Abstract
Influenza virus infection has the potential to induce excess pulmonary inflammation and massive tissue damage in the infected host. Conventional CD4+ and CD8+ as well as nonconventional innate like T cells respond to infection and make an essential contribution to the clearance of virus infected cells and the resolution of pulmonary inflammation and injury. Emerging evidence in recent years has suggested a critical role of local interactions between lung effector T cells and antigen presenting cells in guiding the accumulation, differentiation and function of effector T cells beyond their initial activation in the draining lymph nodes during influenza infection. As such, lung effector CD4+ and CD8+ T cells utilize multiple effector and regulatory mechanisms to eliminate virus infected cells as well as fine tune the control of pulmonary inflammation and injury. Elucidating the mechanisms by which conventional and nonconventional T cells orchestrate their response in the lung as well as defining the downstream events required for the resolution of influenza infection will be important areas of future basic research which in turn may result in new therapeutic strategies to control the severity of influenza virus infection.
Introduction
Influenza virus is a leading cause of upper and lower respiratory infection and constitutes an ongoing threat to global health. Current strategies for influenza prevention and treatment include yearly vaccination and anti-viral drugs. However, frequent changes in the surface antigens of influenza virus due to the antigenic shift and drift allow influenza viruses to escape antibody-mediated immunity following vaccination. Anti-viral treatment is generally only effective during a very short time period early after influenza infection, and furthermore, many circulating influenza virus strains have developed resistance to the current antiviral drugs. Thus, it is of urgent need to understand the pathophysiology and the protective immune responses to influenza virus infection for the development of future preventive and therapeutic means. In this article, we review recent advances in the understanding of the role of adaptive CD4+ and CD8+ T cell immune responses as well as nonconventional T lymphocytes in the protection and recovery of influenza infection with special focuses on the effector mechanisms and the regulation of the T cell responses in the infected site, i.e. the lungs.
Role of CD8+ T cells in anti-influenza immunity
Local control of CD8+ T cell responses in the lung
Following influenza infection, respiratory dendritic cells (DCs), mainly CD103+ DCs, carry influenza antigen and migrate to the draining lymph nodes (dLN) to encounter naive CD8+ (and CD4+) T cells therein [1]. Subsequently, influenza specific CD8+ T cells undergo a stepwise process of activation, proliferation and differentiation to become effector T cells and migrate to the lung to eliminate virus infected cells. Classically, the activation and differentiation of CD8+ T cells are believed to be completed in the secondary lymphoid organs. However, recent advances have suggested that CD8+ effector T cells receive additional signals from local antigen presenting cells (APCs), in particular DCs, to guide their further activation, differentiation and effector activities upon arrival to the lung. Studies from Mcgill et al have demonstrated that the depletion of lung DCs after T cell activation in the dLNs resulted in diminished maintenance and accumulation of CD8+ effector T cells in the lung and thus impaired the clearance of virus in the respiratory tract [2]. Further studies have demonstrated that lung DCs provide cognate antigens and trans-presented IL-15 to promote the proliferation and survival of local effector CD8+ T cells [2,3]. Interestingly, similar to the activation and expansion of naïve CD8+ T cells, Dolfi et al demonstrated that effector T cells require CD28 co-stimulation from lung DCs to sustain their proliferation and survival in the lung [4]. Besides providing signals required for the proliferation and survival of effector T cells, local APCs also produce cytokines to drive further differentiation of CD8+ effector T cells in the lung. For example, effector CD8+ T cells activated in the draining LN are capable of producing high levels of effector cytokine IFN-γ but minimal regulatory cytokine IL-10 [5]. Upon migration to the infected lungs, effector CD8+ T cells acutely acquire the capacity to produce the regulatory cytokine IL-10 to dampen respiratory inflammation associated with the anti-viral immune response (see below) [5]. Lung local APCs, including DCs, macrophages and neutrophils, produce the cytokine IL-27 and play important roles in driving the acquisition process of IL-10 production by lung effector T cells [6]. Thus, the interaction of lung APCs and effector CD8+ T cells in the lung promote further differentiation of effector T cells (i.e. acquisition of a regulatory feature).
Activated CD8+ T cells are capable of producing effector cytokines and cytolytic molecules, however, they do not spontaneous secrete effector cytokines and release cytolytic molecules without further interaction with antigen bearing target cells, including CD45+ APCs and influenza infected epithelial cells localized within the infected respiratory tract [7,8]. Interestingly, effector T cells exhibit differential effector activities when interacted with CD11c+ APCs or epithelial cells. The interaction with lung CD11c+ APCs triggers effector T cells to release both cytolytic molecules and effector cytokines, while the interaction with epithelial cell triggers the release of cytolytic molecules but not effector cytokines [7]. Clearly, both epithelial cells and CD11c+ APCs bear influenza antigen and so can stimulate effector T cells via TCR signaling. However, lung CD11c+ APCs but not epithelial cells express high levels of the co-stimulatory ligands CD80/86 (murine B7.1/.2) molecules and thus can additionally trigger CD28 signaling in effector T cells [7]. Inhibition of CD28 signaling at the time of effector T cell infiltration to the lung acutely abolished effector cytokine production during influenza infection [7]. Thus, anti-viral effector T cell activities in the lung are differentially regulated by the strength of interaction with their target cell types; strong interaction between APCs and effector T cells (involving with both TCR and costimulatory signals) triggers full spectrum of effector T cell activities, while weak interaction only triggers the release of cytolytic molecules to kill target cells. The uncoupling of the release of inflammatory cytokines and the secretion of cytolytic molecules by effector T cells may have important implications in designing novel therapeutics for influenza infection as it is possible in the future to selective inhibit pathogenic cytokine responses of effector T cells but retain their ability to clear virus. However, as CD28 signaling can also promote the survival of effector T cells in the lung [4], such approaches should be also undertaken with extra caution not to affect the accumulation of antiviral T cells in the infected lung.
Effector mechanisms of CD8+ T cells
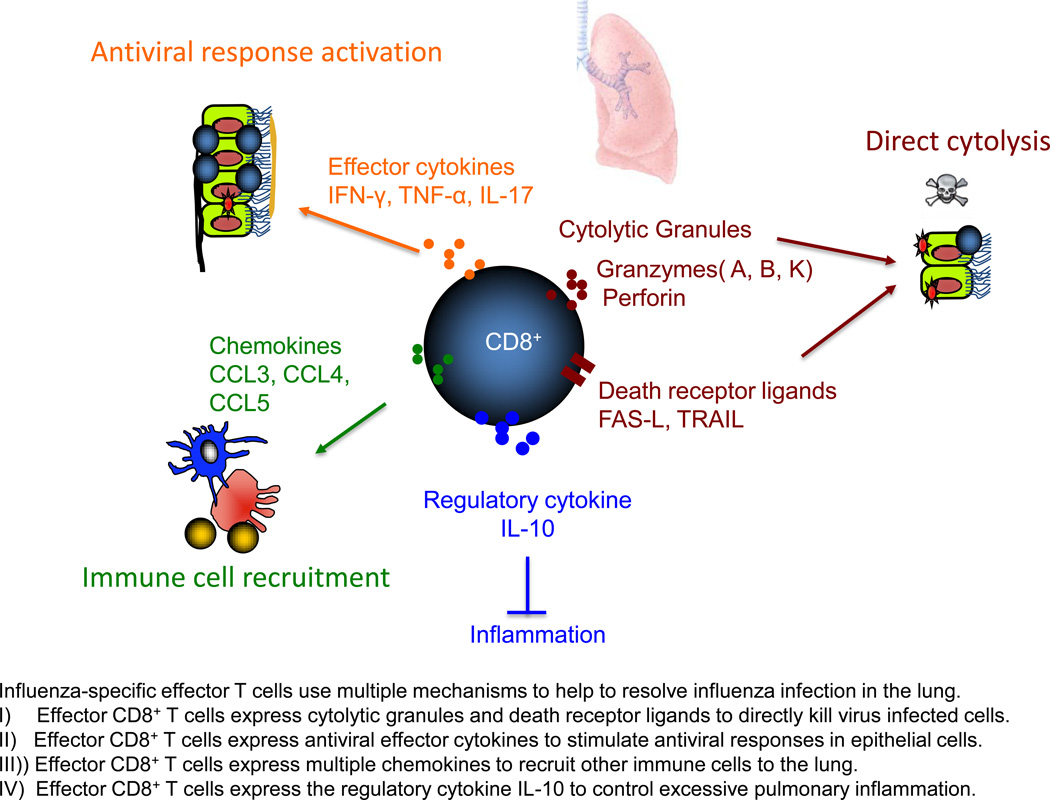
Classically, CD8+ T cells are believed to clear influenza virus infection through Fas/Fas-L and perforin-dependent mechanisms. However, recent studies have suggested that effector T cells may also use other mechanisms to contribute to the resolution of influenza infection in the lung (Figure 1). TRAIL deficient mice exhibited enhanced morbidity and influenza virus titer in response to influenza infection and the transfer of TRAIL deficient CD8+ T cells failed to protect lethal challenge of influenza virus infection [9]. Thus, TRAIL expression by CD8+ T cells appear to contribute to the clearance of influenza infected cells during primary influenza infection. Effector CD8+ T cells also express many anti-viral cytokines and chemokines that could potentially attract additional innate and adaptive immune cells to facilitate viral clearance. A recent report have suggested that effector CD8+ T cells may use multiple redundant effector mechanisms, including direct killing of target cells via perforin and death receptors and the production of effector cytokines and chemokines, to protect against influenza infection [10]. Furthermore, like CD4+ T cells [11], effector CD8+ T cells were able to elicit the activation of an early host innate response and thus provide bystander protection against the virus that could not be recognized by the transferred CD8+ T cells [10]. In addition, besides classical IFN-γ producing Tc1 cells, experiments have established that IL-4 producing Tc2 and IL-17 producing Tc17 cells are able to provide protection to influenza virus infection [12]. Whether these differentially polarized Tc subsets use distinct mechanisms to protect influenza infection warrant further investigation. Likewise, the extent to which Tc2 and Tc17 cells contribute to virus clearance and recovery/immunopathology during experimental (as well as human) influenza infection remains to be determined.
Figure 1.
Control of pulmonary inflammation by CD8+ effector T cells
The control and clearance of influenza virus infection by the innate and adaptive immune system can be accompanied by strong inflammatory responses generated within the infected respiratory tract. The recovery of influenza infection requires that these accompanying inflammatory responses be well controlled to minimize immune-mediated pathology in the process of viral clearance. Multiple immune counter-regulatory mechanisms help to retain the inflammatory responses in control during influenza infection [1]. In particular, as mentioned above, anti-viral effector CD8+ T cells in the lung gain the ability to produce IL-10 during influenza infection. Although multiple cell lineages in the lung can produce IL-10 during influenza infection [13,14], effector CD8+ T cells appeared to be the major producers of IL-10 as determined by CD8+ T cell depletion and in vivo cytokine staining [5]. Importantly, the blockade of IL-10 function, at the time of T cell infiltration to the lung, resulted in excessive pulmonary inflammation and lethal injury [5]. These data suggest that effector CD8+ T cells are able to fine tune respiratory inflammation during influenza infection. Both APC-derived IL-27 and Th-cell derived IL-2 were required for the development of IL-10 producing effector CD8+ T cells in the lung during influenza infection [6]. These IL-10 producing effector CD8+ T cells were characteristic of terminal differentiated effector T cells as they express high levels of effector molecules and the transcription factor Blimp-1. Interestingly, the generation of these IL-10 producing effector T cells are mainly restricted to the primary infection as memory CD8+ T cells progressively lose responsiveness to IL-27 [15].
Role of CD4+ T cells in anti-influenza immunity
The recovery from influenza infection requires the action of both CD8+ and CD4+ T cells. Classically, CD4+ T cells were believed to participate in the anti-viral immune responses by providing the help for CD8+ T and B cell responses to promote viral clearance. During the primary CD8+ T cell responses, CD4+ T cells appeared not critical for the development of CD8+ T cell responses due to the direct activation of DCs by influenza virus [16]. However, CD4+ T cells provide IL-2 to instruct the production of IL-10 by effector CD8+ T cells and thus are required for the full spectrum of cytokine production by effector CD8+ T cells [6]. Recently, a newly described subset of CD4+ T cells, follicular helper T cells (Tfh), were identified as a specialized type of CD4+ T cells to provide the essential help for B cell responses. Influenza infection induces the generation of Tfh cells which can promote germinal center (GC) formation as well as support high affinity antibody production by B cells [17]. Tfh cells induced during influenza infection are for the most part induced within lymphoid organs draining the site of infection, that is mediastinal lymph nodes and requires a novel late activator antigen-presenting cell (LAPC) for optimal induction in the draining LNs [18,19]. LAPCs migrate from the infected lungs to the dLN "late," i.e., 6 d after infection through CXCR3-dependent mechanisms, which is concomitant with Tfh differentiation. Tfh cell development induced by LAPCs required ICOS-ICOSL-dependent signaling [19]. Furthermore, the signaling from IL-6 and IL-21 also controlled the development of Tfh cells during influenza infection [20]. In contrast, IL-2 signaling inhibited the development of Tfh and GC B cell responses during influenza infection [21].
In addition to their role in helping CD8+ T cells and B cells, CD4+ T cells also exhibit direct effector activities against influenza infection. A recent report has identified that lung CD4+ T cells expressed cytolytic molecules such as Granzyme B (Gzmb) and perforin and exhibited direct cytotoxicity against influenza-infected cells [22]. Interestingly, the up-regulation of Gzmb and perforin expression in influenza-specific CD4+ T cells is restricted to the infection site [22], suggesting that the lung microenvironment promotes the generation of these cytotoxic CD4+ T cells. Notably, these Gzmb and perforin-expressing CD4+ T cells express the Th1 signature cytokine IFN-γ, indicating that they can be characterized into broader Th1 lineage [22]. Future studies are needed to identify the molecular mechanisms guiding the development of these cytotoxic CD4+ T cells and to clarify their relationship with classic Th1 cells. Nevertheless, following adoptive transfer, these CD4+ T cells provide both cytolytic and IFN-γ dependent protection again influenza challenge, suggesting these CD4+ T cells employ multiple effector mechanisms to protect and promote recovery from influenza virus infection [22]. Currently the exact physiological function of cytotoxic CD4+ T cells during influenza infection warrants further studies, however, a recent clinical study has implicated that pre-existing CD4+ T cells may directly kill target cells through perfroin dependent mechanisms and in the case of type A influenza infections contribute to protection against infection with heterologous viruses i.e heterosubtypic immunity in humans [23]. Thus, CD4+ T cells are able to protect against influenza infection via multiple mechanisms including both direct cell contact dependent cytolytic effector activity and indirect “help” to other immune cells. An elegant recent study has suggested that these multiple mechanisms of CD4+ T cells may act in a synergistic manner to provide complete protection against influenza virus infection [24].
Role of unconventional T cells in the protective immunity to influenza
Beyond conventional CD4+ and CD8+ T cells, innate like T cells such as NKT cells and γδ T cells were recently shown to play important roles in the recovery of influenza infection. CD1d or invariant NKT cell (Jα18 deficient) deficient mice exhibited enhanced susceptibility to influenza virus infection, and the activation of NK T cells through the injection of α-GalCer protected the host from influenza infection [25,26]. NKT cells exert their function to protect the host through multiple mechanisms. NKT cells were able to suppress the function of myeloid-derived suppressor cells (MDSCs), which promoted anti-viral T cell responses and viral clearance [27]. iNKT cells can also affect the maturation of DCs in the dLN to affect the development of CD8+ T cells responses against influenza infection [28]. Beyond their function in promoting T cell immunity, NKT cells can reduce lung injury induced by influenza infection through the elimination of inflammatory monocytes [29], which were previously shown to be a major contributor to lung injury during influenza infection [1]. Furthermore, NKT cells are a major source of IL-22 and so may help to preserve lung epithelium integrity following influenza infection [30]. Innate-like γδ T cells constitutes a small fraction lymphocytes that express γδ TCR and be able to rapidly respond to infection, potential danger or cellular stress [31]. γδ T cells were originally shown to be able to provide heterotypic immunity against influenza infection. Recently, using a humanized mouse model, it was demonstrated that aminobisphosphonate pamidronate-expanded γδ T cells were able to kill influenza virus–infected cells in vitro and protect humanized mice from lethal influenza infection in vivo [32]. This study raises the possibility that aminobisphosphonate pamidronate and related compounds may be useful as novel therapeutic approaches for protection from and treatment of influenza virus infection in the human.
Conclusion
In this brief review we have identified and described some of the recent findings emerging from the analysis of the role of T lymphocytes in protection and recovery from influenza virus infection. While some of these findings can be extrapolated to infection with other respiratory viruses (and perhaps to a limited extent to the host response to bacterial infection in the respiratory tract), there will be differences in the response of T cells following different species or strains of respiratory viruses. Research within the past two decades has provided us with considerable insight into the processes involved in the induction of innate and adaptive immune responses, the expression of innate and particularly adaptive immune effector activities such as T-cell mediated cytolysis and the mechanisms underlying these processes. An emerging frontier for new research involves understanding the role of the host immune response in orchestrating and regulating the events association with the resolution of infection (i.e. the repair of damage tissue) and in the case of viral and bacterial infections of the respiratory tract, the restoration of normal pulmonary structure and function.
Highlights.
Local interaction between APCs and T cells controls influenza T cell responses
Effector T cells use multiple mechanisms to restrict influenza infection
Target cell types determine effector T cell activities in situ
Nonconventional T cells help to clear virus and limit tissue injury
Acknowledgements
The authors would like to thank M. Hufford for his critical reading of the manuscript. This work was supported by grants from the National Institutes of Health (R21 R21AI09975 to J. Sun; RO1 AI-15068, RO1 AI37293, RO1 HL-33391 and U19AI-83024 to T. J. Braciale).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest have been highlighted as:
•of special interest
•• of outstanding interest
- 1.Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12:295–305. doi: 10.1038/nri3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med. 2008;205:1635–1646. doi: 10.1084/jem.20080314. ••This paper first demonstrated that the interaction of effector CD8+ T cells and local DCs promotes the accumulation of influenza-specific T cells in the lung.
- 3.McGill J, Van Rooijen N, Legge KL. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J Exp Med. 2010;207:521–534. doi: 10.1084/jem.20091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolfi DV, Duttagupta PA, Boesteanu AC, Mueller YM, Oliai CH, Borowski AB, Katsikis PD. Dendritic cells and CD28 costimulation are required to sustain virus-specific CD8+ T cell responses during the effector phase in vivo. J Immunol. 2011;186:4599–4608. doi: 10.4049/jimmunol.1001972. [DOI] [PubMed] [Google Scholar]
- 5. Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. ••This paper first demonstrated that anti-viral effector CD8+ T cells exhibit regulatory functions in the lung by producing IL-10.
- 6.Sun J, Dodd H, Moser EK, Sharma R, Braciale TJ. CD4+ T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nat Immunol. 2011;12:327–334. doi: 10.1038/ni.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hufford MM, Kim TS, Sun J, Braciale TJ. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. J Exp Med. 2011;208:167–180. doi: 10.1084/jem.20101850. ••This paper first demonstrated that effector activities of anti-viral CD8+ T cells are differentially regulated by the target cell types.
- 8.Hufford MM, Richardson G, Zhou H, Manicassamy B, Garcia-Sastre A, Enelow RI, Braciale TJ. Influenza-infected neutrophils within the infected lungs act as antigen presenting cells for anti-viral CD8(+) T cells. PLoS One. 2012;7:e46581. doi: 10.1371/journal.pone.0046581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J Immunol. 2008;181:4918–4925. doi: 10.4049/jimmunol.181.7.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamada H, Bassity E, Flies A, Strutt TM, Garcia-Hernandez Mde L, McKinstry KK, Zou T, Swain SL, Dutton RW. Multiple redundant effector mechanisms of CD8+ T cells protect against influenza infection. J Immunol. 2013;190:296–306. doi: 10.4049/jimmunol.1200571. ••This paper demonstrated that anti-viral CD8+ T cells use multiple mechanisms to control influenza virus infection.
- 11.Strutt TM, McKinstry KK, Dibble JP, Winchell C, Kuang Y, Curtis JD, Huston G, Dutton RW, Swain SL. Memory CD4+ T cells induce innate responses independently of pathogen. Nat Med. 2010;16:558–564. doi: 10.1038/nm.2142. 551p following 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, et al. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun K, Torres L, Metzger DW. A detrimental effect of interleukin-10 on protective pulmonary humoral immunity during primary influenza A virus infection. J Virol. 2010;84:5007–5014. doi: 10.1128/JVI.02408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perona-Wright G, Kohlmeier JE, Bassity E, Freitas TC, Mohrs K, Cookenham T, Situ H, Pearce EJ, Woodland DL, Mohrs M. Persistent loss of IL-27 responsiveness in CD8+ memory T cells abrogates IL-10 expression in a recall response. Proc Natl Acad Sci U S A. 2012;109:18535–18540. doi: 10.1073/pnas.1119133109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson S, Zhan Y, Sutherland RM, Mount AM, Bedoui S, Brady JL, Carrington EM, Brown LE, Belz GT, Heath WR, et al. Selected Toll-like receptor ligands and viruses promote helper-independent cytotoxic T cell priming by upregulating CD40L on dendritic cells. Immunity. 2009;30:218–227. doi: 10.1016/j.immuni.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyden AW, Legge KL, Waldschmidt TJ. Pulmonary infection with influenza A virus induces site-specific germinal center and T follicular helper cell responses. PLoS One. 2012;7:e40733. doi: 10.1371/journal.pone.0040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoo JK, Galligan CL, Virtanen C, Fish EN. Identification of a novel antigen-presenting cell population modulating antiinfluenza type 2 immunity. J Exp Med. 2010;207:1435–1451. doi: 10.1084/jem.20091373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo JK, Fish EN, Braciale TJ. LAPCs promote follicular helper T cell differentiation of Ag-primed CD4+ T cells during respiratory virus infection. J Exp Med. 2012;209:1853–1867. doi: 10.1084/jem.20112256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karnowski A, Chevrier S, Belz GT, Mount A, Emslie D, D'Costa K, Tarlinton DM, Kallies A, Corcoran LM. B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. J Exp Med. 2012;209:2049–2064. doi: 10.1084/jem.20111504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, Randall TD. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown DM, Lee S, Garcia-Hernandez Mde L, Swain SL. Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against lethal influenza virus infection. J Virol. 2012;86:6792–6803. doi: 10.1128/JVI.07172-11. ••This paper demonstrated that influenza infection in the lung induces the development of cytotoxic CD4+ T cells at the site of infection and the transfer of these CD4+ T cells confers both cytokine and cytotoxicity-dependent protection against influenza challenge.
- 23. Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18:274–280. doi: 10.1038/nm.2612. •This paper demonstrated that preexisting CD4+ T cells with cytotoxic potential correlate with disease protection against influenza challenge in humans.
- 24.McKinstry KK, Strutt TM, Kuang Y, Brown DM, Sell S, Dutton RW, Swain SL. Memory CD4+ T cells protect against influenza through multiple synergizing mechanisms. J Clin Invest. 2012;122:2847–2856. doi: 10.1172/JCI63689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho LP, Denney L, Luhn K, Teoh D, Clelland C, McMichael AJ. Activation of invariant NKT cells enhances the innate immune response and improves the disease course in influenza A virus infection. Eur J Immunol. 2008;38:1913–1922. doi: 10.1002/eji.200738017. [DOI] [PubMed] [Google Scholar]
- 26.Kulkarni RR, Haeryfar SM, Sharif S. The invariant NKT cell subset in anti-viral defenses: a dark horse in anti-influenza immunity? J Leukoc Biol. 2010;88:635–643. doi: 10.1189/jlb.0410191. [DOI] [PubMed] [Google Scholar]
- 27. De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–4048. doi: 10.1172/JCI36264. •This paper demonstrated that NKT cells reduce the activities of myeloid-derived suppressor cells and so promote anti-viral T cell responses during influenza infection.
- 28.Paget C, Ivanov S, Fontaine J, Blanc F, Pichavant M, Renneson J, Bialecki E, Pothlichet J, Vendeville C, Barba-Spaeth G, et al. Potential role of invariant NKT cells in the control of pulmonary inflammation and CD8+ T cell response during acute influenza A virus H3N2 pneumonia. J Immunol. 2011;186:5590–5602. doi: 10.4049/jimmunol.1002348. [DOI] [PubMed] [Google Scholar]
- 29.Kok WL, Denney L, Benam K, Cole S, Clelland C, McMichael AJ, Ho LP. Pivotal Advance: Invariant NKT cells reduce accumulation of inflammatory monocytes in the lungs and decrease immune-pathology during severe influenza A virus infection. J Leukoc Biol. 2012;91:357–368. doi: 10.1189/jlb.0411184. [DOI] [PubMed] [Google Scholar]
- 30.Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, Pichavant M, Dumoutier L, Ryffel B, Renauld JC, Gosset P, et al. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J Biol Chem. 2012;287:8816–8829. doi: 10.1074/jbc.M111.304758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girard M. Immunosurveillance and Immunoregulation by γδ T cells. J Invest Dermatol. 2006;126:25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- 32.Tu W, Zheng J, Liu Y, Sia SF, Liu M, Qin G, Ng IH, Xiang Z, Lam KT, Peiris JS, et al. The aminobisphosphonate pamidronate controls influenza pathogenesis by expanding a gammadelta T cell population in humanized mice. J Exp Med. 2011;208:1511–1522. doi: 10.1084/jem.20110226. [DOI] [PMC free article] [PubMed] [Google Scholar]