Abstract
Objectives
To describe first dose and steady-state pharmacokinetics (PK) of dolutegravir (DTG) in blood plasma (BP), seminal fluid (SF), colorectal tissue (RT), and rectal mucosal fluid (RF) of healthy HIV negative men.
Design
A Phase 1, open label, PK study that enrolled 12 healthy men taking DTG 50mg daily for 8d.
Methods
Eleven paired BP samples and 3 SF and RF samples were collected over 24h following first (PK1) and multiple (PK2) dosing. RT biopsies were collected at 1 of 6 time points at PK1 and PK2 to generate composite PK profiles. DTG concentrations were analyzed by validated LC-MS/MS. Noncompartmental PK analysis was conducted with Phoenix WinNonlin v6.3 and Spearman Rank Correlations determined using SAS v9.3.
Results
BP AUCs were similar to previous reports and C24h was 6 - 34 fold greater than the protein adjusted (PA) IC90 of 64 ng/mL. SF exposures were ∼7% of BP, and below the PA-IC90. RT exposures were 17% of BP and ∼2-fold greater than PA-IC90. RF AUCs were ∼2-5% of RT and did not correlate with RT (Rho =0.43, p=0.17). Accumulation of DTG with multiple dosing was observed in BP, SF, and RT.
Conclusions
DTG BP PK were consistent with previously published values. SF concentrations were <7% BP, with SF C24h below the PA-IC90. However, SF protein binding was not measured. Although the AUC of DTG in RT was <20% BP, RT C24h remained ∼ 2-fold higher than the PA-IC90. RF was not a strong surrogate for RT concentrations.
Keywords: dolutegravir, S/GSK1349572, integrase inhibitor, colorectal tissue, seminal fluid, male genital tract
Introduction
Dolutegravir (DTG) is an integrase strand transfer inhibitor currently in phase 3 clinical development for HIV-1 treatment. DTG has potent antiviral activity, a predictable pharmacokinetic (PK) profile in blood plasma (BP) with low intersubject variability (CV% 9-41) compared to raltegravir, and is well tolerated [1-4]. DTG is primarily metabolized by UGT1A1 with a minor route via CYP3A and is also a P-glycoprotein (P-gp) substrate. The 12-15 hour elimination half-life supports once daily dosing and allows for sustained antiretroviral activity without the need for pharmacokinetic boosting [1,2]. The recent SPRING-2 study demonstrated non-inferiority to raltegravir: 88% and 85% of patients, respectively, achieved HIV-1 RNA <50 copies/mL at 48 weeks [4].
The exposure-response relationship for antiretroviral agents (ARV) including DTG and other integrase inhibitors is best described by trough concentrations at 24 hours (C24h) [2,5]. Maintenance of DTG concentrations above the protein-adjusted concentration required for 90% viral inhibition (PA-IC90) across the dosing interval has been used to assess activity. DTG at a dose of 50 mg achieves a steady-state C24h that is approximately 13-fold greater than the PA-IC90 of 64 ng/mL for wild type virus [1].
The purpose of this study is to describe first dose and steady state pharmacokinetics of DTG in seminal fluid (SF), colorectal tissue (RT) and rectal mucosal fluid (RF) compared to blood plasma (BP) in HIV-1 negative men. Although current antiretroviral regimens can decrease blood plasma HIV RNA to <50 copies/mL, the risk of HIV-1 transmission remains, as viral shedding in the male genital tract and colorectum may still occur [6-12]. While a low HIV-1 plasma viral load reduces the risk of heterosexual transmission events, less is known regarding the risk associated with incomplete viral suppression at local sites of transmission [13-14]. Pharmacokinetic requirements to prevent local transmission have not yet been elucidated; however, drugs that achieve high exposure at sites of transmission are expected to make ideal candidates for pre-exposure prophylaxis and treatment as prevention applications by conferring better protection [15]. Understanding the pharmacokinetic behavior of DTG in multiple male biological compartments will inform its role in preventing local viral replication in HIV-infected men as well as its potential in protecting mucosal surfaces against HIV infection. This is the first study of male genital tract and colorectal tissue antiretroviral pharmacokinetics to be performed prior to market approval.
Methods
Study Design
This single center, open-label, prospective PK study evaluated 12 healthy adult males between December 2011 and May 2012. The sample size was chosen to generate PK data adequate for understanding penetration of DTG into the gastrointestinal and genital tract. Subjects received 50 mg oral DTG once daily for 8 days with intensive PK sampling visits after a single dose and at steady state. PK sampling was performed after 7 and 8 doses to allow appropriate SF volumes to be collected and to accommodate procurement of RF. Visits were conducted at the University of North Carolina at Chapel Hill (UNC) in the Clinical and Translational Research Center. The study was approved by the UNC Biomedical Institutional Review Board, operated under FDA IND # 113,425, and was registered with the NIH clinical trial registry (NCT01459315). Dolutegravir (DTG) tablets were provided by ViiV Healthcare. All participants provided written informed consent before commencing study procedures.
Subject Selection
Screening procedures were initiated within 42 days of administration of DTG. Screening procedures consisted of a complete medical history and physical examination, 12-lead electrocardiogram (ECG) with cardiology interpretation and comprehensive laboratory studies (complete blood count with differential, liver function tests, serum chemistries, urinalysis, and urine toxicology). Subjects were screened for active hepatitis B and hepatitis C, HIV, syphilis, gonorrhea, chlamydia and HSV-2. Subjects were eligible to participate if they were natural born males between 18-49 years of age, inclusive at the date of screening, and had a Body Mass Index (BMI) 18-30kg/m2 with total body weight greater than 50kg. Subjects were required to have fully intact genital and gastrointestinal tracts, have no known medication allergies, and not have participated in another drug research study within the prior four months. Subjects were excluded for any clinically significant abnormal lab value, physical examination finding or clinical condition that would interfere with study activities including disorders of the gastrointestinal tract or genital tract. Subjects were required to stop all prescription and nonprescription medications 7 days before and herbal supplements 14 days before study enrollment, and medications could not be restarted until study completion. Subjects were limited to consumption of less than 14 alcoholic beverages per week, and acetaminophen at doses of ≤1g/day.
Subjects abstained from sexual activity or use of intra-rectal products from 72 hours before dosing until follow-up. Subjects abstained from alcohol, Seville oranges and grapefruit juice for 7 days prior to administration of DTG. Additionally, subjects were required to fast for 8 hours prior to DTG administration at PK sampling visits, adhere to a low fiber diet 3 days prior, and a clear liquid diet 12 hours prior to biopsy procedures. Subjects were sequentially assigned to SF and RF collection and RT biopsy times at screening.
Safety Assessments
Vital signs and clinical laboratory testing including a complete lipid panel, serum chemistries and CBC with differential were performed at every visit. Subjects underwent adverse event (AE) assessment on each PK visit day and daily by telephone between inpatient visits. Adverse events were captured by subject self-reporting and completion of a standardized assessment form. All AEs and laboratory values were graded by the study physician according to the National Institute of Health Division of Allergy and Infectious Disease (DAIDS) grading criteria [16]. Subjects were followed until all AEs resolved.
Sample Collection and Processing
Subjects were admitted to the UNC CTRC at least 2 hours prior to their 50 mg DTG dose on day 1 and on day 7. BP was collected in 3mL K2EDTA tubes (BD Diagnostics, Franklin Lakes, NJ) pre-dose and at 1, 2, 3, 4, 5, 6, 8, 12, 18, and 24 hours post-dose on days 1, 7, and 8. Rectal mucosal fluid and seminal fluid were self-collected pre-dose and at two time points per subject on day 1 and three time points per subject on both day 7 and 8 (either 1, 3, 6, 12, 18, or 24 hours post-dose). RF was self-collected by subjects following instruction via insertion of a Dacron swab approximately 1-2 inches into the rectum for 1-2 minutes. For SF collections, participants were provided with self-care instructions, lubricant, visual aids, and a private room then given 30 minutes to procure a sample in a sterile container by masturbation. Colorectal tissue was collected at a single time point per subject (either 1, 3, 6, 12, 18, or 24 hours postdose) on day 1 and on day 7 or 8. Non-targeted endoscopic cold biopsies were taken by flexible sigmoidoscopy (NJS, RDM, ESD) at approximately 10 cm from the anal verge using a spiral approach with 2.8 mm Radial Jaw™ 4 large capacity biopsy forceps (Boston Scientific, Natick, MA). Ten pinch biopsies were taken at each procedure. Subjects were considered evaluable if they were able to provide all RT and 80% of BP, SF and RF samples, excluding pre-dose samples but including 24 hour samples.
K2EDTA tubes were stored on ice and processed within 1 hour of collection by centrifugation at 3000 rpm at 4°C for 10 minutes. BP was transferred to 2 mL cryovials and frozen at -80°C until analysis. RF was frozen immediately in 15 mL conical tubes following collection at -80°C. SF was left to liquefy at room temperature for approximately 1 hour then transferred to 15 mL conical tubes and centrifuged at 5000 rpm at 4°C for 15 minutes. Seminal plasma was then transferred to 2 mL cryovials and frozen at -80°C. RT was flash frozen in liquid nitrogen and stored at -80°C.
Dolutegravir Quantification
Quantification of drug concentrations in all matrices was completed using LC-MS/MS methods validated by the UNC Center for AIDS Research Clinical Pharmacology and Analytical Chemistry Core. DTG in BP was extracted from calibration standards, quality control samples, and study samples using protein precipitation followed by LC-MS/MS analysis. Dolutegravir-d715N (DTG-IS) was used as an internal standard. Thirty microliters of plasma was mixed with 600 μL of acetonitrile containing the internal standard. Following vortex and centrifugation steps, the supernatant was diluted with 50:50 methanol:water prior to LC-MS/MS analysis. DTG was eluted from a Varian (Agilent) Pursuit Diphenyl (2.1 × 50 mm, 3 μm particle size) analytical column. Data were collected using a Sciex Analyst Chromatography Software on an AB Sciex API-5000 triple quadrupole mass spectrometer (AB Sciex, Foster City, CA). Calibration curves were obtained by using a 1/concentration2 weighted linear regression of analyte:internal standard peak area ratio vs. concentration. The calibration range of this assay was 20 to 20,000 ng/mL. All calibrators and quality control samples were within 15% of the nominal value for both within-day and between-day runs. Within-day and between-day precision was < 15%. Recoveries of DTG and its internal standard seen with this methodology were approximately 100%.
DTG was quantified in SF by similar methodology. Thirty microliters of seminal plasma was mixed with 270 μL of acetonitrile containing the internal standard. Following vortex and centrifugation steps, the supernatant was diluted with 50:50 methanol:water and analyzed by LC-MS/MS using the same method as described for BP. The calibration range of the assay was 1 to 1000 ng/mL. All calibrators and quality control samples were within 15% of the nominal value for both within-day and between-day runs. Within-day and between-day precision was < 15%. Recoveries of DTG and its internal standard seen with this methodology were approximately 100%.
In order to extract DTG from RT samples, the tissue samples were homogenized in 1 mL of 80:20 water:acetonitrile. A portion of the resulting homogenate was extracted by protein precipitation with acetonitrile containing DTG-IS. Following vortex and centrifugation steps, a portion of the supernatant was diluted with water. Dolutegravir was detected on an LC-MS/MS system using the same method as described for BP. During method validation, calibration standards were prepared in human vaginal tissue homogenate. QC samples were prepared in human vaginal and rectal tissue homogenates. Method validation results showed that calibration standards prepared in vaginal tissue homogenate could be used successfully to quantitate DTG in rectal tissue samples. For sample analysis, calibration standards and QC samples were prepared in human vaginal tissue homogenate. The dynamic range of the assay was 0.2-200 ng/mL homogenate. The recoveries of DTG and its internal standard were >80% in RT. All calibrators and quality controls samples were within 15% of the nominal value with precision values < 15%. Human saliva was used as a surrogate matrix for analysis of RF. Calibration standards and QC samples were prepared by adding human saliva, fortified at various concentrations with DTG, onto clean swabs to mimic the composition of the study samples. DTG was initally extracted from swabs with 2 mL acetonitrile. A portion of the extract was then mixed with DTG-IS. Following vortex and centrifugation steps, samples were transferred to a 96-well plate for LC-MS/MS analysis using the same method as described for BP. Samples above the calibration range of 0.075 to 75 ng DTG per swab were diluted 20-fold and reanalyzed. All calibrators and quality control samples were within 15% of the nominal value for both within-day and between-day runs. Within-day and between-day precision was < 15%. Recoveries of DTG and its internal standard seen with this methodology were >90%.
Pharmacokinetic and Statistical Analysis
Noncompartmental analysis was performed using Phoenix WinNonlin v6.3 (Certara L.P.; St. Louis, MO). Actual times were used in all PK analyses. Area under the concentration-time curves from 0 to 24 hours (AUC0-24hr) were calculated using the trapezoidal rule with linear up/log down interpolation. Pharmacokinetic estimates were determined at PK1 for SF and both PK1 and PK2 for RT using the geometric mean of concentrations at each time point. As DTG concentrations in some RF samples were below the limit of detection (BLD), composite AUC for RF at PK1 was calculated using median concentrations at each time point. Since RT from two subjects was collected at each time point following single and multiple dosing, composite profiles were used for PK analysis. Spearman Rank Correlations between RF and RT were calculated using SAS 9.3 (SAS Institute Inc; Cary, NC). PK parameters are separated by day and by matrix. BLD concentrations were imputed as 0 and concentrations below the limit of quantification (BLQ) were imputed as half the lower limit of quantification (LLOQ). Accumulation ratios were calculated from the geometric mean AUC0-24h ratio of PK2 to PK1. Data are presented as median (25th–75th percentile) unless otherwise noted.
Results
Demographics
14 participants were enrolled and 12 completed the study. The median age of the 12 evaluable participants was 25.5 (21-44) years. Participants had a median (range) body mass index (BMI) of 25.0 (20.4-31.1) and weight of 78.5 (58.2-107) kg at screening. Seven participants were African American and five were Caucasian. One participant identified his ethnicity as Hispanic or Latino. Fourteen subjects were enrolled and evaluated for safety. Two participants were not able to complete all protocol procedures.
Plasma Concentrations
Single and multiple dose pharmacokinetics of DTG in all matrices are presented in Table 1. DTG achieved BP exposure in males consistent with previous reports. After a single dose, BP DTG median (IQR) AUC0-24h was 31,335 (24,949-39,971) ng*hr/mL, with a median (IQR) t1/2 (calculated within the 24 hour dosing interval) of 12.2 hr (11.2 - 14.2 hr). The observed median (IQR) DTG C24h was 704 (580 - 874) ng/mL, which is approximately 11-fold higher than the protein binding-adjusted IC90 (PA-IC90) of 64 ng/mL. Pharmacokinetic parameters following 7 and 8 days of DTG dosing were comparable, with DTG achieving median (IQR) AUC0-24h accumulation ratios of 1.6 (1.5 - 1.9) and 1.3 (1.0 - 1.6), respectively. Due to pharmacokinetic differences observed between the days, a BP median profile was used for comparison to other matrices. On Day 8, median (IQR) AUC0-24h was 41,320 (28,092-47,219) ng*hr/mL with a median (IQR) t1/2 of 14.2 (12.0 - 16.1) hr, respectively. On Day 8, median (IQR) BP DTG C24h was 954 (554-1093) ng/mL and remained approximately 15-fold above the PA-IC90 of 64 ng/mL. DTG achieved a Cmax of 2285 (1790-2743) ng/mL after a single dose and 2945 (2120-3400) ng/mL after 8 doses. Median (IQR) BP Tmax was 4 (2-5) and 2.5(1-3) hr following a single and 8 doses, respectively. Intersubject variability in Cmax, C24h, and AUC0-24h ranged from 24-43 %CV.
Table 1. Pharmacokinetics of DTG in BP, SF, RF, and RT following single and multiple doses.
| Matrix | t1/2 (h) | Tmax (h) | Cmax (ng/mL, swab or g) | C24h (ng/mL, swab or g) | AUC0-24h (ng*h/mL, swab or g) | AUC0-inf (ng*h/mL, swab or g) | Matrix:BP AUC0-24h ratio | |
|---|---|---|---|---|---|---|---|---|
| BP | Single Dose | 12.2 (11.2-14.2) | 4 (2-5) | 2285 (1790-2743) | 704 (580-874) | 31335 (24948-39970) | 44902 (37253-58911) | - |
| Multiple Doses | 14.1 (12.0-16.1) | 2.5 (1-3) | 2945 (2120-3400) | 954 (554-1093) | 41320 (28092-47219) | 60553 (37785-70036) | - | |
| SF | Single Dose | 7.7 | 12 | 140 | 46.6 | 2018 | 2445 | 0.07 |
| Multiple Doses | 11.5 (8.5-14.8) | 3.5 (3-6) | 244 (190-323) | 57.9 (47.6-94.0) | 3229 (2439-4376) | 4210 (3567-6377) | 0.07 (33) | |
| RF | Single Dose | - | 24 | 17.0 | 17.0 | 91.9 | - | - |
| Multiple Doses | - | 12 (6-12) | 26.5 (8.0-289) | 8.6 (0.9-88.9) | 300 (73.6-3160) | - | - | |
| RT | Single Dose | 4.1 | 6 | 373 | 114 | 5281 | 5890 | 0.17 |
| Multiple Doses | 8.4 | 4 | 418 | 139 | 7596 | 9617 | 0.17 | |
Parameter estimates are presented as median (IQR) or point estimates for parameters generated from composite profiles. BP multiple dose estimates were calculated from concentrations following the last dose. Matrix:BP ratios are presented as the geometric mean AUC0-24h ratio (%CV) for SF after a single dose or geometric mean AUC0-24h ratio for RT and SF after multiple doses.
Seminal Fluid Concentrations
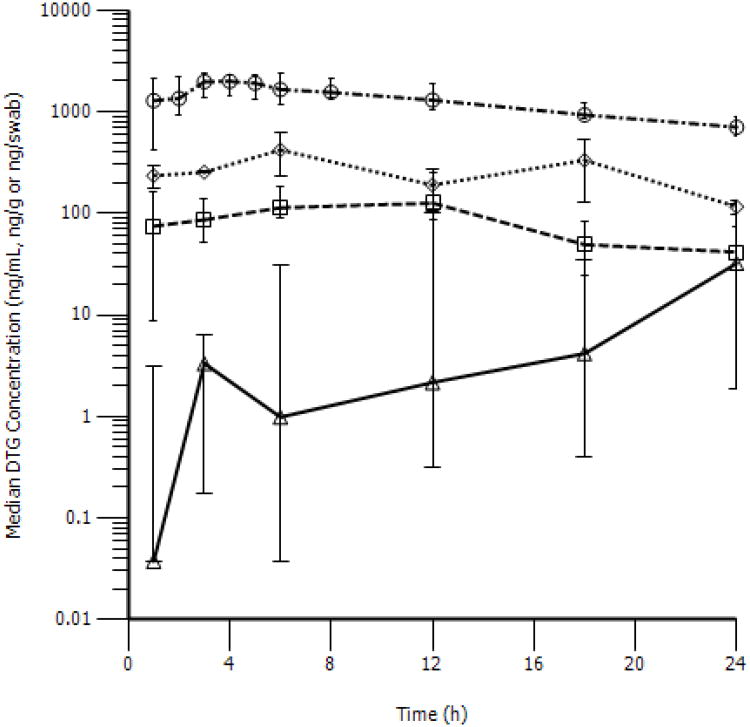
A composite concentration vs. time profile was generated based on the geometric mean concentrations of sparse samples after a single dose of DTG. SF reached a maximum concentration (range) of 139.7 (85.2-254) ng/mL at 11.8 h post-dose with a t1/2 of 7.7 hr. The observed Day 1 SF DTG AUC0-24h was 2,017.7 ng*hr/mL, representing approximately 6.6% BP exposure. SF DTG C24h ranged from 33 ng/mL to 83.4 ng/mL. The observed median (IQR) trough concentration in SF was 41.5 (34.2-73.8) ng/mL. Single dose concentration vs. time profiles for all matrices are presented in Figure 1.
Figure 1. Concentration versus time profiles of DTG in BP, SF, RF, and RT following a single dose.
Blood plasma (○), seminal fluid (□), colorectal tissue (◊), and rectal mucosal fluid (Δ) are presented as median (IQR).
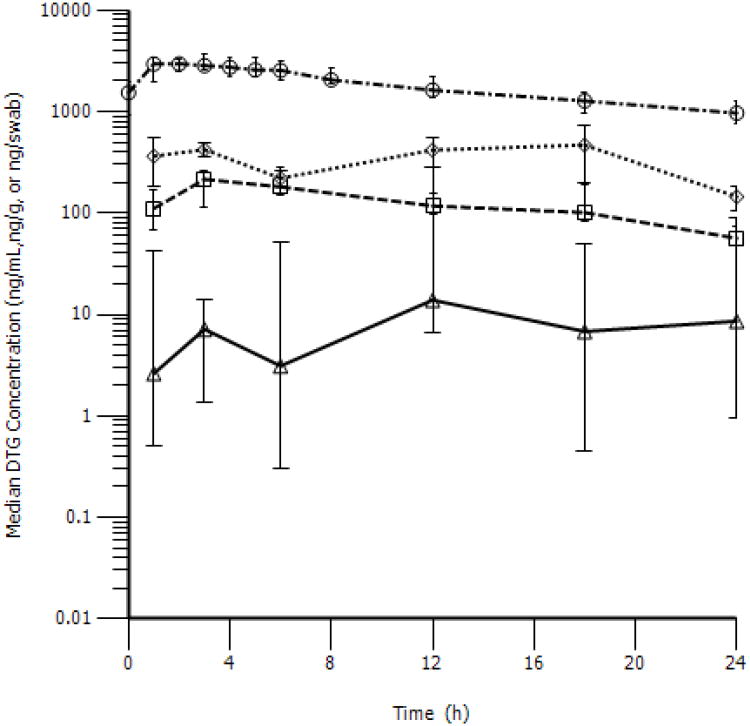
Utilizing a composite analysis of SF collections over Day 7 and 8 of dosing, the median (IQR) AUC0-24h was 3,229 (2439-4376) ng*hr/mL. This resulted in a median (IQR) accumulation ratio (Rac) of 1.6 (1.2-2.2) over 7-8 days, and DTG seminal penetration that was 6.8 (5.5-8.7) % of BP. Peak concentrations of 243.5 (189.5 - 322.5) ng/mL were reached after 3.5 (2.8-5.8) hr. Median C24h was 57.9 (47.6-93.9) ng/mL. SF concentrations reached a Cmax of 243.5 (189.5-322.5) ng/mL after 3.5 (3-6) hr. Concentration vs. time profiles for all matrices following multiple doses are shown in Figure 2.
Figure 2. Concentration versus time profiles of DTG in BP, SF, RF, and RT following multiple doses.
Blood plasma (○), seminal fluid (□), colorectal tissue (◊), and rectal mucosal fluid (Δ) are presented as median (IQR).
Rectal Mucosal Fluid Concentrations
In RF, a peak concentration of 17.0 ng/swab occurred at approximately 24 hours after a single dose. AUC0-24h was 91.9 ng*hr/swab. No terminal elimination phase was observed. In the multiple dose composite profile, a median (IQR) peak concentration of 26.5 (8.0 - 289) ng/mL occurred in RF at 12.1 (6.1-12.1) hr. Median (IQR) RF AUC0-24h was 300.0 (73.6 - 3160) ng*hr/swab and a terminal elimination phase was only quantifiable for 3 subjects. There was no detectable correlation (Spearman rho = 0.43, p = 0.17) between RF concentrations and RT concentrations collected within a 1 hr window across Day 7 and 8. RF exposures were low relative to RT with RF:RT AUC0-24h ratios of 0.018 and 0.047 after single and repeat dosing.
Colorectal Tissue Concentrations
DTG penetration in RT was higher than that observed for either SF or RF. RT AUC0-24h was 5,281 ng/g tissue on Day 1 and 7,596 ng/g tissue on Day 7-8, resulting in RT penetration of 17% compared to BP after both single and multiple dosing. DTG was detected in RT at 1 hour following a single dose and maintained concentrations above the PA-IC90 over the entire dosing interval. C24h was 114.5 ng/g tissue on Day 1 and 139.1 ng/g tissue over Day 7-8. DTG accumulated in RT after multiple doses with an Rac of 1.4.
Safety
Adverse event data includes 14 subjects who received 1 or more doses of DTG. No serious adverse events were reported during this study and all adverse events were ≤ DAIDS grade 2. Two subjects were discontinued for reasons considered unrelated to study drug by the study physician. One subject with a history of generalized anxiety disorder experienced a panic attack of moderate severity during the second PK visit. Study staff was unable to maintain peripheral intravenous access during the first PK visit in another. Non-serious adverse events included headaches (4 subjects) that were considered possibly related to study drug by the investigators. One case of vascular access site soreness was reported and considered to be unrelated to study drug. A single subject experienced transient muscle tightness and the relation to study drug was unknown. No laboratory abnormalities equal to or greater than DAIDS severity grade 2 were observed.
Discussion
DTG pharmacokinetics in BP are consistent with published results, with a relatively long terminal elimination half-life and trough concentrations ranging from 6 to 34 fold above the PAIC90 in all subjects [1,2]. Differences in exposure were observed between Day 7 and 8 of dosing. The higher exposure on Day 7 was likely a food effect, as diet was unrestricted during home dosing on Days 2-6 with likely higher fat content than those given in the research unit. Consistent with this observation, DTG exposures increase from 33% to 66% when taken with food depending on fat content of the meal [17].
DTG SF median trough concentrations are below the PA-IC90 after single and multiple doses. Additionally, SF exposure is only ∼7% of that observed in BP. These low exposures are predicted by the high plasma protein binding of DTG and are similar to results for drugs in other therapeutic classes [16]. The low genital tract penetration is consistent with the SF to BP penetration ratios of other highly protein-bound ARVs including enfuvirtide (0%), lopinavir (5%), nelfinavir (5%), ritonavir (3%), saquinavir (3%), and efavirenz (3%). Protein binding in SF is typically lower than in BP resulting in a higher proportion of available free drug. DTG protein binding in SF was not quantified in this study. Utilizing extremes of SF protein binding in currently documented (8.9% to 97%), free median trough DTG concentrations could theoretically range from 1.2 ng/mL to 37.8 ng/mL after a single dose and from 1.7 ng/mL to 52.7 ng/mL after multiple doses [18,19]. DTG trough concentrations would therefore be greater than 6-fold above the in vitro IC50 of 0.21 ng/mL following a single dose. Despite the low SF concentrations, distribution to this matrix was rapid and drug was quantifiable in all subjects at 1 hour post-dose.
Self-collected RF swabs are unlikely to be a useful surrogate for RT concentrations in future trials. Self-collected swabs of RF were obtained in an effort to identify a substitute for directly obtaining tissue through rectal biopsies. However, the large inter-subject variability in RF DTG exposure is likely due to variation in self-collection by individual subjects, and the lack of a weight or volume correction. Swabs were not corrected for weight or volume due to expected contamination by fecal material, and due to the practicality of how these may be collected outside of an academic research center.
Although DTG distributes rapidly into RT, the overall exposure is 17% of that observed in BP, and lower than the other ARVs ranging from 2.7 (darunavir) to 231 (raltegravir) [18,20]. Despite the low exposure, DTG concentrations in RT remain above the PA-IC90 across dosing intervals following single and multiple doses and trough concentrations remain approximately 2 fold higher than the PA-IC90.
Conclusions
The BP pharmacokinetics of single and multiple doses of DTG in this investigation were similar to previous reports [1,2]. Although DTG rapidly distributes to sites of transmission, DTG exposure in seminal fluid and rectal tissue was considerably lower than BP and other ARVs. Following multiple doses, DTG accumulates in vulnerable fluid and tissue, and maintains concentrations above the PA-IC90 in rectal tissue but only 36% of seminal fluid samples. Further studies are warranted to determine the implications of these findings on prevention.
Acknowledgments
The authors would like to acknowledge Trenton Stevens for his contributions to study visit conduct, the study participants, the UNC Clinical and Translational Research Center, and the UNC Center for AIDS Research.
Flexible sigmoidoscopies and rectal tissue sampling were performed at UNC Hospitals GI Procedures, UNC Healthcare Meadowmont GI Clinic, and Chapel Hill Internal Medicine GI Clinic by Nicholas J Shaheen, Ryan D Madanick, and Evan S Dellon.
WinNonLin was provided to faculty and trainees in the Division of Pharmacotherapy and Experimental Therapeutics, UNC Eshelman School of Pharmacy, by Certara as a member of the Pharsight Academic Center of Excellence Program.
Sources of Funding: The project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Award Number UL1TR000083. Shionogi-Viiv; UNC Center for AIDS Research (5P30AI050410-13; CS, JBD, ADMK), NC TraCS CTSA Grant (UL1TR000083), K23AI077355 (KBP), K23AI093156 (JBD), U01AI095031 (ADMK), R37DK49381 (MSC)
Footnotes
Data presented at the 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, GA, USA, Mar 3-6, 2013.
Conflicts of Interest: Financial support for the study was provided by Shionogi-Viiv Healthcare. Angela Kashuba's spouse is employed by GlaxoSmithKline. The remaining authors have no additional conflicts to declare.
Author Contributions: BN Greener- subject recruitment and study visit conduct, pharmacokinetic and statistical data analysis, and primary author of manuscript
KB Patterson- study visit conduct, clinical study safety officer, critical review of manuscript
HMA Prince- subject recruitment and study visit conduct, critical review of manuscript
C Sykes- analytical data analysis, critical review of manuscript
JL Adams- study visit conduct, pharmacokinetic and statistical data analysis, critical review of manuscript
JB Dumond- pharmacokinetic and statistical data analysis, critical review of manuscript
ES Dellon- study visit conduct, critical review of manuscript
RD Madanick- study visit conduct, critical review of manuscript
NJ Shaheen- study visit conduct, critical review of manuscript
MS Cohen- study design, critical review of manuscript
ADM Kashuba- study design, analytical and pharmacokinetic data analysis, funding, critical review of manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Min S, Song I, Borland J, et al. Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. Antimicrob Agents Chemother. 2010;54:254–258.12. doi: 10.1128/AAC.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Min S, Sloan L, DeJesus E, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS. 2011;25:1737–1745. doi: 10.1097/QAD.0b013e32834a1dd9. [DOI] [PubMed] [Google Scholar]
- 3.Cattaneo D, Gervasoni C, Meraviglia P, et al. Inter- and intra-patient variability of raltegravir pharmacokinetics in HIV-1 infected subjects. J Antimicrob Chemother. 2012;67:460–464. doi: 10.1093/jac/dkr498. [DOI] [PubMed] [Google Scholar]
- 4.Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735–43. doi: 10.1016/S0140-6736(12)61853-4. [DOI] [PubMed] [Google Scholar]
- 5.DeJesus E, Berger D, Markowitz M, et al. Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-naive and treatment-experienced patients. J Acquir Immune Defic Syndr. 2006;43(1):1–5. doi: 10.1097/01.qai.0000233308.82860.2f. [DOI] [PubMed] [Google Scholar]
- 6.Zuckerman RA, Whittington WL, Celum CL, et al. Higher concentration of HIV RNA in rectal mucosa secretions than in blood and seminal plasma, among men who have sex with men, independent of antiretroviral therapy. J Infect Dis. 2004;190(1):156–61. doi: 10.1086/421246. [DOI] [PubMed] [Google Scholar]
- 7.Lorello G, la Porte C, Pilon R, et al. Discordance in HIV-1 viral loads and antiretroviral drug concentrations comparing semen and blood plasma. HIV Med. 2009;10(9):548–54. doi: 10.1111/j.1468-1293.2009.00725.x. [DOI] [PubMed] [Google Scholar]
- 8.Yukl SA, Gianella S, Sinclair E, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010;202(10):1553–61. doi: 10.1086/656722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampinen TM, Critchlow CW, Kuypers JM, et al. Association of antiretroviral therapy with detection of HIV-1 RNA and DNA in the anorectal mucosa of homosexual men. AIDS. 2000;14(5):F69–75. doi: 10.1097/00002030-200003310-00001. [DOI] [PubMed] [Google Scholar]
- 10.Vernazza PL, Troiani L, Flepp MJ, et al. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort. AIDS. 2000;14:117–121. doi: 10.1097/00002030-200001280-00006. [DOI] [PubMed] [Google Scholar]
- 11.Bujan L, Daudin M, Matsuda T, et al. Factors of intermittent HIV-1 excretion in semen and efficiency of sperm processing in obtaining spermatozoa without HIV-1 genomes. AIDS. 2004;18(5):757–766. doi: 10.1097/00002030-200403260-00006. [DOI] [PubMed] [Google Scholar]
- 12.Poles MA, Boscardin WJ, Elliott J, et al. Lack of decay of HIV-1 in gut-associated lymphoid tissue reservoirs in maximally suppressed individuals. J Acquir Immune Defic Syndr. 2006;43:65–8. doi: 10.1097/01.qai.0000230524.71717.14. [DOI] [PubMed] [Google Scholar]
- 13.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 14.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicol MR, Kashuba AD. Pharmacologic opportunities for HIV prevention. Clin Pharmacol Ther. 2010;88(5):598–609. doi: 10.1038/clpt.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute of Allergy and Infectious Diseases, Division of AIDS Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events. US Department of Health and Human Services; [December 28, 2004 with clarification August 2009]. Available at: http://rsc.techres.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf. [Google Scholar]
- 17.Song I, Borland J, Chen S, et al. Effect of food on the pharmacokinetics of the integrase inhibitor dolutegravir. Antimicrob Agents Chemother. 2012;56(3):1627–9. doi: 10.1128/AAC.05739-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown KC, Patterson KB, Jennings SH, et al. Single and Multiple Dose Pharmacokinetics of Darunavir plus Ritonavir and Etravirine in Semen and Rectal Tissue of HIV-Negative Men. J Acquir Immune Defic Syndr. 2012;61(2):138–144. doi: 10.1097/QAI.0b013e31825cb645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown KC, Patterson KB, Malone SA, et al. Single and multiple dose pharmacokinetics of maraviroc in saliva, semen, and rectal tissue of healthy HIV-negative men. J Infect Dis. 2011;203(10):1484–90. doi: 10.1093/infdis/jir059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson KB, Prince HA, Stevens T, et al. Differential penetration of raltegravir throughout gastrointestinal tissue: implications for eradication and cure. AIDS. 2013;27(9):1413–1419. doi: 10.1097/QAD.0b013e32835f2b49. [DOI] [PMC free article] [PubMed] [Google Scholar]