Calcitonin gene–related peptide (CGRP) restricts HIV-1 transfer from Langerhans cells to T cells by decreasing integrin expression and increasing langerin to limit cellular conjugation.
Abstract
Upon its mucosal entry, human immunodeficiency virus type 1 (HIV-1) is internalized by Langerhans cells (LCs) in stratified epithelia and transferred locally to T cells. In such epithelia, LCs are in direct contact with peripheral neurons secreting calcitonin gene–related peptide (CGRP). Although CGRP has immunomodulatory effects on LC functions, its potential influence on the interactions between LCs and HIV-1 is unknown. We show that CGRP acts via its receptor expressed by LCs and interferes with multiple steps of LC-mediated HIV-1 transmission. CGRP increases langerin expression, decreases selected integrins, and activates NF-κB, resulting in decreased HIV-1 intracellular content, limited formation of LC–T cell conjugates, and elevated secretion of the CCR5-binding chemokine CCL3/MIP-1α. These mechanisms cooperate to efficiently inhibit HIV-1 transfer from LCs to T cells and T cell infection. In vivo, HIV-1 infection decreases CGRP plasma levels in both vaginally SHIV-challenged macaques and HIV-1–infected individuals. CGRP plasma levels return to baseline after highly active antiretroviral therapy. Our results reveal a novel path by which a peripheral neuropeptide acts at the molecular and cellular levels to limit mucosal HIV-1 transmission and suggest that CGRP receptor agonists might be used therapeutically against HIV-1.
HIV-1 gains access into the body mainly during sexual intercourse, by crossing epithelial barriers that cover mucosal surfaces of both the male and female genital tracts. In stratified epithelia, such as those of the foreskin and vagina, Langerhans cells (LCs) are among the first cells that capture HIV-1 as a result of their close proximity to the mucosal surface and their ability to bind the HIV-1 envelope glycoprotein subunit gp120 via their specific C-type lectin langerin. Although at low viral concentrations HIV-1 binding to langerin leads to viral internalization and degradation, at higher viral concentrations, the protective effect of langerin is inhibited (de Witte et al., 2007), permitting transfer of internalized intact virions to T cells across LC–T cell conjugates (Ganor et al., 2010; Zhou et al., 2011). Such viral transfer induces extensive replication of the virus in T cells. The natural endogenous host factors that control this process are unknown.
Genital epithelia are innervated by peripheral neurons secreting different neuropeptides. Among these is the 37-aa neuropeptide calcitonin gene–related peptide (CGRP; also termed αCGRP), which is produced by alternative splicing of the calcitonin gene (Rosenfeld et al., 1983) and induces potent vasodilatation (Brain et al., 1985). The CGRP receptor is an assembly of the seven-transmembrane domain G-protein–coupled receptor calcitonin receptor–like receptor (CRLR), an associated single transmembrane domain protein termed receptor activity modifying protein 1 (RAMP1), and an additional intracellular protein termed receptor component protein (RCP) required for functionality (Walker et al., 2010). CGRP might also activate receptors for the related peptides adrenomedullin (i.e., coexpression of CRLR with RAMP2-3) and amylin (i.e., coexpression of the calcitonin receptor with RAMP1-3), which mediate the previously described CGRP type 2 receptor phenotype (Poyner et al., 2002).
CGRP appears as a possible modulator of LC function. CGRP neurons are in direct contact with LCs in the skin, and early observations showed that CGRP inhibits LC antigen presentation to T cells (Hosoi et al., 1993). A later study demonstrated that although CGRP inhibits LC-mediated Th1 antigen presentation and cytokine secretion, it enhanced that of Th2 (Ding et al., 2008). Herein, we hypothesized that CGRP could also interfere with the interactions between LCs and HIV-1. As peripheral neurons are lost upon tissue sampling, such potential interactions were never studied at the mucosal level. Our results show that CGRP affects multiple molecular and cellular events in LCs, resulting in efficient inhibition of HIV-1 transfer from LCs to T cells and T cell infection.
RESULTS AND DISCUSSION
HIV-1 transfer from LCs to T cells
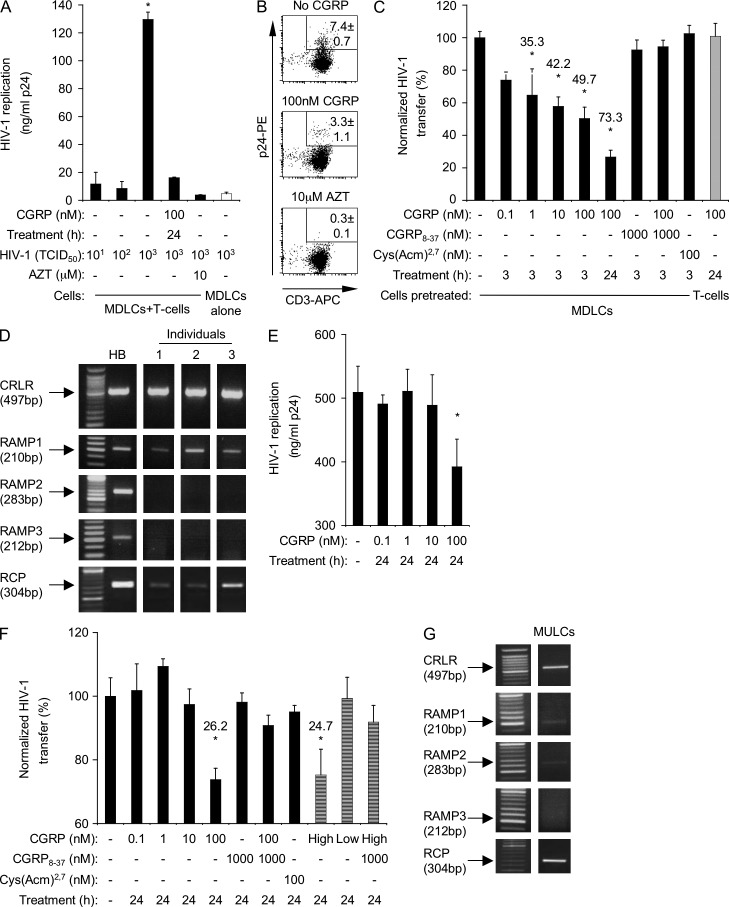
To measure the transfer of HIV-1 from LCs to T cells, we prepared blood monocyte-derived LCs (MDLCs) and pulsed the cells with the HIV-1 molecular clone JRCSF (clade B, R5 tropism). MDLCs were then co-cultured with autologous CD4+ T cells, and HIV-1 replication was measured in the co-culture supernatants 1 wk later by p24 ELISA. In line with previous observations (de Witte et al., 2007), MDLCs inefficiently transferred HIV-1 to T cells at low viral concentrations (Fig. 1 A), corresponding to 101 and 102 tissue culture infectious doses (TCID50). In contrast, at a high HIV-1 concentration of 103 TCID50, MDLCs efficiently transferred the virus to T cells, a process which was significantly abrogated by the antiretroviral drug azidothymidine (AZT; Fig. 1 A). MDLCs pulsed with 103 TCID50 HIV-1 and cultured alone without T cells inefficiently replicated the virus (Fig. 1 A). To confirm these results using a direct read-out for viral replication, the cells were collected at the end of the co-culture period, double-stained for surface CD3 and intracellular p24, and examined by flow cytometry. A clear population of CD3+p24+ infected T cells was detected, which was completely absent when AZT was included during the co-culture period (Fig. 1 B; mean ± SEM percentages of CD3+p24+ cells derived from n = 5 experiments of 7.4 ± 0.7% and 0.3 ± 0.1%, respectively; P < 0.0001). In contrast, when the cells were double-stained for surface CD1a and intracellular p24, a significantly lower proportion of CD1a+ cells was p24+ (1.3 ± 0.2%, n = 5; P < 0.0001 vs. CD3+p24+ cells), confirming the inefficient replication of HIV-1 in MDLCs. These results show that our experimental settings are appropriate for the investigation of HIV-1 transfer from LCs to T cells.
Figure 1.
CGRP inhibits HIV-1 transfer from LCs to T cells. (A and B) MDLCs were left untreated or pretreated for 24 h with 100 nM CGRP. The cells were then pulsed with 101–103 (A) or 103 (B) TCID50 HIV-1 and co-cultured with autologous CD4+ T cells for 1 wk. Untreated MDLCs were also co-cultured with T cells in the presence of AZT or cultured alone (A, open bar). In A, HIV-1 replication was measured in the culture supernatants by p24 ELISA, and results represent mean ± SEM ng/ml p24 from duplicate wells of a representative experiment of n = 9; *, P < 0.0001; ANOVA. In B, the cells were collected at the end of the co-culture period, stained with CD3-APC (surface) and p24-PE (intracellular) mAbs, and examined by flow cytometry; shown are representative FACS plots, with numbers indicating the mean ± SEM percentages of CD3+p24+ infected T cells of n = 5 experiments. (C) MDLCs were left untreated or pretreated, as indicated, for 3–24 h with 0.1–100 nM CGRP, 1,000 nM CGRP8–37 (added alone or 10 min before addition of CGRP), or 100 nM Cys(Acm)2,7. The cells were then pulsed with 103 TCID50 HIV-1 and co-cultured with autologous CD4+ T cells for 1 wk. In some experiments, T cells were pretreated with CGRP and then co-cultured with untreated but HIV-1–pulsed MDLCs (gray bar). HIV-1 replication was measured in the co-culture supernatants by p24 ELISA, and results represent mean ± SEM from n = 9 experiments of HIV-1 transfer normalized against untreated cells serving as the 100% set point; numbers represent percentage of HIV-1 transfer inhibition; *, P = 0.0220 and 0.0003 and P < 0.0001 and 0.0001 for 1 nM/3 h, 10 nM/3 h, 100 nM/3 h, and 100 nM/24 h CGRP-treated versus untreated MDLCs, respectively. (D) RT-PCR images for CRLR, RAMP1-3, and RCP in MDLCs from three individuals with total human brain (HB) RNA serving as positive control. (E and F) MULCs were left untreated or pretreated for 24 h with 0.1–100 nM CGRP, culture supernatants of TT cells containing 70 nM (high) or 4 nM (low) CGRP (F, striped bars), 1,000 nM CGRP8–37 (added alone or 10 min before addition of CGRP), or 100 nM Cys(Acm)2,7, as indicated. The cells were then pulsed with 103 TCID50 HIV-1 and co-cultured with CD4+ T cells for 1 wk. HIV-1 replication was measured in the co-culture supernatants by p24 ELISA, and results of n = 5 experiments are presented as in A and B, respectively; p24 level of untreated MULCs cultured alone was 12.0 ± 0.2 ng/ml; *, P = 0.0315 for MULCs pretreated with 100 nM CGRP versus untreated MULCs (E); *, P = 0.0008 and 0.0338 for MULCs pretreated with 100 nM CGRP and high CGRP versus untreated MULCs, respectively (F). (G) RT-PCR images for CRLR, RAMP1-3, and RCP in MULCs (representative of n = 3 experiments).
MDLCs were next pretreated with CGRP at a concentration range previously found effective in suppressing LC-mediated antigen presentation (0.1–100 nM; Hosoi et al., 1993), before the HIV-1 pulse, and HIV-1 transfer to T cells and T cell infection were measured as described above. Because the efficiency of viral transfer varied when using MDLCs and T cells prepared from different human individuals, the calculated p24 levels in the co-culture supernatants using untreated MDLCs were normalized to 100% for each experiment, allowing for direct comparison. CGRP pretreatment of MDLCs resulted in a dose- and time-dependent inhibition of HIV-1 transfer to T cells, reaching maximal inhibition of 73.3 ± 4.2% after pretreatment with 100 nM CGRP for 24 h (Fig. 1, A–C). Similar inhibition was observed when MDLCs were pulsed with another clade B/R5 HIV-1 molecular clone (ADA; not depicted). The inhibitory effect of CGRP was not a result of increased mortality, as the viability of both untreated and CGRP-treated MDLCs at the end of the co-culture period was similar (∼90% as evaluated by trypan blue exclusion).
Although CGRP modulates several T cell functions (e.g., adhesion and cytokine secretion), HIV-1 transfer was not affected when T cells were pretreated with CGRP and then co-cultured with untreated but HIV-1–pulsed MDLCs (Fig. 1 C). Previous observations also showed that CGRP inhibits antigen presentation to T cells when LCs, but not T cells, are pretreated with CGRP (Hosoi et al., 1993).
The inhibitory effect of CGRP was mediated specifically via the CGRP receptor, as the CGRP receptor antagonist CGRP8–37 abrogated this effect (Fig. 1 C), whereas the linear receptor agonist Cys(Acm)2,7 that acts on related amylin receptors, which are also activated by CGRP (Poyner et al., 2002), was ineffective (Fig. 1 C). In agreement with a potential role of this receptor, RT-PCR showed that MDLCs expressed CRLR, RAMP1, and RCP, the components forming the CGRP receptor (Fig. 1 D). In contrast, MDLCs did not express RAMP2 and RAMP3 (Fig. 1 D).
LCs were also prepared from the human CD34+ acute myeloid leukemia cell line MUTZ-3 (MUTZ-3–derived LCs [MULCs]). At a high HIV-1 concentration of 103 TCID50, MULCs efficiently transferred the virus to T cells; CGRP inhibited such viral transfer, reaching maximal inhibition of 26.2 ± 3.6% after pretreatment of MULCs with 100 nM CGRP for 24 h (Fig. 1, E and F). Endogenous CGRP produced by the thyroid carcinoma cell line TT (Schifter, 1997) exerted an inhibitory effect comparable with that of the synthetic CGRP used above. Hence, pretreatment of MULCs for 24 h with TT culture supernatants containing 70 nM endogenous CGRP resulted in 24.7% inhibition of HIV-1 transfer to T cells, whereas pretreatment with 4 nM endogenous CGRP did not (Fig. 1 F). The inhibitory effects of both synthetic and endogenous CGRP were mediated via the CGRP receptor, as they were abrogated by CGRP8–37, whereas Cys(Acm)2,7 was ineffective (Fig. 1 F). MULCs expressed CRLR, RAMP1, RAMP2, and RCP, but not RAMP3, as shown by RT-PCR (Fig. 1 G). Expression of CRLR and RAMP1 was lower in MULCs relative to MDLCs. Such reduced expression of CRLR and RAMP1, the later necessary for the targeting of CRLR to the plasma membrane (Walker et al., 2010), might provide a possible explanation for the lower ability of CGRP to inhibit HIV-1 transfer to T cells from MULCs versus MDLCs.
Langerin expression and HIV-1 replication
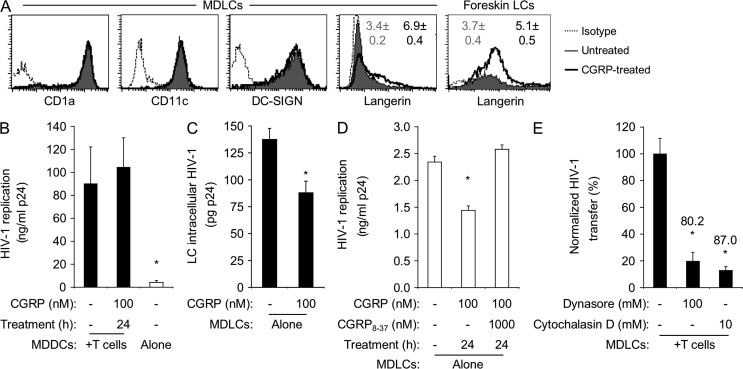
MDLCs differentiated from blood monocytes not only express the LC markers CD1a, CD11c, and langerin, but also DC-SIGN. As DC-SIGN mediates HIV-1 trans-infection of T cells (Geijtenbeek et al., 2000), we investigated its possible role in CGRP-mediated inhibition of HIV-1 transfer. Hence, we prepared in parallel MDLCs and monocyte-derived DCs (MDDCs) using monocytes from the same human individuals and examined the effect of CGRP on DC-SIGN expression on MDLCs and HIV-1 transfer from MDDCs to T cells. CGRP pretreatment (100 nM, 24 h) had no effect on CD1a, CD11c, and DG-SIGN expression on MDLCs (Fig. 2 A) and did not affect HIV-1 transfer from MDDCs to T cells (Fig. 2 B). These results suggest that CGRP-mediated inhibition of HIV-1 transfer does not involve DC-SIGN.
Figure 2.
CGRP increases langerin surface expression and decreases HIV-1 replication. (A) Representative FACS histograms showing surface expression of CD1a, CD11c, DC-SIGN, and langerin in untreated and CGRP-pretreated MDLCs or LCs derived from inner foreskin versus matched isotype controls. Numbers represent mean ± SEM ratios of the MFI for langerin expression (MFI langerin/MFI isotype) derived from n = 15 (MDLCs) or 5 (foreskin LCs) experiments; P < 0.0001 and 0.0352 for CGRP-treated versus untreated LCs, respectively. (B) MDDCs were left untreated or pretreated for 24 h with 100 nM CGRP, pulsed with HIV-1 at 103 TCID50, and either co-cultured with autologous CD4+ T cells or cultured alone (open bar) for 1 wk. HIV-1 replication was measured in the culture supernatants by p24 ELISA. Results represent mean ± SEM ng/ml p24 from n = 4 experiments; *, P = 0.0361 MDDCs alone versus MDDCs + T cells. (C–E) MDLCs were pretreated with CGRP (100 nM, 24 h) alone or after addition of 1,000 nM CGRP8–37 and pulsed with HIV-1 at 103 TCID50. MDLCs were then either incubated with trypsin and lysed immediately, and HIV-1 was measured in the cell lysates (C), or cultured alone in the absence of CD4+ T cells, and HIV-1 was measured in the culture supernatants 1 wk later (D). Untreated MDLCs were also pulsed with HIV-1 at 103 TCID50 in the presence of dynasore or cytochalasin D and co-cultured with autologous CD4+ T cells, and HIV-1 was measured in the co-culture supernatants 1 wk later (E). Results represent mean ± SEM LC intracellular content of HIV-1 (C; pg p24), HIV-1 replication (D; ng/ml p24), or normalized HIV-1 transfer (E; percentage) derived from n = 7, 4, and 3 independent experiments, respectively; numbers in E represent percent inhibition; *, P = 0.0054 and 0.0006 for 100 nM versus no CGRP (C and D); *, P = 0.0145 and 0.0102 for dynasore and cytochalasin D versus untreated MDLCs, respectively (E).
In contrast, CGRP pretreatment (100 nM, 24 h) significantly increased by approximately twofold langerin expression on MDLCs (Fig. 2 A). To substantiate these observations in relevant mucosal in vivo conditions, we evaluated the effect of CGRP on langerin expression in human foreskin mucosa. Using our newly developed ex vivo system, we recently reported that exposure of human adult inner foreskin explants to HIV-1–infected cells results in HIV-1 internalization by LCs, which then transfer HIV-1 to T cells across LC–T cell conjugates (Ganor et al., 2010; Zhou et al., 2011). Here, similar inner foreskin explants were used to evaluate the effect of CGRP pretreatment (100 nM, 24 h) on langerin expression in epidermal single-cell suspensions. In agreement with our aforementioned in vitro observations, CGRP pretreatment also increased langerin expression on inner foreskin epidermal LCs (Fig. 2 A).
Depending on the HIV-1 dose, the interaction between HIV-1 and LCs involves multiple steps, including binding, internalization, degradation, and/or transfer. To investigate the potential impact of CGRP on this complex interaction, untreated or CGRP-pretreated MDLCs were first pulsed with HIV-1. The cells were then incubated with trypsin to remove surface-bound virus as we previously reported (Magérus-Chatinet et al., 2007) and lysed, and the intracellular amounts of HIV-1 were measured in the cell lysates. CGRP pretreatment resulted in a decrease in the intracellular HIV-1 content (Fig. 2 C). In addition, although HIV-1 replication in MDLCs is inefficient (Fig. 1 A), CGRP significantly reduced this low viral replication, an effect which was abrogated by CGRP8–37 (Fig. 2 D). To further sustain these observations, either dynasore (a dynamin inhibitor which prevents the scission of clathrin-coated pits which play a role in langerin recycling) or cytochalasin D (an actin polymerization inhibitor, acting as an endocytosis blocker which increases langerin surface expression; Mc Dermott et al., 2002) was added to untreated MDLCs during the viral pulse period. Both dynasore and cytochalasin D increased langerin surface levels (mean ± SEM fluorescent intensities [MFIs] from n = 4 experiments of 42.8 ± 6.2 [untreated], 91.6 ± 16.3 [dynasore treated], and 108.9 ± 8.0 [cytochalasin D treated] MDLCs; P = 0.0090 and P < 0.0001 vs. untreated MDLCs, respectively; Student’s t test) and inhibited HIV-1 transfer to T cells in a magnitude similar to that of 100 nM CGRP (Fig. 2 E). Hence, CGRP (as well as dynasore and cytochalasin D) increases langerin expression, decreases HIV-1 content and replication in MDLCs, and inhibits HIV-1 transfer from LCs to T cells. These findings might suggest that by increasing langerin cell surface expression, CGRP enhances HIV-1 degradation. Yet, these findings do not rule out the possibility that CGRP might rather decrease HIV-1 uptake, resulting in lower intracellular HIV-1 content and replication. Future studies are needed now to resolve these open questions.
LC–T cell mucosal conjugate formation and T cell infection
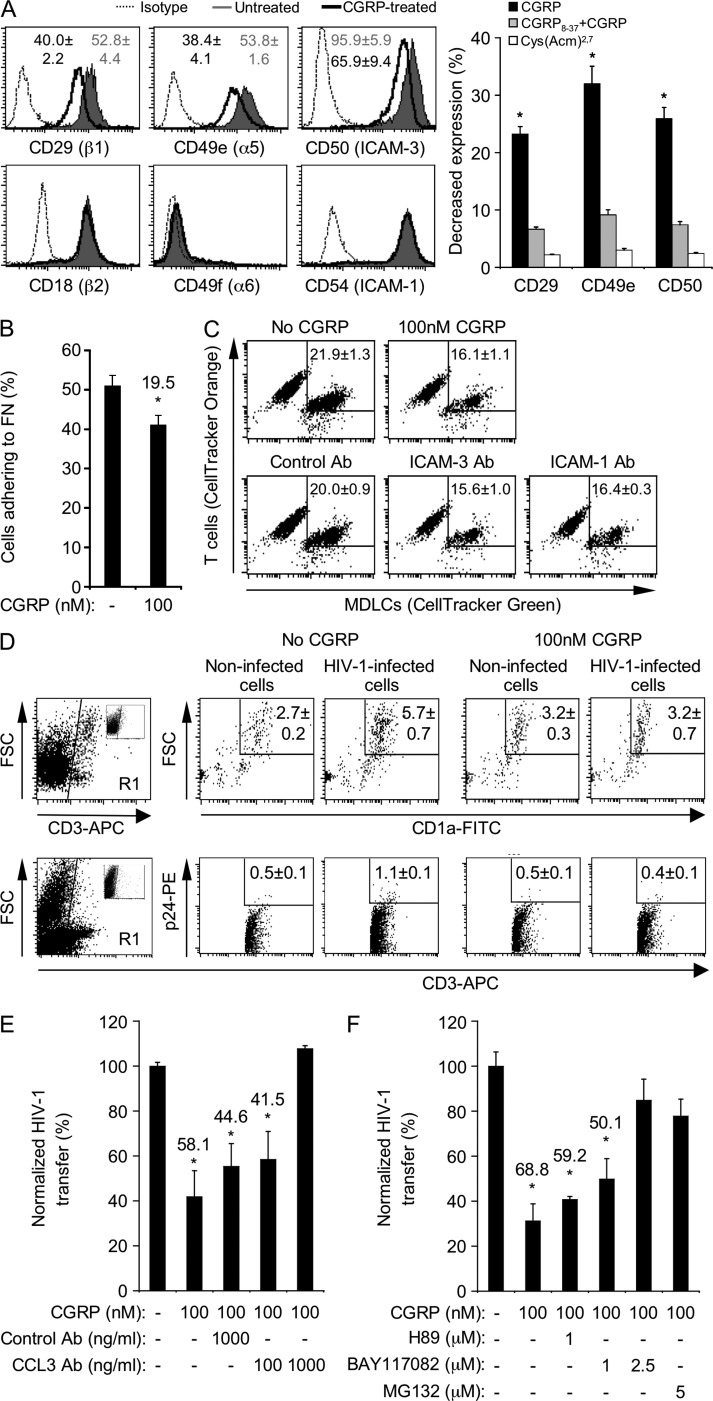
HIV-1 transfer from LCs to T cells takes place across the infectious viral synapse. This transient adhesive LC–T cell cellular conjugate is maintained by specific interactions of various adhesion molecules on the two cell types, such as that between CD11a (LFA-1) on T cells and CD54 (ICAM-1) and especially CD50 (ICAM-3) on LCs (Griffiths et al., 1995). To investigate whether CGRP-mediated inhibition of HIV-1 transfer involves modulation of LCs’ adhesive potential, we evaluated the surface expression levels of several integrins. CGRP pretreatment (100 nM, 24 h) of MDLCs decreased the expression of CD29 (β1), CD49e (α5), and CD50 (ICAM-3), while not affecting that of CD18 (β2), CD49f (α6), and CD54 (ICAM-1) integrins (Fig. 3 A). The inhibitory effect of CGRP was mediated via the CGRP receptor, as it was abrogated by CGRP8–37, whereas Cys(Acm)2,7 was ineffective (Fig. 3 A). Consequently, CGRP pretreatment decreased the percentage of MDLCs adhering to fibronectin-coated plates (Fig. 3 B), a process which is mediated by the β1α5 integrin. CGRP also decreased the percentage of conjugates forming between fluorescently labeled MDLCs and T cells, as did antibodies (Abs) to either ICAM-3 or ICAM-1 (Fig. 3 C). Similar results were obtained when conjugate formation was evaluated with MULCs (not depicted).
Figure 3.
CGRP decreases LC adhesive potential, LC–T cell conjugate formation, and T cell infection and increases CCL3/MIP-1α secretion. (A) Representative FACS histograms showing surface expression of the indicated integrins in untreated or CGRP-pretreated (100 nM, 24 h) MDLCs versus matched isotype controls. Numbers represent mean ± SEM (n = 7) MFI ratios for integrin expression (MFI integrin/MFI isotype) for each surface marker; P = 0.0279 (CD29), 0.0198 (CD49e), and 0.0275 (CD50) for CGRP-treated versus untreated MDLCs, respectively. The graph represents mean ± SEM of the decrease in expression (percentage) of CD29, CD49e, and CD50 after 24-h pretreatment with CGRP alone, 1,000 nM CGRP8–37 + 100 nM CGRP, or 100 nM Cys(Acm)2,7; *, P = 0.0163, 0.0086, and 0.0252 for CD29, CD49e, and CD50 versus untreated MDLCs, respectively. (B) Mean ± SEM (n = 5) percentages of untreated or CGRP-pretreated (100 nM, 3 h) MDLCs adhering in vitro to fibronectin-coated plates; number represents percent decrease; *, P = 0.0265, 100 nM versus no CGRP. (C) Representative FACS profiles showing the percentages of conjugates forming in vitro between CD4+ T cells (CellTracker orange labeled) and MDLCs (CellTracker green labeled). (top) MDLCs were either left untreated or CGRP pretreated (100 nM, 24 h). (bottom) Untreated MDLCs were incubated with CD4+ T cells in the presence of control, ICAM-3, or ICAM-1 Ab. Numbers represent the mean ± SEM (n = 5) percentage of double-positive conjugates; P = 0.0103, 0.0097, and 0.0320 for 100 nM CGRP, ICAM-3 Ab, and ICAM-1 Ab versus no CGRP, respectively. (D) Inner foreskin explants were either left untreated (no CGRP) or CGRP pretreated (100 nM CGRP) for 24 h and exposed for 4 h to either noninfected or HIV-1–infected cells. Shown are representative FACS profiles with numbers representing the mean ± SEM (n = 3) percentages within the entire cell suspensions of either CD3+CD1a+ high FSC LC–T cell conjugates (top, epidermis) or CD3+p24+ cells (bottom, dermis). Cells were first gated on CD3+ cells (R1 gates, left profiles; insets show staining with matched isotype controls). (E and F) HIV-1 transfer experiments in the presence of control Ab or neutralizing Ab to CCL3 (E; n = 3) or the PKA inhibitor H89 or the NF-κB inhibitors BAY117082 and MG132 (F; n = 4). Results are presented as in Fig. 1. *, P = 0.0148, 0.0441, and 0.0229 for pretreatment with CGRP alone, CGRP + control Ab, and CGRP + 100 ng/ml CCL3 Ab versus untreated MDLCs, respectively (E); *, P < 0.0001 and P = 0.0004 and 0.0009 for pretreatment with CGRP alone and in the presence of 1 µM of either H89 or BAY117082 versus untreated MDLCs, respectively (F).
Our recent study demonstrated that exposure of human adult inner foreskin explants to HIV-1–infected cells results in increased formation of LC–T cell conjugates (Zhou et al., 2011). However, upon mucosal tissue sampling, peripheral neurons are sectioned and the role of endogenous neuropeptides could not be studied. Therefore, we further evaluated the effect of CGRP on LC–T cell conjugate formation and T cell infection in these explants. Direct HIV-1 transfer experiments, as described above, could not be performed with LCs from foreskin explants because of limiting cell numbers. In agreement with our recent findings (Zhou et al., 2011), exposure of inner foreskin explants to HIV-1–infected cells increased the percentage of LC–T cell conjugates compared with exposure to noninfected cells (Fig. 3 D, top; mean ± SEM fold percentage LC–T cell conjugates [n = 3] of 2.1 ± 0.1, P = 0.0019; Student’s t test). Moreover, a small but significant proportion of T cells was infected in these explants (Fig. 3 D, bottom; P = 0.0110 for HIV-1–infected vs. noninfected cells [n = 3]; Student’s t test). In contrast, CGRP pretreatment (100 nM, 24 h) abrogated the increase in LC–T cell conjugate formation and T cell infection, mediated by HIV-1–infected cells. Both remained similar to that in explants exposed to noninfected cells (Fig. 3 D).
Of note, the effective concentration of 100 nM CGRP used herein in foreskin explants seems to be within its normal range in the skin (e.g., 10–23 pmol CGRP per gram of mouse skin [Ahmed et al., 1998], which translates to 28–65 nM CGRP, considering that skin contains around 35% water). Together, these results suggest that both in vitro and ex vivo, CGRP restricts T cell infection by decreasing LC–T cell conjugate formation.
CCL3/MIP-1α secretion
CGRP decreases Th1 and increases Th2 cytokine/chemokine secretion by LCs (Ding et al., 2008). Of special interest in the context of HIV-1 are the CCR5-binding chemokines CCL3/MIP-1α, CCL4/MIP-1β, and CCL5/RANTES, which block HIV-1 infection by binding the HIV-1 coreceptor CCR5 on target cells. To investigate whether CGRP-mediated inhibition of HIV-1 transfer might be related to altered secretion of these chemokines, we measured their levels comparatively in culture supernatants of CGRP-treated versus untreated MDLCs and MULCs that were pulsed with HIV-1 and co-cultured for 1 wk with T cells, as described above. These experiments revealed that pretreatment with 100 nM CGRP resulted in increased CCL3/MIP-1α secretion but did not affect that of CCL4/MIP-1β and CCL5/RANTES (mean ± SEM fold secretion from n = 4 experiments of 1.69 ± 0.1 [P = 0.0003; Student’s t test], 0.9 ± 0.2, and 1.2 ± 0.2, respectively). To test whether CGRP-induced elevation of CCL3/MIP-1α secretion contributes to the inhibition of HIV-1 transfer, we added a CCL3/MIP-1α neutralizing Ab during the co-culture period. The CCL3/MIP-1α neutralizing Ab abrogated CGRP-mediated inhibition of HIV-1 transfer from MDLCs to T cells in a dose-dependent manner, whereas a control Ab had no significant effect (Fig. 3 E). Both the control and CCL3/MIP-1α neutralizing Abs had no effect on HIV-1 transfer from untreated MDLCs to T cells (not depicted). In addition, we also included a CCR5-neutralizing Ab (clone 2D7) in other HIV-1 transfer experiments and found that this Ab completely inhibits viral transfer (not depicted). Together, these results clearly show that blocking CCR5 on T cells by either CCL3/MIP-1α or a neutralizing Ab impairs HIV-1 transfer.
As transcriptional up-regulation of the CCL3/MIP-1α gene is mediated by NF-κB, we hypothesized that CGRP-mediated increase in CCL3/MIP-1α secretion results from NF-κB activation. To test this assumption, we treated MDLCs with 100 nM CGRP alone or in the presence of the pharmacological NF-κB inhibitors BAY117082 or MG132 and evaluated viral transfer to T cells as before. MDLCs were also treated with CGRP in the presence of the PKA inhibitor H89, as CGRP also induces PKA activation upon binding to its receptor (Walker et al., 2010). BAY117082 and MG132 abrogated the inhibition of HIV-1 transfer (Fig. 3 F). This abrogation was accompanied by a reduction in CGRP-induced elevation in CCL3/MIP-1α secretion that returned to baseline levels (mean ± SEM fold secretion from n = 4 experiments of 1.0 ± 0.1 for the highest concentration of BAY117082). In contrast, H89 had no effect on HIV-1 transfer from MDLCs to T cells (Fig. 3 F). These results demonstrate that the anti–HIV-1 activity of CGRP in LCs involves specifically the NF-κB, rather than PKA, signaling cascade.
CGRP plasma levels
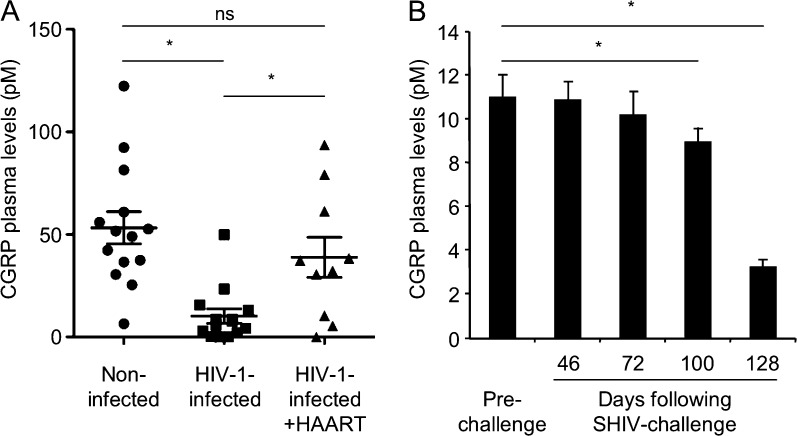
HIV-1 infection is associated in vivo with loss of cutaneous innervation and reduced epidermal nerve fiber density (McCarthy et al., 1995; Zhou et al., 2007). As sensory peripheral nerves innervating the skin/foreskin are reactive for CGRP (Hosoi et al., 1993), we hypothesized that their loss expected to occur upon HIV-1 infection in vivo will result in reduction of CGRP levels. We tested this hypothesis by measuring CGRP in plasma of HIV-1–infected individuals, as foreskin tissues from such patients are difficult to obtain. Although the normal plasma level of CGRP is low (picomolar range) and seems insufficient to operate at the mucosal level (nanomolar range), it serves as a clinical peripheral marker for the deregulation of CGRP in several pathological conditions (e.g., increased in patients with migraine and decreased in patients with hypertension). We found that CGRP levels were significantly decreased in a group of HIV-1–infected patients compared with healthy individuals (Fig. 4 A). In an additional group of HIV-1–infected patients receiving highly active antiretroviral therapy (HAART), CGRP levels were normalized back to baseline levels (Fig. 4 A). To determine whether the reduced levels of plasma CGRP in HIV-1–infected individuals result directly from the presence of the incoming virus early in infection, we also measured CGRP levels in female macaques that were vaginally challenged with SHIV to mimic viral transmission during sexual intercourse. These animals were described in our recent study, which demonstrated the protective efficacy of a gp41-based vaccine active at mucosal sites in protecting against repeated vaginal SHIV challenge (Bomsel et al., 2011). Hence, CGRP plasma levels were measured comparatively in the placebo-vaccinated animals before SHIV challenge and at several later time points at which all animals were infected (see Bomsel et al. [2011] for details regarding the immunization protocol, SHIV doses delivered, and viral loads). This analysis showed that CGRP levels gradually decreased after repeated vaginal SHIV challenges (Fig. 4 B). These results suggest that HIV-1/SHIV infection is associated with reduced circulating levels of CGRP in vivo.
Figure 4.
CGRP plasma levels are decreased in HIV-infected individuals and SHIV-challenged animals. (A) CGRP levels were measured in the plasma of 14 noninfected control individuals, 14 HIV-1–infected individuals, and 10 HIV-1–infected individuals under HAART. Horizontal lines denote the means ± SEM; *, P = 0.0001 HIV-1 infected versus noninfected; *, P = 0.0176 HIV-1 infected + HAART versus HIV-1 infected. (B) Mean ± SEM CGRP levels in the plasma of six female macaques before (prechallenge) and at days 46, 72, 100, and 128 after repeated SHIV challenge. *, P = 0.0133 and 0.0017 for days 100 and 128 after SHIV challenge versus prechallenge, respectively.
We show herein that CGRP efficiently inhibits HIV-1 transfer from LCs to T cells, limits HIV-1 infection of T cells, and is decreased in HIV-1–infected patients and SHIV-challenged animals. CGRP exerts its anti–HIV-1 activities by a combination of several different mechanisms, rather than by a single distinct one, which cooperate together. As such, CGRP possesses some of the properties that characterize cellular HIV-1 restriction factors (Blanco-Melo et al., 2012): CGRP acts autonomously and exhibits antiviral activity in vitro, it employs unique mechanisms to inhibit viral transfer, and its activity might be counteracted in the presence of HIV-1. Yet, in contrast to cellular HIV-1 restriction factors, CGRP exerts its antiviral potential indirectly. We suggest, therefore, that CGRP could be considered a novel indirect HIV-1–limiting factor, which acts at the extracellular rather than intracellular level.
Activation of the CGRP receptor results in Gαs-mediated activation of adenylate cyclase, with a subsequent increase in cAMP and activation of PKA (Walker et al., 2010), which results in relaxation of smooth muscle cells and vasodilatation. Interestingly, a previous study showed that although CGRP increases cAMP production in LCs, such increase alone was not sufficient for inhibiting LC-mediated antigen presentation (Asahina et al., 1995). A later study showed that the inhibition of LC-mediated antigen presentation by CGRP involves the NF-κB pathway (Ding et al., 2007). These findings suggest that different signaling pathways contribute to CGRP-induced vasodilatation in smooth muscle cells versus immunosuppression in LCs. Accordingly, our results show that the NF-κB pathway activated by CGRP mediates HIV-1 transfer inhibition.
CGRP is secreted from peripheral neurons that innervate essentially all organs in the body, including mucosal epithelia. These CGRP neurons are in direct contact with LCs in the skin (Hosoi et al., 1993), as well as with macrophages in the skin and lymphoid organs. CGRP inhibits antigen presentation and cytokine secretion not only in LCs but also in macrophages (Torii et al., 1997). We can therefore speculate that the HIV-1 inhibitory activity of CGRP may be relevant also to later time points during HIV-1 infection after LC migration and viral dissemination in such organs. Additionally, CGRP would potentially control HIV-1 spread within the nervous system itself, where macrophages (as well as microglia) are the principal cells targeted by HIV-1.
CGRP is involved in migraine pathophysiology, in which its plasma levels are increased. Clinical trials have clearly demonstrated the efficacy of CGRP receptor antagonists for the treatment of migraine (Olesen et al., 2004). Based on our results herein, we suggest that enhancing CGRP receptor activation in LCs might provide novel means for limiting HIV-1 spread. Hence, novel CGRP receptor agonists, active especially at mucosal epithelia at targeting LCs, might represent a completely new class of anti–HIV-1 molecules.
Our study not only reveals a novel immunosuppressive effect of CGRP on LC function and its mechanisms of action, but also provides for the first time the evidence that the nervous system can restrict “at a distance” the early events of HIV-1 transmission at the mucosal level. Whether CGRP serves as a natural barrier to HIV-1 transmission and contributes to the rather low degree of infection during sexual intercourse remains an open question.
MATERIALS AND METHODS
Cells.
PBMCs from healthy donors were separated from whole blood by a standard Ficoll gradient. CD14+ monocytes and CD4+ T cells were obtained from PBMCs by negative magnetic selection (STEMCELL Technologies) according to the manufacturer’s instructions. MUTZ-3 cells were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ), and TT cells were obtained from the ATCC. Both cell lines were maintained according to the supplier’s guidelines. MDLCs, MDDCs, and MULCs were prepared and maintained as described previously (Geissmann et al., 1998; Masterson et al., 2002); mean ± SEM percentage of MDLCs expressing surface langerin (see below) was 13.6 ± 4.1% (n = 24).
HIV-1 transfer.
105 MDLCs, 105 MDDCs, and 2.5 × 104 MULCs were pretreated with CGRP, CGRP8–37 (Sigma-Aldrich), Cys(Acm)2,7 (Bachem), or CGRP-containing TT culture supernatants in a 96-well round-bottom plate (200 µl/well final), washed, and pulsed with HIV-1 JRCSF (National Institutes of Health [NIH]) for 2 h (0.1–10 ng corresponding to 101–103 TCID50, prepared as described previously [Ganor et al., 2010; Zhou et al., 2011]). In some experiments, dynasore or cytochalasin D (Sigma-Aldrich) was added during the pulse period at the indicated concentrations. The cells were then incubated with trypsin for 10 min and lysed with NP-40 or washed again and incubated with T cells (3 × 105; autologous for MDLCs). In other experiments, AZT (Sigma-Aldrich), normal goat IgG, neutralizing CCL3/MIP-1α goat Ab (R&D Systems), the NF-κB inhibitors BAY117082 and MG132 (Santa Cruz Biotechnology, Inc.), or the PKA inhibitor H89 (Tocris Bioscience) was included. HIV-1 was measured by p24 ELISA (Innotest; Innogenetics) immediately in the cell lysates or in the co-culture supernatants 1 wk later. The cells were also collected at the end of the co-culture period, double labeled for surface CD3 or CD1a and intracellular p24 expression, and examined by flow cytometry as we recently described (Ganor et al., 2013).
RT-PCR.
Expression of components comprising the CGRP and related receptors was evaluated by RT-PCR as described previously (CRLR and RAMP2 [Sams and Jansen-Olesen, 1998], RAMP1 and RAMP3 [Bailey and Hay, 2006], and RCP [Moreno et al., 2002]); total human brain RNA (Takara Bio Inc.) served as positive control.
Flow cytometry.
MDLCs (105/well) were stained with APC-conjugated mouse anti–human CD1a, CD11c, and DC-SIGN mAbs (BD), PE-conjugated mouse anti–human langerin mAb (clone DCGM4; Beckman Coulter), or 10 µg/ml of the indicated primary mouse anti–human integrin mAbs (ImmunoTools) followed by 1:50 dilution of a secondary FITC-conjugated anti–mouse IgG (Jackson ImmunoResearch Laboratories, Inc.). Cells stained with matched isotype mAbs served as negative control. Each step was performed for 30 min on ice at 50 µl/well. Fluorescent profiles were recorded using a FACSCalibur (BD), and results were analyzed using the CellQuest Pro software (BD).
Adhesive potential and inner foreskin explants.
Adhesion in vitro to fibronectin (Sigma-Aldrich)-coated plates (5,000 MDLCs/well) was performed as we described previously (Ganor et al., 2007). For conjugate formation in vitro, 105 MDLCs were labeled according to the manufacturer’s instructions with CellTracker green (Molecular Probes), pretreated with CGRP, washed, and incubated with 3 × 105 CellTracker orange–labeled T cells for 1 h. Untreated and labeled MDLCs were also incubated with labeled T cells in the presence of mouse anti–human CD50 (ICAM-3) or CD54 (ICAM-1) mAbs (ImmunoTools) at 20 µg/ml. The percentage of double-positive conjugates was measured by flow cytometry. Inner foreskin explants were prepared, inoculated with HIV-1–infected PBMCs, and processed for flow cytometry as we recently described (Zhou et al., 2011).
Ethical approval and human study population.
All human participants were recruited at the Department of Infectious Diseases of the San Raffaele Scientific Institute, Milan, Italy. The institutional review board and the local ethical committee of the San Raffaele Scientific Institute, the Comitato Etico dell’Istituto Scientifico Ospedale San Raffaele, approved the investigations. All human subjects provided informed consents. Animals from which blood was sampled were maintained under guidelines established by the Animal Welfare Act and the NIH Guide for the Care and Use of Laboratory Animals, as we described previously (Bomsel et al., 2011).
A total of 38 plasma samples were selected for the study as follows: (a) 14 samples from healthy individuals not exposed to HIV-1; (b) 14 samples from treatment-naive HIV-1–infected individuals, including seven primary and seven chronic HIV-1–infected patients with mean (and range) of CD4+ T cell count of 330 (149–495)/µl and HIV-1 RNA 1,154,073 (5,169–11,000,000) copies/ml. Primary HIV-1 infection was defined on the basis of the presence of an acute clinical syndrome, a positive test for HIV-1 RNA in plasma, and the presence of less than three positive bands in a Western blot assay; (c) 10 samples from HAART-treated HIV-1–infected patients with mean (and range) of CD4+ T cell count of 738 (317–2,184)/µl, on antiretroviral treatment for at least 24 and not more than 30 mo with chronic and progressive infection, but without previous AIDS defining disease. Samples were matched for sex, age, and risk factors.
Measurement of chemokines and plasma CGRP.
Chemokines levels were measured using a multiplex assay and flow cytometry (Bender MedSystems), as we recently described (Zhou et al., 2011). An Enzyme ImmunoAssay (EIA; Bachem) was used to measure human CGRP plasma levels. A simian ELISA (Cusabio Biotech) was used to measure CGRP plasma levels in six female Macaca mulatta placebo-vaccinated animals, before and at days 46, 72, 100, and 128 (when all animals were infected) after repeated SHIV challenge, as we recently described (Bomsel et al., 2011).
Statistical analysis.
Statistical significance was analyzed by the two-tailed Student’s t test. For comparing human CGRP plasma levels, pairwise comparisons were performed by the nonparametric Mann–Whitney test.
Acknowledgments
Y. Ganor was supported by an Agence Nationale de Recherche sur le SIDA et les Hépatites (ANRS) fellowship; M. Bomsel was supported by a grant from the ANRS. The study was supported by the Bill and Melinda Gates Foundation (grant 53030 to L. Lopalco) and the Italian Ministry of Health, National Program on AIDS (grant 40H15 to L. Lopalco).
All authors report no conflicts of interest.
Footnotes
Abbreviations used:
- Ab
- antibody
- AZT
- azidothymidine
- CGRP
- calcitonin gene–related peptide
- CRLR
- calcitonin receptor–like receptor
- HAART
- highly active antiretroviral therapy
- LC
- Langerhans cell
- MDDC
- monocyte-derived DC
- MDLC
- monocyte-derived LC
- MFI
- mean fluorescent intensity
- MULC
- MUTZ-3–derived LC
- RCP
- receptor component protein
References
- Ahmed A.A., Ahmed M., Theodorsson E., Nordlind K. 1998. Decreased concentrations of CGRP in Leishmania major murine cutaneous leishmaniasis. Neurosci. Lett. 246:149–152 10.1016/S0304-3940(98)00236-5 [DOI] [PubMed] [Google Scholar]
- Asahina A., Moro O., Hosoi J., Lerner E.A., Xu S., Takashima A., Granstein R.D. 1995. Specific induction of cAMP in Langerhans cells by calcitonin gene-related peptide: relevance to functional effects. Proc. Natl. Acad. Sci. USA. 92:8323–8327 10.1073/pnas.92.18.8323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R.J., Hay D.L. 2006. Pharmacology of the human CGRP1 receptor in Cos 7 cells. Peptides. 27:1367–1375 10.1016/j.peptides.2005.11.014 [DOI] [PubMed] [Google Scholar]
- Blanco-Melo D., Venkatesh S., Bieniasz P.D. 2012. Intrinsic cellular defenses against human immunodeficiency viruses. Immunity. 37:399–411 10.1016/j.immuni.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M., Tudor D., Drillet A.S., Alfsen A., Ganor Y., Roger M.G., Mouz N., Amacker M., Chalifour A., Diomede L., et al. 2011. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 34:269–280 10.1016/j.immuni.2011.01.015 [DOI] [PubMed] [Google Scholar]
- Brain S.D., Williams T.J., Tippins J.R., Morris H.R., MacIntyre I. 1985. Calcitonin gene-related peptide is a potent vasodilator. Nature. 313:54–56 10.1038/313054a0 [DOI] [PubMed] [Google Scholar]
- de Witte L., Nabatov A., Pion M., Fluitsma D., de Jong M.A., de Gruijl T., Piguet V., van Kooyk Y., Geijtenbeek T.B. 2007. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 13:367–371 10.1038/nm1541 [DOI] [PubMed] [Google Scholar]
- Ding W., Wagner J.A., Granstein R.D. 2007. CGRP, PACAP, and VIP modulate Langerhans cell function by inhibiting NF-kappaB activation. J. Invest. Dermatol. 127:2357–2367 10.1038/sj.jid.5700858 [DOI] [PubMed] [Google Scholar]
- Ding W., Stohl L.L., Wagner J.A., Granstein R.D. 2008. Calcitonin gene-related peptide biases Langerhans cells toward Th2-type immunity. J. Immunol. 181:6020–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganor Y., Teichberg V.I., Levite M. 2007. TCR activation eliminates glutamate receptor GluR3 from the cell surface of normal human T cells, via an autocrine/paracrine granzyme B-mediated proteolytic cleavage. J. Immunol. 178:683–692 [DOI] [PubMed] [Google Scholar]
- Ganor Y., Zhou Z., Tudor D., Schmitt A., Vacher-Lavenu M.C., Gibault L., Thiounn N., Tomasini J., Wolf J.P., Bomsel M. 2010. Within 1 h, HIV-1 uses viral synapses to enter efficiently the inner, but not outer, foreskin mucosa and engages Langerhans-T cell conjugates. Mucosal Immunol. 3:506–522 10.1038/mi.2010.32 [DOI] [PubMed] [Google Scholar]
- Ganor Y., Zhou Z., Bodo J., Tudor D., Leibowitch J., Mathez D., Schmitt A., Vacher-Lavenu M.C., Revol M., Bomsel M. 2013. The adult penile urethra is a novel entry site for HIV-1 that preferentially targets resident urethral macrophages. Mucosal Immunol. 6:776–786 10.1038/mi.2012.116 [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., Kwon D.S., Torensma R., van Vliet S.J., van Duijnhoven G.C., Middel J., Cornelissen I.L., Nottet H.S., KewalRamani V.N., Littman D.R., et al. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 100:587–597 10.1016/S0092-8674(00)80694-7 [DOI] [PubMed] [Google Scholar]
- Geissmann F., Prost C., Monnet J.P., Dy M., Brousse N., Hermine O. 1998. Transforming growth factor β1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J. Exp. Med. 187:961–966 10.1084/jem.187.6.961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths C.E., Railan D., Gallatin W.M., Cooper K.D. 1995. The ICAM-3/LFA-1 interaction is critical for epidermal Langerhans cell alloantigen presentation to CD4+ T cells. Br. J. Dermatol. 133:823–829 10.1111/j.1365-2133.1995.tb06911.x [DOI] [PubMed] [Google Scholar]
- Hosoi J., Murphy G.F., Egan C.L., Lerner E.A., Grabbe S., Asahina A., Granstein R.D. 1993. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 363:159–163 10.1038/363159a0 [DOI] [PubMed] [Google Scholar]
- Magérus-Chatinet A., Yu H., Garcia S., Ducloux E., Terris B., Bomsel M. 2007. Galactosyl ceramide expressed on dendritic cells can mediate HIV-1 transfer from monocyte derived dendritic cells to autologous T cells. Virology. 362:67–74 10.1016/j.virol.2006.11.035 [DOI] [PubMed] [Google Scholar]
- Masterson A.J., Sombroek C.C., De Gruijl T.D., Graus Y.M., van der Vliet H.J., Lougheed S.M., van den Eertwegh A.J., Pinedo H.M., Scheper R.J. 2002. MUTZ-3, a human cell line model for the cytokine-induced differentiation of dendritic cells from CD34+ precursors. Blood. 100:701–703 10.1182/blood.V100.2.701 [DOI] [PubMed] [Google Scholar]
- Mc Dermott R., Ziylan U., Spehner D., Bausinger H., Lipsker D., Mommaas M., Cazenave J.P., Raposo G., Goud B., de la Salle H., et al. 2002. Birbeck granules are subdomains of endosomal recycling compartment in human epidermal Langerhans cells, which form where Langerin accumulates. Mol. Biol. Cell. 13:317–335 10.1091/mbc.01-06-0300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy B.G., Hsieh S.T., Stocks A., Hauer P., Macko C., Cornblath D.R., Griffin J.W., McArthur J.C. 1995. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 45:1848–1855 10.1212/WNL.45.10.1848 [DOI] [PubMed] [Google Scholar]
- Moreno M.J., Terrón J.A., Stanimirovic D.B., Doods H., Hamel E. 2002. Characterization of calcitonin gene-related peptide (CGRP) receptors and their receptor-activity-modifying proteins (RAMPs) in human brain microvascular and astroglial cells in culture. Neuropharmacology. 42:270–280 10.1016/S0028-3908(01)00176-9 [DOI] [PubMed] [Google Scholar]
- Olesen J., Diener H.C., Husstedt I.W., Goadsby P.J., Hall D., Meier U., Pollentier S., Lesko L.M.; BIBN 4096 BS Clinical Proof of Concept Study Group 2004. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N. Engl. J. Med. 350:1104–1110 10.1056/NEJMoa030505 [DOI] [PubMed] [Google Scholar]
- Poyner D.R., Sexton P.M., Marshall I., Smith D.M., Quirion R., Born W., Muff R., Fischer J.A., Foord S.M. 2002. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 54:233–246 10.1124/pr.54.2.233 [DOI] [PubMed] [Google Scholar]
- Rosenfeld M.G., Mermod J.J., Amara S.G., Swanson L.W., Sawchenko P.E., Rivier J., Vale W.W., Evans R.M. 1983. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 304:129–135 10.1038/304129a0 [DOI] [PubMed] [Google Scholar]
- Sams A., Jansen-Olesen I. 1998. Expression of calcitonin receptor-like receptor and receptor-activity-modifying proteins in human cranial arteries. Neurosci. Lett. 258:41–44 10.1016/S0304-3940(98)00844-1 [DOI] [PubMed] [Google Scholar]
- Schifter S. 1997. Expression of the calcitonin gene family in medullary thyroid carcinoma. Peptides. 18:307–317 10.1016/S0196-9781(96)00169-6 [DOI] [PubMed] [Google Scholar]
- Torii H., Hosoi J., Beissert S., Xu S., Fox F.E., Asahina A., Takashima A., Rook A.H., Granstein R.D. 1997. Regulation of cytokine expression in macrophages and the Langerhans cell-like line XS52 by calcitonin gene-related peptide. J. Leukoc. Biol. 61:216–223 [DOI] [PubMed] [Google Scholar]
- Walker C.S., Conner A.C., Poyner D.R., Hay D.L. 2010. Regulation of signal transduction by calcitonin gene-related peptide receptors. Trends Pharmacol. Sci. 31:476–483 10.1016/j.tips.2010.06.006 [DOI] [PubMed] [Google Scholar]
- Zhou L., Kitch D.W., Evans S.R., Hauer P., Raman S., Ebenezer G.J., Gerschenson M., Marra C.M., Valcour V., Diaz-Arrastia R., et al. ; NARC and ACTG A5117 Study Group 2007. Correlates of epidermal nerve fiber densities in HIV-associated distal sensory polyneuropathy. Neurology. 68:2113–2119 10.1212/01.wnl.0000264888.87918.a1 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Barry de Longchamps N., Schmitt A., Zerbib M., Vacher-Lavenu M.C., Bomsel M., Ganor Y. 2011. HIV-1 efficient entry in inner foreskin is mediated by elevated CCL5/RANTES that recruits T cells and fuels conjugate formation with Langerhans cells. PLoS Pathog. 7:e1002100 10.1371/journal.ppat.1002100 [DOI] [PMC free article] [PubMed] [Google Scholar]