Abstract
Autism and related disorders are increasingly prevalent behavioral syndromes of impaired verbal and nonverbal communication and socialization owing to neurodevelopmental abnormalities. The most recent estimate for the prevalence of autistic disorders is about 1% on a global scale. Etiology of autism is multifactorial and multidimensional that makes therapeutic intervention even harder. Heterogeneity of genetic factors, oxidative stress, autoimmune mechanism, and epigenetic mechanisms complicate the nature of pathogenesis of the disease. Nutraceutical approach to treat this disease is a promising strategy, especially in some areas, it is more attractive than others. This review critically analyzes the roles of vitamins and cofactors, dietary modifications and gut abnormalities, probiotics and prebiotics, phytochemicals, and environmental factors in order to determine the state of evidence in nutraceutical-based autism management practices. This article presents a systematic review of randomized- and placebocontrolled trials to examine the evidence supports the use of autism nutraceu10.1016/j.jsps.2012.10.001ticals. The results will be discussed in the light of all relevant evidence generated from other clinical and exploratory studies.
Keywords: Autism, Autistic spectrum disorders, Nutraceuticals, Vitamins, Diet, Probiotics
1. Introduction
Autistic spectrum disorders (ASDs) are increasingly prevalent neurodevelopmental behavioral syndromes of impaired verbal and nonverbal communication and socialization skills among children. Individuals with ASDs suffer from impairments in social interactions; in language, communication and imagination; and in the range of interests and activities. In the last few decades, refinements of diagnostic techniques have led to the differentiation and variation in traditional use of the term autistic disorder to include autism, Asperger’s syndrome, Rett’s syndrome, and childhood disintegrative disorder. The ASD onset occurs during the first three years of life and has a gender bias with a ratio of 5 males to 1 female (World Health Organization, 1992; Fombonne, 2002; Rapin, 2002; Tonge and Brereton, 2011; Center for Disease Control). Common comorbidities associated with ASDs include gastrointestinal disease and dysbiosis, autoimmunity and mental retardation (Bolte and Poustka, 2002; Sweeten et al., 2003; Buie et al., 2010).
One of 88 children in USA develops any form of ASD, and global prevalence is about 1% (Muhle et al., 2004). In Saudi Arabia, there were 42,500 confirmed cases of autism in the year 2002 (Al-Yafee et al., 2011). In reality, many cases remain undiagnosed in the Arabian Peninsula as suggested by Mostafa et al and other authors as well, who indicate autism prevalence of 1.4 cases per 10,000 children in Oman and 2.9/10,000 in United Arab Emirates (Mostafa, 2011). About 22–30% of children suffering from ASDs also develop seizures without exhibiting underlying pathology. Moreover, about 25% children with ASDs show hypersensitivity-like symptomatology (Theoharides and Zhang, 2011).
Among the ASDs, autism is particularly a severe syndrome characterized by the impairment of reciprocal social interactions and communication development along with extremely restricted and repetitive stereotyped behaviors and corresponding motivational profiles (American Psychiatric Association, 2000). Among the autistics, about 85% suffer from idiopathic autism or primary autism where the exact cause of the disease remains unknown. On the other hand, symptomatic or secondary autism where the causative factor can be determined exists only in 15% of the cases (Sakai et al., 2011).
The exact etiology of autism is unclear, and because of the fact that its pathogenesis starts quite early during embryonic development preventive measures are hard to take. Multifactorial and multidimensional causation of autism include genetic basis and heterogeneity, gastrointestinal pathology, autoimmune complications, inflammation, high level of oxidative stress, decreased ability of the body to detoxify toxins, decreased function of mitochondria, and iatrogenic causes such as vaccinations and food additives. Thus, a number of factors contribute to the pathogenicity of ASDs, of which many are interactive. Autoimmunity to central nervous system exists in many autistic patients (Al-Yadhi and Mostafa, 2011). Oxidative stress from reactive oxygen species is a substantial causative factor for the development and severity of ASDs (Al-Ayadhi et al., 2012).
Genetic studies involving twins, families and genetic associations have revealed a strong genetic correlation in the etiology of autism (Muhle et al., 2004; Folstein and Piven, 1991; Campbell et al., 2006). Heritability in autism exists in as high as 90% of cases. Moreover, twin studies have revealed monozygotic concordance rate of 36–96% as against 0–27% in dizygotic twins (Bakare et al., 2011). Furthermore, genetic heterogeneity behind ASDs is a crucial aspect of their etiology. Also, the potential role of X-linked inheritance has also been demonstrated (Liu et al., 2001; Glessner et al., 2009). Environmental factors also have potentials to contribute significantly to autistic pathogenesis, which besides others, also involve epigenetic mechanisms (Folstein and Piven, 1991; Kinney et al., 2008; Kubota et al., 2012) and even there are some theories from an evolutionary perspective as well (Ploeger and Galis, 2011). Recent studies in animals as well as in humans have identified a vital aspect of gene-environment interaction. An individual with a particular genetic makeup is far more vulnerable to any behavioral disorder such as autism if exposed during the perinatal period to an environmental pathogen or stress (Meaney and Szyf, 2005; Caspi and Moffitt, 2006; Rutter et al., 2006).
1.1. Nutraceuticals and autism
Food is no longer valued from a nutritional point of view only rather it is equally valuable from a health perspective. The use of food or food products in disease prevention or health promotion is an emerging trend that has given rise to the concept of ‘nutraceuticals’. Nutraceuticals is a term first coined in 1989 by the US Foundation for Innovation in Medicine (FIS). FIS defined nutraceuticals as “any substance that is food or a part of food and provides medical or health benefits, including the prevention and treatment of disease” (Brower, 1998; Alissa and Ferns, 2012). General forms of nutraceuticals consist of dietary supplementation (products that supplement the diet such as vitamins, minerals, amino acids, and herbal substances in any composition). Moreover, the term nutraceuticals go a level up in the sense that it also helps in the prevention or treatment of disease by modifying conventional foods like sole meal as against dose based dietary supplements (Kalra, 2003; Pandey et al., 2010).
The use of nutraceuticals in autism management can create a successful integrative model with current treatment to achieve desired results. Nutraceuticals offer several promising benefits that may include promoting healthy gut and lowering body burdens of toxins, reducing excitotoxicity, improving antioxidant capacity, enhancing immunomodulatory systems and minimizing stress and environmental contamination/hazards (Defeat Autism Now (DAN) Project, 2002). Indeed, in 1995, a campaign (Defeat Autism Now) was started by a collaborative network including scientists and physicians with support from parents of autistic children and community. Besides boosting passion for research and practice, to its favor, go a number of scientific publications specifically intervention protocols that guide physicians to manage autistic patients (Levy and Hyman, 2008). Nutraceuticals can significantly advance autism management in a situation where etiological complexity and limitations in earlier interventions hinder therapeutic regimens. Because of their potential benefits, a number of companies have produced several compositions of nutraceuticals available in the market, and there is anecdotal evidence for their efficacies in autistic as well as patients with similar neurological complications. This paper reviews research-based findings about various factors of nutraceuticals in relation to their potentials in autism treatment by consulting the most relevant literature available on this subject.
2. Method
In this systematic analysis, literature was gathered from Pubmed (US National Library of Medicine) /PsycINFO/CINAHL databases using search keywords with restriction to articles published during the last 25 years. Keywords were disease related (autism, autistic spectrum disorders) and nutraceuticals related (nutraceuticals, vitamins, multivitamin/minerals, prebiotics, probiotics, biopterins, gluten, casein, diet, fatty acids, phytochemicals, and toxic). Then disease related terms combined with nutraceuticals related terms, and the result was restricted to control trials. Database suggested corroborations were also examined. Reference list of articles was also explored in search of relevant articles. This review considered only journal articles and proceedings. Search criteria were randomized controlled trial (RCT) and placebo controlled trial (CT) carried out and published in relevance with autism nutraceuticals during 1987–2012. Only eleven research publications could be identified which fulfilled the search criteria. Finally, the systematic review included only eleven selected articles after completing standard steps of identification, selection, analysis, synthesis and compilation. In order to assess the most recent position on the subject, currently proceeding RCTs were also identified, mainly from US National Institute of Health database (clinicaltrials.gov). For more vigorous discussion, other significant and relevant research studies were also included. Interrater reliability was sufficiently strong to promote the overall process of analysis and manuscript preparation.
3. Results
This review of literature has recognized eleven studies reporting either RCT or CT conducted to evaluate the effectiveness, safety and tolerability of several nutraceutical factors for treating one or more forms of ASD. These studies are summarized in Tables 1 and 2 which provide information on intervention, outcome measures, outcome and fundamental limitations. Overall, these interventional studies examined treatment possibilities of multivitamins/minerals (2 RCTs), l-carnitine (2 RCTs), dietary fatty acids (2 RCTs), gluten- and casein-free diet (1 RCT), biopterins (2 CTs), ascorbic acid (1 CT), and prebiotics (1CT). Population size of these studies ranged between 12 and 141 participants and was strongly male biased. Age range of the participants was 3–17 years. In these studies, the most usual dependent variables for interventions were clinical global impressions (CGI), autism treatment evaluation checklist (ATEC), pervasive development disorder behavior inventory (PDDBI), childhood autism rating scale (CARS), Aberrant behavior checklist (ABC), Leiter international performance scale (LIPS), Peabody picture vocabulary tests (PPVT), expressive vocabulary test (EVT), and social responsive scale (SRS). Tolerability measurements conducted by using frequency and intensity of side effects rating (FISER), global rating side effects burden (GRSEB), and patient report to the incidence of side effect (PRISE). Some studies also used biological indicators for measuring a change and carried out laboratory testing.
Table 1.
Summary of randomized controlled trials related to autism nutraceuticals.
| Study | Aspect | Number of subjects | Intervention | Primary outcome measures | Secondary outcome measures | Major findings | Limitations |
|---|---|---|---|---|---|---|---|
| Adams and Holloway (2004) | Multivitamin/mineral supplements in ASD patients | 20 children (3–8 years of age), 18 boys and 2 girls | A gradual increment of multivitamin/mineral suspension (spectrum support II to III) to a maximum dose of 3 ml/5 lb body weight for 3 months | Global impression survey (mothers filled questionnaire) | Vitamins and metabolite levels | Significant improvements in sleep and gut function | Small study size, behavioral assessment through parental assessment only. |
| Adams et al. (2011) | Multivitamin/mineral supplement in patients with autism | 141 (3–60 years of age) 125 males and 16 females | A gradual increment of multivitamin/mineral suspension during 3 month treatment period | ATEC, PDDBI, parental global impressions-revised (PGI-R), severity of autism scale | Metabolic indicators | Significant improvement in reduction of autism symptoms and metabolic indicators. | Shorter study period. Placebo constitution might have affected the results. Some subjects were also on other medication |
| Ellaway et al. (1999) | l-Carnitine treatment to autistic (Rett syndrome) patients | 35 patients | l-Carnitine treatment for 8 months | Rett syndrome motor behavioral assessment, hand apraxia | Patient well-being index | Improvement in patient well-being and hand apraxia specially in girls | Poor identification of predictors of clinical improvement due to a small study size and duration (8 week) |
| Levy and Hyman (2008) | l-Carnitine supplement to ASD patients | 34 children (30 boys and 4 girls) | l-Carnitine at a dose of 50 mg/kg bw/day twice daily for 3 months | CARS, ATEC, CGI, hand muscle test | Lab testing, FISER, GRSEB, PRISE | Significant improvement in CARS, CGI and ATEC scores | Too small study size to observe statistical significance, optimal dosing may not have been achieved |
| Amminger et al. (2007) | Omega-3 fatty acids supplement in autistic children | 13 (5–17 years of age) | 1.5 g/day of omega-3 fatty acids (0.84 g/deicosapentaenoic acid (EPA) + 0.7 g/daydocosahexaenoic acid (DHA) for six weeks | ABC subscales | None | Improved symptoms (hyperactivity and stereotypy) | Small size of the study population |
| Bent et al. (2011) | Omega-3 fatty acids treatment in ASD patients | 27 children (3–8 years of age) | Daily dose of 1.3 g omega-3 fatty acids (EPA + DHA) given as twice daily for12 weeks | ABC-hyperactivity subscale | CGI-Improvement, PPVT, EVT and SRS. Changes in serum omega-3 fatty acids and serum cytokines such as TNF-α | No statistically significant effect of treatment but small treatment effect in hyperactivity reduction. | Small population size and with all subjects exhibiting milder hyperactivity |
| Knivsberg et al. (2002) | Impact of gluten and casein-free diet on autistic patients (one year trial) | 20 children (5–10 years of age) | Gluten- and casein-free diet for one year | Autistic traits (diagnosis of psychotic behavior in children; DIPAB), linguistic abilities, non-verbal cognitive skills (LIPS) | Motor assessment (Movement assessment battery for children) | Significant improvement in reducing the severity of autistic traits | Small population size |
Table 2.
Summary of placebo controlled trials related to autism nutraceuticals.
| Study | Aspect | Number of subjects | Intervention | Primary outcome measures | Secondary outcome measures | Major findings | Limitations |
|---|---|---|---|---|---|---|---|
| Naruse et al. (1987) | Tetrahydro-biopterin treatment in infantile autism | 84 subject (less than 7 years) 62 males and 22 females | Tetrahydrobiopterin was administered at a dose of 1–3 mg/kg bw/day for 12 weeks | Rating scale for abnormal behavior in children | General improvement rating, safety, utility | Found significantly effective for the treatment of autism | Not mentioned |
| Danfors et al. (2005) | Tetrahydro-biopterin treatment in autism | 12 boys (4–7 years of age) | A daily dose of 3 mg/kg body weight for six months | CARS | None | Small non-significant effect on CARS but improvement in social interactions and IQ | Very small population size |
| Dolske et al. (1993) | Ascorbic acid supplement therapy in autism | 18 children | Ascorbic acid at a dose of 8 g/70 kg/day for a period of 10 weeks | Ritvo-Freeman Scale | None | Significant improvement in symptom reduction and sensory motor scores | Small study population |
| Parracho et al. (2010) | Prebiotic WCFS1 treatment in autistic subjects | 3 months | Fecal microbiota, gut function. | Behavioral scores | Significant improvement in stool consistency, behavioral scores and | High interindividual variability and high dropout rate among the participants |
All these studies are bound to various limitations, therefore, are unable to reveal conclusive evidence. Study population size remains the most serious limitation of these studies with an average of about 40 participants, which is inadequate for reliable statistical analysis. In some trials, the duration of intervention appears to be shorter which is also recognized by the researchers. Inconsistencies in results of intervention duration are also evident e.g., Amminger et al. (2007) find improvement in autism symptoms with a 6 week treatment of omega-3 fatty acids (EPA and DHA) while Bent et al. (2011) after treating for 12 weeks with these dietary fatty acids find no significant difference. Evaluation of this intervention in ongoing RCTs is being conducted from 8 to 24 weeks. Dose optimization and other medication intake controls were also reported as limitations that might have played roles altering the results in a couple of studies. Another limitation was the diagnosis of the actual condition which has been reported by at least one study.
As Table 1 summarizes, RCTs which examined the effect of multivitamin-mineral supplementation reported significant benefits of this intervention to ASD patients. Though in the study of Adams and Holloway (Adams and Holloway, 2004) population size was small (20 participants), in Adams et al. (2011) the number of participants was relatively large (141 participants) but the age of the participants ranged between 3 and 60. Two RCTs have evaluated the effectiveness of levocarnitine in ASD patients. Ellaway et al. (1999) reported improvement in hand apraxia, which was seen more in girls, as well as patient well-being benefits. On the other hand, Geier et al. (2011) reported improvement in autism symptoms measured by ATEC, CARS and CGI scores, though study population size was small in both of these RCTs (35 and 34 respectively). Two RCTs are identified that examined the efficacy of omega-3 fatty acids in ASD patients. Amminger et al. (2007) studied children with ASD and found the intervention beneficial as it improved symptoms by reducing hyperactivity and stereotypy while Bent et al. (2011) found no significant difference of treatment in treated and control groups. Again in both studies, the population size was small (17 and 24, respectively). (Knivsberg et al., 2002) conducted a RCT to evaluate the effect of gluten- and casein-free diet for one year in twenty autistic children and reported significant improvement in autism symptoms reduction.
As Table 2 summarizes, two CTs examined the effects of tetrahydrobiopterin treatment. Naruse et al. (1987) with a study population size of 84 participants found this intervention effective in patients with infantile autism while Danfors et al. (2005) with 12 participants (autistic children) reported a small but statistically insignificant effect. In a CT designed to assess the efficacy of ascorbic acid, Dolske et al. found significant improvement in symptom reduction along with improvement in sensory and motor scores with a study population of 18 autistic children (Dolske et al., 1993). Parracho et al. (2010) conducted a CT to evaluate the effectiveness of prebiotics WCFS1 and found it significantly beneficial in treating autism.
Table 3 summarizes currently proceeding RCTs in relation to many autism nutraceutical aspects. Major aspects of these RCTs include vitamins/minerals (7 studies), dietary fatty acids (5 studies), levocarnitine, creatine, cysteine-rich diet and gluten/casein-free diet (one study each). Range of the participants in these RCTs is 16–141.
Table 3.
Currently proceeding randomized controlled trials in the area of autism nutraceuticals.
| Trial Identifier | Title | Intervention | Primary outcome measures | Secondary outcome measures | Enrolled subjects | Status | Executer |
|---|---|---|---|---|---|---|---|
| NCT00273650 | Efficacy study of subcutaneous methyl-B12 (methylcobalamin) in children with autism | Methylcobalamin (25,000 μg/ml), at 64.5 μg/kg or saline placebo s.c. once every 3 days for 6 weeks then subjects cross over for another 6 weeks. After 12 weeks, open label treatment once every 3 days for 6 months. | Clinical global impression (CGI) scale | Neuropsychological test (NEPSY), Aberrant behavior check list (ABC), childhood autism rating scale (CARS), etc. | 35 | Complete | University of California, Davis |
| NCT01230359 | Early nutritional supplement in patients with autism spectrum disorders | Pyridoxine hydrochloride 2.5 g/day for subjects weighing up to 27 kg and 5 g/day for greater than 27 kg for 12 weeks | Improvement in blood parameters | Developmental assessments for ASD | 40 | Complete | Hamad Medical Corp. /Qatar University/Heidelberg Uni. |
| NCT00467818 | Omega-3 fatty acids in the treatment of children with ASDs | Omega 3 fatty acid treatment starts with low doses and based on the weight of individual dosage is increased biweekly. | CGI, ABC, Vineland Adaptive behavior scale (VABS) | Overt aggression scale, parental stress index | 60 | Ongoing | University of Medicine and Dentistry New Jersey/NCCAM |
| NCT01602016 | A folinic acid intervention for autism spectrum disorders | 12 week folinic acid treatment at 1 mg/kg/day for 2 week and then 2 mg/kg/day for 10 weeks. This follows open label extension of both these patterns for 22 weeks | Language improvement (CELF index) | Improved stereotyped behavior and improved social skills | 130 | Ongoing | Arkansas Uni. /Arkansas Children Hospital Research Institute |
| NCT01154894 | Dietary fatty acid improves social impairment in autism spectrum disorders | Subjects over 12 years of age received 6 capsules of Aravita (arachidonic acid and docosaheaenoic acid) and subjects under 12 received 4 capsules for a 16 week period | ABC | Social responsiveness scale (SRS) | 13 | Complete | Ashiya University |
| NCT00672360 | Folate rechallenge | Folic acid treatment at dose 7.6 mg orally twice a day for 4 weeks. | ABC, PDDBI | Plasma folate metabolite levels and site specific DNA methylation | 16 | Ongoing | Baylor College of Medicine |
| NCT01147575 | Effects of creatine supplementation in Rett syndrome | 200 mg creatine monohydrate/kg/day as three doses per day for six months. Treated vs placebo group switch over after 4 week gap. | Global DNA methylation in serum | Metabolic markers of methylation cycle | 21 | Complete | Medical University of Vienna |
| NCT01225198 | Vitamin/mineral supplement for children and adults with autism | A broad spectrum multivitamin/mineral supplement for 12 weeks | Oxidative stress (levels of plasma nitrotyrosine) | Parent global impressions (PGI-R) | 143 | Complete | Arizona State University/Autism Research Institute |
| NCT00090428 | Diet and behavior in young children with autism | Gluten- and casein-free diets for 18 weeks | Safety/efficacy of gluten- and casein-free diet | None | 30 | Unknown | National Institute of Mental Health |
| NCT01366859 | Nutritional intervention in children with autism using protein (immunocal) impact on core areas of behavior | Immunocal (cysteine-rich whey protein) treatment at a dose of 0.5 g/kg for less than 18 kg weight of subjects and 10 g/day for over 18 kg weight subjects for three months. | Behavioral analysis (severity in autism symptoms, communication, and development) | Safety analysis (adverse events recording) | 60 | Ongoing | Nova Southeastern University/Immunotec Inc. |
| NCT01248728 | Omega-3 fatty acids for treatment of young children with autism | Omega-3 fatty acids treatment (first 2 weeks at a dose of 1.875 ml/day and later 3.75 ml/day) for 24 week | PDDBI | CGI, VABS, preschool language scale-4 (PLS-4) | 40 | Ongoing | University of Toronto/EvdokiaAnagnostou |
| NCT01039792 | Trial of methyl-B12 on behavioral and metabolic measures in children with autism | Treatment of methyl-B12 (methylcobalamin) at a dose of 75 μg/kg s.c. once every three days for 8 weeks. | CGI-I | None | 50 | Ongoing | University of San Francisco/University of California, Davis |
| NCT01248130 | Omega-3 fatty acids monotherapy in children and adolescents with ASDs | Treatment of 3 capsules per day (1500 mg omega-3 fatty acids) for 12 weeks. | SRS, CGI-PDD | None | 40 | Ongoing | Massachusetts General Hospital |
| NCT00811083 | Dimercaptosuccinic acid (DMSA) treatment of children with autism and heavy metal toxicity | 4 month treatment of 3 doses of 10 mg DMSA/kg bodyweight for 3 days and then 11 days off | Safety and efficacy | Excretory measurements | 80 | Complete | Southwest College of Naturopathic Medicine |
| NCT00376194 | Mercury chelation to treat autism | Not mentioned | Improvement in social reciprocity | Language skills | 120 | Complete | National Institute of Mental Health |
| ISRCTN 4273114 | A clinical trial of levocarnitine to treat ASD | l-carnitine at a dose of 50 mg/kg bw/day for 3 months | CARS, CGI, ATEC, Hand muscle test | Treatment adherence measurement, side effects, laboratory tests | 30 | Complete | Autism Research Institute |
| JPRN-UMIN 000002650 | Effects of vitamin B6 in children with autism | Pyridoxal 5-phosphate oral dose of 5 mg/kg/day for first 2 weeks followed by a double dose for 2 weeks and switchover | CGI, PPD Autism Society Japan Rating scale | ABC, social maturity scale | 100 | Ongoing | Tohoku University School of Medicine |
| EUCTR2007–006444-21-ES | Effect of 8-week omega-3 fatty acid treatment on oxidative metabolism in patients with ASD | Oral administration of omega-3 fatty acid capsules (350 mg/ml) | Biological testing | CGI, ABC, SRS | Not given | Ongoing | Biomedical Research Foundation of Gregorio Maranon Hospital |
4. Discussion
Though there is scarcity of control trials in the field of autism nutraceuticals, impetus to conduct RCTs is sufficient. There are a number of studies which depict a positive role of vitamins/minerals-based nutraceuticals in treating ASDs. In a case-control study comparing two autism management strategies, the first group of 44 autistic patients, with an age range of 2–28 years, were recommended to take micronutrient supplement containing 14 vitamins, 16 dietary minerals, 3 amino acids, and 3 antioxidants without any medication for autism. On the other hand, the second group of 44 autistic children were recommended conventional medication without supplementation. Patients in both groups improved, but the level of improvement was significantly greater in micronutrient recommended group than in conventional medication group (Mehl-Madrona et al., 2010).
Women who used vitamin supplements during periconceptional period had a lower risk of having autistic children. In a population-based case-control study, mothers of 288 autistic children were less likely to report vitamin intake 3 months before and during the first month of conception compared to the mothers of normal children of the study (Schmidt et al., 2011). Similarly, in a preliminary observatory study, administration of vitamin B12 and glutathione along with low fructose and food additive/color organic diet of ten children (4–10 years of age) for 3–6 months significantly improved social interaction, concentration, writing, language, and behavior (Patel and Curtis, 2007). A meta-analysis of 18 studies revealed that supplementation of vitamin B6 especially in combination with magnesium improved the health conditions of autistic children, though it did not fully cure the disorder (Kidd, 2002; Cornish and Mehl-Madrona, 2008). Autistic children most usually have low levels of vitamin B12 and folate (Ali et al., 2011).
Though these RCTs (Adams and Holloway, 2004; Adams et al., 2011) support the general notion that multivitamins/mineral supplementation remains effective in reducing the symptoms of ASD, there remain several matters to be further evaluated e.g., Adams et al. (2011) suggested a larger study population size for more reliable statistical analysis. Moreover, studies of individual vitamins and other cofactors may also generate useful evidence.
Almost all autistic patients suffer from essential fatty acids deficiency especially omega-3 fatty acids (Vancassel et al., 2001). In an Internet based survey addressing parents of the autistic children, 43% families avail vitamin supplementation and about 28% families supplement omega-3 fatty acid in the diet of autistic children (Richardson, 2004). Whereas, in a special group of 187 autistic children (with verbal apraxia), a combinational supplementation of vitamin E and omega-3 fatty acids produced dramatic improvements in speech, imitation, eye contact, coordination, behavior and sensory function (Morris and Agin, 2009). However, a conclusive beneficial effect of omega-3 fatty acid supplementation has not been achieved in RCTs (Amminger et al., 2007; Bent et al., 2011). After reviewing studies concerning the effect of omega-3 fatty acids on behavior and brain function, Wilczynski-Kwaitek et al. (2009) concluded that data gathered so far are insufficient and lack standardization modalities. Moreover, they lack intermediary as well as endpoint omega 6/3 ratios in plasma lipids in both epidemiological and intervening studies, which warrants a better-designed research in this field.
Autistic children especially those younger than six years of age excrete copious amount of biopterins (cofactor molecules necessary for the biosynthesis of catecholamine and several other pathways) in urine extraordinarily. Cerebrospinal fluid concentration of tetrahydrobiopterin tends to be 42% less in autistic children as compared to normal children (Tani et al., 1994). This is presumably due to depletion of this cofactor in over-activated immune and inflammatory processes (Castellani et al., 2009). Results of the CT conducted by Naruse et al. (1987) revealed significant benefits of tetrahydrobiopterin treatment to autistic children and several other studies of this group strengthen their evidence that reveals that as much as 41–64% of 300 (mild to severe) Japanese autistic children showed encouraging improvement in symptoms upon treatment with tetrahydrobiopterin (Frye et al., 2010).
Naurse et al. (1989) have reported that autistic children under 5 years of age were found to be more responsive to positive effects of tetrahydrobiopterin than their counterparts over five. Moreover, CT conducted by Danfors et al. (2005)has demonstrated improvement in social interaction in tetrahydrobiopterin treated autistic children positively correlates with intelligence quotient of the patients. Frye et al. (2010) after reviewing previous relevant studies expressed that treatment of autistic children with tetrahydrobiopterin has been associated with beneficial effects in language skills, eye contact, sociability, communication and stereotyped behaviors. Also, they stressed on the need for carrying out larger double-blind placebo controlled studies as, so far, data has generated mainly from open labeled potentially biased studies. Furthermore, he recommended studying biological effects along with behavioral outcomes that will improve the understandings of the role of biopterins in ASDs. Moreover, standardization of dose patterns can also help in comparing multiple trials in meta-analyses.
As the autistic children remain unable to catabolize properly casein (milk protein) and gluten (wheat protein) Kidd (2002), Elder et al. (2006), this results in the production of toxic peptides possessing opioid activity capable of crossing the blood brain barrier to contribute in pathogenesis and severity of ASDs (Vojdani et al., 2004). Lucarelli et al. (1995) reported beneficial effects of casein-free diet in about two third of the 36 subjects who were fed cow milk free diet for 8 weeks. Cornish (1998) noticed that 13 of 17 autistic children were consuming excessive amount of milk while intake of many nutrients was lower than normal, when he interviewed the parents of autistic children.
After reviewing a considerable number of studies, Knivsberg et al. (2001) revealed that autistic children who were fed on casein and gluten-free diets grew better than their control counterparts. However, Cornish, 2002 could not find any significant difference between casein and gluten-free autistic children and the control group, though, the sample size was small, results were based on a postal survey, and researcher suggested a longitudinal study to evaluate this dietary intervention. Mulloy et al. (2010) after systematically reviewing 14 studies pertaining to evaluation of effectiveness of gluten- and casein-free diet in ASD patients, concluded that the evidence to support this intervention as an autism treatment strategy is weak. Findings from similar studies suggest that various interactive factors pertaining to diet implementation, gastrointestinal status and immune factors appear to play a role in determining diet responder from diet nonresponder children with ASDs (Pennesi and Klein, 2012). This finding suggests that genetic variations in the etiological factor may also affect the uniformity of this intervention.
High dose probiotics recommendations (e.g., containing Bifidobacteria and Lactobacilli) constitute a serious therapeutic strategy for autism patients as such a treatment enhances the integrity of gut mucosa (Brudnak, 2002) and have been found to produce beneficial effects in alleviating the symptoms of autism (Douglas and Sanders, 2008). Such evidence is further supported by one of the CTs identified in this study (Parracho et al., 2010). Unwanted yeast species, such as Candida, cannot grow in the presence of normal gut flora. Invitro, the addition of tetracycline (antibiotic) to the medium increased the growth of this yeast species, but Candida was reduced in growth when a probiotic species, Lactobacillus plantarum was added to medium (Payne et al., 2003).
Probiotics constituting Lactobacilli and Bifidobacteria are also capable of transforming toxic mercury compounds into metabolites excretable in feces, and thus can also play a vital role in treating autism (Brudnak, 2002). Autistic patients possess weak detoxification capabilities. Therefore, exposure to environmental toxicants can be far more hazardous to them (Curtis and Petel, 2008). For this reason, many practitioners prescribe chelating drugs for heavy metal contamination in autistic patients. Several chelating agents are used including dimercaptosuccinate (DMSA), ethylene diaminetetraacetic acid (EDTA), dimercaprol, and penicillamine. Research studies acknowledge benefits of chelation therapy e.g., DMSA has proven its efficacious potentials in autistic children (Adams et al., 2009) though there are a few occasional reports of fatalities following chelation therapy with EDTA (Brown et al., 2006). Some natural products such as Chinese parsley (Coriandrumsativum) extract which binds metals like lead (Aga et al., 2001) can offer promising prognosis.
Flavonoids like luteolin are also found to inhibit autism like symptoms in mice (Parker-Athill et al., 2009). In an open case series noncontrolled trial, a flavonoid supplement composing of luteolin, quercetin and rutin in a liposomal formulation of olive kernel oil was evaluated in 37 4–14 years old autistic children and was found beneficial in improving gastrointestinal and allergy symptoms, eye contact condition, and social interactions (Theoharides et al., 2012). However, many flavonoids possess antithyroid properties and can also alter normal hypothalamic-pituitary axis. Thus, excessive use of flavonoids during pregnancy can adversely affect fetal brain development (Roman, 2007). Therefore, it is a hazardous class of nutraceuticals for neurodegenerative disorders like ASDs. Diet can be adjusted with required flavonoid depending on the patient’s etiopathogenic factors, conditions and requirements.
Though, the roots of autism cannot be eliminated, the triggering factors can be ameliorated. Therefore, interventions involving dietary manipulations are now increasingly used in treating autism spectrum disorders, and so far results are encouraging. Nutraceuticals for autism treatment need careful execution protocols as every autistic patient is different from other autistic patient. Moreover, it is not an easy task to address etiological heterogeneity of this symptomatology with severe neurological disorder so generally (Shattock and Whiteley, 2000).
Prevalence of autism is rather increasing, and with the development of better diagnostic techniques, groups and subgroups are identified that should be used to classify nutraceuticals of autism, as well. Pregnant women’s nutrition and infant nutrition are the most valuable targets for a preventive nutraceutical strategy as the nutritional, and environmental factors exploit critical periods of development to alter metabolic and endocrine imprinting and associated neural circuitry. Furthermore, physiological alterations drastically modify developmental pathways as has been seen in the case of hypo- and hyperthyroid fetuses in several species including humans. Therefore, besides the use of nutraceuticals for treating autistic patients in order to improve their condition, formulation of safe and pro-health preventive nutraceuticals for pregnant women and infants is also crucial.
This is an area of research which has to deal with many constraints such as sample size, sample uniformity, etiopathogenic uncertainty of the subjects, and longitudinal designs. As the trend suggests more ongoing RCTs (Fig. 1, Table 3), future research is promising for discovering agents to minimize the symptoms of ASD. Furthermore, there is also a need for animal studies in many avenues. Especial focus is required in areas where there exist antagonizing effects of particular nutraceuticals e.g., flavonoids decrease the pathological levels of interleukin-6 (IL-6), and have been found to produce beneficial effects in autistic patients. Also, there are reports of their antithyroid effects during fetal development. Scenario, thus, requires research with more paradigmatic strength as autistic patients are among the most notable clients of nutraceutical industry.
Figure 1.
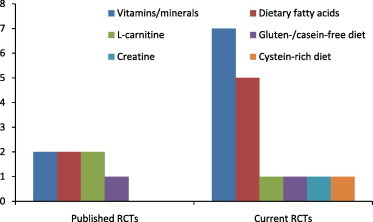
A trend of increasing interest of researchers in conducting RCTs related to autism nutraceuticals.
5. Conclusion
A number of clinical trials with varying designs from open label to randomized double-blind placebo controlled studies have been conducted to evaluate the efficacy and safety of diverse formulations of nutraceutical agents. However, limitations are overwhelming, and, therefore, conclusive evidence awaits further research. Taken into consideration, a nutraceutical approach to manage autism still lacks stronger evidence, though, preliminary evidence is encouraging, but future course will depend on larger and well-designed studies. A huge gap of knowledge and utilization of nutraceuticals needs a supervisory role of leading health regulatory firms like the US Food and Drug Administration (FDA) in order to conduct better clinical trials that can provide reliable evidence for the approval of useful nutraceuticals. So far, we are lucky enough to have good manufacturing practice guidelines from FDA. Barriers like lack of proper formulation, administrative resistance and monitoring difficulties also need attention of policy makers.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adams J.B., Holloway C. Pilot study of a moderate dose multivitamin/mineral supplement for children with autistic spectrum disorder. Journal of Alternative and Complementary Medicine. 2004;10(6):1033–1039. doi: 10.1089/acm.2004.10.1033. [DOI] [PubMed] [Google Scholar]
- Adams J.A., Baral M., Geis E., Mitchell J., Ingram J., Hensley A., Zappia I. Safety and efficacy of oral DMSA therapy for children with autism spectrum disorders: Part B – Behavioral results. BMC Clinical Pharmacology. 2009;9:17. doi: 10.1186/1472-6904-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J.B., Audhya T., McDonough-Means S., Rubin R.A., Quig D., Geis E., Gehn E., Loresto M., Mitchell J., Atwood S., Barnhouse S., Lee W. Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatrics. 2011;11(111) doi: 10.1186/1471-2431-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aga M., Iwaki K., Ueda Y., Ushio S., Masaki N., Fukuda S., Kimoto T., Ikeda M., Kurimoto M. Preventive effect of Coriandrum sativum (Chinese parsley) on localized lead deposition in ICR mice. Journal of Ethnopharmacology. 2001;77(2–3):203–208. doi: 10.1016/s0378-8741(01)00299-9. [DOI] [PubMed] [Google Scholar]
- Al-Ayadhi L.Y., Bacha A.G.B., Kotb M., El-Ansary A.K. A novel study on amyloid β peptide 40, 42 and 40/42 ratio in Saudi autistics. Behavior and Brain Function. 2012;8(4) doi: 10.1186/1744-9081-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A., Waly M.I., Al-Farsi Y.M., Essa M.F., Al-Sharbati M.M., Deth R.C. Hyperhomocysteinemia among Omani autistic children: a case control study. Acta Buiochemica Polonica. 2011;58(4):547–551. [PubMed] [Google Scholar]
- Alissa E.M., Ferns G.A. Functional foods and nutraceuticals in the primary prevention of cardiovascular diseases. Journal of Nutrition and Metabolism. 2012 doi: 10.1155/2012/569486. Article ID 569486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Yadhi L.Y., Mostafa G.A. Low plasma progranulin levels in children with autism. Journal of Neuroinflammation. 2011;8(111) doi: 10.1186/1742-2094-8-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Yafee Y.A., Al-Ayadhi L.Y., Haq S.H., El-Ansary A.K. Novel metabolic biomarkers related to sulfur-dependent detoxification pathways in autistic patients of Saudi Arabia. BMC Neurology. 2011;11:139–145. doi: 10.1186/1471-2377-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Washington, DC: 2000. (American Psychiatric Association, Diagnostic and Statistical Manual-Text Revision (DSMIV-TR TM)). [Google Scholar]
- Amminger G.P., Berger G.E., Schafer M.R., Klier C., Friedrich M.H., Feucht M. Omega-3 fatty acids supplementation in children with autism: a double-blind randomized, placebo-controlled pilot study. Biological Psychiatry. 2007;61:551–553. doi: 10.1016/j.biopsych.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Bakare M.O., Munir K.M., Kinney D.K. Association of hypomelanotic skin disorders with autism: links to possible etiologic role of vitamin-D levels in autism? Hypothesis (Tor) 2011;9(1) doi: 10.5779/hypothesis.v9i1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent S., Bertoglio K., Ashwood P., Bostrom A., Hendren R.L. A pilot randomized controlled trial of omega-3 fatty acids for autism spectrum disorder. Journal of Autism Disorders. 2011;41:545–554. doi: 10.1007/s10803-010-1078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S., Poustka F. The relation between general cognitive level and adaptive behavior domains in individuals with autism with and without co-morbid mental retardation. Child Psychiatry and Human Development. 2002;33:165–172. doi: 10.1023/a:1020734325815. [DOI] [PubMed] [Google Scholar]
- Brower V. Nutraceuticals: poised for a healthy slice of the healthcare market? National Biotechnology. 1998;16:728–731. doi: 10.1038/nbt0898-728. [DOI] [PubMed] [Google Scholar]
- Brown M.J., Willis T., Omalu B., Leiker R. Deaths from hypocalcemia after administration of edetate disodium: 2003–2005. Pediatrics. 2006:118. doi: 10.1542/peds.2006-0858. [DOI] [PubMed] [Google Scholar]
- Brudnak M.A. Probiotics as an adjuvant to detoxification protocols. Medical Hypotheses. 2002;58(5):382–395. doi: 10.1054/mehy.2001.1442. [DOI] [PubMed] [Google Scholar]
- Buie T., Campbell D.B., Fuchs G.J., Furuta G.T., Levy J., Vandewater J. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125(Suppl 1):S1–S18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- Campbell D.B., Sutcliffe J.S., Ebert P.J., Militerni R., Bravaccio C., Trillo S. A genetic variant that disrupts MET transcription is associated with autism. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(45):16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Moffitt T.E. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nature Reviews in Neuroscience. 2006;7(7):583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Castellani M.L., Conti C.M., Kempuraj D.J., Salini V., Vecchiet J., Tete S. Autism and immunity: revisited study. International Journal of Immunopathology and Pharmacology. 2009;22:15–19. doi: 10.1177/039463200902200103. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control. New data on autism spectrum disorder <http://www.cdc.gov/Features/CountingAutism/>.
- Cornish E. A balanced approach towards healthy eating in autism. Journal of Human Nutrition and Diet. 1998;11(6):501–509. [Google Scholar]
- Cornish E. Gluten and casein free diets in autism: a study of the effects on food choice and nutrition. Journal of Human Nutrition and Diet. 2002;15(4):261–269. doi: 10.1046/j.1365-277x.2002.00372.x. [DOI] [PubMed] [Google Scholar]
- Cornish S., Mehl-Madrona The role of vitamins and minerals in psychiatry. Integrative Medicine Insights. 2008;3:33–42. [PMC free article] [PubMed] [Google Scholar]
- Curtis L.T., Petel K. Nutritional and environmental approaches to preventing and treating autism and attention deficit hyperactivity disorder (ADHD): a review. Journal of Alternative and Complementary Medicine. 2008;14(1):79–85. doi: 10.1089/acm.2007.0610. [DOI] [PubMed] [Google Scholar]
- Danfors T., von Knorring A.L., Hartvig P., Langstrom B., Moulder R., Stromberg B. Tetrahydrobiopterin in the treatment of children with autistic disorder: a double-blind placebo-controlled crossover study. Journal of Clinical Psychpharmacology. 2005;25(5):485–489. doi: 10.1097/01.jcp.0000177667.35016.e9. [DOI] [PubMed] [Google Scholar]
- Defeat Autism Now (DAN) Project . Autism Research Institute; San Diego, CA: 2002. (Conference Proceedings, Consensus Reports, Medical Assessment Protocols). [Google Scholar]
- Dolske M.C., Spollen J., McKay S., Lancashire E., Tolbert L. A preliminary trial of ascorbic acid as a supplemental therapy for autism. Progress in Neuro-psychopharmacology, Biology and Psychiatry. 1993;17:765–774. doi: 10.1016/0278-5846(93)90058-z. [DOI] [PubMed] [Google Scholar]
- Douglas L.C., Sanders M.E. Probiotics and prebiotics in dietetics practice. Journal of American Dietitians Association. 2008;108:510–521. doi: 10.1016/j.jada.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Elder J.H., Shankar M., Shuster J., Theriaque D., Burns S., Sherrill L. The gluten-free, casein-free diet in autism: results of a preliminary double-blind clinical trial. Journal of Autism and Developmental Disorders. 2006;36:413–420. doi: 10.1007/s10803-006-0079-0. [DOI] [PubMed] [Google Scholar]
- Ellaway C., Williams K., Leonard H., Higgins G., Wilcken B., Chritodoulou J. Rett syndrome: randomized controlled trial of l-carnitine. Journal of Child Neurology. 1999;14:162–167. doi: 10.1177/088307389901400306. [DOI] [PubMed] [Google Scholar]
- Folstein S.E., Piven J. Etiology of autism: genetic influences. Pediatrics. 1991;87(5):767–773. [PubMed] [Google Scholar]
- Fombonne E. Epidemiological trends in rates of autism. Molecular Psychiatry. 2002;7:S4–S6. doi: 10.1038/sj.mp.4001162. [DOI] [PubMed] [Google Scholar]
- Frye R.E., Huffman L.C., Elliott G.R. Tetrahydrobiopterin as a novel therapeutic intervention for autism. Neurotherapeutics. 2010;7(3):214–249. doi: 10.1016/j.nurt.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier D.A., Kern J.K., Davis G., King P.G., Adams J.B., Young J.L., Geier M.R. A prospective double-blind, randomized clinical trial of levocarnitine to treat autism spectrum disorders. Medical Science Monitoring. 2011;17(6):15–23. doi: 10.12659/MSM.881792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glessner J.T., Wang K., Cai G., Korvatska O., Kim C.E. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra E.K. Nutraceutical – definition and introduction. AAPS Pharmaceutical Science. 2003;5(3):25–26. doi: 10.1208/ps050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd P.M. Autism, an extreme challenge to integrative medicine. Part 2: Medical management. Alternative Medicine Reviews. 2002;7(6):472–499. [PubMed] [Google Scholar]
- Kinney D.K., Miller A.M., Crowley D.J., Huang E., Gerber E. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. Journal of Autism and Developmental Disorders. 2008;38:481–488. doi: 10.1007/s10803-007-0414-0. [DOI] [PubMed] [Google Scholar]
- Knivsberg A.M., Reichelt K.L., Nodland M. Reports on dietary intervention in autistic disorders. Nutrition and Neuroscience. 2001;4(1):25–37. doi: 10.1080/1028415x.2001.11747348. [DOI] [PubMed] [Google Scholar]
- Knivsberg A.M., Reichelt K.L., Hoien T., Nodland M. A randomised, controlled study of dietary intervention in autistic syndromes. Nutritional Neuroscience. 2002;5(4):251–261. doi: 10.1080/10284150290028945. [DOI] [PubMed] [Google Scholar]
- Kubota T., Miyake K., Hirasawa T. Epigenetic understanding of gene-environment interactions in psychiatric disorders: a new concept of clinical genetics. Clinical Epigenetics. 2012;4(1) doi: 10.1186/1868-7083-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S.E., Hyman S.L. Complementary and alternative medicine treatments for children with autism spectrum disorders. Child and Adolescent Psychiatric Clinics of North America. 2008;17(4):803–810. doi: 10.1016/j.chc.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Nyholt D.R., Magnussen P., Parano E., Pavone P. A genomewide screen for autism susceptibility loci. American Journal of Human Genetics. 2001;69:327–340. doi: 10.1086/321980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarelli S., Frediani T., Zingani A., Ferruzzi A., Giardini O., Quintieri F. Food and infantile autism. Panminerva Medica. 1995;37(3):137–141. [PubMed] [Google Scholar]
- Meaney M.J., Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience. 2005;7(2):103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehl-Madrona L., Leung B., Kennedy C., Paul S., Kaplan B.J. Micronutirent versus standard medication management in autism: a naturalistic case-control study. Journal of Child and Adolescent Psychopharmacology. 2010;20(2):95–103. doi: 10.1089/cap.2009.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C.R., Agin M.C. Syndrome of allergy, apraxia, and malabsorption: characterization of a neurodevelopmental phenotype that responds to omega 3 and vitamin E supplementation. Alternative Therapies in Health and Medicine. 2009;15(4):34–43. [PubMed] [Google Scholar]
- Mostafa, A., 2011. Addressing autism in the Arab World. Nature Middle East <http://www.nature.com/nmiddleeast/2011/012345/full/nmiddleeast.2011.147.html>.
- Muhle R., Trentacoste S.V., Rapin I. The genetics of autism. Pediatrics. 2004;113(5):472–486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- Mulloy A., Lang R., O’Reilly M., Sigafoos J., Giulio L., Rispoli M. Gluten-free and casein-free diets in the treatment of autism spectrum disorders: a systematic review. Research in Autism Spectrum Disorders. 2010;4:328–339. [Google Scholar]
- Naruse H., Hayashi T., Takesada M., Nakane A., Yamazaki K., Noguchi T., Watanabe Y., Hayashi O. Therapeutic effect of tetrahydrobiopterin in infantile autism. Proceedings of the Japan Academy. 1987;63:231–233. [Google Scholar]
- Naurse H., Hayashi T., Takesada M., Nakane A., Yamazaki K. Metabolic changes in aromatic amino acids and monoamines in infantile autism and development of new treatment related to the finding. No To Hattatsu (Brain and Development) 1989;21(2):181–189. [PubMed] [Google Scholar]
- Pandey M., Verma R.K., Saraf S.A. Nutraceuticals: new era of medicine and health. Asian Journal of Pharmaceutical and Clinical Research. 2010;3(1):11–20. [Google Scholar]
- Parker-Athill E., Luo D., Bailey A., Giunta B., Tian J., Shytle R.D., Murphy T., Legradi G., Tan J. Flavonoids, a prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose IL-6/MIA associated autism. Journal of Neuroimmunology. 2009;217:20–27. doi: 10.1016/j.jneuroim.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parracho H.M.R.T., Gibson G.R., Knott F., Bosscher D., Kleerebezem M., McCartney A.L. A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. International Journal of Probiotics and Prebiotics. 2010;5(2):69–74. [Google Scholar]
- Patel K., Curtis L.T. A comprehensive approach to treating autism and attention deficit hyperactivity disorder: a prepilot study. Journal of Alternative Complementary Medicine. 2007;13:1091–1097. doi: 10.1089/acm.2007.0611. [DOI] [PubMed] [Google Scholar]
- Payne S., Gibson G., Wynne A., Hudspith B., Brostoff J., Tuohy K. In vitro studies on colonization resistance of the human gut microbiota to Candida albicans and the effects of tetracycline and Lactobacillus plantarum LPK. Current Issues in Intestinal Microbiology. 2003;4(1):1–8. [PubMed] [Google Scholar]
- Pennesi C.M., Klein L.C. Effectiveness of the gluten-free, casein-free diet for children diagnosed with autism spectrum disorder: based on parental report. Nutrition and Neuroscience. 2012;15(2):85–91. doi: 10.1179/1476830512Y.0000000003. [DOI] [PubMed] [Google Scholar]
- Ploeger A., Galis F. Evolutionary approaches to autism – an overview and integration. MJM. 2011;13(2):38–43. [PMC free article] [PubMed] [Google Scholar]
- Rapin I. The autistic spectrum disorders. New England Journal of Medicine. 2002;347(5):302–304. doi: 10.1056/NEJMp020062. [DOI] [PubMed] [Google Scholar]
- Richardson A.J. Long-chained polyunsaturated fatty acids in childhood developmental and psychiatric disorders. Lipids. 2004;39:1215–1222. doi: 10.1007/s11745-004-1350-z. [DOI] [PubMed] [Google Scholar]
- Roman G.C. Autism: transient in utero hypothyroxinemia related to maternal flavonoid ingestion during pregnancy and to other environmental antithyroid agents. Journal of Neurological Sciences. 2007;262(1–2):15–26. doi: 10.1016/j.jns.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Rutter M., Moffitt T.E., Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. Journal of Child Psychology, Psychiatry and Allied Disciplines. 2006;47(3–4):226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Sakai Y., Shaw C.A., Dawson B.C., Dugas D.V., Mohtaseb Z., Hill D.E., Zoghbi Y. Protein interactome reveals converging molecular pathways among autism disorders. Science Translational Medicine. 2011;3(86):86ra49. doi: 10.1126/scitranslmed.3002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R.J., Hansen Rl, Hartiala J., Allayee H., Schmidt L.C., Tancredi D.J., Tassone F., Hertz-Picciotto I. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology. 2011;22:476–485. doi: 10.1097/EDE.0b013e31821d0e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattock P., Whiteley P. Autism Research Unit, University of Sunderland; Sunderland, UK: 2000. (The Sunderland Protocol: A Logical Sequencing of Biomedical Interventions for the Treatment of Autism and Related Disorders). [Google Scholar]
- Sweeten T.L., Bowyer S.L., Posey D.J., Halberstadt C.M., McDougle C.J. Increased prevalence of familial autoimmunity in probands with pervasive developmental disorders. Pediatrics. 2003;112:420. doi: 10.1542/peds.112.5.e420. [DOI] [PubMed] [Google Scholar]
- Tani Y., Fernell E., Watanabe Y., Kanai T., Langstrom B. Decrease in 6R-5,6,7,8-tetrahydrobiopterin content in cerebrospinal fluid of autistic patients. Neuroscience Letters. 1994;181:169–172. doi: 10.1016/0304-3940(94)90586-x. [DOI] [PubMed] [Google Scholar]
- Theoharides T.C., Zhang B. Neuroinflammation, blood brain barrier, seizures and autism. Journal of Neuroinflammation. 2011;8:168. doi: 10.1186/1742-2094-8-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides T.C., Asadi S., Panagiotidou S. A case series of a luteolin formulation (neuroprotek) in children with autism spectrum disorders. International Journal of Immunopathology Pharmacology. 2012;25(2):317–323. doi: 10.1177/039463201202500201. [DOI] [PubMed] [Google Scholar]
- Tonge B., Brereton A. Autism spectrum disorder. Australian Family Physician. 2011;40(9):672–677. [PubMed] [Google Scholar]
- Vancassel S., Durand G., Barthelemy C., Lejeune B., Martineau J., Guilloteau D., Andres C., Chalon S. Plasma fatty acid levels in autisitc children. Prostaglandins, Leukotrines and Essential Fatty Acids. 2001;65(1):1–7. doi: 10.1054/plef.2001.0281. [DOI] [PubMed] [Google Scholar]
- Vojdani A., O’Bryan T., Green J.A., Mccandless J., Woeller K.N., Vojdani E., Nourian A.A., Cooper E.L. Immune response to dietary proteins, gliadin and cerebellar peptides in children with autism. Nutrition Neuroscience. 2004;7(3):151–161. doi: 10.1080/10284150400004155. [DOI] [PubMed] [Google Scholar]
- Wilczynski-Kwaitek A., Singh R.B., De Meester F. Nutrition and behavior: the role of ω3 fatty acids. The Open Nutraceutical Journal. 2009;2:1–10. [Google Scholar]
- World Health Organization . WHO; Geneva: 1992. (The ICD-10 Classification of Mental and Behavioral Disorders (ICD-10)). [Google Scholar]



