Abstract
In order to test the hypotheses that pretreatment metabolic activity in the midbrain and the rostral anterior cingulate may predict remission in response to medications enhancing monoaminergic transmission, we compared relative regional cerebral metabolic rate of glucose (rCMRglu) using positron emission tomography (PET) in medication-free patients with major depression who remitted after 3 months of monoaminergic medication, with non-remitters on the same treatment. [18F]-FDG PET was conducted in a group of 33 drug-free DSM-IV major depression subjects prior to antidepressant treatment. Patients were prescribed paroxetine initially (61%) unless they had failed paroxetine previously. Treatment was then managed by the subjects’ own physician with 91% receiving a selective serotonin reuptake inhibitor and 78% another non-selective monoamine reuptake inhibitor during the 3 months of treatment. Voxel-based parametric brain maps of remitters were compared with maps of non-remitters using SPM2. Remission was defined as a N50% decrease in and a final score of ≤10 on the 24-item Hamilton Depression Rating Scale. We found that treatment remitters have lower activity in a single contiguous brain region (with global maxima in the midbrain, cluster level P=0.013, corrected for multiple comparison (CMC)), prior to treatment, compared with non-remitters to 3 months of community-based monoaminergic antidepressant treatment. Degree of improvement correlated with pretreatment midbrain activity. Pretreatment clinical picture and intensity of treatment did not distinguish remitters. No other area of the brain showed a significant difference between remitters and non-remitters even with CMC completely disabled. Lower relative regional brain activity in the region of monoaminergic nuclei prior to treatment predicts remission in response to 3 months of antidepressant treatment, despite no clinical differences at baseline and no difference in treatment intensity. Brain imaging is a potential objective laboratory technique that may guide treatment selection where clinical methods have not shown promise. Prospective studies are needed to replicate these findings and determine whether outcome prediction is limited to a specific class of antidepressants.
Keywords: Depression, PET, FDG, Brain regions, Remission, Antidepressant treatment, Outcome, Positron emission tomography
1. Introduction
Major depressive disorder (MDD) is one of the leading causes of disability and burden of illness worldwide (Evans and Charney, 2003) and estimated to be second only to cardiovascular disease by 2020 (Michaud et al., 2001). Despite this, the majority of patients with MDD are inadequately treated (Oquendo et al., 1999; Kessler et al., 2003). Even with adequate treatment, response to treatment, defined as a 50% improvement in the Hamilton Depression Rating Scale (HDRS) score, usually occurs in only 50–60% of individuals (Panel, 1999). Rates of remission after antidepressant treatment, defined as both a 50% decline in HDRS and a final 17-item score of <7 or <10 for the 24 item HDRS (Frank et al., 1991), are even lower, only 20%–35% (Mann, 2005). Antidepressants currently in clinical use (selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, selective norepinephrine reuptake inhibitors, and tricyclic antidepressants) primarily target monoaminergic transmission and much evidence has accumulated demonstrating abnormalities of the monoaminergic systems in mood disorders. It is unclear why some patients respond well and others respond poorly to antidepressant treatments that target the monoaminergic system. Nevertheless, prediction of remission in response to monoaminergic antidepressants requires an understanding of the biologic heterogeneity of the etiology and pathophysiology of major depression.
Ten studies, conducted after 1989, utilized F18-fluorodeoxyglucose positron emission tomography (FDG-PET) to map brain regions that predict antidepressant treatment response in MDD (Wu et al., 1992; Little et al., 1996; Buchsbaum et al., 1997; Mayberg, 1997; Brody et al., 1999; Ketter et al., 1999; Kimbrell et al., 1999; Wu et al., 1999; Brannan et al., 2000; Brody et al., 2001; Kennedy et al., 2001; Saxena et al., 2003; Little et al., 2005); these are summarized in Table 1. The rCMRglu in various parts of the temporal lobe region predicted treatment response in 40% of studies (Little et al., 1996; Wu et al., 1999; Saxena et al., 2003; Little et al., 2005), various parts of the anterior cingulate in 60% of the studies (Mayberg 1997; Brody et al., 1999; Wu et al., 1999; Brannan et al., 2000; Kennedy et al., 2001; Little et al., 2005); various parts of the prefrontal cortex (PFC) in 70% of studies (Little et al., 1996; Buchsbaum et al., 1997; Wu et al., 1999; Brody et al., 2001; Kennedy et al., 2001; Saxena et al., 2003; Little et al., 2005). Note however, that in Table 1 the studies we found in the literature do not agree on the exact location (Brodmann area), direction or laterality of the metabolic change predicting response or non-response to treatment. There is no ready explanation for these disagreements, but we speculate that some of these differences may be explained in part by imaging methods and heterogeneity of relatively small patient samples. In addition, all of these studies investigated response to treatment and not remission. Remission is a valuable clinical endpoint because it indicates that treatment has been fully effective; therefore we sought to determine the brain regions associated with remission.
Table 1.
FDG-PET studies of treatment outcome in major depression: table summarizes the 18F-FDG-PET identified in the literature past 1996 that reported on regional brain glucose metabolic rate as a biological predictor of treatment outcome.
| Authors and year | N | Treatment | Imaging analysis | Brain region |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mesiotemporal cortex |
Dorsal
prefrontal cortex |
Ventral
prefrontal cortex |
Anterior cingulate |
Posterior cingulate |
Insula |
Parietal |
Temporal |
Occipital |
Basal ganglia |
Thalamus |
Cerebellum |
Midbrain |
|||||||||||||||||
| L/R/B |
L/R/B |
L/R/B |
L/R/B |
L/R/B |
L/R/B |
L/R/B |
L/R/B |
L/R/B |
L/R/B |
L/R/B |
L/R/B |
L/R/B |
|||||||||||||||||
| Rsp | NR | Rsp | NR | Rsp | NR | Rsp | NR | Rsp | NR | Rsp | NR | Rsp | NR | Rsp | NR | Rsp | NR | Rsp | NR | Rsp | NR | Rsp | NR | Rsp | NR | ||||
| Little et al. (1996) | 11 | Venlafaxine or buproprion (randomized, with cross-over) |
SPM | –/–/↓° | –/–/↓° | –/–/↓° | |||||||||||||||||||||||
| Buchsbaum etal. (1997) | 17 | Sertraline
(placebo-controlled, randomized) |
BMP/correlation | ↑/–/– | |||||||||||||||||||||||||
| Mayberg (1997) | 18 | SSRI, TCA, buproprion | ROI | –/↑/– | –/↓/– | ||||||||||||||||||||||||
| (non-randomized) | SPM | ↓/v/–C | –/–/↓ | –/↑/– | –/↓/– | –/↓/– | |||||||||||||||||||||||
| Brody etal. (1999) | 16 | Paroxetine | ROI/correlation | ↓/–/– | |||||||||||||||||||||||||
| Wu et al. (1999) | 36 | Sleep deprivation | ROI | –/–/↑ | –/↑/– | –/↓/– | –/↓/– | –/↓↑/–D | –/↓/–D | ↑/–/– | –/↓/– | ↓/–/– | –/↓/– | ||||||||||||||||
| *ROI | –/↓/– | ↓/–/– | –/↑/↑A | –/–/↑ | –/↓/– | ||||||||||||||||||||||||
| Brannan et al. (2000) | 53 | Antidepressants | ↑/–/– | –/↑/– | –/–/↑ | ||||||||||||||||||||||||
| Brody et al. (2001) | 24 | Paroxetine or ITP | ROI | –/↑/– | ↑/–/– | ||||||||||||||||||||||||
| (non-randomized) | SPM | –/–/↑ | –/–/↑ | –/↑/– | |||||||||||||||||||||||||
| Kennedy et al. (2001) | 13 | Paroxetine | SPM | ↓/–/–phc | –/↑/– | –/↓/– | |||||||||||||||||||||||
| Saxena et al. (2003) | 27 | Paroxetine | ROI/correlation | –/↓/– | |||||||||||||||||||||||||
| 44B | SPM | –/–/↑ | –/↑/– | ||||||||||||||||||||||||||
| Little et al. (2005) | 20 | Venlafaxine or buproprion | SPM | ↓/–/– | –/–/↓ | –/–/↓ | –/–/↑ | /–/↓E | –/↓/–E | –/↓/– | ↓/–/–F | –/–/↓C | –/–/↑G | ↑/–/–G | –/↓/↓° | –/–/↓C | c | –/–/↑ | |||||||||||
| (randomized, with cross-over) | SPM (absolute) | ↑/–/– | |||||||||||||||||||||||||||
| *SPM | ↓/–/– | ↓/–/– | –/–/↓ | ↑/–/–F | ↓/–/– | ||||||||||||||||||||||||
| *SPM (absolute) | ↓/–/– | ↓/–/– | –/–/↓ | ||||||||||||||||||||||||||
Abbreviations: Comparison group are Controls unless otherwise indicated; *Comparison of Responders to Non-responders; N=number of depressed subjects, L/R/B=Left, Right, Bilateral, BMP=Biomedical Computer Program, SPM=Statistical Parametric Mapping, All SPM data are from normalized rCMRglu unless otherwise indicated. Results of Little et al. (1996) are from both absolute and normalized rCMRglu, A=data from 21 subjects not studied previously, B=MDD +/− comorbid OCD, C=decrease rCMRglu in right Brodmann area 44 and left Brodmann area 46/49 are unique to non-responders, phc=post hoc analysis, D=non-responders express decrease rCMRglu in Brodmann area 38/21, increase rCMRglu is seen in responders, decrease rCMRglu in responders is located in Brodmann area 37, E=non-responders to buproprion demonstrated decrease rCMRglu in right Brodmann area 32, while responders to venlafaxine demonstrated decrease rCMRglu in Brodmann area 32 bilaterally, F=non-responders to buproprion demonstrated decreased rCMRglu in left Brodmann area 4, responders to either medication demonstrated increase rCMRglu in left Brodmann area 40, G=venlafaxine non-responders only, buproprion responders only; ITP=Interpersonal therapy, °=coordinates not reported from SPM analysis.
Brain regions that may predict clinical response may be hypothesized based on the monoamine deficiency hypotheses of depression that posit diminished brain monoaminergic function (Achor, 1959; Kur’Ianova, 1959; Stein, 1961; Koutsky et al., 1962; Bunney and Davis, 1965; Coppen, 1967; Feer, 1967; Schildkraut, 1967; Coppen, 1969; van Praag et al., 1969). We hypothesize that monoamine reuptake inhibitors are more likely to be therapeutic in cases of depression where monoaminergic deficiency is part of the abnormality contributing to the development of the depressive symptoms. If monoaminergic deficiency leads to abnormal glucose uptake, than FDG-PET may predict remission. Another hypothesized abnormality that may predict remission is hyperactivity in the rostral anterior cingulate that is reduced by antidepressant treatment regardless of whether drug or psychotherapy treatment is employed (Mayberg et al., 1997).
We conducted PET-FDG scans on 33 medication-free patients with MDD who were awaiting treatment for a major depressive episode (MDE), and then re-evaluated them after 3 months of naturalistic antidepressant treatment. Remission was defined a priori as a 50% decrease in the 24-item HDRS and a final score of ≤10 (Frank et al., 1991; Bschor et al., 2002; Smith et al., 2002). Baseline rCMRglu was mapped and compared between remitters and non-remitters. As is, evident from Table 1 there are no replicated and validated regions of interest (ROIs) predicting remission in the literature; therefore, we chose an unbiased voxel-based approach to analyze the data in addition to an ROI-based analysis.
2. Methods
2.1. Subjects
Thirty-three patients enrolled in a baseline PET study agreed to be followed during their community-based treatment and were included in this study. All patients met DSM-IV criteria for MDD (American Psychiatric Association. and American Psychiatric Association. Task Force on DSM-IV, 1994) and a current major depressive episode with a score greater than 16 on the 17-item HDRS (Hamilton 1960). The subjects gave written informed consent as approved by the Columbia University Medical Center institutional review board. Demographic data, psychiatric, medical, developmental and family history were recorded on Columbia University Baseline Demographic/History Form. History, physical, and laboratory results were assessed for inclusion/exclusion criteria. Exclusion criteria included other comorbid Axis-I disorders, significant medical conditions (especially those affecting the brain or serotonergic system, e.g.: Cushing’s disease, Alzheimer’s disease, migraine and temporal lobe epilepsy), family history of schizophrenia, pregnancy, the use of medication known to affect serotonin function (list may be provided upon request) or brain activity within 2 weeks of the scan except benzodiazepines (ativan, but not within 3 days of the scan) and electroconvulsive therapy (ECT) within 3 months of the scan. Pre-menopausal women were studied within 14 days of the onset of menses.
2.2. Treatment
Prior to PET scan, patients were medication-free for a minimum of 2 weeks (6 weeks for fluoxetine hydrochloride and 1 month for oral antipsychotic agents). Eleven (6 of 11 in the remitter (R) and 5 of 22 in the non-remitter group (NR)) patients were naïve to psychotropic medication. The R and NR groups did not differ in the class or type of medication taken (Table 2) prior to entering the study or in the number of days off medications prior to scanning (F=0.48, df=15, P=0.8; F=3.46, df=1, P=0.104, respectively).
Table 2.
Characteristics of major depression study sample: comparison of remitters vs. non-remitters.
| Characteristics | Remitters | Non-remitters | P value | |
|---|---|---|---|---|
| Total N | 11 | 22 | ||
| Female N (%) | 4(36.4%) | 17 (77.3%) | 0.052a | |
| Age | 43.2±15.3 | 36.6±10.5 | 0.22 | |
| Years of education | 16.8±3.0 | 15.6±2.3 | 0.25 | |
| Number of previous MDEs | 4.9±5.6 | 3.1±2.4 | 0.33 | |
| Psychotic features (%) | 0 (0%) | 5(22.7)% | 0.14a | |
| HAMD-24 | ||||
| Baseline | 29.6±5.0 | 29.7±7.0 | 0.98 | |
| 3 months | 6.2±2.8 | 25.4±7.7 | <0.001 | |
| Change in HDRS | − 23.5±5.2 | −4.3±9.2 | <0.001 | |
| BDI | ||||
| Baseline | 26.8±9.4 | 32.9±9.8 | 0.11 | |
| 3 months | 4.5±3.7 | 28.1±10.8 | <0.001 | |
| Change in BDI | − 23.4±8.1 | −4.8±10.4 | <0.001 | |
| Treatment intensity (ATHF) | 3.5±0.5 | 3.6±1.3 | 0.74 | |
| On meds at 3 mos months (%) | 10(90.9%) | 21(95.5%) | 1.00a | |
| Average number of pharmacologic agents used | t | χ 2 | ||
|
| ||||
| Antidepressant | 1.50±0.52 | 2.00±0.76 | 0.08 | 1.00a |
| SSRIs | 0.91±0.30 | 0.91±0.61 | 1.00 | 0.64a |
| Paroxetine | 0.45±0.52 | 0.68±0.48 | 0.22 | 0.27a |
| Antidepressant, other | 0.18±0.40 | 0.68±0.72 | 0.04 | 0.67a |
| Tricyclics | 0.36±0.50 | 0.23±0.43 | 0.42 | 0.44a |
| MAOI | 0.09±0.30 | 0.18±0.39 | 0.51 | 0.64a |
| Anticonvulsant/mood stabilizer | 0.18±0.40 | 0.59±0.73 | 0.10 | 0.13a |
| Lithium | 0.09±0.30 | 0.32±0.48 | 0.16 | 0.22a |
| Antipsychotic, atypical | 0.18±0.40 | 0.18±0.39 | 1.00 | 1.00a |
| Antipsychotic, typical | 0.00±0.00 | 0.23±0.53 | 0.17 | 0.28a |
| Benzodiazepine | 0.55±0.69 | 0.73±0.77 | 0.51 | 0.72a |
| Stimulant | 0.00±0.00 | 0.18±0.50 | 0.24 | 0.54a |
| Hypnotic | 0.00±0.00 | 0.05±0.21 | 0.49 | 1.00a |
| ECT | 0.09±0.30 | 0.27±0.46 | 0.32 | 0.38a |
Abbreviations: MDD (Major Depression Disorder); MDE (Major Depressive Episodes); HDRS (Hamilton Depression Rating Scale); ATHF (an automated version of the Antidepressant Treatment History Form; see methods (Oquendo et al., 2003a,b)). All P values are derived from paired t-test unless otherwise noted.
p=Fisher’s Exact P value for cross-tabulation.Values are mean±S.D. unless otherwise noted.
Physicians employed in the General Clinical Research Unit at the New York State Psychiatric Institute treated and monitored inpatients. The decision to treat as outpatient or inpatient was determined based on clinical need. Inpatients were evaluated by their physicians at least three times weekly and were monitored continuously by clinical ward staff. Outpatients were evaluated at least once a week by their community-based psychiatrists. After 4 weeks, all outpatients in active treatment were seen monthly, unless clinical status indicated differently.
All patients were initially treated with paroxetine (61%) except for those who had failed paroxetine in the past or where the clinician felt the patient required ECT. For those initially receiving paroxetine, 20 mg was given daily for 3 weeks and then adjusted over 6 weeks depending on clinical response or side effects up to 60 mg per day. Additional pharmacotherapy was added as deemed necessary by the treating psychiatrist. Table 2 lists the average number of medications used per patient in each class of medication. Adequacy and intensity of treatment were determined by a computerized version (Oquendo et al., 2003a,b) of the Antidepressant Treatment History Form (ATHF) (Sackeim et al., 1990). A therapeutic dose was defined as a score of 3 or greater on a scale of 0 to 5 on this instrument, which scores the adequacy of antidepressant treatment trials for the major pharmacological antidepressant categories and ECT and has been shown to have good reliability and validity (Sackeim et al., 1990; Prudic et al., 1996). For all categories of antidepressants, the minimum duration of the treatment with a specific dose is 4 weeks. The minimum adequate daily dose for the tricyclics is at least 200 mg of imipramine hydrochloride or its equivalent; for SSRIs, the minimum daily dose is at least 20 mg of fluoxetine or its equivalent; for phenelzine, the minimum is at least 61 mg; for venlafaxine, the minimum is 225 mg; for bupropion, the minimum is 300 mg; and for ECT, the minimum is more than six unilateral or bilateral treatments. See the work of Sackeim et al. (1990) for further details. One of the subjects within the remitter group did not have sufficient data to determine intensity of treatment.
2.3. Baseline evaluation and treatment response, remission assessment
Prior to initiating treatment, the severity of disease was measured using the 24-item HDRS and the Beck Depression Inventory (BDI) (Beck and Beamesderfer, 1974) within 24 h of the baseline PET scan and again at 3-months follow-up. The change in rating scales was calculated by subtracting post-treatment scores from pretreatment scores. One baseline and two 3-months BDI scores were not available for analysis. Remission was defined as both a 50% decline in 24-item HDRS and a final score of ≤10 at the 3-month assessment (Frank et al., 1991; Bschor et al., 2002; Smith et al., 2002). All ratings were done by clinicians without knowledge of the medication and not involved in the treatment of the patients.
2.4. Image acquisition and analysis
Acquisitions of PET and MRI images were preformed as previously described (Mann et al., 1996a,b). Five mCi of 18F-FDG were administered and subjects looked at a cross-hair in a quiet room for the first 15 min of the uptake phase. Head movement was restricted in the scanner with an individually molded thermoplastic head holder. Image analysis was performed using Statistical Parametric Mapping software (SPM2; Institute of Neurology, University College of London, London, England) implemented in Matlab 6.5 (The Mathworks Inc, Natick, Mass) (Friston, 1994; Friston et al., 1995), with spatial normalization and adjusting for multiple comparisons (Wright et al., 1995). The effects of global metabolism were controlled by normalizing individual voxel counts to total gray matter counts (global normalization and proportional scaling in SPM2). Smoothing was done with an 8 mm Gaussian Kernel. A t-test on a voxel-by-voxel basis was used to identify clusters where remitters had significantly higher or lower rates of mean rCMRglu than non-remitters, while controlling for age and sex. Thresholds were set a priori to P=0.01 and to P≤0.05 for height and extent thresholds respectively after correction for multiple comparisons by SPM2.
We have previously published the neuroanatomic correlates of the objective psychopathologic components of MDD in the same cohort using the Hamilton Depression Rating Scale (Milak et al., 2005). Subgroups of this study population were the subject of other papers including: a subgroup who had a history of a suicide attempt were the subject of an earlier paper on regional brain correlates of suicidal behavior in major depression (Oquendo et al., 2003a,b); a subgroup with borderline personality disorder (Oquendo et al., 2005); regional brain responses to serotonin in MDD (Anderson et al., 2004); patients with history of familial mood disorder (Kegeles et al., 2003); and a subgroup that received ECT therapy (Nobler et al., 2001). Although this cohort has been studied before, the current article presents new data obtained by completing follow-up and has never been published before.
3. Results
3.1. Demographic and clinical characteristics
Eleven (33%) subjects met remission criteria at 3-month follow-up. Clinical and demographic data are summarized in Table 2. The remitters and non-remitters did not differ on demographic characteristics. There was a trend for more females in the non-remitters, and therefore sex was included in the final model as a nuisance variable. At baseline assessment, there was no difference in the pretreatment severity of MDD between groups on the HDRS (P=0.983) and BDI (P=0.113). There was no group difference in duration of current MDE (U=68.5; z=−1.5; P=0.122). As expected at 3 months, remitters had lower scores on the HDRS (6.2+2.8) and BDI (4.5+3.7) compared with non-remitters (HDRS=25.4+7.7, BDI=28.1+10.8, t=−10.4, −9.0; df=29.3, 27.3; P<0.001, respectively), and showed greater improvement on both HDRS and BDI scores (Table 2).
3.2. Treatment intensity
The adequacy and intensity of treatment as assessed by the ATHF score (Sackeim et al., 1990), and the average number of pharmacologic agents used per subject by medication class, did not differ between remitters and non-remitters during the treatment phase (Table 2).
3.3. Statistical Parametric Mapping analyses
3.3.1. Comparing responders vs. non-responders
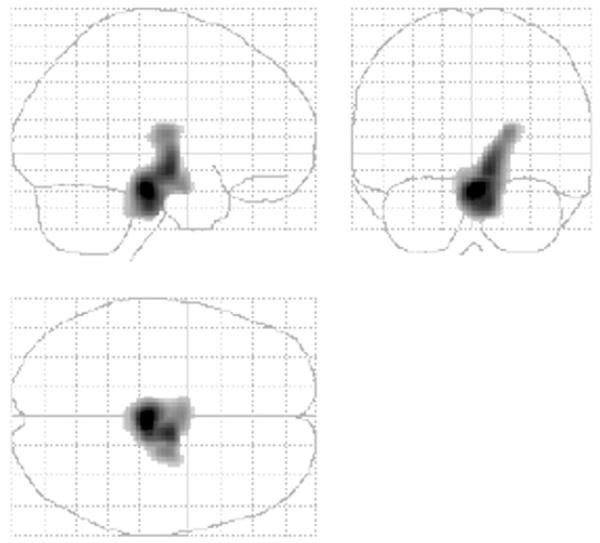
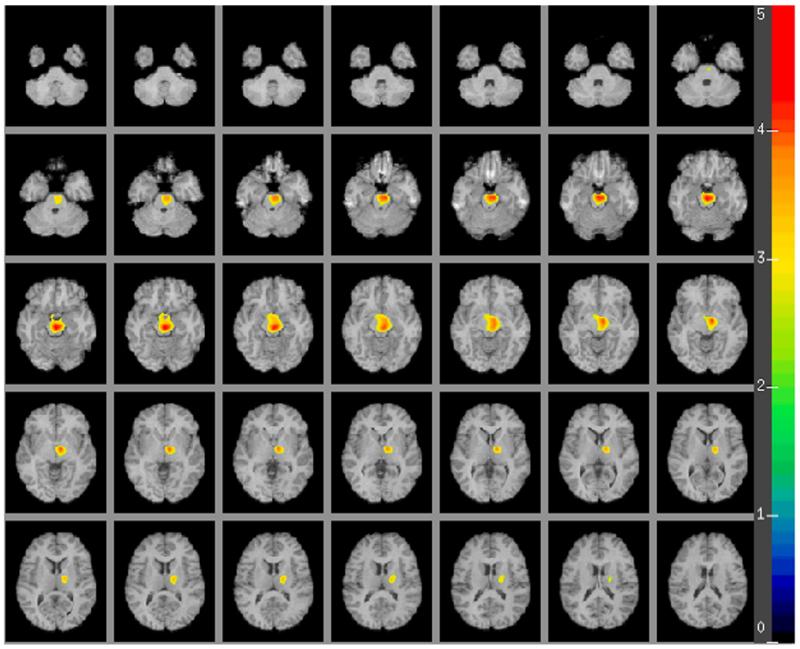
The voxel-based analysis (see Figs. 1 and 2) reveals a single contiguous brain region where remitters have lower mean rCMRglu compared with non-remitters (2498 voxels, cluster level P=0.013 corrected for multiple comparisons, global maximum at Talairach (xyz) coordinates of 2, −24, −20, midbrain). This area encompasses parts of the midbrain, right parahippocampal gyrus, right putamen, right lateral globus pallidus and right thalamus (including the ventral lateral and ventral anterior nuclei). Based on this sample we determined whether higher midbrain metabolic activity may predict non-remission in MDE, finding specificity=0.64; sensitivity=0.82; positive predictive value (PPV)=0.82; negative predictive value (NPV)=0.64 (with sex and midbrain metabolic activity in the logistic regression model).
Fig. 1.
Statistical parametric map showing regions where major depression remitters after three months of treatment had lower rCMRglu than non-remitters. Regions shown as volume in glass brain.
Fig. 2.
A human brain map in major depression depicting the region where antidepressant remitters demonstrate lower regional rates of cerebral glucose metabolism in comparison to the non-remitters. The color scale in the figure indicates the strength (t-score) of the correlation (t-score maps are overlaid on a series of transaxial slices (2 mm apart) of a coregistered MRI scan from 42 mm below to a 26 mm above a line connecting the anterior and posterior commissure).
By conducting a correlation analysis on the entire patient sample restricted to this region of interest described above (SPM2, small search volume correction, SVC) we found a significant correlation between the percent decline in HDRS score in response to treatment and rCMRglu prior to treatment (927 voxels, cluster level r2=0.32; P=0.01 corrected for multiple comparisons, global maximum at Talairach (xyz) coordinates of 0, −24, −24, midbrain).
Brodmann Area 24 relative activity did not differ between groups, although ventral limbic structures on the right side showed lower activity in remitters. Note, however, that we did not exactly replicate the study cited (Mayberg et al., 1997) as they compared responders and non-responders to healthy volunteers and not to each other. Moreover, they reported non-overlapping coordinates in and around the right anterior cingulate (24a/b) x=1 y=44 z=10 z-score=+2.10 for responders and x=10 y=38 z=12 z-score=−3.21 non-responders, where responders were hypermetabolic (24a/b mean rCMLGlc=1270±70) and non-responders hypometabolic (1134±111) compared to controls (1198±85). Nevertheless, in an attempt to avoid the possibility of washing out this finding with excessive correction for multiple comparisons, we repeated the analysis, turning off correction and restricting the search volume to an area located in the same part of BA 24a/b on either side individually, then combined, comparing remitters to non-remitters. Additionally, we surveyed the entire cingulate as well, then separately the entire brain and no suprathreshold voxels were found exceeding a z-score of 1.96 (Mayberg et al., 1997) with either methods (outside of the area reported above in our current results).
3.3.2. Comparing remitters vs. non-remitters
Due to the preponderant use of responders vs. non-responders contrast in studies by others, instead of using remitters vs. non-remitters, we have reanalyzed the data using those definitions. However, we found no area in the brain where responders significantly differed from non-responders, when responder status is evaluated at the 3-months follow-up (with or without correction for multiple comparisons).
4. Discussion
We found a single contiguous region (with a global maximum in the midbrain) in depression where lower pretreatment relative metabolic activity predicts remission after 12 weeks of antidepressant treatment. This distinct difference was observed in the absence of baseline clinical differences and comparable antidepressant treatment in remitters and non-remitters. This finding, if replicated, suggests that brain imaging approach can detect differences, not seen clinically, that forecast clinical outcome and, as such, may be a step towards the development of an objective predictor of antidepressant treatment remission. Future studies need to determine whether brain imaging approaches can predict remission to specific subtypes of antidepressants in order to facilitate selection of the right antidepressant for the individual patient. Such predictors should shorten time to treatment response and remission from initial diagnosis by avoiding the current trial and error approach in antidepressant selection. This approach may reduce disease burden due to depression.
To date there is no biological marker in routine clinical use to predict treatment response or remission. This study shows that, despite the absence of pretreatment clinical differences, this PET scan can detect differences in regional metabolic brain activity that predict remission.
Our findings are consistent with a previous study (Wu et al., 1999) that found lower rCMRglu in responders in the right mesiotemporal cortex, parts of the basal ganglia and midbrain in responders (see Table 1). They also find differences between responders and non-responders in other brain regions, perhaps because they use sleep deprivation as the treatment.
Our study design and outcome measure of remission differ from the studies summarized in Table 1 only in part, because most studies examined degree of clinical improvement. We found that lower rCMRglu in midbrain and nearby regions predict remission, as well as degree of clinical improvement. Other studies identified regions such as prefrontal cortex, anterior cingulate and temporal lobe as related to clinical improvement. Differences in treatment modalities, patient population and scanning methodology may have contributed to differences in identified brain regions. Four studies (Little et al., 1996, 2005; Mayberg et al., 1997; Wu et al., 1999) compared rCMRglu in non-responders versus healthy controls. Some studies enrolled only outpatients (Little et al., 1996, 2005; Brody et al., 1999, 2001; Kennedy et al., 2001; Saxena et al., 2003) whereas others used inpatients exclusively (Mayberg et al., 1997; Wu et al., 1999), and the majority of the studies followed patients for only 6–8 weeks (Little et al., 1996, 2005; Mayberg et al., 1997; Brody et al., 1999; Kennedy et al., 2001; Saxena et al., 2003). Treatments for which response was assessed also varied amongst studies (see Table 1). Two studies enrolled subjects with comorbid Axis-I disorders (Saxena et al., 2003; Little et al., 2005). One included only male subjects (Kennedy et al., 2001).
Imaging methods varied with patients scanned at rest (Mayberg et al., 1997; Brody et al., 1999, 2001; Kennedy et al., 2001; Saxena et al., 2003) or performing variations of a continuous performance task during FDG uptake (Little et al., 1996, 2005; Buchsbaum et al., 1997; Wu et al., 1999). Studies also varied in method of image analysis, employing either a region of interest (Brody et al., 1999; Wu et al., 1999), Statistical Parametric Mapping (SPM) (Little et al., 1996, 2005; Kennedy et al., 2001) or both (Mayberg et al., 1997; Brody et al., 2001; Saxena et al., 2003). Three studies used correlation analysis to find regions that were associated with treatment response, correlating baseline rCMRglu and change in HDRS (Buchsbaum et al., 1997; Brody et al., 1999; Saxena et al., 2003), and Global Assessment Scale (GAS) (Saxena et al., 2003). There are also differences in the methods used to assess treatment response. Studies defined response based on changes in the HDRS (Buchsbaum et al., 1997; Mayberg et al., 1997; Wu et al., 1999; Brody et al., 2001; Kennedy et al., 2001), Clinical Global Impression Scale (CGI) (Little et al., 1996, 2005), or a combination of HDRS with other measures of depression severity, i.e. CGI (Brody et al., 1999) and GAS (Saxena et al., 2003) and set their criteria for response between 40 and 50% decrease in HDRS (Buchsbaum et al., 1997; Mayberg et al., 1997; Brody et al., 1999; Wu et al., 1999; Kennedy et al., 2001), and/or a CGI of moderate to marked response (Little et al., 1996, 2005; Brody et al., 1999). One study (Brannan et al., 2000) defined response as a score of ≤2 on the Lickert scale. Another study included subjects with decreases in the 17-item HDRS; but they were not separated into responders and non-responders (Brody et al., 2001). None of these methodological differences offer a direct explanation of the lack of reproducibility amongst the studies reviewed. An important problem in imaging studies is correction for multiple comparisons; note that most of these studies did not report whether such correction was used and/or how many other contrasts were tested. As mentioned in the Results section, we were not able to reproduce any of the findings listed in Table 1 (with one exception noted above), despite using a variety of different approaches, including one-tailed statistical contrasts without correction for multiple comparisons and/or restricting the search volume to specific regions of interest reported to be significant by studies in Table 1, which suggests that more standardized approaches and larger sample sizes are needed in order to avoid type-one error and sampling bias.
Several SPECT and PET scans using receptor-specific radioligands have found abnormal binding in the brainstem of depressed patients (Drevets et al., 1999, 2007; Morris et al., 1999; Dahlstrom et al., 2000; Willeit et al., 2000; Bhagwagar et al., 2004; Laasonen-Balk et al. 2004; Uusitalo et al., 2004; Newberg et al., 2005; Lehto et al., 2006, 2008; Meyer et al., 2006; Joensuu et al., 2007; Walter et al., 2007; Miller et al., 2008; Reimold et al., 2008). More recently, we and others have begun to scan indices of specific neurotransmitter systems, as predictors of antidepressant response. It has been reported that MDD patients with serotonin transporter (5-HTT) promoter region insertion were more responsive to placebo, as well as more responsive to SSRI than was the short allele group (Rausch et al., 2002). We found that lower serotonin transporter binding predicts poorer antidepressant response (Miller et al., 2006). Innis and colleagues found that higher pretreatment diencephalic 5-HTT availability significantly predicted better treatment response 4 weeks later (Kugaya et al., 2004). Linking these neurotransmitter-specific scan findings to more than just SSRI responses is an important future area of research. We also report elsewhere (Parsey et al., 2006a,b) that non-remission of major depression is associated with higher pretreatment brainstem 5-HT1A binding (midbrain autoreceptors) and relate that to the higher autoreceptor expressing GG genotype. Based on this imaging finding the following model was proposed: the GG polymorphism is associated with greater 5-HT1A gene expression in the midbrain raphe nuclei and hence the higher 5-HT1A binding. As the 5-HT1A receptors are presynaptic auto-inhibitory, more 5-HT1A raphe autoreceptors would result in less serotonin neuron firing and decreased terminal field 5-HT release. This may be followed by a homeostatic upregulation of post-synaptic terminal field 5-HT1A receptors. The GG genotype by itself is also associated with resistance to a variety of antidepressant treatments (Albert 2004; Lemonde et al., 2004).
An important question is, how lower midbrain metabolic activity in remitters, in the present study, may be reconciled with fewer 5-HT1A autoreceptors in the midbrain as predictors of better treatment outcome or, specifically, of remission? Since activating the 5-HT1A receptors causes the opening of the potassium (K+) channels, fewer available 5-HT1A receptors are associated with the closing of the K+ channels in the raphe serotonergic cell bodies. This in turn decreases the workload of the ATP-dependent K+ pump, leading to lower metabolic activity in midbrain of the remitters. A major fraction of cerebral energy production is required for the extrusion of intracellular Na+ that enters during excitation and depolarization of neurons in other parts of the brain, suggesting higher firing leading to higher metabolic rates. However, in the raphe under wakeful rest and uneventful waking activity, serotonergic neurons display slow, clock-like activity of about 1 to 5 spikes/s (Jacobs and Fornal 1993; Frazer and Hensler, 1999). This slow firing barely changes (~1 to 1.25×) the resting workload of the Na/K-ATPase activity (Albers and Siegel, 1999). Therefore remitters with lower somato-dentritic 5-HT1A receptor expression, and consequently closed K+ channels, will have lower midbrain metabolic activity (despite slightly higher firing rates) than non-remitters.
Our results show that some subcortical ventral monoaminergic projection areas such as right parahippocampal gyrus, right putamen, right lateral globus pallidus and right thalamus, also have lower metabolic rates before treatment in remitters. We know from earlier studies that MDD subjects have higher rCMRglu in these regions prior to treatment (Milak et al., 2005), suggesting that those who later turn out to be remitters have less elevation in activity in these regions, prior to treatment, compared with patients who do not remit. In other words, less abnormality in those regions predicts better response. These findings are consistent with transmitter imaging studies of the 5-HT1A and serotonin transporter binding sites where less abnormality predicts better treatment response (Parsey et al., 2006a,b).
It was first reported by Mayberg et al. (1997) that rCMRglu in the anterior cingulate may also be a predictor of favorable treatment response (not remission) by 60% of the studies. Note however, that there is disagreement between these studies in the location and direction of change in rCMRglu that discriminated responders from non-responders (see Table 1), moreover this metabolic abnormality was interpreted as an adaptive or compensatory response (not considered to be causally related) to depression and it was suggested that failure of this compensatory response may underlie poor outcome (Mayberg et al., 1997). We did not detect a relationship of remission or response with the subgenual anterior cingulate, perhaps in part because our study was designed for a different endpoint to improvement, but clearly this difference needs further research.
Limitations
This study involved a naturalistic community-based treatment, making the results more relevant to clinical practice, but as a consequence patients received a variety of treatments. Nevertheless 97% of the patients received antidepressant medications that increase monoaminergic transmission. Future controlled double blind cross-over studies are needed to determine whether brain imaging before treatment can predict improvement regardless of the mechanism by which the treatment enhances monoaminergic transmission, — or the findings described here are specific effects of a particular class of antidepressants.
Acknowledgements
Department of Molecular Brain Imaging and Neuropathology Brain Imaging Division assisted with image analysis and thanks are due to clinicians involved in subject recruitment and clinical ratings. This study was supported in part by NIH Grants: MH40695, MH62185, RR00645 and NARSAD.
References
- Achor RW. Depressions in hypertensive patients. I’ll Heart Bulletin. 1959;8:105–107. [PubMed] [Google Scholar]
- Albers RW, Siegel GJ. 5. Membrane Transport. In: Siegel GJ, editor. Basic neurochemistry : molecular, cellular, and medical aspects. Lippincott Williams & Wilkins; Philadelphia: 1999. p. xxi.p. 1183. [Google Scholar]
- Albert PR. A functional promoter polymorphism of the 5HT1A receptor gene: association with depression and completed suicide. Biological Psychiatry. 2004;55:46S–46S. [Google Scholar]
- American Psychiatric Association. American Psychiatric Association. Task Force on DSM-IV . Diagnostic and Statistical Manual of Mental Disorders : DSM-IV. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Anderson AD, Oquendo MA, Parsey RV, Milak MS, Campbell C, Mann JJ. Regional brain responses to serotonin in major depressive disorder. Journal of Affective Disorders. 2004;82(3):411–417. doi: 10.1016/j.jad.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Modern Problems of Pharmacopsychiatry. 1974;7(0):151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Molecular Psychiatry. 2004;9(4):386–392. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- Brannan SK, Mayberg HS, McGinnis S, Silva JA, Tekell JL, Mahurin RK, Jerabek PA, Fox PT. Cingulate metabolism predicts treatment response: a replication. Biological psychiatry. 2000;47:1S–173S. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Silverman DH, Alborzian S, Fairbanks LA, Phelps ME, Huang SC, Wu HM, Maidment K, Baxter LR., Jr. Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Research. 1999;91(3):127–139. doi: 10.1016/s0925-4927(99)00034-7. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S, Phelps ME, Huang SC, Wu HM, Ho ML, Ho MK, Au SC, Maidment K, Baxter LR., Jr. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Archives of General Psychiatry. 2001;58(7):631–640. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- Bschor T, Adli M, Baethge C, Eichmann U, Ising M, Uhr M, Modell S, Kunzel H, Muller-Oerlinghausen B, Bauer M. Lithium augmentation increases the ACTH and cortisol response in the combined DEX/CRH test in unipolar major depression. Neuropsychopharmacology. 2002;27(3):470–478. doi: 10.1016/S0893-133X(02)00323-8. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Wu J, Siegel BV, Hackett E, Trenary M, Abel L, Reynolds C. Effect of sertraline on regional metabolic rate in patients with affective disorder. Biological Psychiatry. 1997;41(1):15–22. doi: 10.1016/s0006-3223(96)00097-2. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Jr., Davis JM. Norepinephrine in depressive reactions. A review. Archives of General Psychiatry. 1965;13(6):483–494. doi: 10.1001/archpsyc.1965.01730060001001. [DOI] [PubMed] [Google Scholar]
- Coppen A. The biochemistry of affective disorders. British Journal of Psychiatry. 1967;113(504):1237–1264. doi: 10.1192/bjp.113.504.1237. [DOI] [PubMed] [Google Scholar]
- Coppen A. Defects in monoamine metabolism and their possible importance in the pathogenesis of depressive syndromes. British Journal of Psychiatry. 1969;72(2):173–180. [PubMed] [Google Scholar]
- Dahlstrom M, Ahonen A, Ebeling H, Torniainen P, Heikkila J, Moilanen I. Elevated hypothalamic/midbrain serotonin (monoamine) transporter availability in depressive drug-naive children and adolescents. Molecular Psychiatry. 2000;5(5):514–522. doi: 10.1038/sj.mp.4000766. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biological Psychiatry. 1999;46(10):1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nuclear Medicine and Biology. 2007;34(7):865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Charney DS. Mood disorders and medical illness: a major public health problem. Biological Psychiatry. 2003;54(3):177–180. doi: 10.1016/s0006-3223(03)00639-5. [DOI] [PubMed] [Google Scholar]
- Feer H. The catecholamine hypothesis of depressions: further arguments. Comprehensive Psychiatry. 1967;8(1):1–6. doi: 10.1016/s0010-440x(67)80008-7. [DOI] [PubMed] [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Archives of General Psychiatry. 1991;48(9):851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Frazer A, Hensler JG. 13. Serotonin. In: Siegel GJ, editor. Basic neurochemistry: molecular, cellular, and medical aspects. Lippincott Williams & Wilkins; Philadelphia: 1999. p. xxi.p. 1183. [Google Scholar]
- Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Friston KJ. Statistical parametric mapping. In: Thatcher Robert W., Hallett, Mark, et al., editors. Functional neuroimaging: Technical foundations. 1994. pp. 79–93.pp. xxpp. 303 [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends Neurosciences. 1993;16(9):346–352. doi: 10.1016/0166-2236(93)90090-9. [DOI] [PubMed] [Google Scholar]
- Joensuu M, Tolmunen T, Saarinen PI, Tiihonen J, Kuikka J, Ahola P, Vanninen R, Lehtonen J. Reduced midbrain serotonin transporter availability in drugnaive patients with depression measured by SERT-specific [(123)I] nor-beta-CIT SPECT imaging. Psychiatry Research. 2007;154(2):125–131. doi: 10.1016/j.pscychresns.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Malone KM, Slifstein M, Ellis SP, Xanthopoulos E, Keilp JG, Campbell C, Oquendo M, Van Heertum RL, Mann JJ. Response of cortical metabolic deficits to serotonergic challenge in familial mood disorders. American Journal of Psychiatry. 2003;160(1):76–82. doi: 10.1176/appi.ajp.160.1.76. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. American Journal of Psychiatry. 2001;158(6):899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Kimbrell TA, George MS, Willis MW, Benson BE, Danielson A, Frye MA, Herscovitch P, Post RM. Baseline cerebral hypermetabolism associated with carbamazepine response, and hypometabolism with nimodipine response in mood disorders. Biological Psychiatry. 1999;46(10):1364–1374. doi: 10.1016/s0006-3223(99)00210-3. [DOI] [PubMed] [Google Scholar]
- Kimbrell TA, Little JT, Dunn RT, Frye MA, Greenberg BD, Wassermann EM, Repella JD, Danielson AL, Willis MW, Benson BE, Speer AM, Osuch E, George MS, Post RM. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biological Psychiatry. 1999;46(12):1603–1613. doi: 10.1016/s0006-3223(99)00195-x. [DOI] [PubMed] [Google Scholar]
- Koutsky CD, Mulvahill JE, Orbuch MW. Danger of depression associated with Rauwolfia therapy. Journal-lancet. 1962;82:346–349. [PubMed] [Google Scholar]
- Kugaya A, Sanacora G, Staley JK, Malison RT, Bozkurt A, Khan S, Anand A, Van Dyck CH, Baldwin RM, Seibyl JP, Charney D, Innis RB. Brain serotonin transporter availability predicts treatment response to selective serotonin reuptake inhibitors. Biological Psychiatry. 2004;56(7):497–502. doi: 10.1016/j.biopsych.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Kur’Ianova EI. The therapy of patients of hypertension with Rauwolfia serpentina preparations. Part 1. Preliminary results of therapy. Trudy Leningradskogo Sanitarno-GigienicÏeskogo Medicinskogo Instituta. 1959;48:169–183. [PubMed] [Google Scholar]
- Laasonen-Balk T, Viinamaki H, Kuikka JT, Husso-Saastamoinen M, Lehtonen J, Tiihonen J. 123I-beta-CIT binding and recovery from depression. A six-month follow-up study. European Archives of Psychiatry and Clinical Neuroscience. 2004;254(3):152–155. doi: 10.1007/s00406-004-0458-5. [DOI] [PubMed] [Google Scholar]
- Lehto S, Tolmunen T, Joensuu M, Saarinen PI, Vanninen R, Ahola P, Tiihonen J, Kuikka J, Lehtonen J. Midbrain binding of [123I]nor-beta-CIT in atypical depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2006;30(7):1251–1255. doi: 10.1016/j.pnpbp.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Lehto SM, Tolmunen T, Joensuu M, Saarinen PI, Valkonen-Korhonen M, Vanninen R, Ahola P, Tiihonen J, Kuikka J, Lehtonen J. Changes in midbrain serotonin transporter availability in atypically depressed subjects after one year of psychotherapy. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32(1):229–237. doi: 10.1016/j.pnpbp.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Du L, Bakish D, Hrdina P, Albert PR. Association of the C(−1019)G 5-HT1A functional promoter polymorphism with antidepressant response. International Journal of Neuropsychopharmacology. 2004;7(4):501–506. doi: 10.1017/S1461145704004699. [DOI] [PubMed] [Google Scholar]
- Little JT, Ketter TA, Kimbrell TA, Danielson A, Benson B, Willis MW, Post RM. Venlafaxine or bupropion responders but not nonresponders show baseline prefrontal and paralimbic hypometabolism compared with controls. Psychopharmacology Bulletin. 1996;32(4):629–635. [PubMed] [Google Scholar]
- Little JT, Ketter TA, Kimbrell TA, Dunn RT, Benson BE, Willis MW, Luckenbaugh DA, Post RM. Bupropion and venlafaxine responders differ in pretreatment regional cerebral metabolism in unipolar depression. Biological Psychiatry. 2005;57(3):220–228. doi: 10.1016/j.biopsych.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Mann JJ. The medical management of depression. N. Engl. J. Med. 2005;353(17):1819–1834. doi: 10.1056/NEJMra050730. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Malone KM, Diehl DJ, Perel J, Cooper TB, Mintun MA. Demonstration in vivo of reduced serotonin responsivity in the brain of untreated depressed patients. American Journal of Psychiatry. 1996a;153(2):174–182. doi: 10.1176/ajp.153.2.174. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Malone KM, Diehl DJ, Perel J, Nichols TE, Mintun MA. Positron emission tomographic imaging of serotonin activation effects on prefrontal cortex in healthy volunteers. Journal of Cerebral Blood Flow and Metabolism. 1996b;16(3):418–426. doi: 10.1097/00004647-199605000-00008. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic–cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8(4):1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, Young T, Praschak-Rieder N, Wilson AA, Houle S. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Archives of General Psychiatry. 2006;63(11):1209–1216. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- Michaud CM, Murray CJ, Bloom BR. Burden of disease—implications for future research. Jama. 2001;285(5):535–539. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- Milak MS, Parsey RV, Keilp J, Oquendo MA, Malone KM, Mann JJ. Neuroanatomic correlates of psychopathologic components of major depressive disorder. Archives of General Psychiatry. 2005;62(4):397–408. doi: 10.1001/archpsyc.62.4.397. [DOI] [PubMed] [Google Scholar]
- Miller JM, Oquendo MA, Ogden RT, Hu X, Goldman D, Huang YY, Mann JJ, Parsey RV. Brain serotonin transporter and serotonin transporter polymorphism genotype predict one year remission from major depression. Biological Psychiatry. 2006;59(8):98S–98S. [Google Scholar]
- Miller JM, Oquendo MA, Ogden RT, Mann JJ, Parsey RV. Serotonin transporter binding as a possible predictor of one-year remission in major depressive disorder. Journal of Psychiatric Research. 2008 doi: 10.1016/j.jpsychires.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999;10(2):163–172. doi: 10.1006/nimg.1999.0455. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Amsterdam JD, Wintering N, Ploessl K, Swanson RL, Shults J, Alavi A. 123I-ADAM binding to serotonin transporters in patients with major depression and healthy controls: a preliminary study. Journal of Nuclear Medicine. 2005;46(6):973–977. [PubMed] [Google Scholar]
- Nobler MS, Oquendo MA, Kegeles LS, Malone KM, Campbell CC, Sackeim HA. Decreased regional brain metabolism after ect. American Journal of Psychiatry. 2001;158(2):305–308. doi: 10.1176/appi.ajp.158.2.305. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Malone KM, Ellis SP, Sackeim HA, Mann JJ. Inadequacy of antidepressant treatment for patients with major depression who are at risk for suicidal behavior. American Journal of Psychiatry. 1999;156(2):190–194. doi: 10.1176/ajp.156.2.190. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Baca-Garcia E, Kartachov A, Khait V, Campbell CE, Richards M, Sackeim HA, Prudic J, Mann JJ. A computer algorithm for calculating the adequacy of antidepressant treatment in unipolar and bipolar depression. Journal of Clinical Psychiatry. 2003a;64(7):825–833. doi: 10.4088/jcp.v64n0714. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Placidi GP, Malone KM, Campbell C, Keilp J, Brodsky B, Kegeles LS, Cooper TB, Parsey RV, van Heertum RL, Mann JJ. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Archives of General Psychiatry. 2003b;60(1):14–22. doi: 10.1001/archpsyc.60.1.14. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Krunic A, Parsey RV, Milak M, Malone KM, Anderson A, van Heertum RL, John Mann J. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge in major depressive disorder with and without borderline personality disorder. Neuropsychopharmacology. 2005;30(6):1163–1172. doi: 10.1038/sj.npp.1300689. [DOI] [PubMed] [Google Scholar]
- Panel DG. Depression in primary care. Vol. 2 Treatment of Major Depression. US Department of Health and Human Services; Rockville, MD: 1999. Clinical Practice Guideline, Number 5. [Google Scholar]
- Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ. Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006a;31(8):1745–1749. doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ. Higher 5-HT(1A) receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006b doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- Prudic J, Haskett RF, Mulsant B, Malone KM, Pettinati HM, Stephens S, Greenberg R, Rifas SL, Sackeim HA. Resistance to antidepressant medications and short-term clinical response to ECT. American Journal of Psychiatry. 1996;153(8):985–992. doi: 10.1176/ajp.153.8.985. [DOI] [PubMed] [Google Scholar]
- Rausch JL, Johnson ME, Fei YJ, Li JQ, Shendarkar N, Hobby HM, Ganapathy V, Leibach FH. Initial conditions of serotonin transporter kinetics and genotype: influence on SSRI treatment trial outcome. Biological Psychiatry. 2002;51(9):723–732. doi: 10.1016/s0006-3223(01)01283-5. [DOI] [PubMed] [Google Scholar]
- Reimold M, Batra A, Knobel A, Smolka MN, Zimmer A, Mann K, Solbach C, Reischl G, Schwarzler F, Grunder G, Machulla HJ, Bares R, Heinz A. Anxiety is associated with reduced central serotonin transporter availability in unmedicated patients with unipolar major depression: a [(11)C]DASB PET study. Molecular Psychiatry. 2008 doi: 10.1038/sj.mp.4002149. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Prudic J, Devanand DP, Decina P, Kerr B, Malitz S. The impact of medication resistance and continuation pharmacotherapy on relapse following response to electroconvulsive therapy in major depression. Journal of Clinical Psychopharmacology. 1990;10(2):96–104. doi: 10.1097/00004714-199004000-00004. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Ho ML, Zohrabi N, Maidment KM, Baxter LR., Jr. Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. American Journal of Psychiatry. 2003;160(3):522–532. doi: 10.1176/appi.ajp.160.3.522. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders. A review of supporting evidence. International Journal of Psychiatry. 1967;4(3):203–217. [PubMed] [Google Scholar]
- Smith WT, Londborg PD, Glaudin V, Painter JR. Is extended clonazepam cotherapy of fluoxetine effective for outpatients with major depression? Journal of affective Disorders. 2002;70(3):251–259. doi: 10.1016/s0165-0327(01)00352-4. [DOI] [PubMed] [Google Scholar]
- Stein L. Effects and interactions of imipramine, chlorpromazine, reserpine and amphetamine on self-stimulation: possible neurophysiological basis of depression. Recent Advances in Biological Psychiatry. 1961;4:288–309. doi: 10.1007/978-1-4684-8306-2_27. [DOI] [PubMed] [Google Scholar]
- Uusitalo AL, Valkonen-Korhonen M, Helenius P, Vanninen E, Bergstrom KA, Kuikka JT. Abnormal serotonin reuptake in an overtrained, insomnic and depressed team athlete. International Journal of Sports Medicine. 2004;25(2):150–153. doi: 10.1055/s-2004-819952. [DOI] [PubMed] [Google Scholar]
- van Praag HM, Kits TP, Schut T, Dijkstra P. An attempt at indirect evaluation of the noradrenaline hypothesis. Results of a pilot study of the antidepressive qualities of p-chloro-N-methylamphetamine. Behavioral Neuropsychiatry. 1969;1(5):17–24. [PubMed] [Google Scholar]
- Walter U, Hoeppner J, Prudente-Morrissey L, Horowski S, Herpertz SC, Benecke R. Parkinson’s disease-like midbrain sonography abnormalities are frequent in depressive disorders. Brain. 2007;130(Pt 7):1799–1807. doi: 10.1093/brain/awm017. [DOI] [PubMed] [Google Scholar]
- Willeit M, Praschak-Rieder N, Neumeister A, Pirker W, Asenbaum S, Vitouch O, Tauscher J, Hilger E, Stastny J, Brucke T, Kasper S. [123I]-beta-CIT SPECT imaging shows reduced brain serotonin transporter availability in drug-free depressed patients with seasonal affective disorder. Biological Psychiatry. 2000;47(6):482–489. doi: 10.1016/s0006-3223(99)00293-0. [DOI] [PubMed] [Google Scholar]
- Wright IC, McGuire PK, Poline JB, Travere JM, Murray RM, Frith CD, Frackowiak RS, Friston KJ. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage. 1995;2(4):244–252. doi: 10.1006/nimg.1995.1032. [DOI] [PubMed] [Google Scholar]
- Wu J, Buchsbaum MS, Gillin JC, Tang C, Cadwell S, Wiegand M, Najafi A, Klein E, Hazen K, Bunney WE, Jr., Fallon JH, Keator D. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. American Journal of Psychiatry. 1999;156(8):1149–1158. doi: 10.1176/ajp.156.8.1149. [DOI] [PubMed] [Google Scholar]
- Wu JC, Gillin JC, Buchsbaum MS, Hershey T, Johnson JC, Bunney WE., Jr. Effect of sleep deprivation on brain metabolism of depressed patients. American Journal of Psychiatry. 1992;149(4):538–543. doi: 10.1176/ajp.149.4.538. [DOI] [PubMed] [Google Scholar]