Abstract
[Purpose] This study investigated the effect of neurological symptoms and/or signs after the occurrence of neurogenic intermittent claudication (NC) on postural sway during quiet standing of patients with lumbar spinal canal stenosis (LSS). [Subjects and Methods] Thirty-two female patients with LSS at the L4/5 level were studied. We measured the path of center of foot pressure (COP) during quiet standing with eyes open for 30 s using a stabilometer before and after the occurrence of NC. [Results] The total path length of COP (LC) and area surrounded by the outline of the path of COP (AC) significantly increased after NC. Body mass index (BMI) correlated with both the NC rate (after NC/before NC) of LC and that of AC. The average lateral COP displacement from the center of the base of support (COPRL) before NC was located on the asymptomatic side from the center of the base of support in 29 of 32 patients. After NC, COPRL moved to the symptomatic side in 31 patients. [Conclusion] These results suggest that patients with LSS are at risk of falling after NC, especially those with high BMI.
Keywords: Degenerative lumbar spinal canal stenosis, Postural sway, Neurogenic intermittent claudication
INTRODUCTION
According to the ROAD project1), a nationwide cohort study, the estimated number of patients with lumbar spondylosis is 38 million in Japan, and the prevalence is even higher in those over 60 years of age. Lumbar spinal canal or intervertebral foramen stenosis (LSS), a major degenerative lumbar disease causing low back pain in people over age 65 years2), is caused by bulging degenerated intervertebral discs, thickened ligaments or hypertrophy of the facet joints associated with aging3). In addition to decline in physical function with aging, stenosis- induced symptoms and/or signs impair balance4, 5). Stucki et al.6) reported that 66% of older adults with LSS have balance disturbances. However, there are no previous reports of the prevalence of falls among patients with LSS, although many investigators have noted the fall risk of LSS patients.
The main symptom of LSS is neurogenic intermittent claudication (NC). NC is an asymptomatic condition at rest, but after walking patients experience weakness, tiredness, or heaviness of the legs that gradually increases and necessitates discontinuation of walking7). The prevalence of NC reportedly ranges from 46.7 to 94% in patients with LSS2, 8,9,10). NC is thought to be caused by direct compression of the nerve11), ischemia12) or venous stasis13) of the nerve root. Takahashi et al.14) showed that upright walking creates the highest epidural pressure, and that pressure peaks intermittently during the double supporting phase corresponding to maximum forward tilt of the pelvis15) (leads maximum lumbar lordosis). Many patients with LSS tend to walk with their backs flexed and with short steps at a slow speed16). After the occurrence of NC, postural sway is expected to increase markedly compared with before NC. One report described the sway of center of foot pressure (COP) during quiet standing on a force plate after the occurrence of NC. Hanai et al.17) reported the change in COP after NC. COP after NC immediately shifted forward, toward the symptomatic side, and then returned to the initial area (before NC) after 20 minutes of rest. The time required to return to the initial area was longer than that required for the patient’s subjective recovery from symptoms. This observation suggests that neurological deficits persist even when symptoms show relief, and indicate that patients are still at risk of falling. However, Hanai et al. did not analyze COP data quantitatively or statistically due, presumably, to the limitations of their force plate. In this study, we investigated how COP changes during quiet standing before and after the occurrence of NC and demonstrated that LSS patients are at increased risk of falling.
SUBJECTS AND METHODS
Subjects
Thirty-seven female patients (56–83 years old) with LSS were included in this study. The inclusion criteria were female gender, no pain or neurological deficits at rest, the existence of stenosis at the L4/5 level, no past history of spinal surgery, and the absence of neuromuscular, vestibular and cardiopulmonary diseases. We explained the outline of the present study, and all patients agreed to the use of their data in this research.
Methods
We evaluated balance ability using a stabilometer (Gravicoder GS11, ANIMA, Tokyo) with a 20 Hz sampling frequency. Patients stood still in the upright position (to prevent excess trunk bending) with their feet together and eyes open on the center of the stabilometer for 30 s. Patients focused on a 1 cm mark on the wall 2 m away from the place at which they stood during measurement. We used four data sets derived from the stabilometer measurements: LC, the total length of COP; AC, area surrounded by the outline on the path of COP; COPAP, the average COP displacement in the anterior-posterior direction; and COPRL, the average COP displacement in the right-left direction. Values in LC and AC were normalized individual foot length.
After the stabilometric measurements, patients were asked to walk around a 25 m course on flat ground without rest to provoke NC; none utilized walking aids. They were instructed to walk at their prefer speed without excessive anterior trunk bending. After they could not continue walking, the patients rested for one minute. None of the patients were unable to walk because of joint pain in the lower extremities, palpitations or dyspnea. We measured their walking distances (WD). After the one-minute rest, we evaluated the degree of their symptoms employing a visual analogue scale (VAS). Finally, stabilometry was repeated under the same conditions as before the occurrence of NC. During the first stabilometric test, we marked the positions of the tips of both big toes and the heels on the stabilometer to ensure that patients stood in the same position as before NC during the second stabilometric examination.
All data were analyzed with PASW statistics 18.0. The paired t-test was used to compare the LC and AC values of before and after the occurrence of NC. We calculated the NC rates, values after NC/values before NC × 100%, of LC and AC. Pearson’s product moment correlation coefficient was used to examine the relationship between NC rates and other parameters: age, body mass index (BMI), VAS and WD. For all tests, a p value < 0.05 was considered statistically significant.
RESULTS
Five patients were unable to stand quietly for 30s after NC. Therefore, data from the other 32 patients (69.5±7.2 years, BMI of 24.8±2.5 kg/m2) were analyzed for this study. Average walking distance was 314.1±270.8 m, and VAS after one minute rest was 53.3±28.6 mm.
The stabilometry results are shown in Table 1. LC and AC after NC were significantly greater than before NC. Regarding relationships with NC rate, only BMI correlated with both LC (r=0.32) and AC (r=0.36).
Table 1. Stabilometric parameters before and after NC.
| before NC | after NC | |
| LC (cm)* | 2.8 ± 1.1 | 3.2 ± 1.6 |
| AC (cm2)* | 0.2 ± 0.1 | 0.2 ± 0.2 |
| NC rate in LC (%) | 1.6 ± 0.2 | |
| NC rate in AC (%) | 1.3 ± 0.6 | |
| COP | ||
| anterior | 1 | 15 |
| posterior | 31 | 17 |
| COP | ||
| symtomatic side | 3 | 31 |
| asymptomatic side | 29 | 1 |
average ± SD, p<0.001
* COP after NC: direction compared with before NC
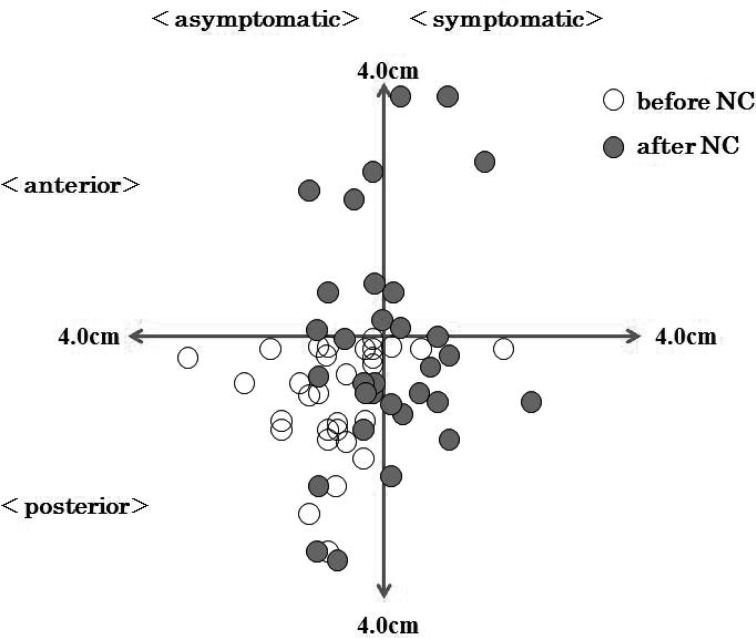
Before NC, COPAP was located posterior to the center of the base of support in 31 of the 32 patients. After NC, COPAP shifted forward in 15 patients (group A) and backward in 17 (group P). The unpaired t-test was used to compare age, BMI, VAS and WD between these two groups. Although there were no significant differences, patients in group P tended to have higher BMI (group A: 24.0±2.4 kg/m2; group P: 25.5±2.5 kg/m2). COPRL before NC was located on the asymptomatic side from the center of the base of support in 29 of the 32 patients. After NC, COPRL shifted to the symptomatic side in 31 patients (Fig. 1).
Fig. 1.
The change in COP before and after NC
DISCUSSION
NC induced by lumbar stenosis leads to muscular weakness, sensory disturbance and/or pain. Although the occurrence of NC diminishes balance ability, few studies have investigated postural sway after the occurrence of NC focusing on fall risk. Our present results show that the sway of COP increases after NC due to neurological deficits. VAS, reflecting subjective pain, did not correlate with the NC rate of either LC or AC. This supports the results of Hanai et al.17) indicating that the time to COP recovery is longer than the time required for subjective recovery from symptoms. Thus, postural sway after NC depends not on symptoms (degree of pain), but rather on neurological signs.
NC rates of both LC and AC correlated with BMI. High body mass has a strong association with increasing postural sway18,19,20). The causes of increased body mass’ influence on postural sway have been reports as adaptation and desensitization of plantar cutaneous receptors18), diminished proprioception in the knee and ankle joints21), and increased ankle torque18). Increased body mass also affects the spine. High BMI is known to be a risk factor of the progression of osteoarthritis in the facet joints22). Vismara et al.23) reported that obesity reduces the range of spinal motion and that obese individuals with chronic low back pain often show increased lumbar lordosis, which would cause NC to occur earlier. According to Geisser24), BMI correlates inversely with WD. These results suggest that high BMI is a risk factor of impaired physical function (balance ability or walking capacity) as well as progress of disease in LSS.
In the present study, we found COPAP was located posterior to the center of the base of support before NC in 31 of our 32 patients. An increase in lumbar lordosis is expected to shift COP forward. Suzuki et al.25) investigated the lumbar lordosis angle (LAA) of patients with LSS by employing standing radiographs. They reported that LSS patients with NC had small LAA as compared with standard values for Japanese. This finding indicates that LSS patients with NC habitually tilt their pelvis backward had in daily life to avoid lumbar stenosis. We speculate that COPAP before NC had a posterior location due to this strategic posture.
After NC, COPAP shifted forward in 15 patients and backward in 17. Patients in group P had higher BMI than those in group A, although the difference was not statistically significant. In healthy subjects, COPAP shifts forward after walking as compared with COPAP before walking17). As BMI correlates inversely with WD24), obese patients might have more severe symptoms after NC than non-obese patients. COPAP would tend to shift backward due to planta forefoot sensory disturbance or toe muscle weakness in group P. One more possible explanation for this result is excessive abdominal mass. It would kinematically lead to lumbar lordosis. Obese patients with LSS may compensate for diminished LAA by tilting their pelvis more backward compared to patients with normal BMI. Although we neither measured abdominal circumference nor evaluated posture, BMI would affect postural control after the occurrence of NC in LSS patients. This suggests that increased COPAP would occur not only because of LSS, but also BMI.
Changes in COPRL were essentially regular showing a shift from the asymptomatic side before NC to the symptomatic side after NC. In normal subjects, COPRL remains focused on the small area near the center after 10 minutes walking on a treadmill18). The shift in COPRL observed in our LSS patients can be explained by patients being unable to compensate on the asymptomatic side, because stenosis-induced pain, perceptual disturbance and/or weakness increased on the symptomatic side. Hanai et al.17) investigated COP displacement after NC, and found that COP shifted to the symptomatic side immediately after NC. Patients with hemiparesis after stroke tend to fall to the paretic side, and about two thirds of femoral neck fractures occur on the paretic side26, 27). Similarly, LSS patients with neurological findings are at risk of falling to the symptomatic side after the occurrence of NC.
This study had three major limitations. First, we did not compare postural sway of LSS patients with healthy individuals or LSS patients without NC. Therefore, we could not show the effect of NC on postural sway distinct from the effect of lumbar degeneration. Second, we did not measure lumbar curvature or abdominal circumference. Finally, the degree of the stenosis-induced neurological signs (sensory disturbance or weakness) other than pain were not evaluated after NC.
In conclusion, when postural sway increases after the occurrence of NC, LSS patients are at risk of falling to the symptomatic side. Not only stenotic signs, but also high BMI is a significant factor increasing postural sway of patients with LSS.
REFERENCES
- 1.Yoshimura N, Murakami S, Oka H, et al. : Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/ osteoporosis against disability study. J Bone Miner Metab, 2009, 27: 620–628 [DOI] [PubMed] [Google Scholar]
- 2.Jöhnsson B, Annertz M, Sjoberg C, et al. : A prospective and consecutive study of surgically treated lumbar spinal stenosis. PartI: Clinical features related to radiographic findings. Spine, 1997, 22: 2932–2937 [DOI] [PubMed] [Google Scholar]
- 3.Szpalski M, Gunzburg R: Lumbar spinal stenosis in the elderly: an overview. Eur Spine J, 2003, 12: S170–S175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin SI, Lin RM: Sensorimotor and balance function in older adults with lumbar nerve root compression. Clin Orthop Relat Res, 2002, 394: 146–153 [DOI] [PubMed] [Google Scholar]
- 5.Iversen MD, Kale MK, Sullivan JT: Pilot case control study of postural sway and balance performance in aging adults with degenerative lumbar spinal stenosis. J Geriatr Phys Ther, 2009, 32: 15–21 [DOI] [PubMed] [Google Scholar]
- 6.Stucki G, Liang MH, Lipson SJ, et al. : Contribution of neuromuscular impairment to physical functional status in patients with lumbar spinal stenosis. J Rheumatol, 1994, 21: 1338–1343 [PubMed] [Google Scholar]
- 7.Porter RW: Spinal stenosis and neurogenic claudication. Spine, 1996, 21: 2046–2052 [DOI] [PubMed] [Google Scholar]
- 8.Hall S, Bartleson JD, Onofrio BM, et al. : Lumbar spinal stenosis. Clinical features, diagnostic procedures, and results of surgical treatment in 68 patients. Ann Intern Med, 1985, 103: 271–275 [DOI] [PubMed] [Google Scholar]
- 9.Radu AS, Menkes CJ: Update on lumbar spinal stenosis. Retrospective study of 62 patients and review of the literature. Rev Rhum Engl Ed, 1998, 65: 337–345 [PubMed] [Google Scholar]
- 10.Goh KJ, Khalifa W, Anslow P, et al. : The clinical syndrome associated with lumbar spinal stenosis. Eur Neurol, 2004, 52: 242–249 [DOI] [PubMed] [Google Scholar]
- 11.Verbiest H: Further experiences on the pathologic influence of a developmental narrowness of the bony lumbar vertebral canal. J Bone Joint Surg Br, 1955, 37-B: 576–583 [DOI] [PubMed] [Google Scholar]
- 12.Blau JN, Louge V: Intermittent claudication of the cauda equina. Lancet, 1961, 277: 1081–1086 [Google Scholar]
- 13.Ooi Y, Mita F, Satoh Y: Myeloscopic study on lumbar spinal canal stenosis with special reference to intermittent claudication. Spine, 1990, 15: 544–549 [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Kagechika K, Takino T, et al. : Changes in epidural pressure during walking in patients with lumbar spinal stenosis. Spine, 1995, 20: 2746–2749 [DOI] [PubMed] [Google Scholar]
- 15.Thurston AJ, Harris JD: Normal kinematics of the lumbar spine and pelvis. Spine, 1983, 8: 199–205 [DOI] [PubMed] [Google Scholar]
- 16.Suda Y, Saitou M, Shibasaki K, et al. : Gait analysis of patients with neurogenic intermittent claudication. Spine, 2002, 27: 2509–2513 [DOI] [PubMed] [Google Scholar]
- 17.Hanai K, Ishii K, Nojiri H: Sway of the center of gravity in patients with spinal canal stenosis. Spine, 1988, 13: 1303–1307 [DOI] [PubMed] [Google Scholar]
- 18.Hue O, Simoneau M, Marcotte J, et al. : Body weight is a strong predictor of postural stability. Gait Posture, 2007, 26: 32–38 [DOI] [PubMed] [Google Scholar]
- 19.Greve J, Alonso A, Bordini AC, et al. : Correlation between body mass index and postural balance. Clinics, 2007, 62: 717–720 [DOI] [PubMed] [Google Scholar]
- 20.Singh D, Park W, Levy MS, et al. : The effects of obesity and standing time on postural sway during prolonged quiet standing. Ergonomics, 2009, 52: 977–986 [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Li JX, Xu DQ, et al. : Proprioception of ankle and knee joints in obese boys and non obese boys. Med Sci Monit, 2008, 14: CR129–CR135 [PubMed] [Google Scholar]
- 22.Kalichman L, Guermazi A, Li L, et al. : Association between age, sex, BMI and CT-evaluated spinal degeneration features. J Back Musculoskelet Rehabil, 2009, 22: 189–195 [DOI] [PubMed] [Google Scholar]
- 23.Vismara L, Menegoni F, Zaina F, et al. : Effect of obesity and low back pain on spinal mobility: a cross sectional study in women. J Neuroeng Rehabil, 2010, 7: 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geisser ME, Haig AJ, Tong HC, et al. : Spinal canal size and clinical symptoms among persons diagnosed with lumbar spinal stenosis. Clin J Pain, 2007, 23: 780–785 [DOI] [PubMed] [Google Scholar]
- 25.Suzuki H, Endo K, Kobayashi H, et al. : Total sagittal spinal alignment in patients with lumbar canal stenosis accompanied by intermittent claudication. Spine, 2010, 35: E344–E346 [DOI] [PubMed] [Google Scholar]
- 26.Ramnemark A, Nyberg L, Borssén B, et al. : Fractures after stroke. Osteoporos Int, 1998, 8: 92–95 [DOI] [PubMed] [Google Scholar]
- 27.Ramnemark A, Nyberg L, Borssén B, et al. : Stroke, a major and increasing risk factor for femoral neck fracture. Stroke, 2000, 31: 1572–1577 [DOI] [PubMed] [Google Scholar]