, we obtained a fully automated PathFinder™ system from IMSTAR. Comets can be scored visually and given arbitrary units, or by semi-automated or fully automated analysis. All three scoring methods generally agree within acceptable limits (3). Visual scoring systematically overestimates low levels of damage, while heavily damaged comets are less efficiently detected with image analysis. Both visual and semi-automated scoring methods are time consuming, strenuous and with number of samples being the bottleneck of the analysis. Therefore, a fully automated comet scoring analysis, such as the IMSTAR PathFinder™ system used by our laboratory, is more efficient. The system is programmed to localise nuclei and analyse the level of DNA strand breaks. Slide preparation is a crucial step in successful analysis. For the microscope to focus properly, the cells have to be localised in one plane and an optimal cell number has to be achieved to simplify the analysis. Scoring of 300–600 cells/sample will increase the likelihood that even small differences in DNA strand break levels are detected (17). The high-throughput method with fully automated scoring allows analysis of several hundreds of samples in 1 week.
We changed the moulding platform to 20-well Trevigen CometSlides™ stained with SYBR® Green for the high-throughput method. We optimised the electrophoresis voltage and length based on available recommendations (2). In agreement with previous findings, we observed that increased electrophoresis time and strength resulted in more DNA strand breaks in the tail. This improved comet readings by the fully automated system and the correlation with the semi-automated system. We currently use electrophoresis strength of 1.15V/cm and 25-min duration.
In conclusion, we have validated the use of frozen cells and tissues in a high-throughput protocol with fully automated scoring of DNA strand breaks using the comet assay. We suggest that a small block of tissue designated for comet analysis should be frozen immediately at tissue collection and kept deep frozen until embedding in agarose. The use of frozen tissues allows for screening of large numbers of samples collected over a longer time span, and furthermore increases the number of samples that can be processed within a day. Use of frozen tissues benefits the use of the comet assay in toxicological studies and will enable the use of the comet assay in prospective epidemiological studies.
Funding
Danish Centre for Nanosafety (grant 20110092173/3); Danish Agency for Science, Technology and Innovation (PhD scholarship to P.J.).
Acknowledgements
Technical assistance from Gitte Kristiansen, Michael Guldbrandsen and Eva Terrida is greatly appreciated. The authors are grateful to Harald Hannerz for statistical analysis of the historical internal controls.
Conflict of interest statement: None declared.
References
- 1. Azqueta A., Lorenzo Y., Collins A. R. (2009). In vitro comet assay for DNA repair: a warning concerning application to cultured cells. Mutagenesis, 24, 379–381 [DOI] [PubMed] [Google Scholar]
- 2. Azqueta A., Gutzkow K. B., Brunborg G., Collins A. R. (2011). Towards a more reliable comet assay: optimising agarose concentration, unwinding time and electrophoresis conditions. Mutat. Res., 724, 41–45 [DOI] [PubMed] [Google Scholar]
- 3. Azqueta A., Meier S., Priestley C., Gutzkow K. B., Brunborg G., Sallette J., Soussaline F., Collins A. (2011). The influence of scoring method on variability in results obtained with the comet assay. Mutagenesis, 26, 393–399 [DOI] [PubMed] [Google Scholar]
- 4. Azqueta A., Gutzkow K. B., Priestley C. C., Meier S., Walker J. S., Brunborg G., Collins A. R. (2013). A comparative performance test of standard, medium- and high-throughput comet assays. Toxicol. In Vitro, 27, 768–773 [DOI] [PubMed] [Google Scholar]
- 5. Azqueta A., Arbillaga L., López de Cerain A., Collins A. (2013). Enhancing the sensitivity of the comet assay as a genotoxicity test, by combining it with bacterial repair enzyme FPG. Mutagenesis, 28, 271–277 [DOI] [PubMed] [Google Scholar]
- 6. Azqueta A., Collins A. R. (2013). The essential comet assay: a comprehensive guide to measuring DNA damage and repair. Arch. Toxicol., 87, 949–968 [DOI] [PubMed] [Google Scholar]
- 7. Ersson C., Möller L. (2011). The effects on DNA migration of altering parameters in the comet assay protocol such as agarose density, electrophoresis conditions and durations of the enzyme or the alkaline treatments. Mutagenesis, 26, 689–695 [DOI] [PubMed] [Google Scholar]
- 8. Ersson C., Møller P., Forchhammer L., et al. (2013). An ECVAG inter-laboratory validation study of the comet assay: inter-laboratory and intra-laboratory variations of DNA strand breaks and FPG-sensitive sites in human mononuclear cells. Mutagenesis, 28, 279–286 [DOI] [PubMed] [Google Scholar]
- 9. Forchhammer L., Bräuner E. V., Folkmann J. K., Danielsen P. H., Nielsen C., Jensen A., Loft S., Friis G., Møller P. (2008). Variation in assessment of oxidatively damaged DNA in mononuclear blood cells by the comet assay with visual scoring. Mutagenesis, 23, 223–231 [DOI] [PubMed] [Google Scholar]
- 10. Forchhammer L., Johansson C., Loft S., et al. (2010). Variation in the measurement of DNA damage by comet assay measured by the ECVAG inter-laboratory validation trial. Mutagenesis, 25, 113–123 [DOI] [PubMed] [Google Scholar]
- 11. Forchhammer L., Ersson C., Loft S., et al. (2012). Inter-laboratory variation in DNA damage using a standard comet assay protocol. Mutagenesis, 27, 665–672 [DOI] [PubMed] [Google Scholar]
- 12. Johansson C., Møller P., Forchhammer L., et al. (2010). An ECVAG trial on assessment of oxidative damage to DNA measured by the comet assay. Mutagenesis, 25, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Møller P., Friis G., Christensen P. H., et al. (2004). Intra-laboratory comet assay sample scoring exercise for determination of formamidopyrimidine DNA glycosylase sites in human mononuclear blood cell DNA. Free Radic. Res., 38, 1207–1214 [DOI] [PubMed] [Google Scholar]
- 14. Møller P. (2006). The alkaline comet assay: towards validation in biomonitoring of DNA damaging exposures. Basic Clin. Pharmacol. Toxicol., 98, 336–345 [DOI] [PubMed] [Google Scholar]
- 15. Møller P., Möller L., Godschalk R. W., Jones G. D. (2010). Assessment and reduction of comet assay variation in relation to DNA damage: studies from the European Comet Assay Validation Group. Mutagenesis, 25, 109–111 [DOI] [PubMed] [Google Scholar]
- 16. Recio L., Kissling G. E., Hobbs C. A., Witt K. L. (2012). Comparison of Comet assay dose-response for ethyl methanesulfonate using freshly prepared versus cryopreserved tissues. Environ. Mol. Mutagen., 53, 101–113 [DOI] [PubMed] [Google Scholar]
- 17. Sharma A. K., Soussaline F., Sallette J., Dybdahl M. (2012). The influence of the number of cells scored on the sensitivity in the comet assay. Mutat. Res., 749, 70–75 [DOI] [PubMed] [Google Scholar]
- 18. Gutzkow K. B., Langleite T. M., Meier S., Graupner A., Collins A. R., Brunborg G. (2013). High-throughput comet assay using 96 minigels. Mutagenesis, 28, 333–340 [DOI] [PubMed] [Google Scholar]
- 19. Al-Salmani K., Abbas H. H., Schulpen S., et al. (2011). Simplified method for the collection, storage, and comet assay analysis of DNA damage in whole blood. Free Radic. Biol. Med., 51, 719–725 [DOI] [PubMed] [Google Scholar]
- 20. Bornholdt J., Dybdahl M., Vogel U., Hansen M., Loft S., Wallin H. (2002). Inhalation of ozone induces DNA strand breaks and inflammation in mice. Mutat. Res., 520, 63–71 [DOI] [PubMed] [Google Scholar]
- 21. Bourdon J. A., Saber A. T., Jacobsen N. R., et al. (2012). Carbon black nanoparticle instillation induces sustained inflammation and genotoxicity in mouse lung and liver. Part. Fibre Toxicol., 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dybdahl M., Risom L., Møller P., et al. (2003). DNA adduct formation and oxidative stress in colon and liver of Big Blue rats after dietary exposure to diesel particles. Carcinogenesis, 24, 1759–1766 [DOI] [PubMed] [Google Scholar]
- 23. Dybdahl M., Risom L., Bornholdt J., Autrup H., Loft S., Wallin H. (2004). Inflammatory and genotoxic effects of diesel particles in vitro and in vivo. Mutat. Res., 562, 119–131 [DOI] [PubMed] [Google Scholar]
- 24. Jackson P., Hougaard K. S., Boisen A. M., et al. (2012). Pulmonary exposure to carbon black by inhalation or instillation in pregnant mice: effects on liver DNA strand breaks in dams and offspring. Nanotoxicology, 6, 486–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson P., Halappanavar S., Hougaard K. S., et al. (2013). Maternal inhalation of surface-coated nanosized titanium dioxide (UV-Titan) in C57BL/6 mice: effects in prenatally exposed offspring on hepatic DNA damage and gene expression. Nanotoxicology, 7, 85–96 [DOI] [PubMed] [Google Scholar]
- 26. Jacobsen N. R., Møller P., Jensen K. A., Vogel U., Ladefoged O., Loft S., Wallin H. (2009). Lung inflammation and genotoxicity following pulmonary exposure to nanoparticles in ApoE-/- mice. Part. Fibre Toxicol., 6, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Madsen A. M., Saber A. T., Nordly P., Sharma A. K., Wallin H., Vogel U. (2008). Inflammation but no DNA (deoxyribonucleic acid) damage in mice exposed to airborne dust from a biofuel plant. Scand. J. Work. Environ. Health, 34, 278–277 [DOI] [PubMed] [Google Scholar]
- 28. Møller P., Wallin H., Vogel U., et al. (2002). Mutagenicity of 2-amino-3-methylimidazo[4,5-f]quinoline in colon and liver of Big Blue rats: role of DNA adducts, strand breaks, DNA repair and oxidative stress. Carcinogenesis, 23, 1379–1385 [DOI] [PubMed] [Google Scholar]
- 29. Saber A. T., Jensen K. A., Jacobsen N. R., Birkedal R., Mikkelsen L., Møller P., Loft S., Wallin H., Vogel U. (2012). Inflammatory and genotoxic effects of nanoparticles designed for inclusion in paints and lacquers. Nanotoxicology, 6, 453–471 [DOI] [PubMed] [Google Scholar]
- 30. Saber A. T., Koponen I. K., Jensen K. A., Jacobsen N. R., Mikkelsen L., Møller P., Loft S., Vogel U., Wallin H. (2012). Inflammatory and genotoxic effects of sanding dust generated from nanoparticle-containing paints and lacquers. Nanotoxicology, 6, 776–788 [DOI] [PubMed] [Google Scholar]
- 31. Saber A. T., Jacobsen N. R., Mortensen A., et al. (2012). Nanotitanium dioxide toxicity in mouse lung is reduced in sanding dust from paint. Part. Fibre Toxicol., 9, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Danielsen P. H., Risom L., Wallin H., Autrup H., Vogel U., Loft S., Møller P. (2008). DNA damage in rats after a single oral exposure to diesel exhaust particles. Mutat. Res., 637, 49–55 [DOI] [PubMed] [Google Scholar]
- 33. Jackson P., Lund S. P., Kristiansen G., Andersen O., Vogel U., Wallin H., Hougaard K. S. (2011). An experimental protocol for maternal pulmonary exposure in developmental toxicology. Basic Clin. Pharmacol. Toxicol., 108, 202–207 [DOI] [PubMed] [Google Scholar]
- 34. Hougaard K. S., Jackson P., Jensen K. A., et al. (2010). Effects of prenatal exposure to surface-coated nanosized titanium dioxide (UV-Titan). A study in mice. Part. Fibre Toxicol., 7, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brunborg G., Søderlund E. J., Holme J. A., Dybing E. (1996). Organ-specific and transplacental DNA damage and its repair in rats treated with 1,2-dibromo-3-chloropropane. Chem. Biol. Interact., 101, 33–48 [DOI] [PubMed] [Google Scholar]