Abstract
NK cells are critical in immune responses against pathogens. However, their role in autoimmunity is still controversial. Here, we demonstrate that neonatal NK cells render newborns more susceptible to neonatal autoimmunity induced by maternal autoantibodies (nAOD); thus, neonatal but not adult NK cells are pathogenic after transfer into NK cell-deficient pups. The inhibitory receptors Ly49C/I are expressed in ~5% of neonatal and ~50% of adult NK cells. Here, we show that the presence of Ly49C/I+ adult NK cells inhibits nAOD induction. Thus, the ontogenetic regulation of Ly49C/I expression determines the propensity to autoantibody induced autoimmunity. In summary, our study provide definitive evidences of a pathogenic role of NK cells in neonatal autoimmunity and also elucidate a novel mechanism by which neonatal NK cells render newborns more susceptible to autoantibody induced autoimmunity.
INTRODUCTION
Approximately 4 million children under the age of 6 months die each year worldwide because of infections (1), revealing that infections early in life are still an important cause of morbidity and mortality. Paradoxically, whereas newborns are more susceptible to infections, they are also more prone to the development of autoimmune diseases. Maternal autoantibodies (autoAb) can cause severe pathology in the fetuses or newborns yet spare the mother. AutoAb to Ro52 or Ro60 in women with lupus or Sjogren’s syndrome are associated with congenital heart block in the progeny (2). Female lupus patients also produce DNA autoAb that can be lethal in neonatal mice when they cross-react with neuron receptors (3). Neonatal myasthenia gravis is associated with maternal AutoAb to fetal acethylcholine receptor (4). Diabetes only occurs after exposure to hen egg lysozyme (HEL) maternal autoAb in mice expressing HEL in pancreatic islets and HEL specific T cell receptor (5). Despite the substantial evidence of a preferential pathogenicity of autoAb in neonates, the mechanisms involved in newborn predisposition to autoimmunity remain unknown.
Neonatal tolerance (6) is insufficient to explain newborn susceptibility to infections since neonatal and adult lymphocytes mount similar responses under the appropriate conditions (7). Importantly, we showed that depending on the host expression of the target Ag (zona pellucida 3, ZP3), the neonatal immune response diverged from tolerance to autoimmunity (8). Thus, the ZP3 peptide (335-342) induced a Th2-deviated tolerance to the “foreign” pZP3 in male mice, while triggered autoimmune ovarian disease (AOD) in female mice.
We have investigated a model of neonatal autoimmunity that resembles human congenital heart block since it also is mediated by maternal autoAb that are harmless to the mother but induce pathology in the newborns. Neonatal AOD (nAOD) is induced in neonates by maternal autoAb to pZP3 (9). Although ZP3 autoAb form immune complexes on the zona pellucida of both adult and neonatal oocytes, ovarian inflammation occurs only when ZP3 autoAb exposure is initiated within the first 5 days of life (9). The requirement of NK cells, FcgRIII and IFNg in nAOD (9, 10) suggests NK cells mediated Ab-dependent cell-mediated cytotoxicity. Importantly, we found that NK cells promote the inductive and effector T cell response, highlighting a crucial interaction between the innate and the adaptive immune responses (9). To further study the T cell requirement in nAOD, we turned to mice deficient in T and B cells. Surprisingly, these mice also developed severe nAOD independently of the adaptive immune response. Using the latter model we made three novel and striking observations: 1) neonatal susceptibility to autoimmunity is restricted to unique properties of the neonatal innate response, 2) we identified neonatal NK cells as a critical determinant for newborn propensity to autoimmunity, and 3) the acquisition of the inhibitory Ly49C/I receptors on adult NK cells renders adult mice resistant to the development of autoimmunity.
MATERIAL AND METHODS
Mice and Abs
C57BL/6 (B6, H-2b), A/J (H-2a), BALB/c (H-2d), and (C57BL/6×A/J) F1 (B6AF1, H-2a×b) mice were obtained from the National Cancer Institute (Frederick, MD). B6 mice deficient in Rag1, b2m, Foxn1 (Nude), Prkdc (Scid), BALB/c Scid and B6.AKR (H-2k) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). B6 Rag2/IL-2rg−/− mice came from Taconic Farms (Germantown, NY). (B6×B6.AKR) F1 (B6.kb, H-2k×b) and B6AF1 Nude mice were generated in our laboratory (11). CD1d−/− B6 mice were a gift from Dr. Bendelac (University of Chicago). Mice were bred and housed in specific pathogen-free facilities following the Animal Care and Use Committee Guidelines of the University of Virginia. The following investigators provided the following mAbs: Dr. Yokoyama (Washington University) NKG2D Ab (C7); Dr. Ortaldo (National Cancer Institute) Ly49C/I depleting Ab (5E6); and Dr. Ravetch (Rockefeller University) FcgRIV Ab (9E9). Asialo GM1 Ab came from Cedarlane laboratories (Canada).
Peptides and mAb production
A ZP3 mAb (1G2 clone, IgG2b) was generated in B6AF1 mice immunized with the chimeric peptide 2 (12) in CFA. Ab binding to ZP3 (330-342) peptide and native ZP3 was screened by ELISA and immunofluorescence, respectively. IgG was produced at the Lymphocyte Culture Core (University of Virginia).
nAOD induction, histopathology and nAOD severity grading
nAOD was induced by feeding with milk from mothers immunized with CP2 in CFA (9), or by i.p. injection of 100 ug of ZP3 mAb (1G2 clone) on days 3 and 5 (day 0 being the day of birth). Mice were euthanized at 2 wks of age, ovaries were fixed in Bouin’s and pathology was graded as unknown samples as described (10).
NK cell isolation and transfer
NK cells were isolated from the spleen of neonatal (1-6 days old), juvenile (4-5 wks old) and adult (≥ 8 wks old) B6 mice using D×5 Microbeads (Miltenyi Biotec, Auburn, CA). The purity of neonatal and adult NK cell ranged from 80-95%. In NK cell reconstitution experiments, 1-5 day-old Rag2/IL-2rg−/− recipients were injected i.p. with 2-5×105 neonatal NK cells or 5×105 NK cells isolated from juvenile or adult mice.
Flow cytometry analysis
Spleen cells were stained with Abs to: CD49b (D×5), NK1.1 (PK136), Ly49C/I (5E6), Ly49I (YLI-90), CD122 (TM-b1), NKp46 (29A1.4) and CD3 (145-2C11). Cells were analyzed on a 5-color FACScan or a 6 color FACSCanto I flow cytometer (BD Biosciences), and data were analyzed with FlowJo software (Tree Star).
Statistical analysis
Statistical differences between 2 samples were determined by the non-parametric Mann-Whitney U test. Fisher’s exact test was used to assess differences in disease incidence. Significance was considered at p<0.05.
RESULTS AND DISCUSSION
NK cells are obligatory in T cell-independent (TI) nAOD
Maternal ZP3 autoAb induced a high frequency of T cell dependent nAOD (TD-nAOD) in wild-type (wt) (B6×A/J)F1 (B6AF1) and (B6×B6.AKR)F1 (B6.kb) mice (Fig. 1A, C) but minimal disease in B6 and BALB/c mice (Fig. 1B, D). In contrast, Nude, Scid, Rag1−/− and Tcra−/− mice developed high frequency of nAOD regardless of the mouse strain (Fig. 1A-D). Maternal ZP3 Ab and ZP3 mAb recognize the same ZP3 B cell epitope and induced comparable nAOD (Fig. 1E). Here, we took advantage of this new TI-nAOD model to investigate the properties of the neonatal innate system that predisposes newborns to autoimmunity. We studied primarily B6 Rag−/− mice injected with ZP3 mAb.
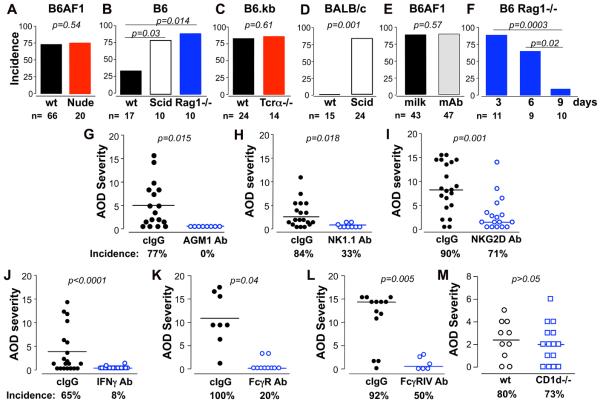
Figure 1. Analysis of the cellular and molecular requirements in TI-nAOD pathogenesis.
mice deficient in T and B cells develop high incidence of ovarian inflammation when exposed to maternal ZP3 autoAb, and requires NK cells, IFNg and FcgRIII. A-D, T cell deficient (Nude and TCRα−/−; A and C) and T/B cell deficient (Scid and Rag1−/−; B and D) mice develop frequent TI-nAOD; whereas wt B6 and BALB/c mice are low or non-responders to ZP3 maternal Ab (B, D). E, Comparable incidences of TD-nAOD in B6AF1 pups after ZP3 Ab+ milk feeding or ZP3 mAb injection. F, B6 Rag1−/− mice develop TI-nAOD when exposed to ZP3 mAb on day 3 or day 6 but not on day 9 after birth. G-L, Analysis of TI-nAOD induction after treatment with control IgG (cIgG) or Ab to: (G) Asialo GM1 [AGM1] (NK cell depletion), (H) NK1.1 (NK cell depletion), (I) NKG2D (NK cell activating receptor blockade), (J) IFNg, (K) FcgRIIB/III mAb, or (L) FcgRIV. (M) CD1d−/− and wt B6.kb mice develop comparable TD-nAOD. nAOD was induced by maternal ZP3 Ab (A-D, M) or ZP3 mAb injection (F-L). G-M, n= 6-21 animals pooled from 3-4 independent experiments.
Similar to TD-nAOD (9, 10), TI-nAOD occurred when ZP3 mAb was injected on days 3 or 6 after birth, but not on day 9 (Fig. 1F). Therefore, neonatal innate response alone can confer propensity to nAOD. As previously shown in TD-nAOD (9, 10), Rag1−/− mice with TI-nAOD showed different degrees of ovarian inflammation, oocyte loss and atrophy (Supplemental Fig. 1). NK cell depletion (Fig. 1G, H) or NK cell activation blockade (Fig. 1I) significantly reduced the incidence and severity of TI-nAOD, indicating that NK cells are critical in TI-nAOD. In addition, NKG2D+ NK cells were detected nearby and inside the ovarian follicles of mice with TI-nAOD (Supplemental Fig. 1), suggesting oocyte-specific NK cell-dependent cytolysis. As in TD-nAOD (9, 10), IFNg and FcgRIII were also critical in TI-nAOD; thus TI-nAOD was inhibited by mAb to IFNg and mAb to FcgRIIB/III (Fig. 1J and K). Strikingly, TI-nAOD induction was also dependent on FcgRIV (Fig. 1L), an activating IgG Fc receptor expressed on dendritic cells, macrophages and neutrophils but not on NK cells (13). Therefore, TI-nAOD induction must also depend on other innate cells. Finally, NKT cells are redundant in nAOD because ovarian inflammation occurred in Nude, Rag1−/− and Tcra−/− mice (Fig. 1A, B, C), and also in CD1d−/− B6.kb mice, deficient in iNKT cells (Fig. 1M).
Therefore, both TD-nAOD and TI-nAOD models depend on NK cells, IFNg and FcgRIII and share the same neonatal time window for disease induction. Moreover, our findings indicate that neonatal mice can mount a robust innate immune response to tissue-associated immune complexes, sufficient to cause severe organ-specific injury. Our data demonstrate that a T cell response is critical in TD-nAOD but dispensable in TI-nAOD. This differential T cell requirement is likely due to the superior innate cell function in T and B cell deficient mice over wt mice (14), and the capacity of T cells to dampen the innate response (15).
Ontogenetic differences in NK cell function determines neonatal susceptibility to TI-nAOD
To investigate whether NK cell function explains neonatal propensity to TI-nAOD, we next compared the capacity of neonatal and adult NK cells to induce nAOD by adoptive transfer into Rag2/IL2rg−/− mice that lack T, B and NK cells. As expected, Rag2/IL-2rg−/− mice were resistant to TI-nAOD induction (Fig. 2A), supporting the critical role of NK cells in nAOD. However, the reconstitution of Rag2/IL-2rg−/− pups with NK cells isolated from 1-6 day-old wt mice restored ovarian inflammation (Fig. 2A). Remarkably, minimal disease was detected when we transferred NK cells from 7-9 or 11-12 day-old donors (Fig. 2A), and NK cells from >4 wk-old donors failed to induce nAOD. Thus, the ontogenetic properties of NK cells to restore nAOD mirror the neonatal time window for nAOD induction (Fig. 1F). The failure of adult NK cells to induce nAOD is not due their poor survival since we recovered similar numbers of donor neonatal and adult NK cells from the recipients (Fig. 2B). Importantly, NK cell-depleted splenic cells no longer reconstituted nAOD in Rag2/IL-2rg−/− pups (Fig. 2C) indicating that IL2rg+ non-NK cells residing in the neonatal spleen cannot induce nAOD. Both TD-nAOD (10) and TI-nAOD (Fig. 1J) pathogenesis is IFNg dependent. The failure of IFNg−/− neonatal NK cells to induce nAOD in Rag2/IL-2rg−/− pups (Fig. 2A) indicates that the neonatal NK cell is a critical source of IFNg. Therefore, we demonstrated that the unique properties of neonatal NK cells render newborns susceptible to TI-nAOD, that IFNg produced by the neonatal NK cells is required for TI-nAOD.
Figure 2. Neonatal but not adult NK cells induce TI-nAOD.
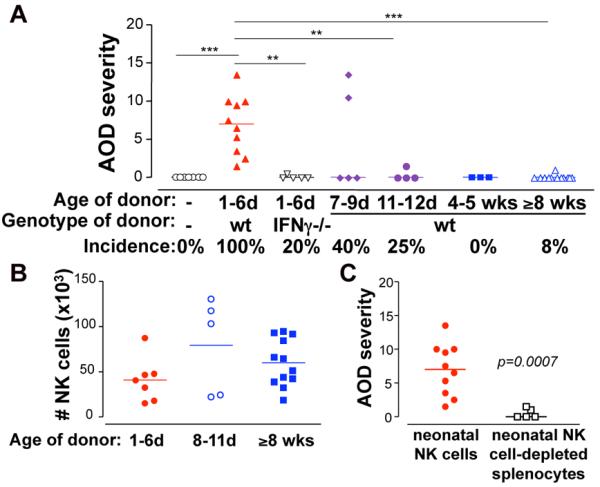
A, TI-nAOD is restored in 1-5 day-old Rag2/IL2rg−/− recipients after reconstitution with 2-5×105 wt but not with IFNg−/− splenic NK cells isolated from neonatal (1-6 days [d]) donors or 5×105 NK cells from >4 wk old wt donors. B, Similar number of NK cells were recovered from the spleen of Rag2/IL2rg−/− mice at 2 wks of age. Gated on live, CD122+ D×5+ NK1.1+ cells. C, NK cell-depleted neonatal spleen cells do not restore TI-nAOD in Rag2/IL-2rg−/− mice. Spleen cells from 1-6 day-old wt mice were depleted ex-vivo with D×5 and NK1.1 Abs using magnetic microbeads. The frequency of residual D×5+ NK1.1+ NK cells was 0.05-0.06%. TI-nAOD was induced by ZP3 mAb; n= 3-12 mice pooled from 2-7 independent experiments. **p<0.01; ***p<0.0001.
The challenging adoptive transfer strategy used here required age synchronization of cell donors and recipients, and >20 neonatal donors were required to transfer 2-5×105 NK cells per recipient. This limited the number of Rag2/IL-2rg−/− pups that could be reconstituted with wt or IFNg−/− neonatal NK cells to 1 or 2 per experiment. Thus, these data were obtained from multiple experiments (3-7) in order to achieve a total of 5-10 mice per group, and they were also used in Fig. 2C and Fig. 3.
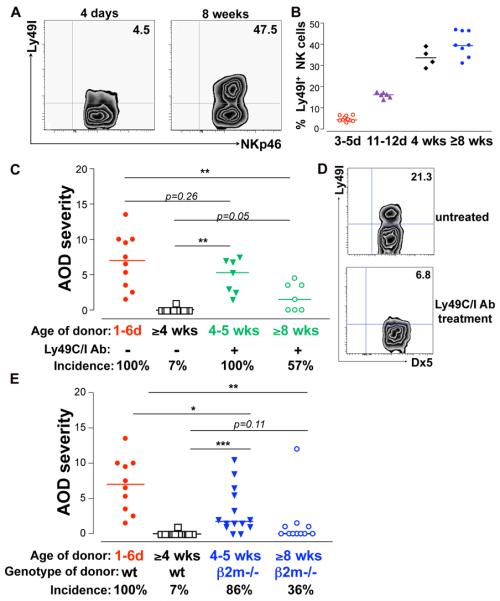
Figure 3. The late ontogeny of Ly49C/I expression on NK cells explains adult resistance to TI-nAOD.
A, 4 day (d)-old wt B6 mice show reduced frequency of splenic Ly49I+ NK cells compared to adult (8 wk-old) wt B6 mice. Gated on live, NKp46+, NK1.1+, CD3− cells. B, Summary of the frequency of Ly49I+ NK cells in wt B6 as described in (A). C, Ovarian pathology in Rag2/IL-2rg−/− mice reconstituted with 1×106 NK cells from wt donors of different ages, with or without co-injection of 50 ug of Ly49C/I depleting mAb. D, Representative contour plots showing the percentage of Ly49I+ NK cells in the spleen from 2 wk-old Rag2/IL-2rg−/− recipients reconstituted with 4 wk-old NK cells without (upper panel) or with Ly49C/I mAb (lower panel). Gated on live, CD122+ NK1.1+ D×5+ cells. E, Ovarian pathology in Rag2/IL-2rg−/− mice reconstituted with 0.5×106 NK cells from b2m−/− donors of different ages. C and E, TI-nAOD was induced by ZP3 mAb. n= 4-14 mice pooled from 2-7 independent experiments. *p<0.05; **p<0.01; ***p<0.0001.
The late ontogeny of Ly49C/I expression on NK cell explains the adult resistance to nAOD
We next studied the influence of the inhibitory Ly49 receptors on TI-nAOD induction since they control NK cell function (16) and their expression is ontogenetically regulated (17-18). We focused on Ly49C/I that recognize Kb (MHC I of B6 mice). As previously reported, ~5% of neonatal NK cells expressed Ly49C/I, whereas ~30% of juvenile and ~50% of adult NK cells were Ly49C/I+ (Fig. 3A and B). This differential expression of Ly49C/I on NK cells during ontogeny, and the observation that only neonatal NK cells induce nAOD (Fig. 2A), lead us to propose that Ly49C/I negative NK cells isolated from older mice might also induce nAOD. To test this, we injected Rag2/IL-2rg−/− neonates reconstituted with NK cells from juvenile or adult donors along with Ly49C/I depleting mAb (Fig. 3C). This led to in vivo depletion of the Ly49C/I+ NK cells in the recipients (Fig. 3D). Strikingly, the residual Ly49C/I negative NK cells from the 4-5 wk-old juvenile donors (Fig. 3C) induced severe nAOD similar to neonatal NK cells (Fig. 3C). These results suggest that Ly49C/I negative NK cells from juvenile mice retain the pathogenic properties of the neonatal NK cells. Because the pathogenicity of the juvenile Ly49C/I negative NK cell subset was revealed only after depleting Ly49C/I+ NK cells, our data also suggest that Ly49C/I+ NK cells regulate the Ly49C/I negative NK cell subset, at least in the context of nAOD induction. In addition, Ly49C/I negative NK cells from >8 wk-old adult mice also induced ovarian inflammation, but it was milder and infrequent (Fig. 3C). This indicates that the pathogenic potential of the Ly49C/I negative NK cells decline as the mice age; nonetheless, it is still controlled by the Ly49C/I+ NK cells.
We next examined the capacity of MHC I deficient NK cells to induce nAOD. In wt mice, the MHC I – Ly49 interactions occur both amongst the NK cells (in cis and trans) and between NK cells and host cells. Instead, when b2m−/− NK cells are infused into Rag2/IL-2rg−/− pups, MHC I – Ly49 binding only occur between NK cells and host cells. Figure 3E shows that NK cells from b2m−/− juvenile mice induced nAOD; however, disease rescue was partial since 9/14 (64%) mice showed mild ovarian inflammation. Because b2m−/− NK cells can overcome their hyporesponsiveness in an MHC I sufficient environment, we believe that the partial disease rescue is due to the NK cell inhibition by the interaction of Ly49 with host MHC I+ cells. In both Fig. 3C and 3E we observed a reduction in the pathogenicity of NK cells from 8 wk-old to 4 wk-old donors. This might be due to the differential expression of activating receptors and/or inhibitory receptors between adult and juvenile Ly49C/I negative NK cells
In summary, we have developed a new model of TI-nAOD, which allowed us to clearly demonstrate that neonatal NK cells are functional, and, in fact, they are superior to adult NK cells in nAOD induction. Using multiples approaches, we showed that neonatal NK cells, and their production of IFNg, are required for nAOD pathogenesis. More importantly, we showed that the low expression of Ly49C/I on neonatal NK cells renders newborns more susceptible to nAOD. Thus, the properties of the neonatal innate response are the basis for neonatal propensity to autoimmunity.
We previously showed that regulatory T cells also control neonatal susceptibility to nAOD since their elimination allows nAOD induction even when pups were exposed to ZP3 autoAb on day 9 of life (10). This indicates multiple levels of regulation in preventing autoimmunity, which are present in the adult, but not in neonatal mice. We envision that adult resistance to autoimmunity relies first, at the innate level, on the acquisition of Ly49 receptors by NK cells, and second, at the adaptive level, on the regulatory T cells. Thus, both TD-nAOD and TI-AOD models have provided valuable information to dissect the mechanisms of neonatal propensity to autoimmunity. In this context, it is important that both models share the neonatal time window for disease induction, and that NK cells, FcgRIII and IFNg are equally critical.
In the steady state, Ly49 negative NK cells are hypo-responsive; however, they can acquire full effector functions under inflammatory conditions (29-31). Accordingly, Ly49C/I negative adult NK cells can respond to the ovarian immune complexes and induce nAOD. Because this only occurs after the elimination of Ly49C/I+ NK cells, we speculate that the Ly49C/I+ NK cell subset can inhibit the activation of Ly49C/I negative NK cells. Previous reports showed that Ly49 receptors in mice (22) and killer cell Ig-like receptors (KIR) in humans (23) intrinsically restrain NK cell activation. However, our data suggest a novel extrinsic inhibitory effect. The cellular and molecular mechanisms of this regulation will require further investigation, and it might involve competition between the two NK cell subsets and dendritic cell interaction and priming (24).
Both promoting and protecting roles have been attributed to NK cells in autoimmunity in animal models (25). In human autoimmune diseases, a lower expression of KIR correlates with a higher predisposition to autoimmunity (26). Moreover, in a model of myasthenia gravis in adult mice, NK cells and FcgRIII are critical for disease pathogenesis (27). Thus, nAOD appears to recapitulate the mechanisms of other clinically relevant diseases.
To our knowledge, we have shown for the first time, how the differential expression of the Ly49 inhibitory receptors during ontogeny can impact NK cell function, thus explaining neonatal propensity to immune complex-mediated autoimmune disease. Importantly, we established that the acquisition of the Ly49 receptors restrains adult NK cell function and renders older mice resistant to autoimmunity.
Supplementary Material
Acknowledgements
We thank Joyce Nash and Virginia Rubianes for expert technical assistance and Dr. Michael Brown for helpful suggestions and critical reading of the manuscript.
This study was supported by NIH grant RO1 AI 51420. CR is supported by the NIH training grant 5T32 DK007769-13.
REFERENCES
- 1.UNICEF . The State of the World’s Children 2009: Maternal and Newborn Health. United Nations Children’s Fund; New York: 2009. [Google Scholar]
- 2.Lindop R, Arentz G, Thurgood LA, Reed JH, Jackson MW, Gordon TP. Pathogenicity and proteomic signatures of autoantibodies to Ro and La. Immunol. Cell Biol. 2012;90:304–309. doi: 10.1038/icb.2011.108. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Zhou D D, Lee J, Niu H, Faust TW, Frattini S, Kowal C, Huerta PT, Volpe BT, Diamond B. Female mouse fetal loss mediated by maternal autoantibody. J. Exp. Med. 2012;209:1083–1089. doi: 10.1084/jem.20111986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardnerova M, Eymard B, Morel E, Faltin M, Zajac J, Sadovsky O, Tripon P, Domergue M, Vernet-der Garabedian B, Bach JF. The fetal/adult acetylcholine receptor antibody ratio in mothers with myasthenia gravis as a marker for transfer of the disease to the newborn. Neurology. 1997;48:50–54. doi: 10.1212/wnl.48.1.50. [DOI] [PubMed] [Google Scholar]
- 5.Silva DG, Daley SR, Hogan J, Lee SK, Teh CE, Hu DY, Lam KP, Goodnow CC, Vinuesa CG. Anti-islet autoantibodies trigger autoimmune diabetes in the presence of an increased frequency of islet-reactive CD4 T cells. Diabetes. 2011;60:2102–2111. doi: 10.2337/db10-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 7.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garza K, Griggs ND, Tung KSK. Neonatal injection of an ovarian peptide induces autoimmune ovarian disease in female mice: requirement of endogenous neonatal ovaries. Immunity. 1997;6:89–96. doi: 10.1016/s1074-7613(00)80245-9. [DOI] [PubMed] [Google Scholar]
- 9.Setiady YY, Samy ET, Tung KSK. Maternal autoantibody triggers de novo T cell-mediated autoimmune disease. J. Immunol. 2003;170:4656–4664. doi: 10.4049/jimmunol.170.9.4656. [DOI] [PubMed] [Google Scholar]
- 10.Setiady YY, Pramoonjago P, Tung KSK. Requirements of NK cells and proinflammatory cytokines in T cell-dependent neonatal autoimmune ovarian disease triggered by immune complex. J. Immunol. 2004;173:1051–1058. doi: 10.4049/jimmunol.173.2.1051. [DOI] [PubMed] [Google Scholar]
- 11.Samy ET, Wheeler KM, Roper RJ, Teuscher C, Tung KSK. Cutting edge: Autoimmune disease in day 3 thymectomized mice is actively controlled by endogenous disease-specific regulatory T cells. J. Immunol. 2008;180:4366–4370. doi: 10.4049/jimmunol.180.7.4366. [DOI] [PubMed] [Google Scholar]
- 12.Lou Y, Ang J, Thai H, McElveen F, Tung KSK. A zona pellucida 3 peptide vaccine induces antibodies and reversible infertility without ovarian pathology. J. Immunol. 1995;155:2715–2720. [PubMed] [Google Scholar]
- 13.Nimmerjahn F, Ravetch JV. FcgRs in health and disease. Curr. Top. Micobiol. Immunol. 2011;350:105–125. doi: 10.1007/82_2010_86. [DOI] [PubMed] [Google Scholar]
- 14.Kim KD, Zhao J, Auh S, Yang X, Du P, Tang H, Fu YX. Adaptive immune cells temper initial innate responses. Nat. Med. 2007;13:1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, Schneider P, Tschopp J. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009;460:269–273. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- 16.Elliott JM, Yokoyama WM. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 2011;32:364–72. doi: 10.1016/j.it.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorfman JR, Raulet DH. Acquisition of Ly49 receptor expression by developing natural killer cells. J. Exp. Med. 1998;187:609–618. doi: 10.1084/jem.187.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubota A, Kubota S, Lohwasser S, Mager DL, Takei F. Diversity of NK cell receptor repertoire in adult and neonatal mice. J. Immunol. 1999;163:212–216. [PubMed] [Google Scholar]
- 19.Ortaldo JR, Winkler-Pickett R, Wiegand G. Activating Ly-49D NK receptors: expression and function in relation to ontogeny and Ly-49 inhibitor receptors. J. Leukoc. Biol. 2000;68:748–756. [PubMed] [Google Scholar]
- 20.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 22.Orr M, Murphy W, Lanier L. Unlicensed natural killer cells dominate the response to cytomegalovirus infection. Nat. Immunol. 2010;11:321–327. doi: 10.1038/ni.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarek N, Le Luduec JB, Gallagher MM, Zheng J, Venstrom JM, Chamberlain E, Modak S, Heller G, Dupont B, Cheung NK, Hsu KC. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J. Clin. Invest. 2012;122:3260–3270. doi: 10.1172/JCI62749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barreira da Silva R, Münz C. Natural killer cell activation by dendritic cells: balancing inhibitory and activating signals. Cell. Mol. Life Sci. 2011;68:3505–18. doi: 10.1007/s00018-011-0801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi FD, Zhou Q. Natural killer cells as indispensable players and therapeutic targets in autoimmunity. Autoimmunity. 2011;44:3–10. doi: 10.3109/08916931003782122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schleinitz N, Vély F, Harlé JR, Vivier E. Natural killer cells in human autoimmune diseases. Immunology. 2010;131:451–458. doi: 10.1111/j.1365-2567.2010.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi FD, Wang HB, Li H, Hong S, Taniguchi M, Link H, Van Kaer L, Ljunggren HG. Natural killer cells determine the outcome of B cell-mediated autoimmunity. Nat. Immunol. 2000;1:245–251. doi: 10.1038/79792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.