Abstract
What the causes of aging are and which factors define lifespan are key questions in the understanding of aging. Here, it is argued that cellular life involves (i) inevitable accumulation of damage resulting from imperfectness and heterogeneity of every cellular process, and (ii) dilution of damage when cells divide. While severe damage is cleared by protective systems, milder damage can only be diluted, due to high cost of accuracy, greater number of damage forms than protective systems, and the constraints inherited from unicellular life. This also applies to cancer cells, which are particularly dependent on damage dilution. Restriction on cell division necessarily leads to aging. Interventions that extend lifespan act through metabolic reprogramming, thereby changing damage composition and the rate of damage accumulation. Thus, heterogeneity leading to the myriad of mild damage forms represents the cause of aging, whereas the processes that affect damage landscape and accumulation are lifespan regulators.
The cause of aging is fundamentally different from the control of lifespan
The cause of aging is one of the most fundamental questions in biology, yet it is rarely addressed experimentally. Currently, there is no understanding what the cause of aging is and how it is different from lifespan control. Imagine a river, and lifespan as the time necessary for the water to flow from the headspring to ocean. One can extend that time by building a dam or routing the river into a longer path, but these approaches would tell nothing about the reason the water flows along the river (which is gravity). Similarly, approaches that extend lifespan [1] are not necessarily informative about the causes of aging. The cause of aging is fundamentally different from the control of lifespan. It is often discussed that aging is caused by the accumulation of damage that leads to dysregulation of cellular processes and eventually to cell and organism’s demise [2,3]. However, what this damage is, why and how it is generated, and why it is not cleared in cells by protective systems has not been satisfactorily answered.
The concept of damage accumulation was initially proposed for errors in transcription and translation [4–8] and the damage caused by reactive oxygen species [9,10], and evolved into a series of related damage-centric theories of aging [11]. However, this idea has largely lost its appeal due to the findings that genetic manipulation of factors that control accumulation of certain types of damage often does not lead to changes in lifespan [12,13].
Damage is an inevitable consequence of infidelity and heterogeneity of cellular life
For many years, a dominant thought has been that damage is inflicted primarily by reactive oxygen species, and that identity of the damage is implied by the existence of enzymes that deal with it. For example, superoxide dismutase evolved to protect against superoxide anion radical. A prominent idea was also that transcription and translation errors may lead to error catastrophe [4–8]. However, there is no reason to think there would not be numerous other forms of damage, or that the damage forms resulting from reactive oxygen species and errors in protein synthesis are necessarily more dangerous than other types of damage. Some damage forms, because of their low abundance, might not have the corresponding protective systems, whereas potentially lethal damage forms may be largely cleared by efficient protective systems. Another, and a much more important, misconception relates to the idea of perfect specificity and accuracy of cellular reactions and interactions. The implication is that damage is generated only by a handful of cellular processes, such as respiration, transcription, translation, etc.
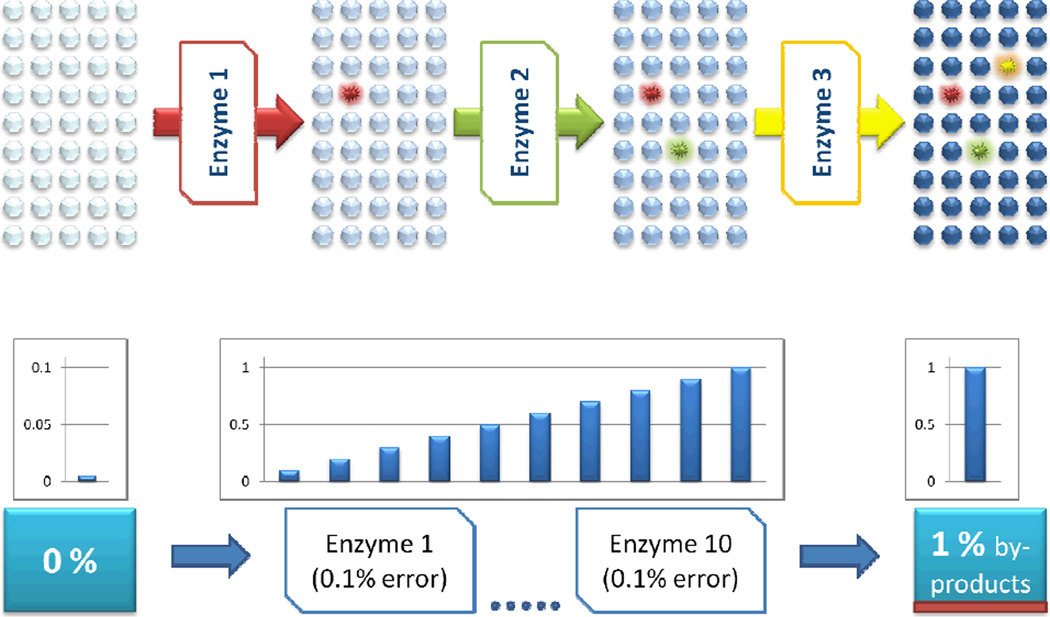
However, inaccuracy and infidelity of cellular reactions and interactions and heterogeneity of cellular metabolism are fundamental features of biology [14]. Enzymes have remarkable specificity, but they are not perfect and necessarily produce by-products. For example, consider a pathway of 10 consecutive reactions catalyzed by enzymes, each converting 99.9% of its substrate to product, which would represent a very impressive specificity. However, when only 100 metabolite molecules pass through this pathway, 1 molecule would be lost to at least 10 different by-products resulting from 0.1% inaccuracy in each step. Significant flux through this pathway can easily make by-products more abundant that the product itself. Consider now that this applies to every reaction in the cell. Anybody who has done organic synthesis knows that chemical reactions produce by-products. Chemists care about reaction yields, whether these reactions are catalytic or not, and the by-products of such reactions are as expected at the end of chemical synthesis as the products themselves. By the same token, enzymes, while being highly efficient catalysts, are not 100% accurate, and yield by-products. Enzymes are constrained by conformational flexibility, promiscuity, occasional mutations, environmental perturbations, and imperfectness as they are built from only 20 amino acid types.
However, catalytic imperfectness of enzymes is just one reason for heterogeneity and accumulation of unwanted products. Every reaction and every macromolecular interaction in the cell are imperfect, as macromolecular specificity is inherently restricted by infidelity, transient interactions and intracellular fluxes. There is stochasticity and cell-to-cell variability in gene expression [15,16], and differences in protein levels, modifications, and even length due to imperfect translation termination [17]. A substantial fraction of protein molecules possess phenotypic mutations as a result of errors in protein synthesis [18]. Transcription, translation and other major metabolic processes introduce heterogeneity in a myriad of different forms. These same principles lead to single cell heterogeneity [19]. More generally, genetic, stochastic and environmental variance will lead to heterogeneity and noise in cellular components, producing unintended molecular species at every level. For brevity, we call these products of heterogeneity and infidelity as damage, but the term encompasses any unintended and erroneous molecular species and interactions. Because of numerous molecular forms of these species, we refer to them as damage forms throughout the paper. Although damage is generally minimized by natural selection, the constraints discussed above result in the trade-off between accuracy and specificity on one hand and their costs on the other. Damage is the inevitable consequence of life because of heterogeneity. What does happen to all this damage in the cell?
Repair and removal of damage
Various damage forms would unequally affect cell (organism) fitness. Severe damage can be detrimental, so organisms that do not protect themselves from this damage will be removed by natural selection, and those that survive would be armed with the evolved protective and repair systems. With regard to severe damage forms, every “superoxide” has its “superoxide dismutase.” DNA damage [20] would be one prominent example of severe damage that is cleared by DNA repair machinery. However, milder forms of damage would be too numerous and will slowly accumulate during organism’s life. Not only would they not be subject to natural selection, but it would simply be impossible to remove and repair all this damage through the development of specific clearance systems, as there are a greater number of damage forms than protective systems. In addition, numerous protective systems would be too costly, and they would themselves produce damage that has to be dealt with. Thus, much of the damage that accumulates in cells consists of numerous forms of mild damage.
Both severe and mild damage forms may differ between organisms and even between different cell types within the same organism. If during evolution a certain type of damage becomes more severe, it would stimulate the development of a protective system, whereas if the damage becomes milder (no longer affecting fitness), the corresponding clearance system may be lost. For example, most unicellular organisms have an enzyme that repairs an oxidized form of methionine, methionine-R-sulfoxide, but this enzyme was lost in animals [21]. In this regard, identity of mild damage forms and the ever changing damage landscape are the issues central to the understanding of the aging process. There is also a beneficial side in heterogeneity and damage accumulation as they provide an important resource for innovation [14]. For example, some compounds generated as metabolic by-products may become substrates for novel pathways, whereas protein promiscuity may lead to the development of new protein functions.
Damage dilution is a basic strategy of cellular life: its prevention leads to aging
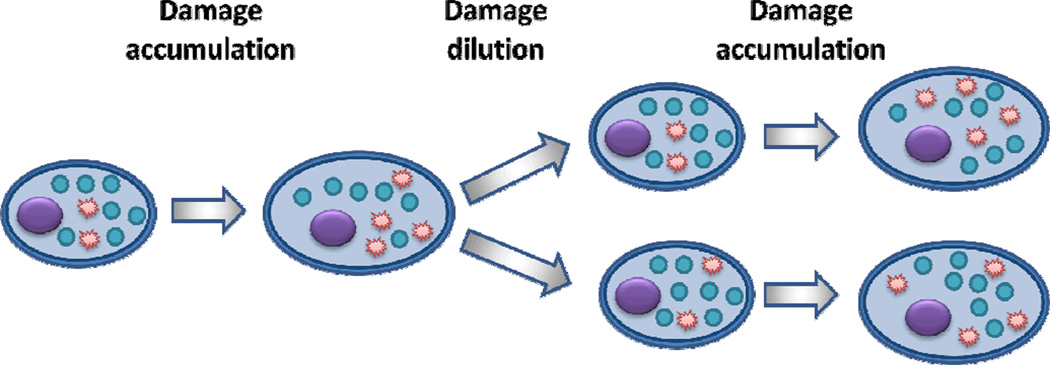
Since it is not possible to remove or repair all damage forms, there is seemingly no escape from damage accumulation and eventual damage overload. However, Nature has found an elegant solution to this problem that allows cells to survive by only dealing with severe damage. The solution is dilution! Damage is simply diluted when cells divide. This way, cells can optimize their metabolism to maintain a balance between generation of damage and its dilution. By diluting damage, they no longer need to develop costly protective systems to deal with the myriad of mild damage forms. Therefore, damage dilution is the most basic and essential strategy that sustains cellular life. It is the default process of all life forms, from single cells to the most complex organisms. Damage can be diluted symmetrically, like in most unicellular organisms, or asymmetrically, for example, during differentiation of mammalian cells and the budding of yeast [22,23]. Many truly symmetrically dividing cells do not age and may have unlimited lifespan. An unlimited lifespan may even theoretically be in a multicellular organism that continuously produces all types of its somatic cells from stem cells. Asymmetric division typically leads to unequal damage distribution, ultimately resulting in senescence of a mother cell, or one of daughter cells that inherits more damage. Recent evidence shows that some forms of damage may be distributed asymmetrically even in seemingly symmetrically dividing cells, such as bacteria and human cells in culture [24,25], implying existence of mechanisms that make room for the young from within the old [26]. This may be another illustration of the damage dilution strategy.
Damage dilution must have been an essential strategy since the first forms of cellular life, and this process continued for billions of years. For prokaryotic symmetrically dividing cells, it is not necessary to deal with all forms of cellular damage. As long as damage accumulation is balanced by damage dilution, there is no evolutionary pressure for the development of numerous protective strategies. However, in multicellular organisms, somatic cell division is limited by apoptosis and various checkpoints. These organisms must have inherited the damage dilution strategy from their unicellular ancestors, but the occurrence of post-mitotic cells in these organisms has led to senescence. Aging is also the result of the damage dilution constraints inherited from the prokaryotic world. At the same time, lifespan of certain non-dividing cells (e.g., neurons and cardiomyocytes in some mammals) extended to last for more than a hundred years. By adjusting control of damage accumulation, the lifespan of non-dividing cells may be further increased, but sooner or later, these cells will face senescence due to damage overload. The use of non-dividing cells may have evolved as a protection against cancer, but the consequence of such protection is aging.
Lifespan is modulated by the landscape of molecular damage and the rate of its accumulation
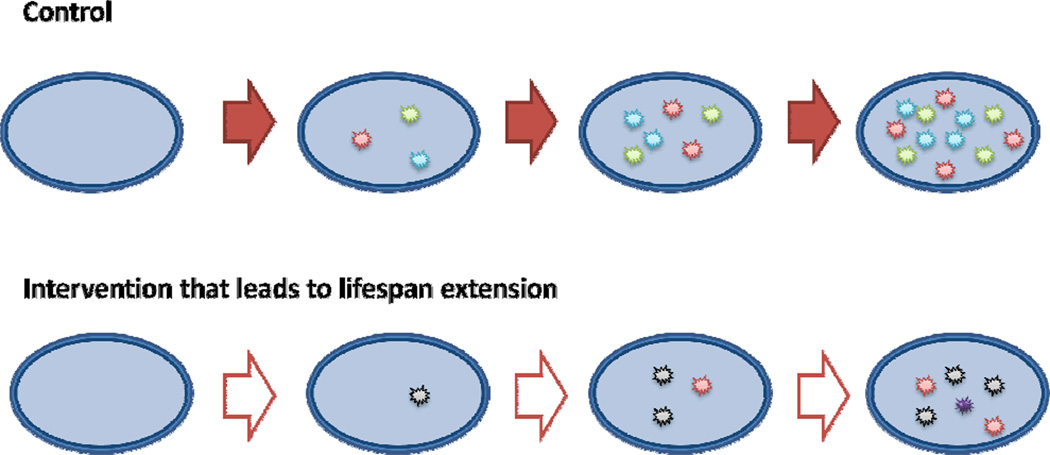
The idea of damage dilution helps explain why various independent treatments and gene manipulations can extend lifespan. It also explains how lifespan is regulated. Longevity of species can both increase and decrease during evolution by adjusting systems that deal with with the rates of damage generation and removal, but also by changing the damage landscape. Nutrient conditions and treatments (e.g., caloric restriction, rapamycin) [27,28] and gene manipulations [29,30] that extend lifespan not only delay accumulation of damage, but also change cellular metabolism, such that different damage forms accumulate. This altered spectrum of cellular damage explains the paradox of why longevity is sometimes associated with increased metabolic rate, increased oxygen use or increased oxidative damage. Indeed, oxidative damage is just one form of molecular damage, and its accumulation is not representative of cumulative damage, except at certain conditions. It may increase, decrease or even reverse upon changes in metabolic states, with unpredictable consequences on lifespan. For example, under caloric restriction conditions the yeast Saccharomyces cerevisiae increases respiration and decreases the flux through glycolysis, increasing lifespan, but also elevating reactive oxygen species [31]. In this example, oxidative damage is elevated as a consequence of respiration, even if the cumulative damage is restructured and decreased. Higher levels of oxidative stress were also observed in young naked mole rats compared to mice, but they did not further increase with the increase in age of this animal, whereas in mice they did [32]. In fact, many longevity-promoting interventions lead to the formation of reactive oxygen species, yet they also activate protective mechanisms against other damage forms [33]. Thus, different metabolic states of the cell lead to different lifespans by both generating different forms of molecular damage and accumulating damage at different rates.
Insights into the logic of cellular life
The concept of damage dilution provides insights into the logic of cellular life in diverse biological systems. For example, mammalian cells have a limited replicative potential, known as the Hayflick limit [34], whereas stem cells can divide without the limit. In stem cells, symmetrical division would equally dilute damage, and as long as damage accumulation is balanced by dilution these cells may divide an unlimited number of times. On the other hand, many differentiated cells accumulate damage (e.g., in the form of telomere shortening, oxidative modifications, etc.) and eventually die. Although these cells also symmetrically divide and equally dilute the damage, their damage accumulates faster than it is diluted, leading to clonal senescence. Mammalian cells also have systems of checks and breaks, which activate apoptotic processes. These systems evolved to control and limit cell division potential, thereby coordinating division with that of other cells and protecting against cancer. These checks and breaks may be overcome by certain manipulations, e.g., those that lead to induced pluripotency. The unlimited cell division supported by damage dilution seems to be the default process, so removal of apoptotic and other cell division-blocking systems may bring cells to this state.
Cancer may also be viewed as a condition that removes these breaks. Like during symmetrical division of unicellular organisms, the metabolic strategy of cancer cells is to dilute damage. Cellular protective systems kill newly emerged cancer cells, until these systems fail themselves due to accumulated damage. Cancer is manifested in mutations, which directly or indirectly disrupt apoptotic and other protective systems. DNA damage dysregulates cellular metabolism in cancer cells [35], so they accumulate damage faster than normal cells. Many types of normal cells evolved to be mostly in the non-dividing state, whereas cancer cells are forced to dilute their damage in order to survive. If they divide sufficiently rapidly, they may even lose some of their protective systems, which are no longer needed. Consistent with this idea, cancer cells are generally more susceptible than normal cells to treatments that slow down cell division and lead to additional damage (e.g., chemotherapy, radiotherapy). Cancer is intimately linked to aging. As damage accumulates in cells during aging, these cells are removed by apoptotic, immune and other protective systems, resulting in cell depletion. However, aging of these protective systems (e.g., mutations in them) allows some of the aging cells to restructure their metabolism and escape. Such cells include cancer cells. With regard to damage accumulation and dilution, cancer is the disease of aging.
Conclusions
Reactions and interactions, such as those catalyzed by enzymes, inevitably produce by-products and other unwanted products in cells. These and many other forms of cellular damage, generated due to infidelity, heterogeneity and biological noise, will necessarily accumulate during cellular life. The damage forms are too numerous to be removed by designated clearance systems, which themselves produce damage. The strategy to deal with this problem involves evolution of systems that protect against severe damage and dilution of mild damage during cell division. Thus, damage dilution is a central biological strategy that sustains cellular life. It frees resources from damage removal to biological innovation. However, innovation that produces non-dividing cells will necessarily lead to damage overload, senescence, and death. The idea of damage accumulation also explains regulation of lifespan by various interventions, including gene manipulations and dietary treatments: they affect cellular metabolism thereby both changing the pattern of damage forms and the rate of their accumulation. This concept provides insights into the logic of cellular life beginning with primordial organisms, the cause of aging in multicellular and asymmetrically dividing organisms, the control of damage landscape by approaches that extend lifespan, and the strategy used by cancer cells to deal with the vulnerabilities caused by damage accumulation. In this concept, heterogeneity leading to inevitable damage accumulation is the cause of aging, and the control of damage forms and the rate of their accumulation is the regulation of lifespan.
To put these ideas into perspective, novelty of our concept includes the following: (i) logical separation of the cause of aging and regulation of lifespan; (ii) damage dilution as a central strategy of cellular life that balances damage accumulation and allows cells to deal only with severe damage. This strategy was inherited from the prokaryotic world; (iii) inevitable accumulation (due to infidelity, biological noise and heterogeneity) of slightly deleterious, mild damage, which is the single cause of aging. Any individual damage forms, such as oxidative and DNA damage, represent only a subset of cumulative damage; and (iv) control of lifespan by metabolic reprogramming, whereby both modulating the pattern of accumulated damage and affecting damage accumulation. This is the basis for dietary and genetic interventions that extend lifespan.
Fig. 1. Enzyme-generated damage.
Enzymes inevitably generate by-products. Consider a pathway of 10 enzymes, each with 99.9% accuracy, which would represent a very impressive specificity. However, when only 100 molecules pass through this pathway, 1 molecule would be lost to numerous by-products resulting from 0.1% inaccuracy in each step. Significant flux through this pathway can easily make by-products more abundant than the product itself.
Fig. 2. Damage dilution.
Cells deal with the accumulation of mild damage (shown in red) by diluting it during cell division.
Fig. 3. Control of lifespan by regulating rate and pattern of damage accumulation.
Interventions that extend lifespan both change the landscape of damage in cells (i.e. different damage is accumulated) and decrease the rate of its accumulation.
References
- 1.Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Partridge L, Gems D. Mechanisms of ageing: public or private? Nat. Rev. Genet. 2002;3:165–175. doi: 10.1038/nrg753. [DOI] [PubMed] [Google Scholar]
- 3.Sinclair DA, Oberdoerffer P. The ageing epigenome: damaged beyond repair? Ageing Res Rev. 2009;8:189–198. doi: 10.1016/j.arr.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orgel LE. Ageing of clones of mammalian cells. Nature. 1973;243:441–445. doi: 10.1038/243441a0. [DOI] [PubMed] [Google Scholar]
- 5.Orgel LE. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc. Natl. Acad. Sci. USA. 1963;49:517–521. doi: 10.1073/pnas.49.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orgel LE. The maintenance of the accuracy of protein synthesis and its relevance to ageing: a correction. Proc. Natl. Acad. Sci. USA. 1970;67:1476. doi: 10.1073/pnas.67.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hopfield JJ. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc. Natl. Acad. Sci. USA. 1974;71:4135–4139. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danchin A. Natural selection and immortality. Biogerontology. 2009;10:503–516. doi: 10.1007/s10522-008-9171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 10.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Kirkwood TB, Kowald A. The free-radical theory of ageing - older, wiser and still alive: Modelling positional effects of the primary targets of ROS reveals new support. Bioessays. 2012 doi: 10.1002/bies.201200014. in press. [DOI] [PubMed] [Google Scholar]
- 13.Blagosklonny MV. Aging: ROS or TOR. Cell Cycle. 2008;7:3344–3354. doi: 10.4161/cc.7.21.6965. [DOI] [PubMed] [Google Scholar]
- 14.Tawfik DS. Messy biology and the origins of evolutionary innovations. Nat. Chem. Biol. 2010;6:692–696. doi: 10.1038/nchembio.441. [DOI] [PubMed] [Google Scholar]
- 15.Bahar R, et al. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441:1011–1014. doi: 10.1038/nature04844. [DOI] [PubMed] [Google Scholar]
- 16.Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- 17.Chittum HS, et al. Rabbit beta-globin is extended beyond its UGA stop codon by multiple suppressions and translational reading gaps. Biochemistry. 1998;37:10866–10870. doi: 10.1021/bi981042r. [DOI] [PubMed] [Google Scholar]
- 18.Willensdorfer M, Burger R, Nowak MA. Phenotypic mutation rates and the abundance of abnormal proteins in yeast. PLoS Comput. Biol. 2007;3:e203. doi: 10.1371/journal.pcbi.0030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, Bodovitz S. Single cell analysis: the new frontier in 'omics'. Trends Biotechnol. 2010;28:281–290. doi: 10.1016/j.tibtech.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freitas AA, de Magalhães JP. A review and appraisal of the DNA damage theory of ageing. Mutat Res. 2011;728:12–22. doi: 10.1016/j.mrrev.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Le DT, et al. Functional analysis of free methionine-R-sulfoxide reductase from Saccharomyces cerevisiae. J. Biol. Chem. 2009;284:4354–4364. doi: 10.1074/jbc.M805891200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguilaniu H, Gustafsson L, Rigoulet M, Nyström T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- 23.Shcheprova Z, Baldi S, Frei SB, Gonnet G, Barral Y. A mechanism for asymmetric segregation of age during yeast budding. Nature. 2008;454:728–734. doi: 10.1038/nature07212. [DOI] [PubMed] [Google Scholar]
- 24.Ackermann M, Stearns SC, Jenal U. Senescence in a bacterium with asymmetric division. Science. 2003;300:1920. doi: 10.1126/science.1083532. [DOI] [PubMed] [Google Scholar]
- 25.Fuentealba LC, Eivers E, Geissert D, Taelman V, De Robertis EM. Asymmetric mitosis: Unequal segregation of proteins destined for degradation. Proc. Natl. Acad. Sci. USA. 2008;105:7732–7737. doi: 10.1073/pnas.0803027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binder PM, Danchin A. Life's demons: information and order in biology. What subcellular machines gather and process the information necessary to sustain life? EMBO Rep. 2011;12:495–499. doi: 10.1038/embor.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon length of the life span and upon the ultimate body size. J. Nutr. 1935;10:63–75. [PubMed] [Google Scholar]
- 28.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apfeld J, Kenyon C. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell. 1998;95:199–210. doi: 10.1016/s0092-8674(00)81751-1. [DOI] [PubMed] [Google Scholar]
- 30.Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- 31.Lin SJ, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 32.Pérez VI, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc. Natl. Acad. Sci. USA. 2009;106:3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011;51:327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 35.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]