NOD2 signaling maintains intestinal intraepithelial lymphocytes via recognition of gut microbiota and IL-15 production.
Abstract
NOD2 functions as an intracellular sensor for microbial pathogen and plays an important role in epithelial defense. The loss-of-function mutation of NOD2 is strongly associated with human Crohn’s disease (CD). However, the mechanisms of how NOD2 maintains the intestinal homeostasis and regulates the susceptibility of CD are still unclear. Here we found that the numbers of intestinal intraepithelial lymphocytes (IELs) were reduced significantly in Nod2−/− mice and the residual IELs displayed reduced proliferation and increased apoptosis. Further study showed that NOD2 signaling maintained IELs via recognition of gut microbiota and IL-15 production. Notably, recovery of IELs by adoptive transfer could reduce the susceptibility of Nod2−/− mice to the 2,4,6-trinitrobenzene sulfonic acid (TNBS)–induced colitis. Our results demonstrate that recognition of gut microbiota by NOD2 is important to maintain the homeostasis of IELs and provide a clue that may link NOD2 variation to the impaired innate immunity and higher susceptibility in CD.
Pattern recognition receptors, including NOD-like receptors (NLRs), TLRs, and RIG-I–like receptor et al., play a key role in the innate immune response by recognizing pathogen-associated molecular patterns derived from a diverse collection of microbial pathogens (Janeway and Medzhitov, 2002; Meylan et al., 2006). NOD2, a family member of NLRs, functions as an important intracellular sensor for intracellular bacteria and can detect peptidoglycan through the recognition of muramyl dipeptide (MDP; Meylan et al., 2006; Elinav et al., 2011). After binding with MDP, NOD2 recruits adaptor protein RIP2 to activate NF-κB and initiate a proinflammatory response (Meylan et al., 2006). Furthermore, NOD2 was the first identified gene strongly associated with susceptibility to Crohn’s disease (CD; Hugot et al., 2001; Ogura et al., 2001), which affects over one million people in North America and Europe and is characterized by diarrhetic colitis and chronic, relapsing inflammation. Although it has been suggested that the loss-of-function mutation of NOD2 leads to reduced antimicrobial resistance and impaired innate immunity in CD (Comalada and Peppelenbosch, 2006), how NOD2 mutation causes compromised host defense and contributes to the pathogenesis of this disease remains elusive.
The intestinal intraepithelial lymphocytes (IELs) are mostly T cells dispersed as single cells within the epithelial cell layer and located at the interface between outside and the body. Unlike lymphocytes in spleen, blood, or lymph node, IELs comprise >70% of CD8+ T cells and include greater numbers of TCRγδ+ T cells (Cheroutre, 2004). In addition, a large fraction of these cells express a CD8αα homodimer, which is essentially absent from the circulation. IELs display an “innate” or “memory-like” phenotype and play an important role in the homeostasis of intestinal mucosa (Cheroutre, 2004), and loss of them results in impaired intestinal barrier function and more severe colitis in mice (Hoffmann et al., 2001; Chen et al., 2002; Olivares-Villagómez et al., 2008; Li et al., 2011), suggesting the protective role of IELs in inflammatory bowel diseases. The impaired intestinal barrier function and increased susceptibility to colitis have also been shown in Nod2−/− mice (Barreau et al., 2007; Penack et al., 2009). However, whether NOD2 affects the homeostasis of IELs is unclear. In this study, we investigated the role of Nod2 signaling in the maintenance of IELs. Our data demonstrate that IELs, especially the “unconventional” TCRγδ+ and CD8αα+TCRαβ+ IELs, are significantly lost when Nod2 signaling is absent. Our data also indicate that loss of IELs contributes to the high susceptibility of Nod2−/− mice to 2,4,6-trinitrobenzene sulfonic acid (TNBS)–induced colitis.
RESULTS
Selectively reduced IEL subsets in Nod2−/− mice
To investigate whether NOD2 signaling has an impact on the homeostasis of IELs, we first examined the numbers and subpopulation percentages of IELs in Nod2−/− mice. The total number of IELs in Nod2−/− mice was reduced significantly in the small intestine and colon (approximately fourfold; Fig. 1, A and B) as compared with wild-type or Nod1−/− mice. We further examined whether Nod2 deletion affected the lymphocytes in other immune organs, and the results showed that the total numbers were normal in the thymus or spleen of Nod2−/− mice (Fig. 1, C and D), suggesting that Nod2 deletion specifically affects the maintenance of intestinal IELs.
Figure 1.
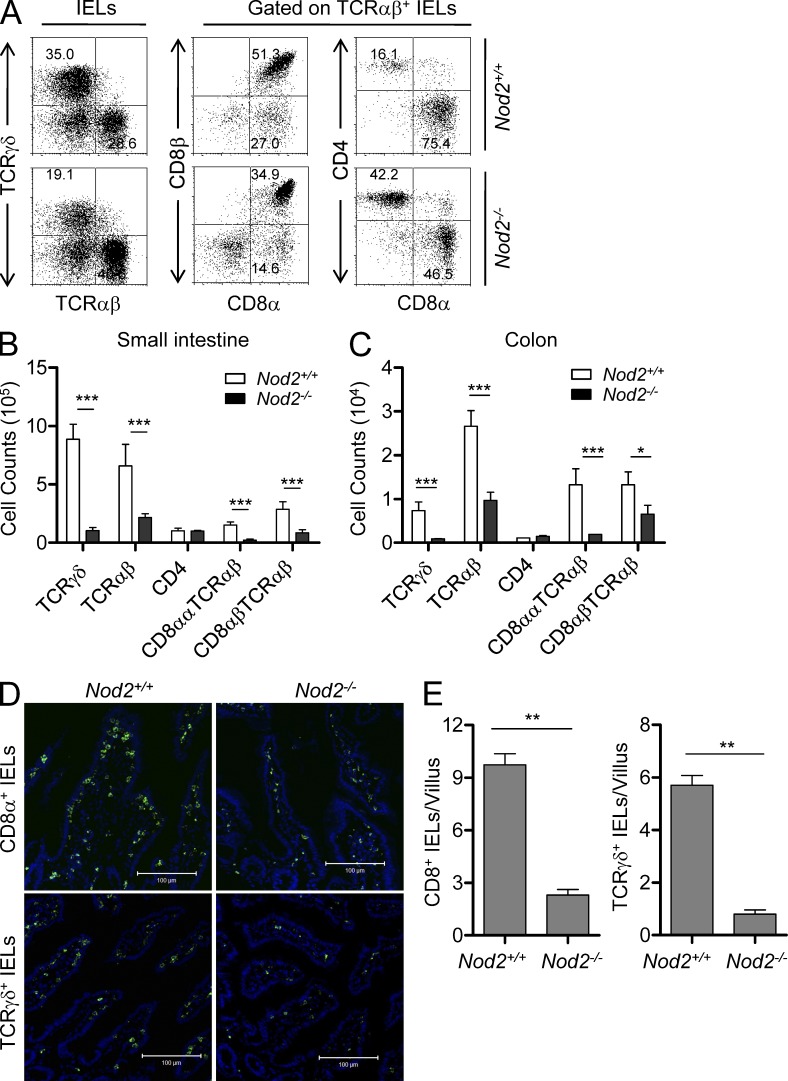
Loss of IELs in Nod2−/− mice. (A and B) The numbers of IELs in the small intestine (A) and colon (B) of mutant mice and individual control mice. **, P < 0.01; ***, P < 0.001. (C and D) The numbers of total thymocytes (C) and splenocytes (D) of mutant mice and individual control mice. Horizontal bars indicate the mean. 20–25 mice per group from three independent experiments.
IELs are simply classified as TCRγδ+, CD8αα+TCRαβ+, CD8αβ+TCRαβ+, and CD4+TCRαβ+ IELs. TCRγδ+ and CD8αα+TCRαβ+ IELs are regarded as unconventional T cells bearing innate-like phenotypes (Cheroutre, 2004; Cheroutre and Lambolez, 2008). Although CD8αβ+TCRαβ+ IELs are conventional T cells, the majority of them exhibit memory-like phenotype (Cheroutre, 2004). Further analysis of small intestinal IELs in Nod2−/− mice revealed that the unconventional TCRγδ+ (approximately eightfold) and CD8αα+TCRαβ+ IELs (approximately sixfold) were dramatically reduced and the memory-like CD8αβ+TCRαβ+ IEL subset was significantly reduced (approximately threefold), whereas the CD4+ IEL subset was normal (Fig. 2, A and B). Similar results were also observed in the colon of Nod2−/− mice (Fig. 2 C). The loss of CD8+ and TCRγδ+ IELs in Nod2−/− mice was also confirmed by staining of CD8α and TCRγδ in sections of small intestine (Fig. 2, D and E). These data thus indicate a critical and selective role for Nod2 in the homeostasis of TCRγδ+ and CD8αα+TCRαβ+ IELs.
Figure 2.
Selectively reduced IEL subsets in Nod2−/− mice. (A) IELs from Nod2+/+ or Nod2−/− mice were stained as indicated. Numbers in the dot plots indicate the percentage of cells represented in the quadrant. (B and C) The absolute numbers of the indicated IEL subsets in the small intestine (B) or colon (C) of individual mice. *, P < 0.05; ***, P < 0.001. 20–25 mice per group from three independent experiments. (D) Tissue samples from the small intestine of Nod2−/− or Nod2+/+ mice were stained with anti-TCRγδ or anti-CD8α (green) antibody and DAPI (blue). Bars, 100 µm. (E) Quantification of anti-TCRγδ or anti-CD8α staining in the villi of Nod2−/− or Nod2+/+ mice. Data were from 50 villi per sample. **, P < 0.01. Representative of three experiments. Error bars indicate SEM.
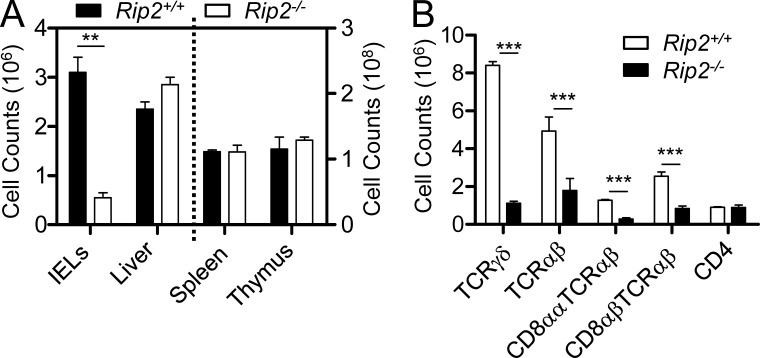
RIP2 is a critical adaptor protein for NOD2 to initiate downstream signaling. To confirm the role of Nod2 signaling in the maintenance of intestinal IELs, we examined Rip2−/− mice. Similar to the results observed in Nod2−/− mice, the TCRγδ+ and CD8αα+TCRαβ+ IELs were also dramatically reduced in Rip2−/− mice as compared with control mice (Fig. 3, A and B). Collectively, these results indicate that Nod2 signaling plays a critical and selective role for the maintenance of IELs in the intestine.
Figure 3.
Loss of IELs in Rip2−/− mice. (A) Quantification of total numbers of small intestinal IELs, liver lymphocytes, splenocytes, and thymocytes in wild-type mice and Rip2−/− mice. **, P < 0.01. (B) The absolute numbers of the indicated IEL subsets in wild-type mice and Rip2−/− mice. ***, P < 0.001. Five mice per group. Representative of three experiments. Error bars indicate SEM.
Nod2 signaling is not required for the development of IELs in the thymus
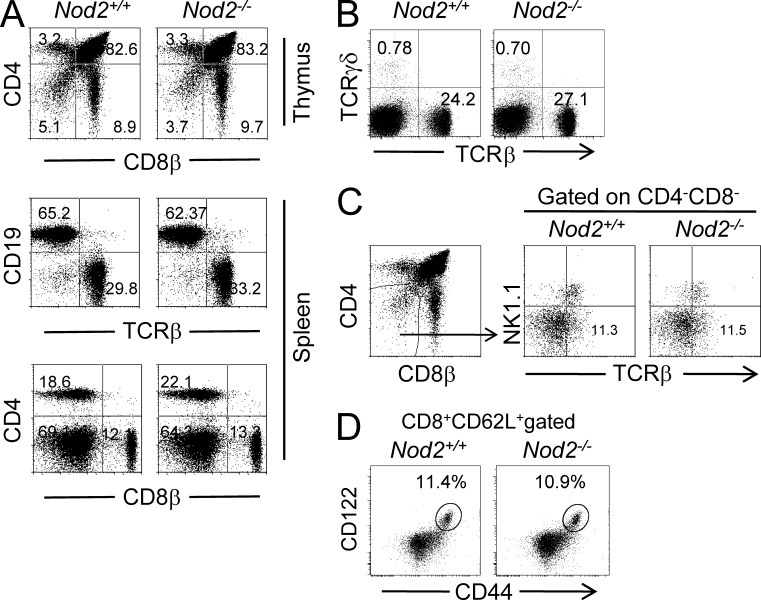
Because mice with Nod2 deletion lack IELs, especially TCRγδ+ and CD8αα+TCRαβ+ IELs, we investigated whether Nod2 signaling had an impact on the development of IELs in the thymus. First, our results showed that the percentages of T and B cells were normal in the thymus or spleen of Nod2−/− mice (Fig. 4 A), suggesting that Nod2 deletion has no impact on the development of conventional lymphocytes in the thymus. Despite early studies suggesting that IELs can develop extrathymically, more recent studies indicate that the majority of them are derived from thymic precursors, at least in the presence of thymus (Cheroutre and Lambolez, 2008). We then examined the frequencies of TCRγδ+ T cells in the spleen, and no differences were observed in Nod2−/− mice as compared with control mice (Fig. 4 B). Recent evidence suggests that CD8αα+TCRαβ+ IELs are derived from CD4−CD8− (double negative [DN]) NK1.1−TCRαβ+ thymocytes, which can give rise to CD8αα+TCRαβ+ IELs when transferred into Rag2−/− mice (Gangadharan et al., 2006). We therefore investigated whether this putative IEL precursor population was affected by Nod2 deletion. The analysis showed that DN NK1.1−TCRαβ+ thymocytes were present in normal frequencies in Nod2−/− mice (Fig. 4 C). These results indicate that Nod2 deficiency has no effect on the development of TCRγδ+ and CD8αα+TCRαβ+ IELs in the thymus and Nod2 signaling affects the maintenance of these IELs, possibly by impacting the gut environment. This idea was also supported by the results showing that the numbers and frequencies of CD8+ memory T cells in the spleen were normal (Fig. 4 D), whereas the memory-like CD8αβ+TCRαβ+ IELs were reduced in Nod2−/− mice. These results suggest that Nod2 signaling is not required for the development of IELs in thymus.
Figure 4.
Normal subsets in the thymus and spleen of Nod2−/− mice. (A) The thymocytes and splenocytes from Nod2+/+ or Nod2−/− mice were stained as indicated. (B) The splenocytes from Nod2−/− mice were stained as indicated. (A and B) Numbers in the dot plots indicate the percentage of cells represented in the quadrant. (C) Analysis of putative thymic IEL precursors in Nod2+/+ and Nod2−/− mice. Total thymocytes were stained as indicated. DN thymocytes were analyzed for NK1.1 and TCRβ expression. Numbers in the quadrants indicate percentages. (D) The splenocytes from Nod2−/− mice were stained as indicated. The percentages of memory CD8+ T cells are shown. Five mice per group. Representative of two or three experiments.
IELs display reduced proliferation and increased apoptosis in Nod2−/− mice
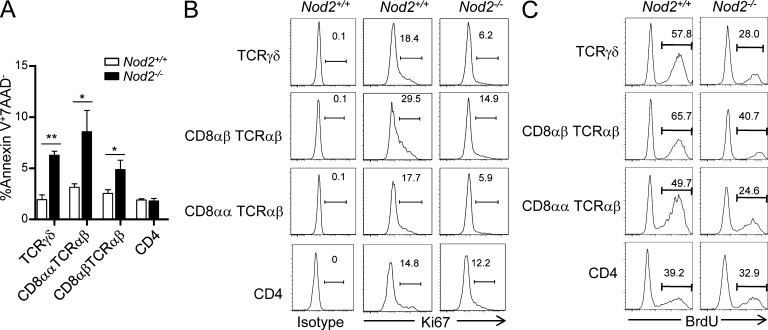
To further explore the reason for loss of IELs in Nod2−/− mice, we examined whether Nod2 deletion affected the proliferation and apoptosis of IELs. The isolated IELs from Nod2−/− mice were stained with Ki67 and annexin-V as the marker for proliferation and apoptosis, respectively. TCRγδ+, CD8αα+TCRαβ+, and CD8αβ+TCRαβ+ IELs showed poorer proliferation and higher apoptosis in Nod2−/− mice, whereas the CD4+ IELs were normal (Fig. 5, A and B). The poorer proliferation of TCRγδ+, CD8αα+TCRαβ+, and CD8αβ+TCRαβ+ IELs was also confirmed by BrdU incorporation assay (Fig. 5 C). These results were consistent with loss of IEL subsets in Nod2−/− mice, suggesting that Nod2 regulates the homeostasis of IELs by affecting their proliferation and survival.
Figure 5.
Nod2 is required for the homeostasis of IELs. (A) Bar graph represents the percentage of apoptotic (annexin-V+7AAD−) IEL subsets in Nod2+/+ and Nod2−/− mice. Error bars indicate SEM. *, P < 0.05; **, P < 0.01. (B) Ki67 expression on IEL subsets of Nod2+/+ and Nod2−/− mice. (C) Nod2+/+ and Nod2−/− mice were injected with BrdU (1.8 mg/mouse) and then fed with BrdU in drinking water (0.8 mg/ml). After 4 d, BrdU incorporation in the indicated IEL subsets was analyzed by flow cytometry. Experiments were performed in at least three mice per group. One representative experiment out of two or three is shown.
Nod2 signaling maintains IELs via recognition of gut microbiota
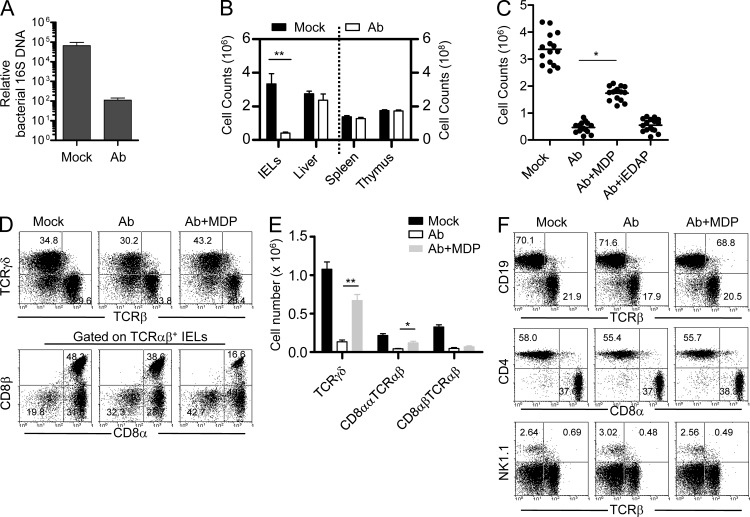
NOD2 is an intracellular sensor not only for microbial but also for commensal bacteria (Kobayashi et al., 2005; Petnicki-Ocwieja et al., 2009). Nod2 expression in the intestine is dependent on the presence of gut microbiota, and Nod2−/− mice have an increased load of commensal resident bacteria (Petnicki-Ocwieja et al., 2009). Moreover, the commensal bacteria in the gut are also required for the maintenance of IELs (Kawaguchi et al., 1993), but the mechanisms remain unclear. Thus, we investigated whether the gut microbiota maintained IELs via NOD2 signaling. To deplete gut microbiota in the intestine of mice, we fed mice with a cocktail of antibiotics after birth (Rakoff-Nahoum et al., 2004). After 6 wk, the load of bacteria was reduced significantly in antibiotic-treated mice (Fig. 6 A). Consistent with the results observed in germ-free mice, depletion of the gut microbiota reduced the IEL numbers in the intestine but had no effect on the lymphocytes from other immune organs, including thymus, spleen, and liver (Fig. 6 B). Importantly, the total numbers of IELs could be recovered significantly by supplementation of NOD2 agonist MDP in drinking water (Fig. 6 C). In contrast, supplementation of iEDAP, an agonist for NOD1, did not increase the IELs in gut microbiota–depleted mice (Fig. 6 C). Further analysis of IEL subsets in MDP-supplemented mice revealed that NOD2 activation by MDP treatment selectively and significantly recovered TCRγδ+ and CD8αα+TCRαβ+ IELs, whereas it didn’t have an effect on CD8αβ+TCRαβ+ IELs (Fig. 6, D and E). We also analyzed the subpopulations of lymphocytes, including B, CD4+ T, CD8+ T, NKT, and NK cells in the spleen of gut microbiota–depleted mice, and no differences were observed (Fig. 6 F). Collectively, these results indicate that NOD2 signaling maintains the IELs via sensing of gut microbiota.
Figure 6.
Nod2 signaling maintains IELs via recognition of gut microbiota. (A) Bacterial 16S DNA were determined by quantitative PCR and normalized to total mouse genomic DNA in the same pellets of mice treated with antibiotics (Ab) for 6 wk. (B) Quantification of total numbers of small intestinal IELs, liver lymphocytes, splenocytes, and thymocytes in antibiotic (Ab)-treated and control mice (Mock). **, P < 0.01. (C) Quantification of total numbers of small intestinal IELs in mice treated with antibiotics alone (Ab) or in the presence of MDP (Ab + MDP) or iEDAP (Ab + iEDAP). Horizontal bars indicate the mean. *, P < 0.05. (D and E) The percentages (D) or absolute numbers (E) of the indicated IEL subsets in the small intestine of mice treated with antibiotics alone (Ab) or in the presence of MDP (Ab + MDP) or iEDAP (Ab + iEDAP) are shown. *, P < 0.05; **, P < 0.01. At least 15 mice per group. (F) Mice were treated with antibiotics (Ab) with or without the presence of MDP, and the splenocytes were stained as indicated. Numbers in the dot plots indicate the percentage of cells represented in the quadrant. Three to five mice per group. Representative of three experiments. Error bars indicate SEM.
Nod2 signaling in the hematopoietic system–derived APCs maintains IELs
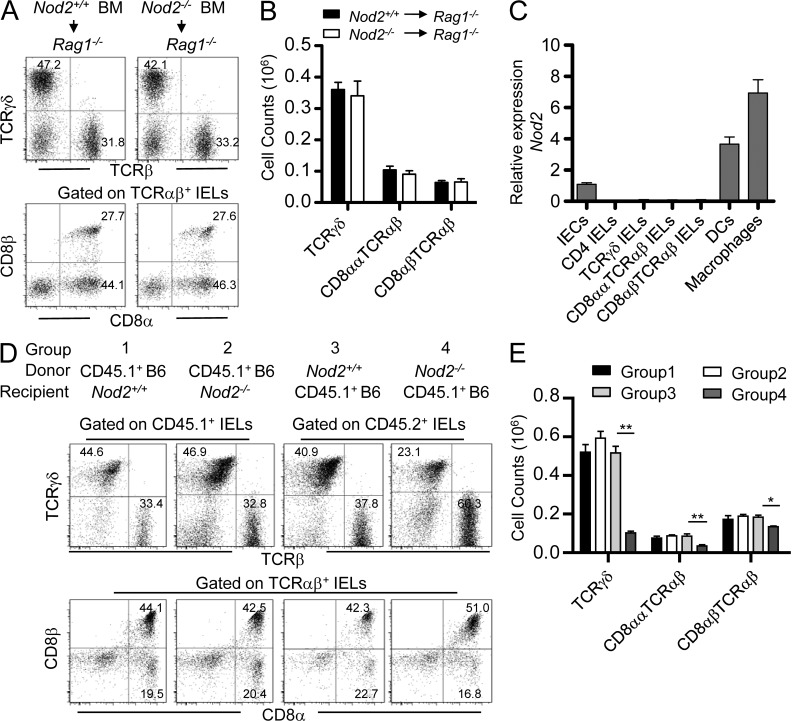
To further investigate how Nod2 signaling maintains IELs, we then determined whether IEL-intrinsic or -extrinsic mechanisms were responsible for the specific reduction in IELs. Adoptive transfer of BM cells from Nod2+/+ mice and Nod2−/− mice both reconstituted IELs in Rag1-deficient hosts (Fig. 7, A and B), suggesting that IEL-intrinsic Nod2 signaling is not required for the homeostasis of IELs. This was consistent with the results showing that no expression of Nod2 was detected in IELs (Fig. 7 C). As Nod2 was expressed in parenchymal cells, including intestinal epithelial cells (IECs), and some hematopoietic system–derived APCs, such as macrophages and DCs (Fig. 7 C), we then determined whether Nod2 signaling in hematopoietic or parenchymal cells is responsible for the maintenance of IELs. When BM cells from CD45.1+ WT mice were transferred into lethally irradiated Nod2+/+ or Nod2−/− mice, after 8 wk, there was no difference for the reconstitution of IELs (Fig. 7, D and E), suggesting that Nod2 signaling in parenchymal cells, including IECs, is not responsible for the homeostasis of IELs. When BM cells from Nod2+/+ or Nod2−/− mice were transferred into lethally irradiated CD45.1+ WT mice, after 8 wk, TCRγδ+ and CD8αα+TCRαβ+ in mice transferred with Nod2−/− BM cells were poorly reconstituted compared with mice transferred with Nod2+/+ BM cells (Fig. 7, D and E), suggesting that Nod2 signaling in hematopoietic cells is required for the homeostasis of IELs. Because IEL-intrinsic Nod2 signaling was not involved (Fig. 7, A and B), these results indicate that Nod2 signaling in the hematopoietic system–derived APCs, such as macrophages or DCs, might be critical for the homeostasis of IELs.
Figure 7.
Nod2 signaling in the hematopoietic system–derived APCs maintains IELs. (A and B) FACS analysis of IELs of the indicated BM chimeras of Nod2+/+ or Nod2−/− BM cells into irradiated Rag1−/− mice. The percentages (A) or absolute numbers (B) of the indicated IEL subsets are shown. (C) Isolated IECs, sorted IEL subsets, macrophages, and DCs were determined by quantitative PCR, and the relative expression of Nod2 is shown. (D and E) FACS analysis of IELs of the indicated BM chimeras of CD45.1+ WT BM cells into irradiated Nod2+/+ or Nod2−/− mice, or Nod2+/+ or Nod2−/− BM cells into irradiated CD45.1+ WT mice. The percentages (D) or absolute numbers (E) of the indicated IEL subsets are shown. Numbers in the dot plots indicate the percentage of cells represented in the quadrant. *, P < 0.05; **, P < 0.01. Five mice per group. Representative of two experiments. Error bars indicate SEM.
Impaired IL-15 expression results in the loss of IELs in Nod2−/− mice
IL-15 is an NF-κB target gene, and more importantly, the IL-15 enrichment environment in the gut is critical for the survival and homeostasis of IEL subsets (Cao et al., 1995; Washizu et al., 1998). We thus investigated whether Nod2 signaling affected the proliferation and apoptosis of IELs via IL-15. First, exogenous IL-15 administration recovered the IEL loss in Nod2−/− mice partially (Fig. 8 A), suggesting that the impaired expression of IL-15 might be responsible for the loss of IELs in Nod2−/− mice. Consistent with the results in Nod2−/− mice, Il-15−/− mice showed reduced TCRγδ+, CD8αα+TCRαβ+, and CD8αβ+TCRαβ+ IELs but normal CD4+ IELs (Fig. 8 B). Importantly, MDP supplementation could not recover the IEL loss in Il-15−/− mice. We then examined the expression of IL-15 in IECs, intestinal macrophages, and DCs and found that macrophages and DCs expressed higher IL-15 and in the basal condition (Fig. 8 C). As expected, the basal or MDP-induced expression of IL-15 in macrophages was decreased when Nod2 was absent (Fig. 8 D). Furthermore, our results showed that MDP treatment up-regulated IL-15 expression in macrophages in a Nod2- and Rip2-depdent manner (Fig. 8 E). Because MDP supplementation rescued the loss of IELs in gut microbiota–depleted mice, we also examined whether MDP could recover the IL-15 expression. As expected, gut microbiota–depleted mice showed lower IL-15 expression in macrophages, whereas MDP treatment restored the expression of IL-15 (Fig. 8 F), suggesting that Nod2 signaling might maintain the expression of IL-15 via recognition of microbiota. These results indicate that the reduced IL-15 expression contributes to the loss of IELs in Nod2−/− mice.
Figure 8.
Impaired IL-15 expression results in the loss of IELs in Nod2−/− mice. (A) Nod2−/− mice were treated with IL-15 for 2 wk, and the absolute numbers of the indicated small intestinal IEL subsets are shown. (B) The absolute numbers of the indicated small intestinal IEL subsets in Il-15−/− mice or Il-15−/− mice treated with MDP for 4 wk. (C) cDNA from IECs, intestinal macrophages, or DCs was assayed for Il-15 expression by quantitative PCR. Expression levels were normalized to IECs. (D) Nod2−/− mice were injected with 0.5 mg/kg LPS or 5 mg/kg MDP, and after 3 h, intestinal macrophages were isolated and sorted for analyzing Il-15 expression by quantitative PCR. (E) Sorted intestinal macrophages from mutant mice were treated with 50 µg/ml MDP for 24 h, and supernatants were analyzed by ELISA for IL-15. (F) cDNA from sorted intestinal macrophages of mice treated with antibiotics alone (Ab) or in the presence of MDP (Ab + MDP) was assayed for Il-15 expression by quantitative PCR. Expression levels were normalized to the nontreated group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Experiments were performed in at least three mice per group. One representative experiment out of two or three is shown. Error bars indicate SEM.
IEL loss contributes to the high susceptibility to TNBS-induced colitis in Nod2−/− mice
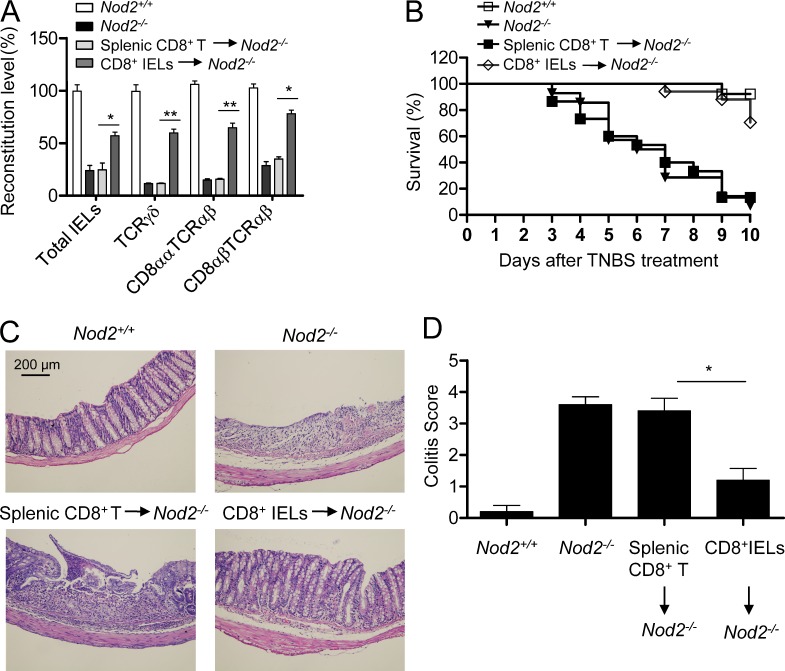
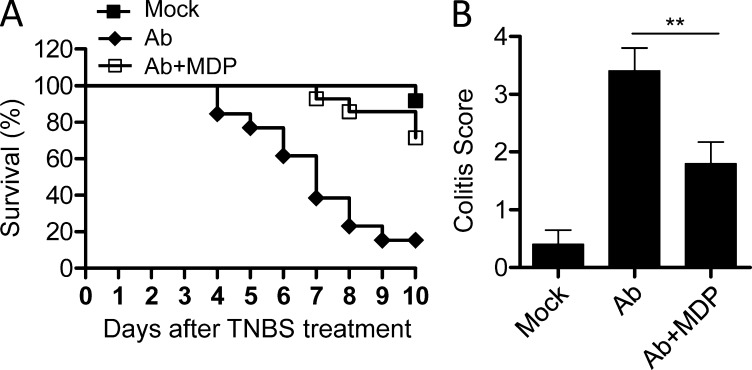
IELs inhabit the intestinal epithelial barriers and provide a first line of defense against environmental challenges or pathogen invading via stimulating repair of damaged epithelia and limiting bacterial penetration (Cheroutre, 2004). Loss of TCRγδ+ IELs or CD8αα+TCRαβ+ IELs in mice aggravates colitis in several animal models and results in impaired ability to control bacterial overgrowth (Ninomiya et al., 2000; Hoffmann et al., 2001; Olivares-Villagómez et al., 2008), suggesting a critical role of IELs in the mucosal defense and epithelial homeostasis. Similarly, Nod2−/− mice are susceptible to the colitis induced by TNBS (Barreau et al., 2007; Penack et al., 2009), but the mechanisms are unclear. We thus studied whether the loss of IELs contributed to the high susceptibility of Nod2−/− mice to colitis. To do this, we transferred CD8+ IELs and splenic CD8+ T cells from wild-type mice to Nod2−/− mice and found that adoptive transfer of CD8+ IELs could recover the IEL loss in Nod2−/− mice partially (Fig. 9 A). Consistent with previous studies (Barreau et al., 2007; Penack et al., 2009), challenge of Nod2−/− mice with TNBS resulted in higher mortality and colitis scores as compared with control mice (Fig. 9, B–D). Moreover, when Nod2−/− mice were transferred with CD8+ IELs from wild-type mice to recover the loss of IELs by Nod2 deletion, the mortality and histology damage were alleviated significantly (Fig. 9, B–D), suggesting that recovery of IELs in Nod2−/− mice can reduce the high susceptibility of these mice to colitis. Furthermore, consistent with the finding that MDP supplementation restored the IELs partially in gut microbiota–depleted mice, MDP treatment reduced the colitis induced by TNBS in these mice (Fig. 10, A and B). Collectively, these results indicate that the loss of IELs in Nod2−/− mice caused by failing to recognize gut microbiota contributes to the impaired innate immune defense and high susceptibility to colitis in these mice.
Figure 9.
IEL loss contributes to the high susceptibility to TNBS-induced colitis in Nod2−/− mice. (A) Sorted splenic CD8+ T cells or CD8+ IELs from wild-type mice were transferred into Nod2−/− mice to reconstitute IELs, and 3 d later, the numbers of total IELs or indicated IEL subsets were counted and the reconstitution levels were normalized to the corresponding numbers of total IELs or indicated IEL subsets in Nod2+/+ mice. *, P < 0.05; **, P < 0.01. Three mice per group. (B–D) Sorted splenic CD8+ T cells or CD8+ IELs from wild-type mice were transferred into Nod2−/− mice, and 3 d later, the mice were treated with TNBS, and the survival rate (B), H&E staining of colon sections (C), and colitis scores (D) are shown. *, P < 0.05. 13–17 mice per group. One representative experiment out of two is shown. Error bars indicate SEM.
Figure 10.
MDP supplementation reduces the susceptibility of gut microbiota–depleted mice to TNBS-induced colitis. (A and B) Mice were treated with antibiotics (Ab) with or without the presence of MDP and then treated with TNBS, and the survival rate (A) and colitis scores (B) are shown. 12–15 mice per group. **, P < 0.01. One representative experiment out of two is shown. Error bars indicate SEM.
DISCUSSION
We show here that Nod2 signaling is important to maintain IELs in the intestine. Mice with Nod2 or Rip2 deletion lacked IELs, especially the unconventional TCRγδ+ IELs and CD8αα+TCRαβ+ IELs, in the small intestine and colon. In contrast, the lymphocytes in thymus, spleen, and liver remained normal. In Nod2−/− mice, the residual IELs displayed reduced proliferation and increased apoptosis. Furthermore, we found that Nod2 signaling maintained IELs via recognition of gut microbiota because supplementation of NOD2 agonist MDP recovered the IELs in gut microbiota–depleted mice. The loss of IELs in Nod2−/− mice was caused by the impaired expression of IL-15 in APCs, and supplementation of IL-15 rescued the IEL loss caused by Nod2 deletion. Importantly, recovery of IELs by adoptive transfer of IELs to Nod2−/− mice could reduce the susceptibility of the mice to TNBS-induced colitis. Thus, our results demonstrate a previously unrecognized role for Nod2 signaling in the homeostasis of IELs and probably provide a new clue for the observed impaired host defense in Nod2−/− mice and patients with CD.
Nod2−/− mice have been reported to be more susceptible to oral infection with Listeria monocytogenes (Kobayashi et al., 2005), suggesting the important role of NOD2 signaling in epithelial defense. The current knowledge for the mechanism of defective epithelial defense in Nod2−/− mice is that these mice show impaired production of defensins by Paneth cells (Kobayashi et al., 2005). However, the defensin defect in Nod2−/− mice is limited, and only several, but not all, defensins are modestly decreased, questioning the role of these limited changes on relevant antimicrobial defense in the intestine (Kobayashi et al., 2005). In this study, we show that IELs are dramatically reduced in Nod2−/− mice. IELs play a critical role for maintaining the integrity of the epithelial barrier, and loss of TCRγδ+ IELs results in higher bacterial penetration of the intestinal mucosa (Ismail et al., 2011). The higher permeability for bacteria translocation in the intestinal mucosa is also observed in Nod2−/− mice (Barreau et al., 2007), suggesting that the defect of IELs may play an important role in the intestinal barrier dysfunction in Nod2−/− mice.
Although the etiology of CD is poorly understood, increasing evidence from genetics, functional studies on innate immunity, and therapeutic trials on patients suggest that CD results from impaired innate immunity and dysfunction of mucosal barrier (Comalada and Peppelenbosch, 2006; Marks et al., 2006). The genetic association of NOD2 with CD established a critical link between innate immunity and the development of the disease, but the underlying mechanisms remain controversial. The CD-associated NOD2 variants show impaired ability to recognize microbial components and lack of activation of NF-κB in monocytes (Hugot et al., 2001). However, the higher NF-κB activity is observed in patients with CD (Podolsky, 2002). Defensin levels are noted to be lower in the Paneth cells of patients with mutant NOD2 (Wehkamp et al., 2005), but recent work has shown that defensin deficiency in CD is independent of the NOD2 genotype (Simms et al., 2008). In this study, our results suggest that IEL dysregulation caused by loss of function of NOD2 may favor the onset of CD. Indeed, multiple studies have described a decreased level of the TCRγδ+ IELs in patients with CD (Fukushima et al., 1991; Lee et al., 1997). This idea is also supported by the results showing that loss of TCRγδ+ IELs or CD8αα+TCRαβ+ IELs aggravates colitis in the mouse model (Hoffmann et al., 2001; Olivares-Villagómez et al., 2008).
Gut microbiota resides in the intestine and has a beneficial effect ranging from aiding in metabolism to competing with invasive pathogens (Sonnenburg et al., 2006; Honda and Littman, 2012). Increasing evidence from germ-free mice or antibiotic-treated mice reveals that gut microbiota is important for the lymphoid tissue development in the intestine (Abt and Artis, 2009). IELs are a unique T cell population located between IECs and function as a critical component of the epithelial barrier. IELs reduced dramatically in germ-free mice (Guy-Grand et al., 1991; Kawaguchi et al., 1993; Suzuki et al., 2002), suggesting the important role of gut microbiota in the maintenance of IELs. In this study, we demonstrate that gut microbiota–derived products, probably MDP, can signal NOD2 to maintain IELs. Thus, our data provide a new mechanism through which gut microbiota controls the homeostasis of IELs. However, it is unclear which bacterial species are associated with the homeostasis of IELs observed in this study. It will be important to define the commensal bacterial species that elicit this effect.
NOD2 has an important role in host defense against the invasive intracellular pathogen, including L. monocytogenes (Kobayashi et al., 2005); however, little is known whether NOD2 can recognize commensal bacteria. In this study, we demonstrate that Nod2 maintains IELs via recognition of gut microbiota, suggesting the important role of Nod2 in sensing of commensal bacteria. Interestingly, the expression of Nod2 in the intestine is dependent on the presence of gut microbiota (Petnicki-Ocwieja et al., 2009). In summary, our results demonstrate that NOD2 signaling by sensing of gut microbiota is important for maintaining the homeostasis of IELs and suggest that IEL loss may contribute to the impaired innate immunity and higher susceptibility to mucosal damage in Nod2−/− mice and patients with CD.
MATERIALS AND METHODS
Mice.
Nod2−/−, Rip2−/−, and Myd88−/− mice were described previously (Adachi et al., 1998; Kobayashi et al., 2002, 2005). Nod1−/− mice were provided by Millennium Pharmaceuticals. Il15−/− mice were originally provided by Immunex. CD45.1+ and Rag1−/− mice were purchased from the Jackson Laboratory. All mice were from a C57BL/6 background. The littermates of the mutant mice were used as control. All animal experiments were approved by a local ethics committee (the Ethics Committee of the University of Science and Technology of China; Service Vétérinaire Cantonal, Lausanne, Switzerland).
Cell preparations.
Thymocyte and splenocyte suspensions were prepared by grinding the organs through mesh filters. IELs were isolated as previously described (Jiang et al., 2010). In brief, Peyer’s patches were removed, and then the small intestine or colon was opened longitudinally and cut into 1-cm-long pieces. After washing the specimens in PBS containing 100 U/ml penicillin and 100 µg/ml streptomycin twice, the pieces were then stirred at 37°C in prewarmed Dulbecco’s modified Eagle’s medium containing 100 U/ml penicillin, 100 µg/ml streptomycin, and 5% FCS for 30 min. The supernatants were then separated by a 40–70% Percoll density gradient (GE Healthcare), and the cells that layered between the 40–70% fractions were collected as IELs. After IEL isolation, tissues were shook in PBS containing 1.3 mM EDTA at 37°C for 30 min. This step was repeated and the supernatants were discarded. LPLs were then isolated after digestion in RPMI 1640 supplemented with 100 U/ml collagenase (Sigma-Aldrich), 1 mM CaCl2, 1 mM MgCl2, and 5% FCS at 37°C for 30 min. Released cells were then washed in PBS containing 5% FCS and subjected to Percoll fractionation as described above for isolation of IELs. Intestinal macrophages (F4/80+) and DCs (CD11b−CD11c+) were purified from LPLs by cell sorting. IEC isolation and culture were performed as previously described (Zhou et al., 2007).
Antibodies and flow cytometry.
The following mAb conjugates were used: TCRγδ (GL3)-FITC, -PE, and -allophycocyanin; TCRβ (H57)-PE-Cy5, -allophycocyanin, and -allophycocyanin-eFluor780; CD4 (GK1.5)-FITC, and -PE-Cy7; CD8α (53.6.7)-PE-Cy7, -allophycocyanin, and -allophycocyanin-eFluor780; CD8β (H35)-FITC, -PE, and -PE-Cy7; CD19(1D3)-PE; CD44 (IM781) -allophycocyanin-Cy7; CD62L (Mel-14)-FITC; CD122 (IL-2Rβ, TM-β1)-PE; and CD161 (PK136)–peridinin chlorophyll protein–Cy5.5. All antibodies were obtained from BD or eBioscience. BD Canto or Verse cytometers were used for flow cytometry, and data were analyzed using WinMDI or FlowJo (Tree Star). Fluorescence-activated cell sorting was performed using a FACSAria flow cytometer (BD).
Cell proliferation and apoptosis analysis.
Cell proliferation and apoptosis assay were performed as previously described (Jiang et al., 2010). In brief, IELs were labeled with the appropriate mAb and then fixed and permeabilized using the BD Perm&Fix kit (BD) and subsequently stained with anti-Ki67-FITC (B56; BD) for 1 h. After washing, cells were analyzed on a flow cytometer (Verse; BD). For analysis of apoptosis, cells were stained with an annexin-V staining kit according (BD) to the manufacturer’s protocol.
Gut microbiota depletion and MDP reconstitution.
The gut microbiota was depleted by feeding antibiotics in drinking water as previously described (Rakoff-Nahoum et al., 2004). In brief, mice were provided 1 g/liter ampicillin (Sigma-Aldrich), 500 mg/liter vancomycin (Sigma-Aldrich), 1 g/liter neomycin sulfate (Sigma-Aldrich), and 1 g/liter metronidazole (Sigma-Aldrich) in drinking water for 6 wk before treatment with TNBS. The depletion effects were evaluated by bacteria 16S rRNA gene quantification as previously reported (Reikvam et al., 2011). Mice were supplemented with MDP or iEDAP (InvivoGen) drinking water at a fixed concentration of 1 µM from the age of 2 wk for 4 wk to reconstitute MDP or iEDAP in the intestine.
Colitis model.
Induction of TNBS (Sigma-Aldrich) colitis was performed as described previously (Scheiffele and Fuss, 2002). Severity of colitis was assessed using a semiquantitative scoring system (Scheiffele and Fuss, 2002).
Immunofluorescence.
Frozen sections of tissues were stained with FITC-CD8α or FITC-TCRγδ antibodies (eBioscience) and then mounted using ProLong Gold reagent with DAPI (Invitrogen). Confocal images were obtained using an LSM 700 microscope (Carl Zeiss), and for image analysis we used the LSM software (Carl Zeiss).
ELISA.
Tissues were homogenized, and then the lysates were assayed for mouse IL-15 (eBioscience) according to the manufacturer’s instructions.
Adoptive transfer.
For prevention of chemical-induced colitis, CD8+ IELs or splenic T cells were isolated from wild-type mice and sorted and then were intravenously transferred into Nod2−/− mice (200 µl PBS/107 cells). 3 d later, the mice were used to analyze IELs or induce colitis using TNBS or DSS.
For BM chimera experiments, Nod2+/+ or Nod2+/+ BM cells were intravenously transferred into Rag12/2 mice, or CD45.1+ WT BM cells were intravenously transferred into lethally irradiated (12 Gy given 1 d before adoptive transfer) Nod2+/+ or Nod2+/+ mice, or Nod2+/+ or Nod2+/+ or Nod2+/+ BM cells were intravenously transferred into lethally irradiated CD45.1+ WT mice. After 8 wk, IELs were analyzed by flow cytometry.
In vivo treatment with IL-15.
Mice were injected intraperitoneally with PBS or PBS containing human IL-15 (5 µg/mouse; R&D Systems) once daily for 2 wk. IELs were harvested 24 h after the last injection of IL-15.
Real-time PCR.
The Il-15 and Nod2 primers were obtained from SAbiosciences. Real-time PCR using SYBR was performed on a LightCycler (Roche) as previously described (Jiang et al., 2010).
Hematoxylin and eosin (H&E) staining.
For histology, colon tissue was fixed in 10% neutral-buffered formalin and embedded in paraffin. 5-µm sections were affixed to slides, deparaffinized, and stained with H&E. Morphological changes in the stained sections were examined under light microscopy (BX53; Olympus).
Statistical analysis.
Data are expressed as mean ± SEM. Differences were analyzed by Student’s t test (with 95% confidence interval). P-values <0.05 were considered significant.
Acknowledgments
We thank Yonglin Chen and Jianlin Geng for technical assistance and Dr. Shizuo Akira (Osaka University, Osaka, Japan) for providing the Myd88−/− mice.
Work in Prof. Zhou’s laboratory was supported by the National Natural Science Foundation of China (81273318, 81222040, and 31200659), the National Basic Research Program of China (2013CB944904), the Doctoral Fund of Ministry of Education of China (20123402120001 and 20123402110010), the One Hundred Person Project (R. Zhou) and Chinese Academy of Sciences (CAS) President’s fund (R. Zhou) from the CAS, the Fundamental Research Funds for the Central Universities (R. Zhou and W. Jiang), the Anhui Provincial Natural Science Foundation, and the start-up funds (R. Zhou and W. Jiang) from the University of Science and Technology of China. Work in Prof. Tschopp’s laboratory was supported by grants from the Swiss National Science Foundation, the National Center of Competence in Research in Molecular Oncology, the Institute for Arthritis Research, the Foundation Louis-Jeantet, and the EU-FP7 program APO-SYS. Work in Prof. Wei’s laboratory was supported by the National Basic Research Program of China (2013CB531406).
The authors have no conflicting financial interests.
Author contributions: W. Jiang performed the majority of the experiments for this work; X. Wang, B. Zeng, L. Liu, and A. Tardivel performed experiments. W. Jiang, H. Wei, H.R. MacDonald, J. Han, J. Tschopp, Z. Tian, and R. Zhou designed the research. W. Jiang, Z. Tian, and R. Zhou wrote the manuscript. R. Zhou supervised the project.
Footnotes
Abbreviations used:
- CD
- Crohn’s disease
- DN
- double negative
- IEC
- intestinal epithelial cell
- IEL
- intraepithelial lymphocyte
- MDP
- muramyl dipeptide
- TNBS
- 2,4,6-trinitrobenzene sulfonic acid
References
- Abt M.C., Artis D. 2009. The intestinal microbiota in health and disease: the influence of microbial products on immune cell homeostasis. Curr. Opin. Gastroenterol. 25:496–502 10.1097/MOG.0b013e328331b6b4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi O., Kawai T., Takeda K., Matsumoto M., Tsutsui H., Sakagami M., Nakanishi K., Akira S. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 9:143–150 10.1016/S1074-7613(00)80596-8 [DOI] [PubMed] [Google Scholar]
- Barreau F., Meinzer U., Chareyre F., Berrebi D., Niwa-Kawakita M., Dussaillant M., Foligne B., Ollendorff V., Heyman M., Bonacorsi S., et al. 2007. CARD15/NOD2 is required for Peyer’s patches homeostasis in mice. PLoS ONE. 2:e523 10.1371/journal.pone.0000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Shores E.W., Hu-Li J., Anver M.R., Kelsall B.L., Russell S.M., Drago J., Noguchi M., Grinberg A., Bloom E.T., et al. 1995. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 2:223–238 10.1016/1074-7613(95)90047-0 [DOI] [PubMed] [Google Scholar]
- Chen Y., Chou K., Fuchs E., Havran W.L., Boismenu R. 2002. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc. Natl. Acad. Sci. USA. 99:14338–14343 10.1073/pnas.212290499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheroutre H. 2004. Starting at the beginning: new perspectives on the biology of mucosal T cells. Annu. Rev. Immunol. 22:217–246 10.1146/annurev.immunol.22.012703.104522 [DOI] [PubMed] [Google Scholar]
- Cheroutre H., Lambolez F. 2008. The thymus chapter in the life of gut-specific intra epithelial lymphocytes. Curr. Opin. Immunol. 20:185–191 10.1016/j.coi.2008.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comalada M., Peppelenbosch M.P. 2006. Impaired innate immunity in Crohn’s disease. Trends Mol. Med. 12:397–399 10.1016/j.molmed.2006.07.005 [DOI] [PubMed] [Google Scholar]
- Elinav E., Strowig T., Henao-Mejia J., Flavell R.A. 2011. Regulation of the antimicrobial response by NLR proteins. Immunity. 34:665–679 10.1016/j.immuni.2011.05.007 [DOI] [PubMed] [Google Scholar]
- Fukushima K., Masuda T., Ohtani H., Sasaki I., Funayama Y., Matsuno S., Nagura H. 1991. Immunohistochemical characterization, distribution, and ultrastructure of lymphocytes bearing T-cell receptor gamma/delta in inflammatory bowel disease. Gastroenterology. 101:670–678 [DOI] [PubMed] [Google Scholar]
- Gangadharan D., Lambolez F., Attinger A., Wang-Zhu Y., Sullivan B.A., Cheroutre H. 2006. Identification of pre- and postselection TCRalphabeta+ intraepithelial lymphocyte precursors in the thymus. Immunity. 25:631–641 10.1016/j.immuni.2006.08.018 [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Cerf-Bensussan N., Malissen B., Malassis-Seris M., Briottet C., Vassalli P. 1991. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J. Exp. Med. 173:471–481 10.1084/jem.173.2.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J.C., Peters K., Henschke S., Herrmann B., Pfister K., Westermann J., Zeitz M. 2001. Role of T lymphocytes in rat 2,4,6-trinitrobenzene sulphonic acid (TNBS) induced colitis: increased mortality after gammadelta T cell depletion and no effect of alphabeta T cell depletion. Gut. 48:489–495 10.1136/gut.48.4.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Littman D.R. 2012. The microbiome in infectious disease and inflammation. Annu. Rev. Immunol. 30:759–795 10.1146/annurev-immunol-020711-074937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugot J.P., Chamaillard M., Zouali H., Lesage S., Cézard J.P., Belaiche J., Almer S., Tysk C., O’Morain C.A., Gassull M., et al. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 411:599–603 10.1038/35079107 [DOI] [PubMed] [Google Scholar]
- Ismail A.S., Severson K.M., Vaishnava S., Behrendt C.L., Yu X., Benjamin J.L., Ruhn K.A., Hou B., DeFranco A.L., Yarovinsky F., Hooper L.V. 2011. Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc. Natl. Acad. Sci. USA. 108:8743–8748 10.1073/pnas.1019574108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C.A., Jr, Medzhitov R. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216 10.1146/annurev.immunol.20.083001.084359 [DOI] [PubMed] [Google Scholar]
- Jiang W., Ferrero I., Laurenti E., Trumpp A., MacDonald H.R. 2010. c-Myc controls the development of CD8alphaalpha TCRalphabeta intestinal intraepithelial lymphocytes from thymic precursors by regulating IL-15-dependent survival. Blood. 115:4431–4438 10.1182/blood-2009-11-254698 [DOI] [PubMed] [Google Scholar]
- Kawaguchi M., Nanno M., Umesaki Y., Matsumoto S., Okada Y., Cai Z., Shimamura T., Matsuoka Y., Ohwaki M., Ishikawa H. 1993. Cytolytic activity of intestinal intraepithelial lymphocytes in germ-free mice is strain dependent and determined by T cells expressing gamma delta T-cell antigen receptors. Proc. Natl. Acad. Sci. USA. 90:8591–8594 10.1073/pnas.90.18.8591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Inohara N., Hernandez L.D., Galán J.E., Núñez G., Janeway C.A., Medzhitov R., Flavell R.A. 2002. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 416:194–199 10.1038/416194a [DOI] [PubMed] [Google Scholar]
- Kobayashi K.S., Chamaillard M., Ogura Y., Henegariu O., Inohara N., Nuñez G., Flavell R.A. 2005. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 307:731–734 10.1126/science.1104911 [DOI] [PubMed] [Google Scholar]
- Lee H.B., Kim J.H., Yim C.Y., Kim D.G., Ahn D.S. 1997. Differences in immunophenotyping of mucosal lymphocytes between ulcerative colitis and Crohn’s disease. Korean J. Intern. Med. 12:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Innocentin S., Withers D.R., Roberts N.A., Gallagher A.R., Grigorieva E.F., Wilhelm C., Veldhoen M. 2011. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 147:629–640 10.1016/j.cell.2011.09.025 [DOI] [PubMed] [Google Scholar]
- Marks D.J., Harbord M.W., MacAllister R., Rahman F.Z., Young J., Al-Lazikani B., Lees W., Novelli M., Bloom S., Segal A.W. 2006. Defective acute inflammation in Crohn’s disease: a clinical investigation. Lancet. 367:668–678 10.1016/S0140-6736(06)68265-2 [DOI] [PubMed] [Google Scholar]
- Meylan E., Tschopp J., Karin M. 2006. Intracellular pattern recognition receptors in the host response. Nature. 442:39–44 10.1038/nature04946 [DOI] [PubMed] [Google Scholar]
- Ninomiya T., Takimoto H., Matsuzaki G., Hamano S., Yoshida H., Yoshikai Y., Kimura G., Nomoto K. 2000. Vgamma1+ gammadelta T cells play protective roles at an early phase of murine cytomegalovirus infection through production of interferon-gamma. Immunology. 99:187–194 10.1046/j.1365-2567.2000.00938.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y., Bonen D.K., Inohara N., Nicolae D.L., Chen F.F., Ramos R., Britton H., Moran T., Karaliuskas R., Duerr R.H., et al. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 411:603–606 10.1038/35079114 [DOI] [PubMed] [Google Scholar]
- Olivares-Villagómez D., Mendez-Fernandez Y.V., Parekh V.V., Lalani S., Vincent T.L., Cheroutre H., Van Kaer L. 2008. Thymus leukemia antigen controls intraepithelial lymphocyte function and inflammatory bowel disease. Proc. Natl. Acad. Sci. USA. 105:17931–17936 10.1073/pnas.0808242105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penack O., Smith O.M., Cunningham-Bussel A., Liu X., Rao U., Yim N., Na I.K., Holland A.M., Ghosh A., Lu S.X., et al. 2009. NOD2 regulates hematopoietic cell function during graft-versus-host disease. J. Exp. Med. 206:2101–2110 10.1084/jem.20090623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petnicki-Ocwieja T., Hrncir T., Liu Y.J., Biswas A., Hudcovic T., Tlaskalova-Hogenova H., Kobayashi K.S. 2009. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc. Natl. Acad. Sci. USA. 106:15813–15818 10.1073/pnas.0907722106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D.K. 2002. Inflammatory bowel disease. N. Engl. J. Med. 347:417–429 10.1056/NEJMra020831 [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 118:229–241 10.1016/j.cell.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Reikvam D.H., Erofeev A., Sandvik A., Grcic V., Jahnsen F.L., Gaustad P., McCoy K.D., Macpherson A.J., Meza-Zepeda L.A., Johansen F.E. 2011. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS ONE. 6:e17996 10.1371/journal.pone.0017996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele F., Fuss I.J. 2002. Induction of TNBS colitis in mice. Curr. Protoc. Immunol. Chapter 15:19. [DOI] [PubMed] [Google Scholar]
- Simms L.A., Doecke J.D., Walsh M.D., Huang N., Fowler E.V., Radford-Smith G.L. 2008. Reduced alpha-defensin expression is associated with inflammation and not NOD2 mutation status in ileal Crohn’s disease. Gut. 57:903–910 10.1136/gut.2007.142588 [DOI] [PubMed] [Google Scholar]
- Sonnenburg J.L., Chen C.T., Gordon J.I. 2006. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 4:e413 10.1371/journal.pbio.0040413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Jeong K.I., Itoh K., Doi K. 2002. Regional variations in the distributions of small intestinal intraepithelial lymphocytes in germ-free and specific pathogen-free mice. Exp. Mol. Pathol. 72:230–235 10.1006/exmp.2002.2433 [DOI] [PubMed] [Google Scholar]
- Washizu J., Nishimura H., Nakamura N., Nimura Y., Yoshikai Y. 1998. The NF-kappaB binding site is essential for transcriptional activation of the IL-15 gene. Immunogenetics. 48:1–7 10.1007/s002510050393 [DOI] [PubMed] [Google Scholar]
- Wehkamp J., Salzman N.H., Porter E., Nuding S., Weichenthal M., Petras R.E., Shen B., Schaeffeler E., Schwab M., Linzmeier R., et al. 2005. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc. Natl. Acad. Sci. USA. 102:18129–18134 10.1073/pnas.0505256102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Wei H., Sun R., Zhang J., Tian Z. 2007. NKG2D recognition mediates Toll-like receptor 3 signaling-induced breakdown of epithelial homeostasis in the small intestines of mice. Proc. Natl. Acad. Sci. USA. 104:7512–7515 10.1073/pnas.0700822104 [DOI] [PMC free article] [PubMed] [Google Scholar]