Abstract
Background
The HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial showed that among patients with heart failure (HF), regular exercise confers a modest reduction in the adjusted risk for all-cause mortality or hospitalization.
Objective
This study determined whether greater volumes of exercise were associated with greater reductions in clinical events.
Methods
Patients randomized to the exercise training arm of HF-ACTION who were event-free at 3 mo after randomization were included (n= 959). Median follow-up was 28.2 months. Clinical end points were all-cause mortality or hospitalization and cardiovascular mortality or HF hospitalization.
Results
A reverse J-shaped association was observed between exercise volume and adjusted clinical risk. Based on Cox regression, exercise volume was not a significant linear predictor but was a logarithmic predictor (p=0.03) for all-cause mortality or hospitalization. For cardiovascular mortality or HF hospitalization, exercise volume was a significant (p=0.001) linear and logarithmic predictor. Moderate exercise volumes of 3 to <5 and 5 to <7 MET-hr per week were associated with reductions in subsequent risk that exceeded 30%. Exercise volume was positively associated with the change in peak oxygen uptake at 3 months (r=0.10; p=0.005).
Conclusions
In patients with chronic systolic HF, volume of exercise is associated with the risk for clinical events, with only moderate levels (3–7 MET-hr per week) of exercise needed to observe a clinical benefit. Although further study is warranted to confirm the relationship between volume of exercise completed and clinical events, our findings support the use of regular exercise in the management of these patients.
Clinical Trial Registry: http://clinicaltrials.gov/ct2/show/NCT00047437
Keywords: exercise training, dose response, cardiac rehabilitation
Introduction
Among patients with coronary heart disease, cardiac rehabilitation (CR) reduces the risk for all-cause mortality and cardiovascular (CV) mortality approximately 20% and 25%, respectively (1–3). Studies involving exercise training in patients with heart failure (HF) also demonstrate a benefit on clinical events (4–7). However, despite the important contribution that these studies made toward the care of patients with CV disease, the question remains as to the relation between the volume of exercise completed and magnitude of reduction in risk for experiencing a subsequent clinical event.
Suaya et al. observed that the adjusted relative risk for mortality was reduced almost 20% in patients with coronary heart disease who attended ≥ 25 sessions of CR, versus patients who attended ≤ 24 or fewer sessions (8). Similarly, among Medicare beneficiaries with HF who attended 36 versus 12 CR sessions, all-cause mortality and myocardial infarction were both reduced approximately 18% (9). Finally, Taylor et al (2) dichotomized exercise dose based on patients completing more or less than 1000 units of exercise and reported no association between dose of exercise and risk for clinical outcomes.
The HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial randomized 2331 patients with HF due to systolic dysfunction to usual care alone versus usual care plus aerobic exercise training and provides an opportunity to elucidate the relation between exercise volume (EV) and clinical outcomes. Specifically, we identified a subset of 959 patients randomized to the exercise training arm that were event-free for at least 3 months. We tested the hypothesis that EV has an inverse association, either linear or logarithmic, with the adjusted risk for subsequent clinical events. We used a logarithmic transformation to account for skewness in the EV data and adjusted the risk for important baseline variables to control for confounding with baseline health status. We also estimated the adjusted risk and the change in exercise capacity associated with five different categories of EV. Our primary outcome was all-cause mortality or hospitalization and our secondary outcomes were the disease-specific end point of CV mortality or HF hospitalization and change in exercise capacity as measured by peak oxygen uptake (VO2)
METHODS
Study Design and Patients
The HF-ACTION trial enrolled patients (left ventricular ejection fraction ≤35%) with New York Heart Association class II to IV symptoms despite optimal therapy; the design and primary results have been previously reported (4,10). The protocol was approved by the Institutional Review Board or Ethics Committee for each center and patients provided written informed consent.
Exercise Training Protocol
All patients received an education manual that recommended 30 minutes of moderate intensity activity most days of the week (11). Patients randomized to the exercise arm were scheduled to participate in supervised walking or stationary cycling 3 days per week. After completing 18 sessions, patients were asked to add a 2-day per week home-based exercise program. They were fully transitioned to a 5-day per week home-based exercise program after completing 36 supervised sessions. The duration for supervised exercise was 30 minutes; intensity was initially set at a heart rate of 60% of heart rate reserve (i.e., peak heart rate − resting heart rate × 0.6 + resting heart rate) (12) and titrated to 70% of heart rate reserve. This intensity corresponds to 70% of peak VO2 reserve (12) and is effective for improving exercise capacity in patients with HF taking a beta-adrenergic blocking agent (13). Home exercise was prescribed at 40 minutes at 60–70% of heart rate reserve. Each patient received a heart rate monitor (Polar USA, Inc, New York) to facilitate adherence. Among patients in whom heart rate was an invalid measure of exercise intensity (e.g., atrial fibrillation), the Borg rating of perceived exertion scale was used and set at a level of 12–14 (i.e., fairly light to somewhat hard).
Exercise Volume
We used MET-hr per week to quantify EV, which is the product of exercise intensity (metabolic equivalents, MET; where 1 MET is ~3.5 of mL of O2·kg−1·min−1) and the hours of exercise per week. For example, for the patient whose peak MET level during their last cardiopulmonary exercise test was 5 and then exercise trained, on average, at 60% intensity over the three month interval, they would be exercising at an intensity of ~ 3 METs (14). If this patient trained ~ 1.5 hours per week (i.e., 30 minutes, 3 days per week), their EV would be ~ 4.5 MET-hr per week.
MET-hr per week data recorded during months 1–3 was derived from each supervised exercise session or, for home-based exercise, from self-reported activity logs and telephone follow-up calls. Telephone calls were scheduled every 2 weeks for 9 months and then monthly through month 24.
Exercise Testing
Cardiopulmonary exercise testing was performed prior to and three months following randomization. Patients were tested using a modified Naughton treadmill protocol or ramp (10 W·min−1) stationary cycle protocol. During testing patients were encouraged to achieve a rating of perceived exertion of > 17 (very hard) on the Borg scale and a respiratory exchange ratio > 1.10.
End Points
The primary outcome in this analysis was a composite of all-cause mortality or hospitalization. The secondary clinical outcome was CV mortality or HF hospitalization. Due to the nature of the intervention, blinding subjects and site investigators was not possible. However, all deaths, and other clinical end points to first HF hospitalization, were adjudicated by a Clinical End Points Committee. Cardiopulmonary exercise test data were forwarded to a core laboratory for analysis. Peak VO2 was defined as the highest VO2 for a given 15 to 20 second interval within the last 90 seconds of exercise or the first 30 seconds of recovery.
Statistics
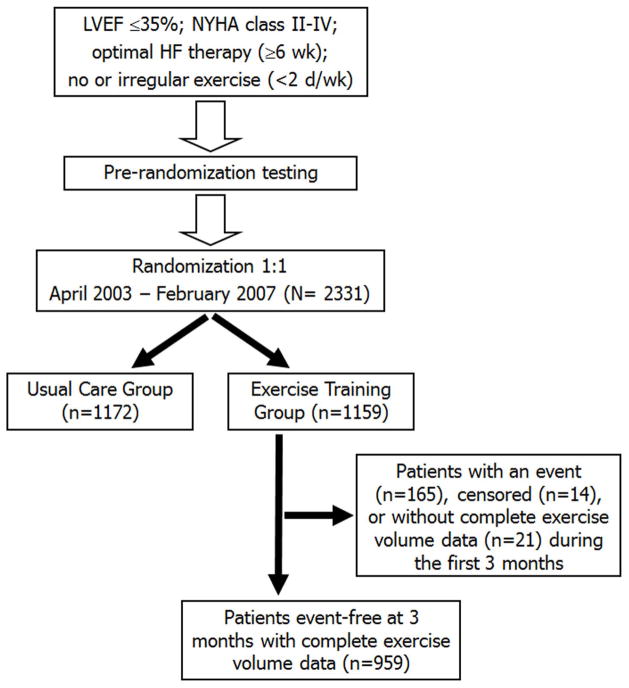
The associations between EV, measured in MET-hr per week during months 1–3, and the clinical end points were assessed using the Cox proportional hazards model, which modeled the time-to-first occurrence of each end point. Only those patients randomized to the exercise training arm of HF-ACTION who did not experience the primary end point during the first 3 months were included in this analysis (n=959) (Figure 1). This inclusion criterion was used to ensure an opportunity for at least 3 months of exercise training for patients in the current analysis. Also, any patient who experienced the primary end point during months 1–3, by definition, reached the end point before the reporting of EV at 3 months. As a result, in the time-to-first event Cox model analyses a subject’s EV could not be used to predict an end point that already occurred. Consequently, the Cox analyses used 3 months after randomization as time zero, a so-called “landmark” analysis (15).
Figure 1. Study Flow Chart.
Flow of patients through the trial to the 3-month event-free group.
Sixty candidate baseline variables, including the variables considered for the HF-ACTION risk model (16), were considered for covariate adjustment. The SAS procedure PROC MI was used to create 5 complete data sets with imputed values to fill in missing values among candidate predictors. With the 5 completed data sets, the SAS procedure PROC MIANALYZE was used in conjunction with PROC PHREG, and backward variable selection was used to identify the adjustment variables for EV in the Cox analyses.
MET-hr per week was first included as a linear term in the covariate-adjusted Cox models. Because MET-hr per week had a skewed distribution, the hypothesis was tested using a logarithmic transformation [log2(MET-hr per week+1) to decrease the influence of outliers (17,18). Multivariate likelihood ratios were used to determine if the linear Cox model or the logarithmic transformed Cox model provided a better model fit.
To estimate the relation between EV and clinical outcome, we also divided the subjects into 5 categories: 0 to <1 (n=144), 1 to <3 (n=234), 3 to <5 (n=233), 5 to <7 (n=178), and ≥7 (n=170) MET-hr per week. These categories were chosen so as to have a reasonable number of subjects in each category, while at the same time retaining clinical relevance. Using 0 to 1 MET-hr per week as the reference category, we then computed the unadjusted and adjusted hazard ratios for the 4 remaining exercise categories.
Cumulative event rates were calculated using Kaplan-Meier methods. Covariate adjusted relative risks were expressed as Cox model hazard ratios with 95% confidence intervals. Change in peak VO2 was linearly regressed on EV to assess the association between the two variables. Also the median (25th, 75th percentiles) change in peak VO2 was computed for each of the 5 categories of EV. Analyses were performed using SAS (version 8.2, SAS Institute Inc, Cary, North Carolina) and the R Design Library (version 2.9.2, The R Foundation for Statistical Computing). Statistical significance was set at the two-tailed alpha .05 level, with no adjustment for multiple comparisons. All P values are based on the Wald chi-square statistic. Discrete variables are expressed as percent and continuous variables as median and inter-quartile range.
RESULTS
Patient Characteristics and Follow-Up
Table 1 shows baseline characteristics for patients included in our primary analysis involving patients event-free at 3 months after randomization. Among these patients the median duration of follow-up was 28.2 (18.0, 40.0; 25th, 75th percentile) months. Median EV at 3 months was 3.9 (1.9, 6.2) MET-hr per week.
Table 1.
Selected Baseline Characteristics of Patients Event-Free for at Least 3 Months
| Characteristics | Descriptive Statistic (n = 959) |
|---|---|
| Age, median (IQR), y | 59 (51–67) |
|
| |
| Female sex | 297 (31) |
|
| |
| Race* | |
| Black or African American | 306/943 (32) |
| White | 589/943 (62) |
| Other | 48/943 (5) |
|
| |
| NYHA class* | |
| II | 626 (65) |
| III | 323 (33) |
| IV | 10 (1) |
|
| |
| Ischemic etiology of heart failure | 480 (50) |
|
| |
| Ejection fraction, median (IQR), % | 25 (20–30) |
|
| |
| Diabetes mellitus | 307 (32) |
|
| |
| Previous myocardial infarction | 390 (41) |
|
| |
| Hypertension | 591/954 (62) |
|
| |
| Atrial fibrillation or flutter | 204 (21) |
|
| |
| Beck Depression Inventory II score, median (IQR) | 8 (5–15) |
|
| |
| Systolic blood pressure, median (IQR), mmHg | 112 (100–126) |
|
| |
| Diastolic blood pressure, median (IQR), mmHg | 70 (60–78) |
|
| |
| Baseline medications | |
| ACE inhibitor or ARB | 917 (96) |
| B-Blocker | 904 (94) |
| Aldosterone receptor antagonist | 431 (45) |
| Loop diuretic | 728 (76) |
| Digoxin | 415 (43) |
|
| |
| Implantable cardioverter-defibrillator | 396 (17) |
|
| |
| Biventricular pacemaker | 182 (19) |
|
| |
| Cardiopulmonary Exercise Test | |
| Peak oxygen uptake, median (IQR), mL·kg−1·min−1 | 14.5 (11.6–17.8) |
|
| |
| Peak respiratory exchange ratio, median (IQR) | 1.08 (1.01–1.16) |
Unless otherwise indicated, values reflect n (percent of patients); percentages may not sum to 100 because of rounding.
Indicates the number of patients/number of patients with non-missing data.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; IQR, interquartile range; NYHA, New York Heart Association.
Clinical End Points
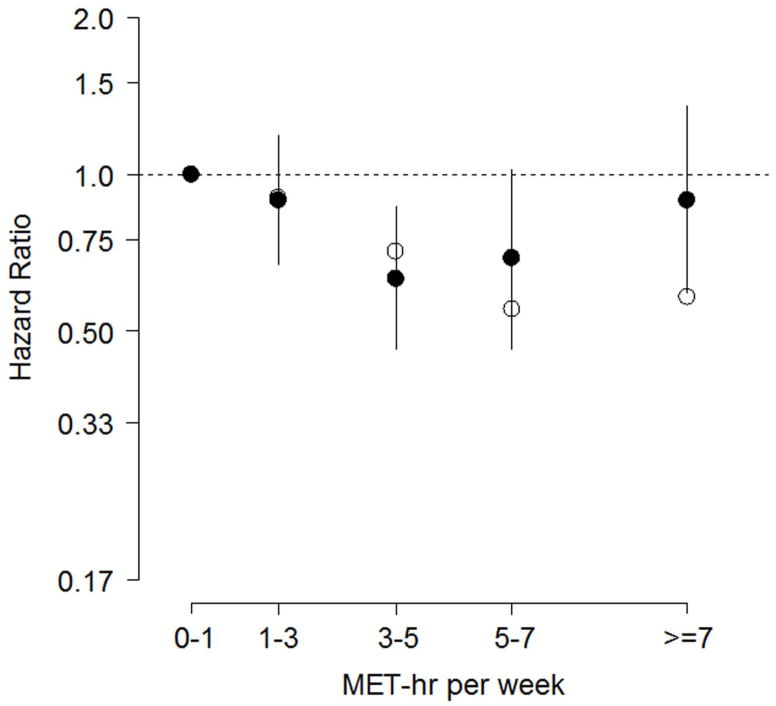
As seen in Table 2, MET-hr per week was not a linear predictor of our primary end point (i.e., all-cause mortality or hospitalization; P=0.18). However, MET-hr per week was a significant logarithmic predictor of all-cause mortality or hospitalization (P = 0.03). The reason for this difference can be seen in Figure 2, where the adjusted hazard ratio, as a function of MET-hr per week, is somewhat reverse J-shaped. Specifically, when compared to an EV of 0 to <1 MET-hr per week, moderate EV of 3 to <5 and 5 to <7 MET-hr per week were associated with reductions in adjusted risk that approximated 37% and 31%, respectively. Consequently, the logarithmic model for MET-hr per week better captured the adjusted risk than did the linear model, which was confirmed by the multivariate likelihood ratios (226 vs. 223, respectively). It is also important to note from Figure 2 that while the adjusted hazard ratios were reverse J-shaped, the largest decrease in the unadjusted hazard ratio occurred for EV ≥ 5 MET-hr per week.
Table 2.
Covariate Adjusted* P-values for Exercise Volume (MET-hr per week) as a Linear or Logarithmic-Transformed† Predictor of Clinical Events in Patients Event-Free for at Least 3 Months (n = 959).
| Clinical Event | Model for MET-hr per week | P Value |
|---|---|---|
| All-cause mortality or hospitalization (582 events) | ||
| linear | .18 | |
| logarithmic | .03 | |
|
| ||
| Cardiovascular mortality or heart failure hospitalization (250 events) | ||
| linear | .001 | |
| logarithmic | <.001 | |
Adjustment variables: Weber Class; gender; serum sodium (≥ 136 mEq·L−1); ventricular conduction on resting electrocardiogram prior to exercise test; mitral regurgitation grade; resting heart rate; prescribed a beta-adrenergic-blocking agent; Kansas City Cardiomyopathy Questionnaire total symptom, social limitation, and symptom stability scores; left ventricular ejection fraction; loop diuretic dose (truncated above 90 mg.day−1 furosemide equivalent); blood urea nitrogen (truncated at 47 mg·dL−1); Beck Depression Inventory II Score (truncated at 8); geographic region; peak respiratory exchange ratio on cardiopulmonary exercise test (truncated above 1.3); marital status; employment status; etiology of heart failure.
The logarithmic transformation was Log2(MET-hr per week + 1).
Figure 2. Hazard Ratios for All-cause Mortality or Hospitalization.
Among patients event-free for at least three months, adjusted hazard ratios (filled circles, log scale) for all-cause mortality or hospitalization with 95% confidence intervals; reference category is 0–1 MET-hr per week. Unadjusted hazard ratios are plotted with open circles.
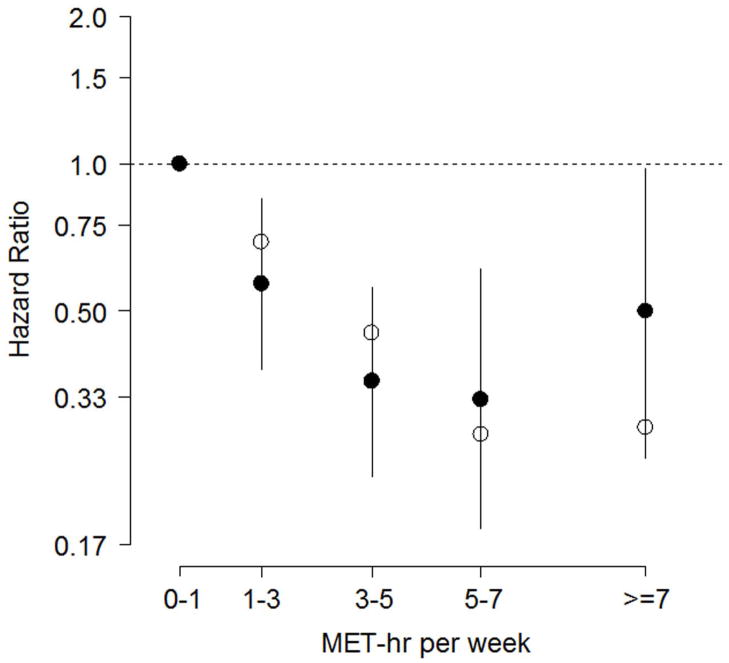
For the disease-specific end point of CV mortality or HF hospitalization, MET-hr per week was both a significant linear (P=0.001) and logarithmic (P<0.001) predictor of subsequent risk (Table 2). As shown in Figure 3, the adjusted risk reductions associated with the moderate EV of 3 to <5 MET-hr per week and 5 to <7 MET-hr per week were 64% and 67%, respectively. The greatest reductions in unadjusted risk were again observed at the higher EV. For CV mortality or HF hospitalization, the logarithmic model for MET-hr per week better captured the adjusted risk than did the linear model (multivariate likelihood ratios: 280 vs. 276, respectively).
Figure 3. Hazard Ratios for Cardiovascular Mortality or Heart Failure Hospitalization.
Among patients event-free for at least three months, adjusted hazard ratios (filled circles, log scale) for cardiovascular mortality or heart failure hospitalization with 95% confidence intervals; reference category is 0–1 MET-hr per week. Unadjusted hazard ratios are plotted with open circles.
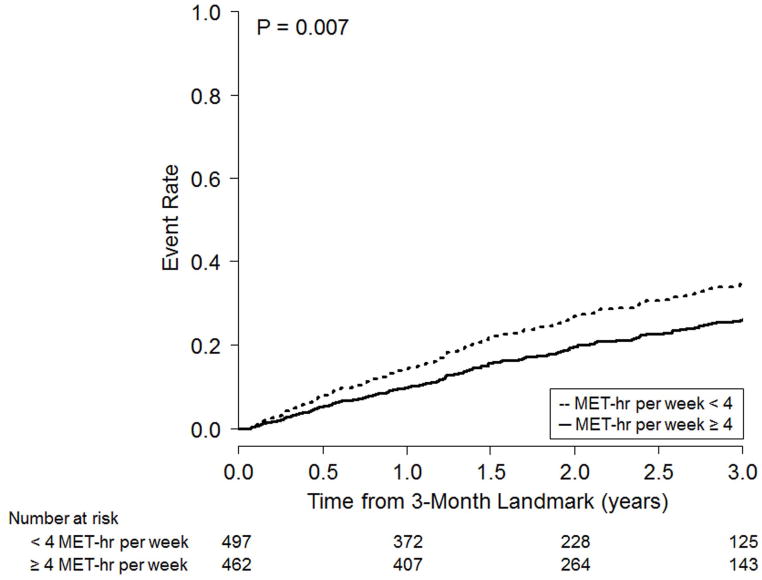
Figure 4 shows the adjusted Kaplan-Meier event rate curves for the two clinical end points, dichotomized based on EV during months 1–3: < 4 MET-hr per week or ≥ 4 MET-hr per week. We chose 4 MET-hr per week because this approximated the median EV for months 1–3.
Figure 4. Adjusted Kaplan-Meier Curves for Clinical Outcomes.
Adjusted Kaplan-Meier curves for all-cause mortality or hospitalization (left panel) and cardiovascular mortality or heart failure hospitalization (right panel) in patients event-free for at least 3 months, stratified at the median exercise volume of 4 MET-hr per week.
Exercise Capacity
There was a significant association between the EV completed by subjects during the first 3 months of the trial and change in exercise capacity at 3 months (r=0.10; p=0.005, n=853). The median (25th, 75th percentiles) changes in peak VO2 for patients exercising 0 to <1, 1 to <3, 3 to <5, 5 to <7, and ≥7 MET-hr per week were −0.4 (−1.2, 1.0), 0.6 (−0.7, 2.1), 0.5 (−0.7, 2.4), 0.9 (−0.5, 2.5), and 0.9 (−0.5, 2.9) mL·kg−1·min−1, respectively.
DISCUSSION
This study provides new and important information concerning the association between the volume of exercise completed per week and subsequent risk for clinical events in a large cohort of stable outpatients with systolic HF. When treated as a continuous variable, EV was not significant (P = 0.18) when modeled as a linear predictor for all-cause mortality or hospitalization, but was significant when modeled as a logarithmic predictor (P = 0.03) because the latter better expressed the association between EV and the primary end point. The reason for the improved fit using the logarithmic model was due to the fact that the adjusted risk resembled a reverse J-shaped function of EV, where moderate EV of 3 to <5 and 5 to <7 MET-hr per week were associated with reductions in subsequent risk that exceeded 30% (Figures 2–3).
For the CV mortality or HF hospitalization end point, EV treated as a continuous variable was a significant predictor regardless of whether it was modeled linearly or logarithmically. This is because of the greater effect that EV had on CV mortality or HF hospitalization (versus all-cause mortality or hospitalization). This finding is consistent with the main HF-ACTION trial (4), in which exercise training had its greatest effect on CV mortality or HF hospitalization, suggesting a greater specificity for reducing HF-related end points. Although we can only speculate as to the mechanism(s) responsible for the favorable impact of exercise training on clinical outcomes in patients with HF, especially its more favorable effect on the HF-specific end point, numerous studies show that regular exercise improves much of the pathophysiology that is unique to HF including changes in central (19,20), inflammatory (21–24), neuro-endocrine (25,26), endothelial (19,27,28), and skeletal muscle (29,30) function.
For both clinical end points, we observed that an EV ≥7 MET-hr per week was associated with a smaller decrease in adjusted risk than both the 3 to < 5 MET-hr per week and 5 to < 7 MET-hr per week groups (Figures 2 and 3). This was because (a) peak VO2, categorized via Weber class (31), was highly associated with clinical outcome (adjusted p<0.0001) and (b) a higher EV was associated with a higher peak VO2. Indeed, the median peak VO2 at baseline for our 0 to < 1, 1 to < 3, 3 to <5, 5 to <7 and ≥7 MET-hr per week categories were 13.1, 12.6, 13.4, 16.0, and 17.7 mL·kg−1·min−1, respectively. Since the adjusted risk associated with EV represents the ability of EV to independently predict clinical outcome, we observe that EV had less ability to independently predict clinical outcome in the ≥7 MET-hr per week group than it did for other groups (e.g., 3 to < 5 MET-hr per week or 5 to < 7 MET-hr per week groups). Nevertheless, there was a larger decrease in unadjusted risk as EV increased because EV reflects better baseline health, rather than the independent predictive ability of EV.
The above findings showing an association between EV (treated as a continuous variable or across all 5 categories) and clinical end points is consistent with prior observational studies in older coronary patients that treated the amount of exercise completed in a dichotomous manner (8,9). Hammill and coworkers observed that among Medicare beneficiaries with HF who attended 36 versus 12 CR sessions, all-cause mortality was reduced by approximately 18% (9). Combining the observations from our study with those of prior studies that involved exercise in patients with HF (4–7,9), it seems reasonable to suggest that a moderate volume of exercise training (e.g., 3 to 7 MET-hr per week) is safe and associated with a reduction in risk for important clinical end points.
Among all 1159 patients randomized to the exercise group in the HF-ACTION trial, only ~40% reported exercising at or above the protocol prescribed minimum number of minutes per week during the first 12 months (4). Therefore, this study may provide insight regarding how the EV completed by patients participating in the trial might have contributed to the modest improvement in clinical outcomes reported in the main outcomes paper. Clearly, maintaining an exercise program can be a challenge for the patient with HF, a behavior that is influenced both by pre-existing co-morbidities (32) and practitioners that often do not routinely assess a patient’s health beliefs and self-efficacy for change (33). However, our observation that a large decrease in adjusted risk for both clinical end points occurred among patients training at only 3–5 MET-hr per week (Figures 2–3) may be a potentially important motivating strategy, especially when counseling sedentary patients with HF who experience exercise intolerance and are reluctant to exercise. For reference, 4 MET-hr per week approximates walking at just 1.7 miles per hour for 26 minutes, 4 times per week.
The EV completed by patients during the first 3 months of the trial was significantly associated with the change in peak VO2 measured at 3 months. Across all 5 categories of EV the median increases in exercise capacity were modest and below the 1 to 4 mL·kg−1·min−1 increases reported for patients with HF taking evidence-based therapy (13). However, among patients exercise training at 5 or more MET-hr per week the median increase in peak VO2 was 0.9 mL·kg−1·min−1, a value that approaches what is often considered a clinically meaningful change (i.e., > 6% or 1 mL·kg−1·min−1) (34,35). Initial reports (36,37) involving patients with HF suggest that higher intensity interval training may be an alternate method to attain a higher volume of exercise, with the potential to yield a greater improvement in peak VO2.
Limitations
Despite the large sample size, the rigorous collection of EV and clinical event data, and the blind adjudication of end points, there are potential limitations associated with this study. First, it is possible that selection bias, such as those patients who expressed interest in exercise, were healthier, or better adhered to medical treatment plans, might have been more physically able and likely to undertake the exercise regimen. Nevertheless, the association between EV and clinical events persisted after adjusting for major variables associated with clinical status. A sufficiently powered prospective trial that randomly assigned subjects to different EV is warranted to establish a causal relationship.
Second, although this study was a planned secondary analysis in the HF-ACTION protocol, it is a retrospective analysis of prospectively collected data. Therefore, some of the associations we observed between EV and outcome may be partly due to the presence or absence of other factors that independently affect clinical events. Although we controlled for the influence of many of these potentially confounding variables, our models still may not have included variables that are also related to adherence to exercise or the clinical end points.
Third, due to missing data for some of the clinical variables measured at baseline, we used multiple imputation as a means to include a greater number of candidate variables when conducting the multivariable analyses. It is unknown whether multiple imputation influenced bias, versus simply omitting variables with incomplete data, although the imputation methods we employed have been shown to reduce bias in many situations (38).
Conclusions
Among patients with chronic systolic HF on evidence based therapy, our results extend the findings from the main HF-ACTION trial by estimating the adjusted hazard ratios (i.e., independent risk reductions) associated with various levels of EV completed during the first 3 months after randomization. We found that the adjusted hazard ratio as a function of EV was reverse J-shaped and due to this association, EV was not a significant linear predictor for all-cause mortality or hospitalization but was significant as a logarithmic predictor. For CV mortality or HF hospitalization, EV was a significant linear predictor and logarithmic predictor. Moderate EV between 3 and 7 MET-hr per week were associated with reductions in adjusted risk that exceeded 30%. Although further study is warranted to confirm the relations between EV and clinical outcomes, our findings support the use of moderate, regular exercise in the management of patients with chronic systolic HF.
Acknowledgments
Financial Support: This work was supported by the National Heart, Lung and Blood Institute (NHLBI), National Institutes of Health (NIH), Bethesda, Maryland.
Abbreviations
- EV
exercise volume
- CR
cardiac rehabilitation
- CV
cardiovascular
- HF
heart failure
- MET
metabolic equivalent
Footnotes
Conflict of interest: Keteyian: Received grants or funding, personal income for consulting, and honoraria from the NIH, GE Healthcare, and Amgen; Leifer: Employee of NHLBI Houston-Miller: None; Kraus: Received grants or funding, personal income for consulting, and honoraria from the NIH, GE Healthcare, and Amgen; Brawner: None; O’Connor: Received grants or funding, personal income for consulting, and honoraria from the NIH, GE Healthcare, and Amgen; Whellan: Received grants or funding, personal income for consulting, and honoraria from the (NIH, GE Healthcare, and Amgen; Cooper: Employee of NHLBI; Fleg: Employee of NHLBI itzman: Received grants or funding, personal income for consulting, and honoraria from the NIH, GE Healthcare, and Amgen; Cohen-Solal: Received grants or funding from Servier, Astra Zeneca, Menarini, Takeda, Amgen, IPSEN Genzyme, GE Health Care, Novartis, Sorin; Piña: Received grants or funding, personal income for consulting, and honoraria from the NIH, GE Healthcare, and Amgen
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lavie CJ, Milani RV. Cardiac rehabilitation and exercise training in secondary coronary heart disease prevention. Prog Cardiovasc Dis. 2011;53:397–403. doi: 10.1016/j.pcad.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systemic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116:682–692. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Giannuzzi P, Temporelli PL, Marchiolo R, et al. GOSPEL Investigators. Global secondary prevention strategies to limit event recurrence after myocardial infarction. Arch Intern Med. 2008;168(20):2194–2204. doi: 10.1001/archinte.168.20.2194. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor CM, Whellan DJ, Lee KL, et al. HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure HF-ACTION randomized controlled trial; HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med. 2004;116:693–706. doi: 10.1016/j.amjmed.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Piepoli MF, Davos C, Francis DP, Coats AJ. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BM J. 2004;328:189–196. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies EJ, Moxham T, Rees K, et al. Exercise based rehabilitation for heart failure. Cochrane Database of Systematic Reviews. 2010;(4):Art. No.: CD003331. doi: 10.1002/14651858.CD003331.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Suaya JA, Stason WB, Ades PA, Normand SLT, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardio. 2009;54:25–33. doi: 10.1016/j.jacc.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 9.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;121:63–70. doi: 10.1161/CIRCULATIONAHA.109.876383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whellan DJ, O’Connor CM, Lee KL, et al. HF-ACTION Trial Investigators. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–11. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Jessup M, Abraham WT, Casey DE, et al. writing on behalf of the 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult Writing Committee. 2009 Focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 12.American College of Sports Medicine. American College of Sports Medicine’s Guidelines for Exercise Testing and Prescription. 8. Philadelphia, PA: Lippincott Williams & Wilkins; 2010. p. 156. [Google Scholar]
- 13.Keteyian SJ. Exercise training in congestive heart failure: risks and benefits. Progress in Cardiovascular Diseases. 2011;53:419–428. doi: 10.1016/j.pcad.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Howley ET. Type of activity: resistance, aerobic and leisure versus occupational physical activity. Med Sci Sports Exerc. 2001;33(Suppl 6):S364–S369. doi: 10.1097/00005768-200106001-00005. [DOI] [PubMed] [Google Scholar]
- 15.Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4:363–371. doi: 10.1161/CIRCOUTCOMES.110.957951. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor CM, Whellan DJ, Wojdyla D, Leifer E, Clare RM, Ellis SJ, Fine LJ, Fleg JL, Zannad F, Keteyian SJ, Kitzman DW, Kraus WE, Rendall D, Piña IL, Cooper LS, Fiuzat M, Lee KL. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circ Heart Fail. 2012;5:63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller R. Beyond ANOVA: Basics of Applied Statistics. CRC Press LLC; Boca Raton, FL: 1997. [Google Scholar]
- 18.D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 19.Erbs S, Hollriegel R, Linke A, et al. Exercise training in patients with advanced chronic heart failure (NYHA IIIb) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left ventricular function. Circ Heart Failure. 2010;3:486–494. doi: 10.1161/CIRCHEARTFAILURE.109.868992. [DOI] [PubMed] [Google Scholar]
- 20.Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients. J Am Coll Cardiol. 2007;49:2329–2336. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 21.Gielen S, Adams V, Mobius-Winkler S, et al. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42:861–868. doi: 10.1016/s0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 22.LeMaitre JP, Harris S, Fox KAA, Denvir M. Change in circulating cytokines after 2 forms of exercise training in chronic stable heart failure. Am Heart J. 2004;147:100–105. doi: 10.1016/j.ahj.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Larsen AI, Aukrust P, Aarsland T, Dickstein K. Effect of aerobic exercise training on plasma levels of tumor necrosis factor alpha in patients with heart failure. Am J Cardiol. 2001;88:805–808. doi: 10.1016/s0002-9149(01)01859-8. [DOI] [PubMed] [Google Scholar]
- 24.Adamopoulos S, Parissis J, Karatzas D, et al. Physical training modulates proinflammatory cytokines and the soluble Fas/soluble Fas ligand system in patients with chronic heart failure. J Am Coll Cardiol. 2002;39:653–663. doi: 10.1016/s0735-1097(01)01795-8. [DOI] [PubMed] [Google Scholar]
- 25.Roveda F, Middlekauff HR, Rondon MUPB, et al. The effects of exercise training on sympathetic neural activation in advanced heart failure. J Am Coll Cardiol. 2003;42:854–860. doi: 10.1016/s0735-1097(03)00831-3. [DOI] [PubMed] [Google Scholar]
- 26.Braith RW, Welsch MA, Feigenbaum MS, Kluess HA, Pepine CJ. Neuroendocrine activation in heart failure is modified by endurance exercise training. J Am Coll Cardiol. 1999;34:1170–1175. doi: 10.1016/s0735-1097(99)00339-3. [DOI] [PubMed] [Google Scholar]
- 27.Linke A, Schoene N, Gielen S, et al. Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J Am Coll Cardiol. 2001;37:392–397. doi: 10.1016/s0735-1097(00)01108-6. [DOI] [PubMed] [Google Scholar]
- 28.Hambrecht R, Fiehn E, Weigl C, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–2715. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 29.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure. JAMA. 2000;283:3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 30.Duscha BD, Schulze PC, Robbins JL, et al. Implications of chronic heart failure on peripheral vasculature and skeletal muscle before and after exercise training. Heart Fail Rev. 2008;13:21–37. doi: 10.1007/s10741-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 31.Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic heart failure. Circulation. 1982;65:1213–1223. doi: 10.1161/01.cir.65.6.1213. [DOI] [PubMed] [Google Scholar]
- 32.Corvera-Tindel T, Doering LV, Gomez T, Dracup K. Predictors of noncompliance to exercise training in heart failure. Cardiovascular Nursing. 2004;19(4):269–277. doi: 10.1097/00005082-200407000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Bandura A. The Exercise of Control. New York, NY: W.H. Freemand and Company; 1997. Self-Efficacy. [Google Scholar]
- 34.Keteyian SJ, Brawner CA, Ehrman JK, Ivanhoe R, Boehmer JP, Abraham WT. PEERLESS-HF Trial Investigators. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise parameters: implications for clinical trials and clinical practice. Chest. 2010;138:950–5. doi: 10.1378/chest.09-2624. [DOI] [PubMed] [Google Scholar]
- 35.Corrà U, Mezzani A, Bosimini E, Giannuzzi P. Prognostic value of time-related changes of cardiopulmonary exercise testing indices in stable chronic heart failure: a pragmatic and operative scheme. Eur J Cardiovasc Prev Rehabil. 2006;13:186–92. doi: 10.1097/01.hjr.0000189807.22224.54. [DOI] [PubMed] [Google Scholar]
- 36.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–94. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 37.Freyssin C, Verkindt C, Prieur F, Benaich P, Maunier S, Blanc P. Cardiac Rehabilitation in Chronic Heart Failure: Effect of an 8-Week, High-Intensity Interval Training Versus Continuous Training. Arch Phys Med Rehabil. 2012 Mar 21; doi: 10.1016/j.apmr.2012.03.007. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2. New Jersey: Wiley-Interscience; 2002. [Google Scholar]