A subset of PSGL-1 is constitutively associated with L-selectin and signals through Src family kinases to activate LFA-1, which regulates neutrophil slow rolling and recruitment.
Abstract
Neutrophils are recruited from the blood to sites of inflammation, where they contribute to immune defense but may also cause tissue damage. During inflammation, neutrophils roll along the microvascular endothelium before arresting and transmigrating. Arrest requires conformational activation of the integrin lymphocyte function–associated antigen 1 (LFA-1), which can be induced by selectin engagement. Here, we demonstrate that a subset of P-selectin glycoprotein ligand-1 (PSGL-1) molecules is constitutively associated with L-selectin. Although this association does not require the known lectin-like interaction between L-selectin and PSGL-1, the signaling output is dependent on this interaction and the cytoplasmic tail of L-selectin. The PSGL-1–L-selectin complex signals through Src family kinases, ITAM domain–containing adaptor proteins, and other kinases to ultimately result in LFA-1 activation. The PSGL-1–L-selectin complex–induced signaling effects on neutrophil slow rolling and recruitment in vivo demonstrate the functional importance of this pathway. We conclude that this is a signaling complex specialized for sensing adhesion under flow.
Neutrophils are essential to mammalian survival. Defects in neutrophil adhesion, recruitment, chemotaxis, or function lead to shortened lifespan because of bacterial and fungal infections. Among all leukocytes, neutrophils are probably the most efficient at achieving adhesion under flow at wall shear stress levels up to 20 dyn/cm2. Here, we demonstrate that P-selectin glycoprotein ligand-1 (PSGL-1) and L-selectin interact on the cell surface of neutrophils and that this complex is necessary to trigger integrin activation after engagement of the PSGL-1–L-selectin complex by P- or E-selectin expressed on the inflamed endothelium.
PSGL-1 is the main selectin receptor on neutrophils. It binds all three selectins (P-, E-, and L-selectin). P- and L-selectin bind closely apposed and overlapping sites located near the N terminus of PSGL-1 (Zarbock et al., 2011). In contrast, E-selectin binds to poorly defined sites closer to the plasma membrane (Zarbock et al., 2011). Engagement of PSGL-1 leads to intracellular signaling events including activation of different signaling molecules and integrins (Miner et al., 2008). However, the role of PSGL-1 signaling has remained elusive because, under physiological conditions in vivo, ligation of PSGL-1 by P-selectin does not lead to appreciable neutrophil activation and adhesion (Ley et al., 1995). This finding suggests that PSGL-1 may not be the entire recognition system for selectins. However, PSGL-1 may be the limiting receptor because it is expressed at only 25,000 copies per neutrophil (Kappelmayer et al., 2001).
L-selectin is the only selectin expressed on neutrophils. It is quite abundant at ∼100,000 copies per neutrophil (Lewinsohn et al., 1987). Like PSGL-1 (Zarbock et al., 2011), L-selectin is localized to the tips of neutrophil microvilli (Bruehl et al., 1996). Upon neutrophil activation, L-selectin is rapidly shed from the cell surface, allowing neutrophils to migrate normally after extravasation (Venturi et al., 2003). Inhibiting L-selectin shedding promotes neutrophil activation and adhesion (Hafezi-Moghadam et al., 2001). Although the cytoplasmic tail of L-selectin contains no known signaling motifs (Kansas, 1992), L-selectin ligation clearly triggers signaling events (Waddell et al., 1995). L-selectin ligation may induce integrin activation, but not all studies are well controlled (Sikorski et al., 1996; Giblin et al., 1997).
It has been shown (Zarbock et al., 2008; Yago et al., 2010) that PSGL-1 on neutrophils recruits FcRγ and DAP-12, which are phosphorylated by Fgr to recruit spleen tyrosine kinase (Syk). Syk is required for SLP-76 activation, which subsequently activates Bruton’s tyrosine kinase (Btk; Block et al., 2012). Activated Btk and adhesion and degranulation promoting adaptor protein regulate two pathways (Mueller et al., 2010; Block et al., 2012). One pathway is phosphoinositide-3-kinase γ (PI3Kγ; Mueller et al., 2010) and P-Rex-1 (Herter et al., 2013) dependent, and the other pathway comprises phospholipase C γ2 (PLCγ2), p38 mitogen-activated protein kinase, and Ras-related protein 1a (Mueller et al., 2010; Stadtmann et al., 2011). Both signaling pathways result in extension of the integrin lymphocyte function–associated antigen 1 (LFA-1), enabling it to transiently bind its ligand intercellular adhesion molecule 1 (ICAM-1) in vitro (Kuwano et al., 2010) and in vivo (Zarbock et al., 2007). LFA-1 extension requires talin-1 (Lefort et al., 2012) and is detectable by conformation-specific antibodies like KIM127 or NKI-L16.
Here, we show that L-selectin is constitutively associated with a subset of PSGL-1, Lyn, Fgr, Hck, DAP12, and FcRγ. This complex results in LFA-1 extension when PSGL-1 engages E- or P-selectin under shear stress. The lectin-like interaction between PSGL-1 and L-selectin and the cytoplasmic tail of L-selectin are required for LFA-1 extension through this pathway.
RESULTS AND DISCUSSION
L-selectin and PSGL-1 form a complex in primary neutrophils
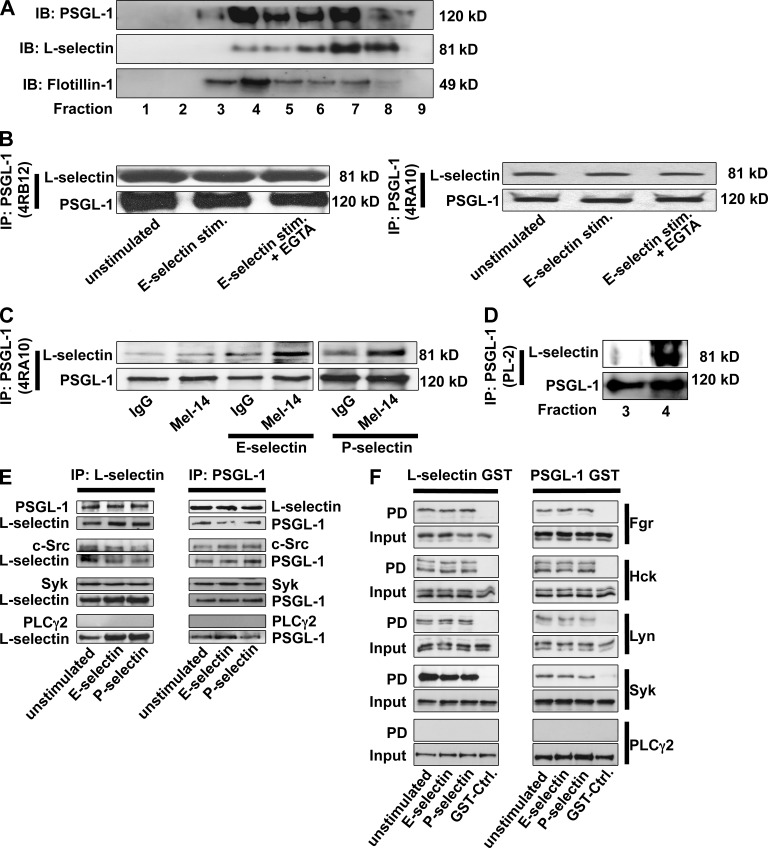
First, we investigated whether L-selectin and PSGL-1 are localized in the same lipid raft fractions on neutrophils. By using a sucrose gradient, we showed that a small amount of PSGL-1 and L-selectin colocalizes in some lipid raft fractions (Fig. 1 A). Next, we tested whether L-selectin coimmunoprecipitates with PSGL-1. This was the case in BM-derived neutrophils (Fig. 1, B and C). 4RA10, an mAb to PSGL-1 that blocks its P- and L-selectin binding site, was as effective as 4RB12, a nonblocking mAb. The Western blot signal was not affected by whether neutrophils were stimulated by adhesion to E-selectin under shear conditions, suggesting that the interaction is constitutive. Blocking the lectin domain of L-selectin by an anti–L-selectin antibody (MEL-14) did not disrupt its interaction with PSGL-1 (Fig. 1 C). Furthermore, we demonstrated that L-selectin and PSGL-1 interact at least in one lipid raft fraction (Fig. 1 D). By performing coimmunoprecipitation in primary neutrophils, we demonstrated that Src family kinases (SFKs) and Syk interact with PSGL-1 and L-selectin (Fig. 1 E). To determine which of the three SFKs expressed in neutrophils interact with the cytoplasmic tails of PSGL-1 and L-selectin, the cytoplasmic domains of PSGL-1 and L-selectin were fused to GST (Fig. 1 F) and incubated with cell lysates of primary neutrophils. The cytoplasmic tails of PSGL-1 and L-selectin interact with Fgr, Hck, Lyn, and Syk (Fig. 1 F). PLCγ2 does not interact with the cytoplasmic tail of PSGL-1 and L-selectin (Fig. 1 F).
Figure 1.
L-selectin and PSGL-1 form a complex in primary neutrophils. (A) Neutrophil lysate was centrifuged in a discontinuous sucrose gradient. Fractions collected from the top were analyzed by Western blotting (n = 3). (B and C) Lysates of WT BM-derived neutrophils (in the presence or absence of an anti–L-selectin antibody), which were plated on uncoated (unstimulated) or selectin–coated coverslips for 10 min, were immunoprecipitated (IP) with either a blocking (4RA10) or a nonblocking (4RB12) PSGL-1 antibody, followed by immunoblotting (IB) with an L-selectin antibody (n = 3). (D) Lipid fractions were immunoprecipitated with an anti–PSGL-1 antibody, followed by immunoblotting with an L-selectin antibody (n = 3). (E) Neutrophil lysates were immunoprecipitated with either an anti–L-selectin or an anti–PSGL-1 antibody, followed by immunoblotting with an antibody against PSGL-1, L-selectin, c-Src, Syk, or PLCγ2 (n = 3). (F) GST or GST fusion proteins containing the cytoplasmic tail of PSGL-1 or L-selectin were bound to glutathione–Sepharose beads and incubated with neutrophil cell extracts. The proteins, which were pulled down (PD), were analyzed by Western blot with antibodies against Fgr, Hck, Lyn, Syk, and PLCγ2 (n = 3).
The association between PSGL-1 and L-selectin increases after stimulation
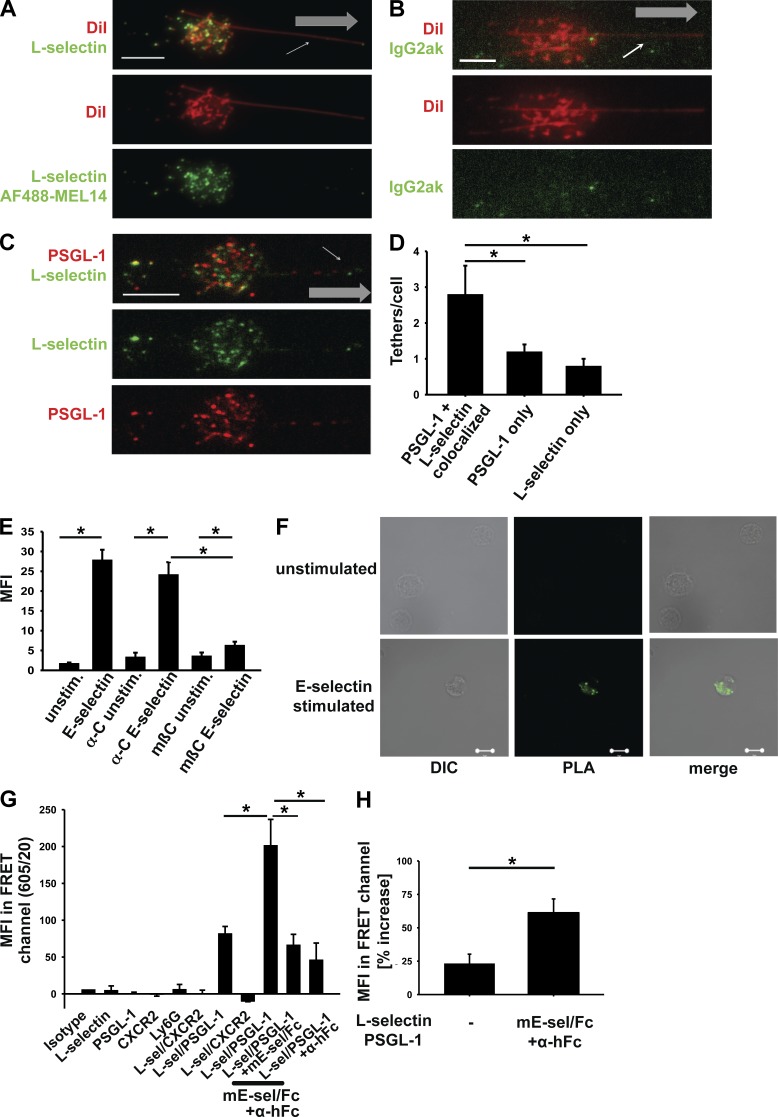
To assess colocalization of PSGL-1 with L-selectin at the microscopic level (∼200-nm resolution), we interrogated neutrophils rolling on P-selectin with quantitative dynamic footprinting (qDF), a method based on total internal reflection fluorescence (TIRF; Sundd et al., 2012). This method interrogates the parts of the rolling neutrophil in close contact with the adhesive substrate. We used Mel14 antibody to L-selectin and 4RB12 antibody to PSGL-1. Binding was specific (Fig. 2, A and B). As expected, PSGL-1 and L-selectin colocalized in many microvilli and tether anchor points. Surprisingly, some microvilli appeared to express L-selectin only or PSGL-1 only (Fig. 2, C and D). This suggests that, although PSGL-1 and L-selectin are localized in lipid rafts on the tips of microvilli, they are not necessarily localized in the same rafts and may be sorted into these different rafts by different cellular mechanisms.
Figure 2.
The association between PSGL-1 and L-selectin increases after stimulation. (A–C) Representative images of neutrophils rolling on a P-selectin–coated substrate using qDF microscopy. Cells were stained with an antibody for L-selectin (Mel-14, Alexa Fluor 488 coupled; A) or an IgG2aκ control antibody (Alexa Fluor 488 coupled; B) and DiI for visualizing the cell membrane (n = 3). The small arrows in A–C signify a ‘sling’ at the front of a rolling neutrophil. Gray arrows indicate the direction of flow. (C) Cells were stained with an Alexa Fluor 488–Mel14 antibody to L-selectin and an Alexa Fluor 568–4RB12 antibody to PSGL-1 showing the distribution of these two molecules on the cell surface during rolling in a flow chamber coated with P-selectin (n = 3). (D) Quantification of the number of tethers per cell positive for L-selectin or PSGL-1 or showing a colocalization of L-selectin and PSGL-1 (n = 3). (E and F) Representative images (F) and quantification (E) of the MFI of a PLA of unstimulated and E-selectin–stimulated BM-derived neutrophils from WT mice in the presence or absence of methyl-β-cyclodextrin or its inactive analogue α-cyclodextrin (n = 3). Using antibodies of different species (4RB12 for PSGL-1 and N-18 for L-selectin) and species-specific secondary antibodies coupled to a DNA strand, a reaction using rolling circle amplification and ligation is started when the two molecules are in close proximity. A fluorescent signal is produced when two tested molecules are within ∼40 nm of each other. DIC, differential interference contrast. (G and H) Quantification of the MFI of FRET experiments comparing unstimulated and E-selectin–stimulated neutrophils. FRET was tested by flow cytometry between L-selectin (labeled by anti–L-selectin antibody LAM1-101 antibody coupled to Alexa Fluor 488) and PSGL-1 (labeled by an anti–PSGL-1 antibody 4RB12 coupled to Alexa Fluor 488) and as a negative control, L-selectin and CXCR2 (n = 3). (D, E, G, and H) Data are presented as means ± SEM. *, P < 0.05. Bars, 5 µm.
Because PSGL-1 engagement by selectins is known to be required for the signaling event (Zarbock et al., 2007), we used a proximity ligation assay (PLA) to probe how closely PSGL-1 and L-selectin were associated. This assay produces a fluorescent signal when the two molecules are within ∼40 nm of each other, and the strength of the signal does not increase when the distance is shorter. We found that PSGL-1 and L-selectin were not within 40 nm of each other under resting conditions but moved to within this distance after ligation with E-selectin (Fig. 2, E and F). To investigate whether selectin engagement induces coalescence of lipid rafts, we repeated the PLA in the presence and absence of methyl-β-cyclodextrin or its inactive analogue α-cyclodextrin. Disrupting lipid rafts by methyl-β-cyclodextrin diminished the increased proximity between PSGL-1 and L-selectin after selectin engagement (Fig. 2 E). Fluorescence resonance energy transfer (FRET) quantitatively probes the distance between two molecules, and the signal is inversely proportional to the sixth power of the distance. We labeled PSGL-1 with an antibody coupled to Alexa Fluor 568 and L-selectin with an antibody coupled to Alexa Fluor 488 and performed FRET in mouse BM neutrophils by flow cytometry (Lefort et al., 2012). A FRET signal was detectable in nonstimulated cells, and its intensity significantly increased upon ligation with E-selectin (Fig. 2, G and H). L-selectin did not show FRET with CXCR2. At least some of the PSGL-1 molecules were within ∼5 nm of L-selectin molecules, and this number of molecules increased or their distance significantly decreased with E-selectin stimulation. Collectively, the qDF and FRET data indicate that some of the PSGL-1 and L-selectin constitutively exist in close proximity to each other and this proximity increases after selectin engagement. The biochemistry data together with the signals detected by the PLA and FRET assay demonstrate that some PSGL-1 constitutively interacts with L-selectin and that this interaction increases after selectin engagement.
L-selectin and PSGL-1 are required for LFA-1–dependent slow leukocyte rolling in vitro and in vivo mediated by selectins
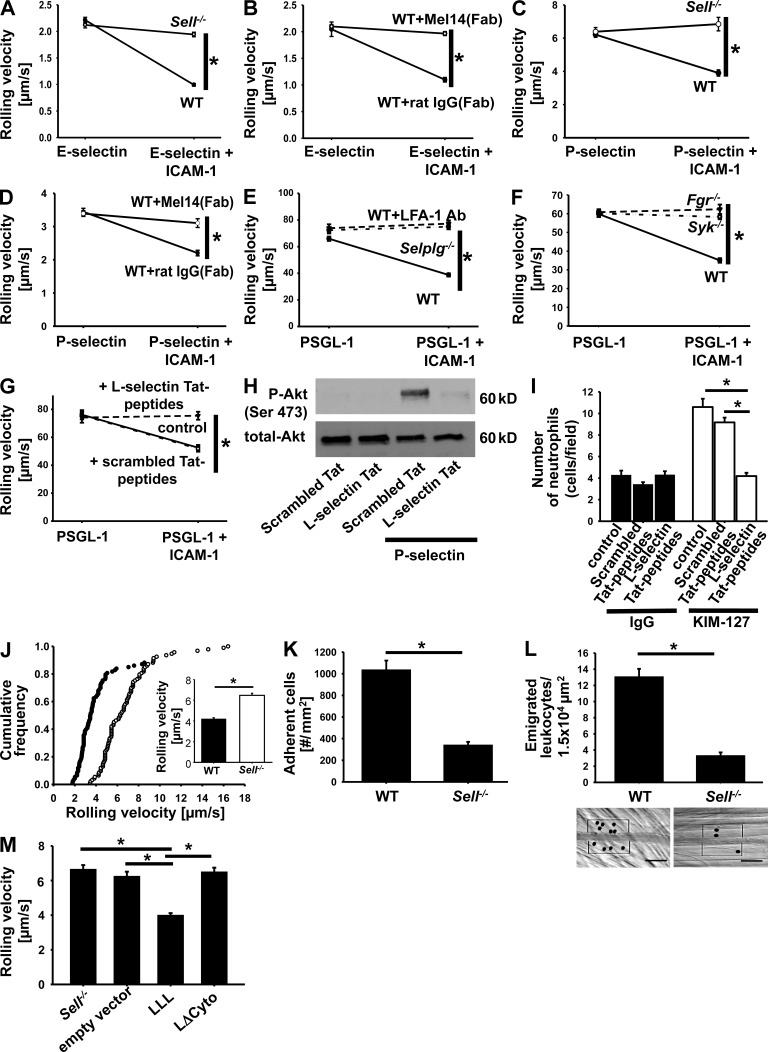
WT neutrophils rolled on E-selectin at ∼2 µm/s, and this rolling velocity was reduced to ∼1 µm/s when ICAM-1, a ligand for LFA-1, was added to the substrate (Fig. 3 A). This reduction of rolling velocity is LFA-1 dependent (Zarbock et al., 2007). The ICAM-1–dependent velocity reduction was completely abolished in Sell−/− neutrophils (Fig. 3 A). Similarly, the reduction of the rolling velocity was abolished by a blocking antibody to L-selectin (MEL-14) but not by isotype control (Fig. 3 B). The rolling velocities on P-selectin were higher (Fig. 3, C and D), and blocking or eliminating L-selectin completely abolished the velocity drop induced by adding ICAM-1 to the substrate.
Figure 3.
L-selectin and PSGL-1 are required for LFA-1–dependent slow leukocyte rolling in vitro and in vivo mediated by selectins. (A–D) Rolling velocities on E-selectin and E-selectin/ICAM-1 (A and B) or P-selectin and P-selectin + ICAM-1 (C and D) of neutrophils from WT (n = 3) and Sell−/− (n = 3) mice (A and C) or neutrophils from WT mice treated with Fab fragments of an IgG (n = 3) or a monoclonal L-selectin antibody (n = 3; B and D). (E) Rolling velocity of isolated WT neutrophils in the presence (n = 3) or absence of an anti–LFA-1 mAb (n = 3) and Selplg−/− neutrophils (n = 3) on PSGL-1 and PSGL-1 + ICAM-1. (F) Rolling velocity of neutrophils from Syk−/− (n = 3), Fgr−/− (n = 3), and WT (n = 3) mice on PSGL-1 and PSGL-1 + ICAM-1. (G) Rolling velocities were determined for untreated or TAT peptide–pretreated isolated human neutrophils (n = 3). (A–G) Data are presented as means ± SEM. (H) Whole blood–derived human neutrophils were plated on uncoated (unstimulated) or selectin-coated wells for 10 min, and then lysates were prepared. Representative Western blots of total lysates of neutrophils pretreated with scrambled or L-selectin–TAT peptides showing the phosphorylation of Akt. Total lysates were immunoblotted with antibodies to phosphorylated Akt (Serin473) or total Akt (n = 3). (I) The number of adherent cells on a reporter antibody–coated flow chamber was determined for untreated or TAT peptide–pretreated isolated human neutrophils (n = 3). Data are presented as means ± SEM. (J–L) Intravital microscopy of postcapillary venules in the murine cremaster muscle, 2 h after intrascrotal TNF injection. (J) Cumulative histogram of the rolling velocities of WT (closed circles) and Sell−/− (open circles) neutrophils after treatment with PTx and a monoclonal P-selectin antibody (n = 3). Inset data are means ± SEM. (K and L) Adherent cells (K) and the number of extravasated cells (L) in WT and Sell−/− mice were determined after PTx injection 2 h before the experiment (n = 3). Data are presented as means ± SEM. (L) Representative reflected light oblique transillumination pictures of postcapillary venules of PTx-pretreated WT and Sell−/− mice 2 h after TNF application. Transmigrated leukocytes are encircled. Bars, 50 µm. (M) Mixed chimeric mice were generated by injecting retrovirally transduced hematopoietic stem cells (L-selectin–WT construct, LLL; LΔcyto construct, LΔcyto; empty vector; and not transfected cells, Sell−/−) from Sell−/− mice into lethally irradiated WT mice. Mean rolling velocity ± SEM of untransduced and transduced leukocytes in inflamed cremaster muscle venules of mixed chimeric mice (n = 3) treated with PTx and a monoclonal blocking P-selectin antibody is shown. *, P < 0.05.
To test whether L-selectin engagement alone was sufficient to induce slow rolling, we coated flow chambers with recombinant PSGL-1, a ligand for L-selectin. Characteristic of L-selectin–mediated rolling, the rolling velocity was high (∼60 µm/s). In WT neutrophils, this rolling velocity was reduced by ∼50% when ICAM-1 was added to the PSGL-1 substrate, and this reduction was LFA-1 dependent because an antibody to LFA-1 completely blocked it (Fig. 3 E). Interestingly, neutrophils isolated from Selplg−/− mice lacking PSGL-1 failed to reduce their rolling velocity on PSGL-1 and ICAM-1, suggesting that both L-selectin and PSGL-1 were required for signaling and LFA-1 extension, even on an L-selectin ligand.
Fgr (Zarbock et al., 2008) and Syk (Zarbock et al., 2007) are known to be required for PSGL-1–induced slow rolling, but the involvement of L-selectin was not tested previously. To test whether L-selectin used similar signaling components, we compared neutrophil rolling on PSGL-1 alone or co-immobilized with ICAM-1. Both Fgr−/− and Syk−/− neutrophils were unable to reduce their rolling velocity (Fig. 3 F), confirming that the PSGL-1 and L-selectin signaling pathways converged on the same kinases. To address which part of L-selectin was required for signaling, we introduced the soluble L-selectin cytoplasmic tail into neutrophils as TAT peptides. L-selectin cytoplasmic tail, but not a scrambled peptide, completely blocked the reduction of rolling velocity induced by co-immobilizing ICAM-1 with PSGL-1 (Fig. 3 G). Furthermore, we demonstrated that the L-selectin cytoplasmic tail abolished selectin-induced signaling (Fig. 3 H). We conclude that the soluble L-selectin tail competes with endogenous L-selectin and thus prevents signaling. To more directly test the role of L-selectin cytoplasmic tail in LFA-1 extension, we used human neutrophils. KIM127 is a mAb that reports extension of human LFA-1 (Robinson et al., 1992). When KIM127 was co-immobilized with PSGL-1 in the flow chamber, untreated human neutrophils (control) or neutrophils treated with a scrambled L-selectin tail peptide immobilized at significantly higher levels than background (Fig. 3 I). L-selectin tail peptide completely abolished this, suggesting that overexpression of the L-selectin cytoplasmic tail effectively prevents L-selectin–induced extension of LFA-1.
To test whether L-selectin was also required for slow leukocyte rolling in vivo, we investigated venules of the mouse cremaster muscle. In this model, rolling is induced by injecting TNF and is dependent on P- and E-selectin. We injected mice with pertussis toxin (PTx) to block Gαi-dependent chemokine receptor signaling and mAb RB40.34 to block P-selectin so that rolling was only dependent on E-selectin, LFA-1, and ICAM-1 as described previously (Zarbock et al., 2007). The rolling velocity in WT mice was ∼4 µm/s but was increased to 6 µm/s in Sell−/− mice (Fig. 3 J). The number of adherent leukocytes was dramatically reduced by 70% in this model (Fig. 3 K), as was the number of leukocytes that had emigrated into the perivascular tissue (Fig. 3 L). This defect was not apparent when chemokine receptor signaling was left intact (not depicted), suggesting that the L-selectin–dependent activation pathway was redundant with chemokine-dependent activation. To assess the importance of the L-selectin cytoplasmic tail in vivo, we reconstituted L-selectin–deficient neutrophils with a WT (LLL) or mutated L-selectin construct (LΔcyto; this construct lacks the last 11 aa of the cytoplasmic tail of L-selectin) and performed intravital microscopy of the M. cremaster 2 h after intrascrotal TNF injection. Neutrophils reconstituted with LΔcyto showed an elevated rolling velocity in vivo compared with L-selectin–deficient neutrophils reconstituted with the LLL construct (Fig. 3 M), suggesting that the cytoplasmic tail of L-selectin is required for downstream signaling and slow leukocyte rolling. A smaller, but still significant effect on P-selectin–dependent rolling velocity was also seen in mice in which E-selectin was blocked (not depicted). Collectively, these functional data show that L-selectin is required for PSGL-1–dependent integrin activation, slow rolling, and leukocyte recruitment in vitro and in vivo.
SFK activity and downstream signaling is dependent on L-selectin
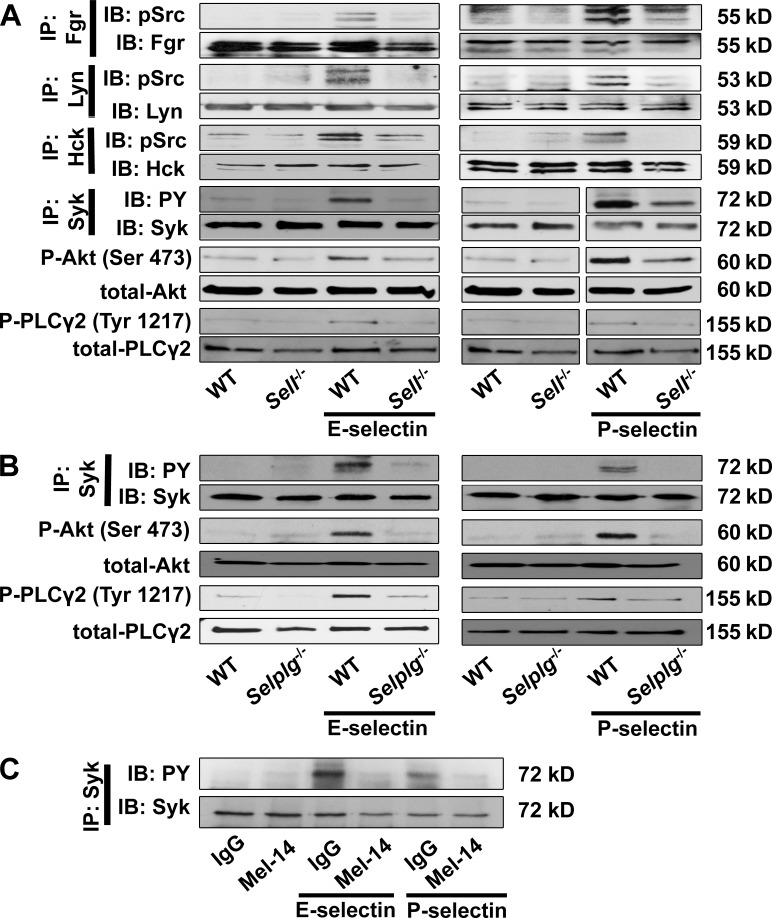
To test the signaling events downstream of PSGL-1 engagement in the presence or absence of L-selectin, we used neutrophil adhesion under shear stress. Lysed adherent cells were immunoprecipitated for the SFKs expressed in neutrophils and then probed with an antibody to Y416 pSrc. As expected, Fgr, Lyn, and Hck were all phosphorylated by incubation on E- or P-selectin under shear (Fig. 4 A) but not without shear (not depicted). Fgr, Hck, and Lyn phosphorylation was completely abrogated in Sell−/− neutrophils (Fig. 4 A). When Syk was immunoprecipitated and blotted for PY, we found the expected phosphorylation, which was completely abrogated in Sell−/− neutrophils on E- and P-selectin (Fig. 4 A).
Figure 4.
SFK activity and downstream signaling is dependent on L-selectin. (A and B) BM-derived neutrophils were plated on uncoated (unstimulated) or E-selectin– or P-selectin–coated coverslips for 10 min, and then lysates were prepared. Representative Western blots of either immunoprecipitations or total lysates of neutrophils of WT and Sell−/− mice showing the phosphorylation of Fgr, Lyn, and Hck and the downstream molecules Syk, PLCγ2, and Akt. (A) Lysates were immunoprecipitated (IP) with an Fgr, Hck, Lyn, or Syk (n = 3 each) antibody followed by immunoblotting (IB) with a pSrc (Tyr416), a general phosphotyrosine (4G10) antibody, or total Fgr, Hck, Lyn, or Syk antibodies. Total lysates were immunoblotted with antibodies to phosphorylated PLCγ2 (Tyr1217), total PLCγ2, phosphorylated Akt, and total Akt (n = 3 each). (B) Lysates of neutrophils of WT and Selplg−/− mice were immunoprecipitated or immunoblotted, demonstrating the phosphorylation of Syk, PLCγ2, and Akt. Lysates were immunoprecipitated with a Syk (n = 3) antibody, followed by immunoblotting with a general phosphotyrosine (4G10) antibody or a total Syk-antibody. Total lysates were immunoblotted with antibodies to phosphorylated PLCγ2 (Tyr1217), total PLCγ2, phosphorylated Akt, or total Akt (n = 3 each). (C) Lysates of WT neutrophils pretreated with Fab fragments of an anti–L-selectin antibody or an isotype control were immunoprecipitated, demonstrating the phosphorylation of Syk. Lysates were immunoprecipitated with a Syk (n = 3) antibody followed by immunoblotting with a general phosphotyrosine (4G10) antibody or a total Syk-antibody (n = 3).
Akt, Syk, and PLCγ2 are phosphorylated in WT, but not Sell−/−, neutrophils after stimulation with E- or P-selectin (Fig. 4 A). Collectively, we conclude that L-selectin is required for the known signaling events induced by PSGL-1 engagement under shear stress. The dependence on L-selectin is as absolute as the dependence on PSGL-1 because phosphorylation of Syk, Akt, and PLCγ2 was also completely abolished after stimulating Selplg−/− neutrophils with E- or P-selectin (Fig. 4 B). Blocking the lectin domain of L-selectin by MEL-14 abolished Syk phosphorylation (Fig. 4 C), suggesting that the lectin domain is required for downstream signaling after selectin engagement.
Because we find that the cytoplasmic tail of L-selectin can effectively block signaling through PSGL-1 or L-selectin, it is likely that the cytosolic domain of L-selectin is involved in assembling the PSGL-1–L-selectin complex. L-selectin ligation has previously been reported to trigger signaling events (Waddell et al., 1995). However, these data are difficult to interpret because intact antibodies were used for L-selectin cross-linking, which may have activated Fc receptors, and because none of the assays were performed under physiological shear stress. Based on our current data, we think that PSGL-1 is required for L-selectin signaling, and vice versa, L-selectin is required for PSGL-1 signaling in neutrophils. Therefore, we propose a complex consisting of at least L-selectin and PSGL-1.
Our findings reported here most likely explain the severe neutrophil recruitment defect seen in mice treated with an antibody to L-selectin (Lewinsohn et al., 1987) or an L-selectin–Ig fusion protein (Watson et al., 1991). Our new findings are also consistent with the neutrophil recruitment defect seen in Sell−/− mice (Arbonés et al., 1994). These previous studies had triggered a search for an endothelial ligand for L-selectin in nonlymphoid venules, but this putative ligand has remained elusive (Axelsson et al., 2012). The proposed role of L-selectin as a required transmembrane signaling molecule in the PSGL-1–L-selectin complex provides an attractive alternative explanation for these findings. It remains to be seen whether other immune cells coexpressing L-selectin and functional PSGL-1 can assemble PSGL-1–L-selectin–like receptor complexes. We think the PSGL-1–L-selectin complex–mediated LFA-1 activation is, in part, redundant with chemokine-mediated LFA-1 activation. It is possible that PSGL-1–L-selectin complex–dependent events dominate neutrophil recruitment in some tissues and organs, and chemokine-dependent events may dominate in others.
MATERIALS AND METHODS
Animals.
8–12-wk-old C57BL/6 (JANVIER), Sell−/− (Arbonés et al., 1994), Selplg−/− (Xia et al., 2002), Fgr−/−(Zarbock et al., 2008), and Syk−/− (Mueller et al., 2010) mice were housed in a special pathogen-free facility. The Animal Care and Use Committees of the La Jolla Institute for Allergy and Immunology and the University of Münster approved all animal experiments.
BM chimeras were generated by i.v. injection of unfractionated fetal liver cells of Syk−/− mice into lethally irradiated WT mice (9.5 Gy). Experiments were performed 6–8 wk after transplantation (Zarbock et al., 2007).
L-selectin TAT fusion mutants.
In brief, isolated human blood neutrophils were incubated with TAT fusion mutants (GenScript, 2 µM, 37°C, 30 min) and subsequently used in flow chamber experiments. Cells were incubated either with an L-selectin TAT peptide, containing the intracellular tail of L-selectin plus a TAT sequence (YGRKKRRQRRRGRRLKKGKKSKRSMNDPY) or a scrambled control peptide (YGRKKRRQRRRGPRMGRKRKKLSYNKKSD). Both peptides were FITC conjugated (N-terminal conjugation).
Intravital microscopy.
Mice were anesthetized using injection of 125 mg/kg ketamine hydrochloride (Sanofi Winthrop Pharmaceuticals) and 12.5 mg/kg xylazine (Tranqui Ved, Phonix Scientific) i.p., and the cremaster muscle was prepared for intravital imaging as previously described (Zarbock et al., 2007, 2008; Mueller et al., 2010; Block et al., 2012). Postcapillary venules with a diameter between 20 and 40 µm were investigated. To determine selectin-mediated slow rolling, adhesion, and transmigration in vivo, mice were injected intrascrotally with 500 ng TNF (R&D Systems) and optional with 4 µg PTx i.v. (Sigma-Aldrich) 2 h before the preparation of the cremaster muscle. In experiments investigating E-selectin– or P-selectin–mediated slow rolling, mice received a blocking anti–E-selectin (9A9) or anti–P-selectin antibody (RB40.34; 30 µg/mouse i.v.). Intravital microscopy was performed on an upright microscope (Axioskop; Carl Zeiss) with a 40× 0.75 NA saline immersion objective. Leukocyte rolling velocity and leukocyte adhesion were determined by transillumination intravital microscopy, whereas leukocyte extravasation was investigated by reflected light oblique transillumination microscopy as previously described (Mueller et al., 2010; Block et al., 2012). Recorded images were analyzed off-line using ImageJ (National Institutes of Health) and AxioVision (Carl Zeiss) software. Emigrated cells were determined in an area 75 × 100 µm to each side of a vessel (representing 1.5 × 104 µm2 tissue area). The microcirculation was recorded using a digital camera (Sensicam QE; Cooke). Blood flow centerline velocity was measured using a dual photodiode sensor system (Circusoft Instrumentation). Centerline velocities were converted to mean blood flow velocities as previously described (Zarbock et al., 2007, 2008; Mueller et al., 2010; Block et al., 2012).
Hematopoietic stem cell isolation and retroviral transduction.
Transduction of hematopoietic stem cells was performed as previously described (Block et al., 2012). The retroviral constructs pMIG–L-selectin (LLL) and pMIG-LΔcyto (provided by G.S. Kansas, Northwestern University, Chicago, IL) were used (Dwir et al., 2001). The transduced cells were injected i.v. into lethally irradiated WT mice (9.5 Gy). Intravital microscopy experiments were performed 6–8 wk after BM transplantation, and transduced neutrophils in the microcirculation of the transplanted mice were identified as GFP+ cells.
Flow chamber systems.
To investigate the rolling velocity of murine neutrophils on E- or P-selectin, we used a previously described flow chamber system (Zarbock et al., 2007, 2008; Mueller et al., 2010; Block et al., 2012). Rectangular glass capillaries (20 × 200 µm) were filled either with 2.5 µg/ml E-selectin (R&D Systems) or 20 µg/ml P-selectin (R&D Systems) alone or in combination with ICAM-1 (2 µg/ml in combination with E-selectin, 5 µg/ml in combination with P-selectin; R&D Systems) for 2 h and then blocked for 2 h using 1% casein (Thermo Fisher Scientific). One side of the chamber was connected to a PE 10 tubing (BD) and inserted into a mouse carotid artery. The other side of the chamber was connected to a PE 50 tubing (BD) and used to control the wall shear stress in the capillary. One representative field of view was recorded for 1 min using an SW40/0.75 objective and a digital camera (Sensicam QE).
In some experiments, blood was collected by cardiac puncture and incubated with Fab fragments (10 µg/ml) of a blocking anti–L-selectin antibody (Mel-14; rat IgG2aκ) or Fab fragments of a rat IgG antibody. In some experiments, WT mice received 30 µg Fab fragments of a blocking anti–L-selectin antibody (Mel-14; rat IgG2aκ) or Fab fragments of a rat IgG antibody before the experiment.
To investigate the rolling velocity of human blood neutrophils or murine BM-derived neutrophils on PSGL-1, we used a parallel plate flow chamber system (GlycoTech) coated either with 5 µg/ml PSGL-1 alone (disulfide-linked homodimer; R&D Systems) or in combination with 5 µg/ml ICAM-1 (R&D Systems). Murine and human neutrophils were isolated using the EasySep negative cell isolation systems (EasySep mouse/human neutrophil enrichment kit; STEMCELL Technologies) according to the manufacturer’s instructions.
To investigate the integrin conformation on human blood neutrophils, the parallel plate flow chamber system was coated with 5 µg/ml PSGL-1 in combination with 25 µg/ml of the β2-integrin reporter antibody (KIM-127) or 25 µg/ml of a mouse IgG antibody. The number of adherent cells per field of view was determined.
Isolation of lipid raft proteins.
Proteins located in lipid rafts were isolated exactly as described previously (Scheel-Toellner et al., 2004). In brief, 5 × 107 neutrophils were lysed on ice in Triton-containing buffer (10 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1 mM NaVO4, 10 µg/ml of pepstatin A, aprotinin, and leupeptin, 4 mM AEBSF, and 1% Triton X-100). Cell debris was removed, and the lysate was adjusted to 42% sucrose, transferred to a 15-ml polypropylene centrifuge tube, overlaid with lysis buffer containing 30% and 5% sucrose, respectively, and spun at 200,000 g for 16–18 h. Fractions of 1.25 ml were eluted from the centrifuge tube, and the proteins were collected by TCA precipitation before analysis by Western blotting for the presence of L-selectin and PSGL-1. Raft fractions were identified using Flotillin-1 as a positive marker of lipid rafts.
qDF microscopy.
qDF microscopy was performed as described previously (Sundd et al., 2012). In brief, a 1.5-mm-thick polydimethylsiloxane (PDMS) chip with microchannels was sealed against a coverslip using a magnetic clamp. The microchannels were connected to an inlet. The exposed surface of the glass was coated by incubating with 2 µg/ml of murine P-selectin–Fc (R&D Systems) at room temperature for 30 min. After the incubation, the surface of the coverslip was blocked by incubating with 1% casein in PBS for 30 min at room temperature.
Murine neutrophils were isolated from BM of WT mice by negative selection (EasySep mouse neutrophil enrichment kit; STEMCELL Technologies) and stained with Alexa Fluor 488–conjugated anti–L-selectin mAb (Mel14) and Alexa Fluor 568–conjugated anti–PSGL-1 mAb (4RB12) or an Alexa Fluor 488–conjugated IgG control antibody (rat IgG2aκ). Microscopy was performed using an inverted TIRF microscope (IX71; Olympus) to study the footprints of rolling neutrophils. The used wall shear stress was 10 dyn/cm2. For data analysis, SlideBook Software was used as previously described (Sundd et al., 2012).
FRET.
BM from C57BL6/J mice was isolated in Hanks’ balanced salt solution containing CaCl2, MgCl2, and 0.5% rat serum. BM cells were labeled with Alexa Fluor 647–conjugated anti-Ly6G mAb, DyLight488-conjugated anti–L-selectin mAb (clone LAM1-101, nonblocking; provided by T.F. Tedder, Duke University, Durham, NC), Alexa Fluor 568–conjugated anti–PSGL-1 mAb (clone 4RB12), and/or Alexa Fluor 568–conjugated anti-CXCR2 mAb (R&D Systems). Flow cytometry was performed using an LSR-II (BD) and the following laser-filter combinations: 488ex/525em for DyLight488, 561ex/610em for Alexa Fluor 568, 640ex/670em for Alexa Fluor 647, and 488ex/605em for the FRET channel (DyLight488 > Alexa Fluor 568). Mean fluorescence intensity (MFI) of Ly6G+ neutrophils was acquired for all channels. The FRET channel was corrected for bleed-through using single-label controls.
PLA.
The PLA was performed according to the manufacturer’s protocol (OLINK Bioscience). In brief, unstimulated or E-selectin–stimulated (10 min, rotating conditions on E-selectin–coated glass coverslips) WT neutrophils (untreated or α-cyclodextrin or methyl-β-cyclodextrin treated) were fixed with EtOH (80%, 4°C, 10 min). After three washing steps using TBS, slides were blocked with the manufacturers blocking solution for 30 min. An anti–L-selectin mAb (N-18) and anti–PSGL-1 mAb (4RB12) were used as primary antibodies (1 h, 4°C). After a washing step, species-specific secondary antibodies were added as PLA probes and incubated for 1 h. For detection of the fluorescent signal, ligation, amplification, and washing steps were performed according to the manufacturer’s instructions.
E- and P-selectin engagement with PSGL-1.
For biochemical assays, BM-derived WT, Sell−/−, and Selplg−/− neutrophils or human whole blood–derived neutrophils were isolated, suspended in PBS (containing 1 mM each CaCl2 and MgCl2), and left untreated or were pretreated with 1 mM EGTA. Subsequently the cells were incubated under rotating conditions (65 rpm) for 10 min on 3 µg/ml E-selectin– or 5 µg/ml P-selectin–coated coverslips in multiwell plates (Zarbock et al., 2008; Mueller et al., 2010; Block et al., 2012). Cells were lysed in RIPA buffer, and lysates were boiled with sample buffer (10 min, 95°C) or incubated with Sepharose A/G beads (Santa Cruz Biotechnology, Inc.) and anti-Fgr, anti-Hck, anti-Lyn, anti-Syk, anti–L-selectin (Santa Cruz Biotechnology, Inc.), or anti–PSGL-1 (4RB12 or 4RA10) antibody for 4 h at 4°C. Beads were washed four times, and bound proteins were eluted by adding boiling sample buffer. Cell lysates and immunoprecipitates were run on 10% SDS-PAGE and immunoblotted using antibodies against phosphotyrosine (4G10; EMD Millipore), Akt, phospho-Akt (Ser473), PLCγ2, phospho-PLCγ2 (Tyr1217), and phospho-Src (Tyr416; all from Cell Signaling Technology) and L-selectin, Fgr, Hck, Lyn, and Syk (Santa Cruz Biotechnology, Inc.). Immunoblots were developed using an ECL system (GE Healthcare). Densitometric quantification was performed using ImageJ software.
Pull-down of proteins from cell extracts with GST fusion proteins.
The GST–PSGL-1 (provided by F. Sanchez-Madrid, Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain) and GST–L-selectin constructs containing the full-length cytoplasmic region of PSGL-1 or L-selectin were generated by PCR (Urzainqui et al., 2002). The pull-down of proteins from HL-60 cells extracts was performed as previously described (Urzainqui et al., 2002).
Statistics.
Statistical analysis was performed with SPSS Statistics (version 21.0; IBM). Differences between the groups were evaluated by one-way ANOVA, Student-Newman-Keuls test, or Student’s t test where appropriate. Data are presented as mean ± SEM, and P < 0.05 was considered statistically significant.
Acknowledgments
We thank Tomas F. Tedder for providing the anti–L-selectin antibody (LAM1-101), Francisco Sanchez-Madrid for providing the GST–PSGL-1 construct, Geoffrey S. Kansas for providing the L-selectin constructs, Nicole Hillebrand for designing figures, and Alan “Rick” Horwitz (University of Virginia, Charlottesville, VA) for critical reading of the manuscript.
The work is supported by the German Research Foundation (ZA428/5-1 and ZA428/8-1 to A. Zarbock).
The authors declare no competing financial interests.
Author contributions: A. Stadtmann designed and performed most of the experiments, analyzed the results, and prepared the manuscript; G. Germena performed biochemistry experiments; M. Boras and K. Buscher generated constructs; H. Block performed some flow chamber experiments and the PLA; P. Sundd and C.I. Fisher performed the TIRF experiments; C. Lefort and J. Rossaint performed the FRET experiments; A. Urzainqui provided valuable reagents; B. Gelschefarth performed biochemistry assays; and V. Gerke, K. Ley, and A. Zarbock provided overall supervision, helped design all of the experiments, and prepared the manuscript.
Footnotes
Abbreviations used:
- FRET
- fluorescence resonance energy transfer
- MFI
- mean fluorescence intensity
- PLA
- proximity ligation assay
- PTx
- pertussis toxin
- qDF
- quantitative dynamic footprinting
- SFK
- Src family kinase
- Syk
- spleen tyrosine kinase
- TIRF
- total internal reflection fluorescence
References
- Arbonés M.L., Ord D.C., Ley K., Ratech H., Maynard-Curry C., Otten G., Capon D.J., Tedder T.F. 1994. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1:247–260 10.1016/1074-7613(94)90076-0 [DOI] [PubMed] [Google Scholar]
- Axelsson J., Xu D., Kang B.N., Nussbacher J.K., Handel T.M., Ley K., Sriramarao P., Esko J.D. 2012. Inactivation of heparan sulfate 2-O-sulfotransferase accentuates neutrophil infiltration during acute inflammation in mice. Blood. 120:1742–1751 10.1182/blood-2012-03-417139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block H., Herter J.M., Rossaint J., Stadtmann A., Kliche S., Lowell C.A., Zarbock A. 2012. Crucial role of SLP-76 and ADAP for neutrophil recruitment in mouse kidney ischemia-reperfusion injury. J. Exp. Med. 209:407–421 10.1084/jem.20111493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl R.E., Springer T.A., Bainton D.F. 1996. Quantitation of L-selectin distribution on human leukocyte microvilli by immunogold labeling and electron microscopy. J. Histochem. Cytochem. 44:835–844 10.1177/44.8.8756756 [DOI] [PubMed] [Google Scholar]
- Dwir O., Kansas G.S., Alon R. 2001. Cytoplasmic anchorage of L-selectin controls leukocyte capture and rolling by increasing the mechanical stability of the selectin tether. J. Cell Biol. 155:145–156 10.1083/jcb.200103042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblin P.A., Hwang S.T., Katsumoto T.R., Rosen S.D. 1997. Ligation of L-selectin on T lymphocytes activates beta1 integrins and promotes adhesion to fibronectin. J. Immunol. 159:3498–3507 [PubMed] [Google Scholar]
- Hafezi-Moghadam A., Thomas K.L., Prorock A.J., Huo Y., Ley K. 2001. L-selectin shedding regulates leukocyte recruitment. J. Exp. Med. 193:863–872 10.1084/jem.193.7.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herter J.M., Rossaint J., Block H., Welch H., Zarbock A. 2013. Integrin activation by P-Rex1 is required for selectin-mediated slow leukocyte rolling and intravascular crawling. Blood. 121:2301–2310 10.1182/blood-2012-09-457085 [DOI] [PubMed] [Google Scholar]
- Kansas G.S. 1992. Structure and function of L-selectin. APMIS. 100:287–293 10.1111/j.1699-0463.1992.tb00874.x [DOI] [PubMed] [Google Scholar]
- Kappelmayer J., Kiss A., Karászi E., Veszprémi A., Jakó J., Kiss C. 2001. Identification of P-selectin glycoprotein ligand-1 as a useful marker in acute myeloid leukaemias. Br. J. Haematol. 115:903–909 10.1046/j.1365-2141.2001.03179.x [DOI] [PubMed] [Google Scholar]
- Kuwano Y., Spelten O., Zhang H., Ley K., Zarbock A. 2010. Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils. Blood. 116:617–624 10.1182/blood-2010-01-266122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort C.T., Rossaint J., Moser M., Petrich B.G., Zarbock A., Monkley S.J., Critchley D.R., Ginsberg M.H., Fässler R., Ley K. 2012. Distinct roles for talin-1 and kindlin-3 in LFA-1 extension and affinity regulation. Blood. 119:4275–4282 10.1182/blood-2011-08-373118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn D.M., Bargatze R.F., Butcher E.C. 1987. Leukocyte-endothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes. J. Immunol. 138:4313–4321 [PubMed] [Google Scholar]
- Ley K., Bullard D.C., Arbonés M.L., Bosse R., Vestweber D., Tedder T.F., Beaudet A.L. 1995. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 181:669–675 10.1084/jem.181.2.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J.J., Xia L., Yago T., Kappelmayer J., Liu Z., Klopocki A.G., Shao B., McDaniel J.M., Setiadi H., Schmidtke D.W., McEver R.P. 2008. Separable requirements for cytoplasmic domain of PSGL-1 in leukocyte rolling and signaling under flow. Blood. 112:2035–2045 10.1182/blood-2008-04-149468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller H., Stadtmann A., Van Aken H., Hirsch E., Wang D., Ley K., Zarbock A. 2010. Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) gamma2 and PI3Kgamma pathways. Blood. 115:3118–3127 10.1182/blood-2009-11-254185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.K., Andrew D., Rosen H., Brown D., Ortlepp S., Stephens P., Butcher E.C. 1992. Antibody against the Leu-CAM beta-chain (CD18) promotes both LFA-1- and CR3-dependent adhesion events. J. Immunol. 148:1080–1085 [PubMed] [Google Scholar]
- Scheel-Toellner D., Wang K., Craddock R., Webb P.R., McGettrick H.M., Assi L.K., Parkes N., Clough L.E., Gulbins E., Salmon M., Lord J.M. 2004. Reactive oxygen species limit neutrophil life span by activating death receptor signaling. Blood. 104:2557–2564 10.1182/blood-2004-01-0191 [DOI] [PubMed] [Google Scholar]
- Sikorski M.A., Staunton D.E., Mier J.W. 1996. L-selectin crosslinking induces integrin-dependent adhesion: evidence for a signaling pathway involving PTK but not PKC. Cell Adhes. Commun. 4:355–367 10.3109/15419069609010778 [DOI] [PubMed] [Google Scholar]
- Stadtmann A., Brinkhaus L., Mueller H., Rossaint J., Bolomini-Vittori M., Bergmeier W., Van Aken H., Wagner D.D., Laudanna C., Ley K., Zarbock A. 2011. Rap1a activation by CalDAG-GEFI and p38 MAPK is involved in E-selectin-dependent slow leukocyte rolling. Eur. J. Immunol. 41:2074–2085 10.1002/eji.201041196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundd P., Gutierrez E., Koltsova E.K., Kuwano Y., Fukuda S., Pospieszalska M.K., Groisman A., Ley K. 2012. ‘Slings’ enable neutrophil rolling at high shear. Nature. 488:399–403 10.1038/nature11248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzainqui A., Serrador J.M., Viedma F., Yáñez-Mó M., Rodríguez A., Corbí A.L., Alonso-Lebrero J.L., Luque A., Deckert M., Vázquez J., Sánchez-Madrid F. 2002. ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity. 17:401–412 10.1016/S1074-7613(02)00420-X [DOI] [PubMed] [Google Scholar]
- Venturi G.M., Tu L., Kadono T., Khan A.I., Fujimoto Y., Oshel P., Bock C.B., Miller A.S., Albrecht R.M., Kubes P., et al. 2003. Leukocyte migration is regulated by L-selectin endoproteolytic release. Immunity. 19:713–724 10.1016/S1074-7613(03)00295-4 [DOI] [PubMed] [Google Scholar]
- Waddell T.K., Fialkow L., Chan C.K., Kishimoto T.K., Downey G.P. 1995. Signaling functions of L-selectin. Enhancement of tyrosine phosphorylation and activation of MAP kinase. J. Biol. Chem. 270:15403–15411 10.1074/jbc.270.25.15403 [DOI] [PubMed] [Google Scholar]
- Watson S.R., Fennie C., Lasky L.A. 1991. Neutrophil influx into an inflammatory site inhibited by a soluble homing receptor-IgG chimaera. Nature. 349:164–167 10.1038/349164a0 [DOI] [PubMed] [Google Scholar]
- Xia L., Sperandio M., Yago T., McDaniel J.M., Cummings R.D., Pearson-White S., Ley K., McEver R.P. 2002. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J. Clin. Invest. 109:939–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yago T., Shao B., Miner J.J., Yao L., Klopocki A.G., Maeda K., Coggeshall K.M., McEver R.P. 2010. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling. Blood. 116:485–494 10.1182/blood-2009-12-259556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A., Lowell C.A., Ley K. 2007. Spleen tyrosine kinase Syk is necessary for E-selectin-induced alpha(L)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 26:773–783 10.1016/j.immuni.2007.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A., Abram C.L., Hundt M., Altman A., Lowell C.A., Ley K. 2008. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcRγ to induce slow leukocyte rolling. J. Exp. Med. 205:2339–2347 10.1084/jem.20072660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A., Ley K., McEver R.P., Hidalgo A. 2011. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 118:6743–6751 10.1182/blood-2011-07-343566 [DOI] [PMC free article] [PubMed] [Google Scholar]