Abstract
Objective
To summarize the efficacy and safety profile of Tocilizumab (TCZ), a humanized monoclonal antibody against interleukin-6 (IL-6), approved for the treatment of rheumatoid arthritis (RA).
Methods
A systematic literature review was conducted to identify English language articles within Pubmed and the Cochrane Library from January 1989 to August 2011 reporting results from phase III TCZ double-blinded randomized controlled trials (RCT), non-controlled clinical trials and open-label extensions with duration ≥ 6 months. Study outcomes had to include at least one of the following: American College of Rheumatology (ACR) 20, 50, 70; tender/swollen joint count, HAQ disability, radiographic outcomes and drug persistence. Phase II RCTs were included only if they contained relevant information not available in phase III RCTs. To review TCZ pharmacology, relevant studies were selected to evaluate pharmacokinetics and pharmacodynamics.
Results
Ten published clinical trials (7 phase 3, 3 phase 2) for TCZ were retrieved (7,833 articles initially identified) and 31 from Cochrane library. Compared to MTX monotherapy, TCZ 8 mg/Kg monotherapy had higher rates of ACR20 (p<0.001), ACR50 (p=0.002) and ACR70 (p<0.001) scores at week 24. TCZ 8mg/Kg IV + oral MTX had a higher ACR20 response rate than placebo + oral MTX in patients with RA that failed to respond to MTX or anti-TNF therapy (p<0.001). Patients on TCZ 8mg/Kg had less radiographic progression on Sharp-Genant Score, (85% had no progression) than the control group (67% had no progression, p<0.001). The rate of serious infections was 4.7 events /100 patient years of exposure in the TCZ groups. A greater frequency of neutropenia, thrombocytropenia, hyperlipidemia, and transaminitis was observed with TCZ compared to placebo.
Conclusion
The short term efficacy and safety profile of TCZ is promising. Additional long term safety data are needed to better characterize the risk-benefit profile of this agent.
Keywords: tocilizumab, rheumatoid arthritis, IL-6, MRA, juvenile idiopathic arthritis
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune inflammatory disease characterized by persistent synovitis and progressive destruction of cartilage and bone (1). It is associated with progressive joint damage, pain, fatigue, disability, as well as, the elevation of acute phase reactants like C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) (2-5). It is a common disease affecting about 1% of adults aged >35 years and over 2% of adults age >60 years in the United States (US) (6-7). Similar prevalence figures have been reported worldwide (7-8). Even though the cause of RA is not fully understood, pro-inflammatory cytokines like tumor necrosis factor (TNF), interleukin-1 (IL-1) and IL-6 play an important role in disease pathogenesis (3-4). More than a decade of experience with anti-TNF therapy has shown these agents to be effective in a significant proportion of patients with RA. However, at least two-thirds of RA patients have a partial but incomplete response to anti-TNF therapy (9). Tocilizumab (TCZ) has been introduced as a new approach to the treatment of RA since it targets the IL-6 pathway, which is important in the pathogenesis of RA.
IL-6 is a pleiotropic cytokine with a wide range of biological activities in immune regulation, hematopoiesis, inflammation and oncogenesis. Its activities are shared by IL-6 related cytokines such as leukemia inhibitory factor (LIF), cilliary neutrophic factor (CNTF) and oncostatin M. There are two different IL-6 driven signaling pathways. One is mediated by membrane-bound IL-6 receptor (mIL-6R, CD 126) (10-11) via activation of glycoprotein 130 (gp130); the second via proteolytic cleavage of the mIL-6R that leads to the generation of a soluble receptor for IL-6 (sIL-6R). sIL-6R is able to bind to IL-6 and can stimulate cells that lack endogenous mIL-6R (12-14). IL-6 is produced by various cell types, including T-cells, B-cells, monocytes, fibroblasts, endothelial cells, and synovial cells (10, 15). Higher levels of IL-6 have been found in the synovium of patients with RA (16). IL-6 can stimulate pannus formation through increased vascular endothelial growth factor (VEGF) expression and increase bone resorption as a result of osteoclastogenesis (5, 17). Systemic effects of IL-6 include regulation of acute-phase protein synthesis, as well as hepcidin production and stimulation of the hypothalamo-pituitary-adrenal axis, the latter two actions leading to anemia and fatigue respectively (5).
TCZ, a humanized anti-IL-6 receptor monoclonal antibody, represents a promising new treatment strategy in patients with RA and is currently approved in the US for RA patients that have failed at least one anti-TNF therapy. TCZ prevents IL-6 from binding to both mIL-6R and sIL-6R, thereby blocking the proinflammatory effects of IL-6 (15, 18). The purpose of this article is to review the pharmacology of TCZ and present results from pivotal trials regarding efficacy, safety and tolerability of TCZ in patients with RA.
Methodology
To review the pharmacology of TCZ, relevant studies were selected as part of a narrative review to determine its pharmacokinetics and pharmacodynamic properties. Relevant information was extracted from the identified articles and their references to identify pertinent publications including meta-analyses, review articles and pharmacologic studies.
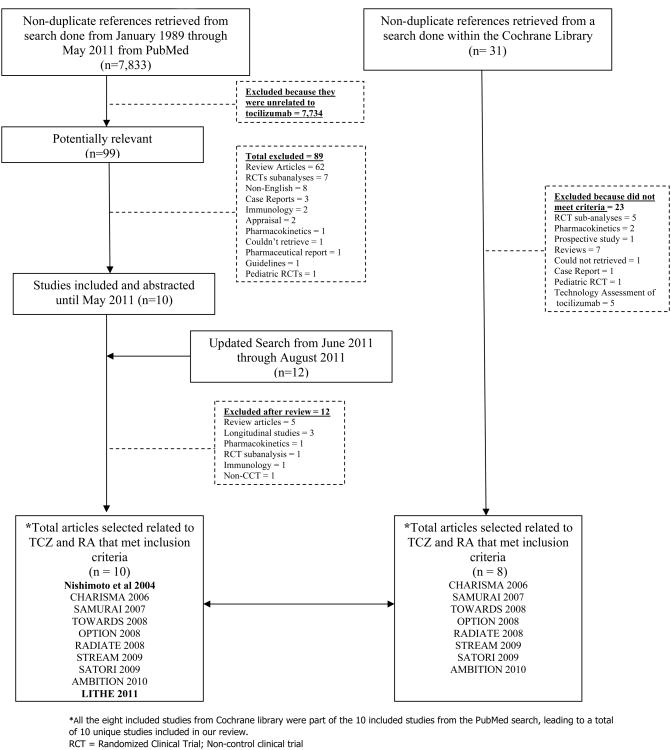
To establish the evidence base for the remainder of the review, a systematic literature review using PubMed and the Cochrane Library was conducted to identify English language articles reporting results from randomized controlled (RCT), controlled clinical trials (CCT) of TCZ, non-control clinical trials and their open-label extensions following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines for systematic review (19). The literature search used was performed by our group as part of the 2008 American College of Rheumatology (ACR) recommendations for the treatment of RA (20) and the ongoing 2012 update. As part of the systematic literature review for the RA guidelines, we searched Pubmed from January 1989 through March 2010. for six older biologics (etanercept, infliximab, adalimumab, abatacept, anakinra, rituximab) and three newer biologics (golimumab, certolizumab, tocilizumab). From April 2010 through May 2011 we searched PubMed for TCZ and RA articles only which added up to a total of 7,833 potential articles for RA and TCZ from combined Pubmed searches from January 1989 through May 2011. An update to this search was made from May 2011 through August 2011 with 11 new potential articles. An additional search was done within the Cochrane library for tocilizumab using the same terms used in the pubmed search until August 2011 (n = 31) (See online supplement Appendix 1 for more details). Risk of bias for each of the selected RCTs was assessed according to the Review Manager (RevMan) version 5.1 used to prepare Cochrane systematic reviews (21) . Agreement between the authors was established to finalize the assessment of risk of bias. A flow diagram for study selection is provided in Figure 1 and the risk of bias for each clinical trial retrieved is presented in Appendix 2 (online supplement).
Figure 1.
Flow chart of literature search for biologic agents from January 1989 through August 2011 within PubMed and the Cochrane Library. RCT randomized controlled trials; CCT = controlled clinical trials. CHARISMA = Chugai Humanized Anti-Human Recombinant Interleukin-6 Monoclonal Antibody; SAMURAI = Study of Active Controlled Monotherapy Used for Rheumatoid Arthritis, an IL-6 Inhibitor; TOWARD = Tocilizumab in Combination With Traditional DMARD Therapy; OPTION Tocilizumab Pivotal Trial in Methotrexate Inadequate Responders; RADIATE = Research on Actemra Determining efficacy after Anti-TNF failurEs; STREAM = Safety and Efficacy of Tocilizumab, an anti-IL-6 receptor monoclonal antibody, in Monotherapy, in Patients With Rheumatoid Arthritis; SATORI = Study of Active Controlled TCZ Monotherapy for RA Patients with and Inadequate Response to Methotrexate; AMBITION = Actemra Versus Methotrexate Double-Blind Investigative Trial in Monotherapy; LITHE = Tocilizumab Safety and the Prevention of Structural Joint Damage. *All the 8 included studies from Cochrane library were part of the 10 included studies from the PubMed search, leading to a total of 10 unique studies included in our review.
As inclusion criteria, the studies required the TCZ trials to be conducted for an RA population with a comparator group and contain outcomes for one or more of the following: ACR20, 50, 70; tender/swollen joint count, health assessment questionnaire (HAQ) disability, radiographic outcomes and drug persistence. For adverse events and safety, the studies must have had one or more of the following: drug terminations, adverse events, serious adverse events, infections, serious infections (defined by need for intravenous antibiotics or hospitalization) and selected specific morbidities (e.g. infusion or injection site reaction, cancer, heart failure, auto-antibody production) and mortality. (See online supplement Appendix 3 for further details on inclusion/exclusion criteria and search strategy). We searched for the following terms: IL-6, actemra, tocilizumab, rheumatoid arthritis, IL-6 receptor inhibitor, joint damage, radiographic damage, myeloma receptor antibody (MRA) and clinical trials.
The phase III clinical trials were prioritized for more extensive discussion for this review over phase II trials since these provided more extensive data on efficacy and safety. Non-phase III clinical trials were selected and reviewed only if they provided unique information for dosing, long-term safety or radiographic outcomes that were not contained in any phase III clinical trials.
Results
Pharmacology of IL-6 receptor antagonist, tocilizumab
TCZ is a recombinant humanized monoclonal antibody of the IgG1 subclass against the IL-6 receptor. Its molecular weight is approximately 150 kd (22), and it binds to sIL-6R in a dose-dependent manner and saturates the receptor at approximately 0.1 μg/mL. It also competitively inhibits IL-6 binding to sIL-6R; complete inhibition is seen at approximately 4 μg/mL (23).
The pharmacokinetics of intravenous (IV) TCZ have been determined using a population pharmacokinetic analysis on a database composed of 1,793 patients with RA treated with one-hour infusions of 4 and 8 mg/Kg TCZ IV every 4 weeks for 24 weeks (24-25). TCZ is metabolized by the reticuloendothelial system like endogenous IgG. The half-life is concentration-dependent (first order kinetics), up to 11 days for 4mg/Kg IV and up to 13 days for 8mg/Kg IV every 4 weeks at steady-state (26). The maximum concentration increases in proportion to increased dosages. Following intravenous administration, it undergoes biphasic elimination from the circulation. At higher concentration the elimination is predominantly linear, while at a low TCZ concentration the clearance is non-linear (25-26).
In patients with RA the central volume of distribution was 3.5 liters, and the peripheral volume of distribution 2.9 liters, resulting in a volume of distribution at steady state of 6.4 liters (25-26). The area under the curve (AUC) at 28 days for TCZ 4 mg/Kg IV was 13 ± 6 mg·hour/mL and the minimum (Cmin) and maximum (Cmax) concentrations were 1.47 ± 2.07 and 88 ± 41, respectively. For TCZ 8 mg/Kg IV the AUC at 28 days was 34 ± 15 mg·hour/mL and the Cmin and Cmax of 9.52 ± 10.1 and 181 ± 85, respectively (27). Maintenance of higher through levels of serum TCZ seems to be important to achieve clinical efficacy as reported in a small study (1).
Efficacy and Safety of TCZ from Clinical Trials
Using results from the systematic literature review, ten published clinical trials for TCZ were retrieved (double blind, open label extensions and single blinded studies), including 7 randomised phase III clinical trials (28-34), one phase II European clinical trial (35), a Japanese 5 year extension study of an initial phase II trial (36), and phase II Japanese clinical trial (37). These studies were selected from the initial 7,833 articles on biologics identified from January 1989 through August 2011 in the PubMed library. Within the Cochrane library, a total of 31 articles related to TCZ and RA were retrieved until August 2011 and 8 out of the 10 articles retrieved and selected in PubMed were also retrieved in the Cochrane library. No additional study within Cochrane met inclusion criteria for this review.
Among the phase II clinical trials retrieved, we included the “Chugai Humanized Anti-Human Recombinant Interleukin-6 Monoclonal Antibody (CHARISMA) study” (35) since this study provided information on the doses of TCZ that were subsequently used in the phase III studies. “The safety and efficacy of TCZ, in monotherapy, in patients with RA” (STREAM) study, was an open label, long term 5-year extension trial following an initial 3-month randomized phase II Japanese trial (36) that provided more extensive information regarding long-term safety. A multicenter 3-month clinical trial conducted in Japan (37) of TCZ monotherapy at 2 different doses 4mg/Kg and 8 mg/Kg versus placebo (no actual therapy) showed TCZ had better over the placebo group. ACR20 response was better in the 2 TCZ arms compared to placebo (p < 0.001 for both doses). ACR50 response rate was higher compared to placebo (p < 0.001 for both doses of TCZ). ACR70 was also significantly different from placebo (p = 0.001 for TCZ 4 mg/Kg and p = 0.002 for TCZ 8 mg/Kg).
There were seven phase III clinical trials (28-34) that evaluated the efficacy, safety profile, and radiographic progression of TCZ in patients with RA. The patient population, short term safety profile, efficacy of TCZ, inflammatory markers, (i.e. CRP, ESR) and clinical responses based on Disease Activity Score (DAS28) and American College of Rheumatology (ACR) parameters that were measured in the phase III clinical trials are summarized in Table 1.
Table 1. Efficacy of Phase III Clinical Trials.
| Name, Phase, Duration | OPTION Phase 3 RCT 24 wk | TOWARD Phase 3 RCT 24 wk | RADIATE Phase 3 RCT 24 wk | AMBITION Phase 3 RCT 24 wk | LITHE Phase 3 RCT 52 wk | SAMURAI† Phase 3 RCT 52 wk | SATORI Phase 3 RCT 24 wk | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | Moderate to severe RA, MTX-IR | Moderate to severe RA, DMARD-IR | Moderate to severe RA, anti-TNF-IR | Moderate to severe RA, not MTX failures | RA < 5 years, DMARD-IR | Moderate to severe RA, DMARD-IR | Moderate to severe RA, MTX-IR | |||||||||
| Treatment | TCZ 4mg/kg + MTX | TCZ 8mg/kg + MTX | PBO + MTX | TCZ 8mg/kg + DMARDs | PBO + DMARDs | TCZ 4mg/kg + MTX | TCZ 8mg/kg + MTX | PBO + MTX | MTX | TCZ 8mg/kg monotherapy | MTX +PBO | TCZ 8mg/kg | TCZ 8mg/kg | MTX 8 mg every week | TCZ 8mg/kg + DMARDs | MTX 8 mg q week DMARDs |
| ACR20, % | 48a | 59a | 27 | 61a | 25 | 30a | 50a | 10 | 53 | 70a | 27 | 56b | 78c | 34 | 80c | 25 |
| ACR50, % | 32a | 44a | 11 | 38a | 9 | 17a | 29a | 4 | 34 | 44a | 10 | 32b | 64c | 10 | 49 | 10 |
| ACR70, % | 12a | 22a | 2 | 21a | 3 | 5 | 12b | 1 | 15 | 28a | 2 | 13b | 44c | 30 | 6 | |
| DAS28 remission (<2.6), % | 13b | 27a | 0.8 | 30a | 3 | 8 | 30a | 2 | 12 | 34 | 7 | 8b | 59c | 3 | 43c | 2 |
| TSS (vdH) (mean change from baseline at week 52) | ND | ND | ND | ND | ND | 1.39 | 0.29a | 2.3b | 6.1 | ND | ||||||
| HAQ-DI (mean change from baseline) | −0.52d | −0.55d | −0.34 | −0.5a | −0.2 | −0.31b | −0.39a | −0.05 | −0.5 | −0.7 | 52d,e | 63b,e | −0.50c | −0.13 | −0.40b | −0.18 |
| CRP (mean change from baseline) | −16.6d | −25.1a | −3.5 | −2.20a | −0.27 | Not normalized | Normalized (<0.3 mg/dL) | Not normalized | −1.9 | −2.8 | NR | NR | NR | NR | ||
| FACIT* (Mean Change from Baseline) | 7.3b | 8.6a | 4.0 | 8a | 3.6 | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| SF-36** (Mean Change from Baseline) | 9.7a | 9.5a | 5 | 8.9a | 4.1 | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| VEGF*** (Mean Change from Baseline) | ND | ND | ND | ND | ND | ND | −347c | −74 | ||||||||
p<0.0001,
p<0.01,
p<0.001
p <0.05 vs control
TCZ: tocilizumab, MTX: methotrexate, anti-TNF: TNF antagonist, DMARD: disease modifying anti-rheumatic drug, IR: inadequate response, PBO: placebo, TSS (vdH): van der Heijde modified total Sharp score, HAQ-DI: Health Assessment Questionnaire – Disability Index, CRP: C-reactive protein, ND: not determined, NR: not reported
modified HAQ, % patients with decrease ≥ 0.3 units
FACIT = Functional Assessment of Chronic Illness Therapy-Fatigue
SF-36 = Short Form 36 (physical)
VEGF vascular endothelial growth factor;
ACR20, 50, 70, DAS28 and mHAQ assessed unblinded
The ACR definition of improvement in RA was the primary efficacy measure in the TCZ trials. The ACR response criteria is a composite outcome derived from a core set of seven measures, summarizing the improvement (i.e. change) in disease activity from baseline. Although somewhat oversimplified, ACR response is defined by a decrease of ≥20, 50 or 70% in a formula that includes tender and swollen-joint counts, the patient's and the physician's global assessments of disease activity, patient's assessment of pain, health assessment questionnaire (HAQ) disability score (HAQ-DI), and an acute-phase reactants (CRP or ESR) (38-39). It is currently a key criterion for regulatory decisions by the US Food and Drug Administration (FDA) with respect to anti-rheumatic drugs that seek an indication to reduce the signs and symptoms of RA.
Clinical response to tocilizumab: (Phase III studies)
The Study of active controlled TCZ monotherapy for RA patients with inadequate response to MTX (SATORI) (33) besides evaluating the efficacy and safety of TCZ, the investigators also studied the effect of TCZ on VEGF. This trial consisted of 2 arms, TCZ 8 mg/Kg monotherapy every 4 weeks and MTX 8 mg monotherapy PO every week throughout the 24 weeks of the study without concomitant folic acid supplementation. At 24 weeks 80% of the patients receiving TCZ achieved ACR20 versus 25% in the MTX group (p < 0.001). The ACR50 and ACR70 response rates in the TCZ group were higher than in the control group at all time points from week 4 in both TCZ doses compared to placebo. The reduction in DAS28 (p < 0.001) and modified HAQ (mHAQ) (p < 0.05) was greater for the TCZ group versus placebo. TCZ also caused a greater reduction in VEGF levels compared to placebo (p < 0.001) which is thought to be a major contributor to angiogenesis and pannus formation in patients with RA (5).
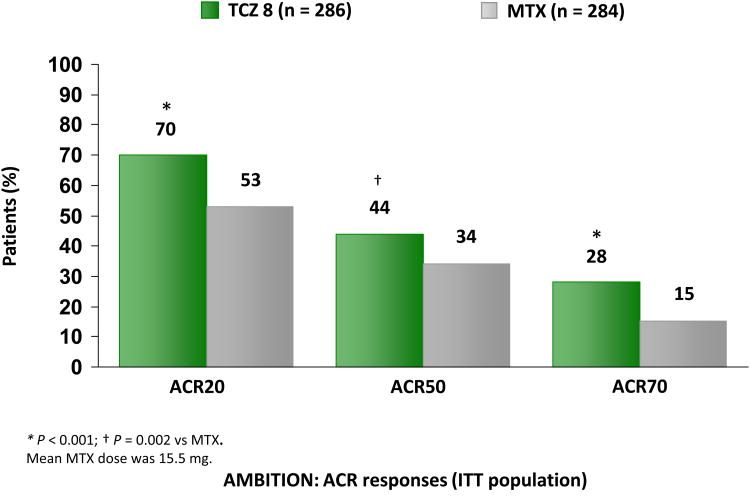
In four out of the five multinational phase III clinical trials, patients were required to have had an inadequate response to oral methotrexate (MTX), disease-modifying anti-rheumatic drugs (DMARDs) but not have failed anti-TNF therapy. In the fifth trial, the TCZ versus MTX double-blind investigative trial in monotherapy (AMBITION) trial (30), the efficacy and safety profile of TCZ 8mg/Kg IV monotherapy was compared to oral MTX 7.5-20 mg every week in moderate to severe RA for whom treatment with MTX or biological agents had not previously failed. Approximately 66% of the patients in the trial were MTX naïve. Patients in the TCZ 8mg/Kg IV monotherapy group had a better ACR20, 50 (p < 0.001 and p < 0.002, respectively) response than those on the oral MTX 7.5-20 mg every week group at the end of 24 weeks (Figure 2). TCZ 8mg/Kg also was superior to oral MTX as monotherapy in improving disease activity parameters including DAS28 and functional ability assessed by HAQ-DI.
Figure 2.
Tocilizumab (TCZ) response rate compared with methotrexate (MTX) at 24 weeks in patients who were treated previously with MTX. The ACR response is defined by a decrease of ≥ 20%, 50%, or 70% in a formula that includes tender and swollen joint counts, the patient's and the physician's global assessments of disease activity, patient's assessment of pain, HAQ-DI score, and acute-phase reactants (CRP or ESR). ACR = American College of Rheumatology; ITT = intention-to-treat. *P< 0.001; †P = 0.002 versus MTX. The mean MTX dose was 15.5 mg.29
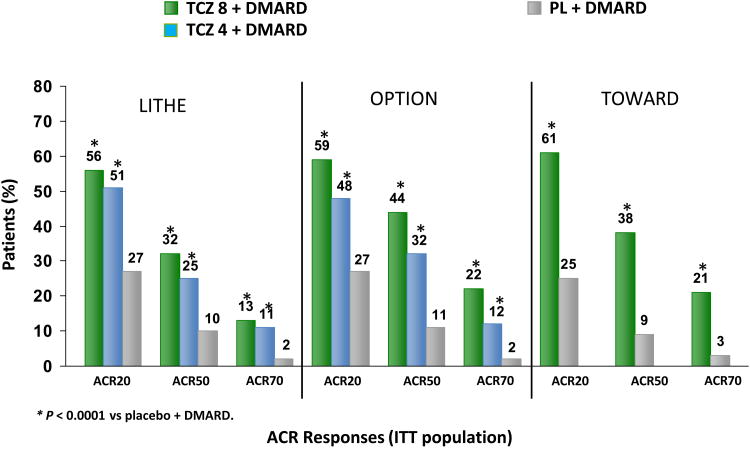
TCZ in combination with traditional DMARD therapy (TOWARD) (29), the TCZ pivotal trial in MTX inadequate responders (OPTION) (28) and the TCZ safety and the prevention of structural joint damage (LITHE) (32) studies evaluated TCZ 4 and 8 mg/Kg IV in combination with oral MTX 7.5-20 mg every week (28, 32) or in combination with another DMARD (29) in patients with moderate to severe RA. In the TOWARD (29) study the most commonly used DMARD at baseline was MTX. The patient population included those who had an incomplete response to previous MTX/DMARDs therapy and was biologic-naïve or on anti-TNF therapy (as long as they did not have an incomplete response to anti-TNF). The duration of these studies was 24 weeks (28-29) and 2 years (32), respectively and the RA disease duration ≥6 months. The primary end point was the proportion of patients who achieved an ACR20/50/70 at 24 weeks resulting in a 60%, 40%%, 20% response respectively. (Figure 3) Other clinical response based on DAS28, HAQ-DI, Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT), and Short Form-36 (SF-36) at 24 weeks was superior and statistically significant (See table 1 for results) in the TCZ groups of these trials. Different doses of TCZ were also tested (4mg/Kg and 8mg/Kg), with the 8mg/Kg dose yielding numerically superior ACR response rates compared to placebo.
Figure 3.
American College of Rheumatology (ACR) response rates with tocilizumab (TCZ) in patients at 24 weeks at 4 and 8 mg/kg plus disease-modifying antirheumatic drugs (DMARD) in the LITHE (Tocilizumab Safety and the Prevention of Structural Joint Damage), OPTION (Tocilizumab Pivotal Trial in Methotrexate Inadequate Responders), and TOWARD (Tocilizumab in Combination With Traditional DMARD Therapy) trials. The ACR response is defined by a decrease of ≥ 20%, 50%, or 70% in a formula that includes tender and swollen joint counts, the patient's and the physician's global assessments of disease activity, patient's assessment of pain, HAQ-DI score, and acute-phase reactants(CRP or ESR). *P < 0.0001 versus placebo plus DMARD. ITT = intention-to-treat. †In the LITHE study, the difference between the tocilizumab 4 mg/kg plus MTX versus placebo plus MTX groups was significant at P < 0.05; for this comparison, however, the test fell after the break in hierarchical chain of the algorithm; therefore, statistical significance is not claimed.27,28,31,39
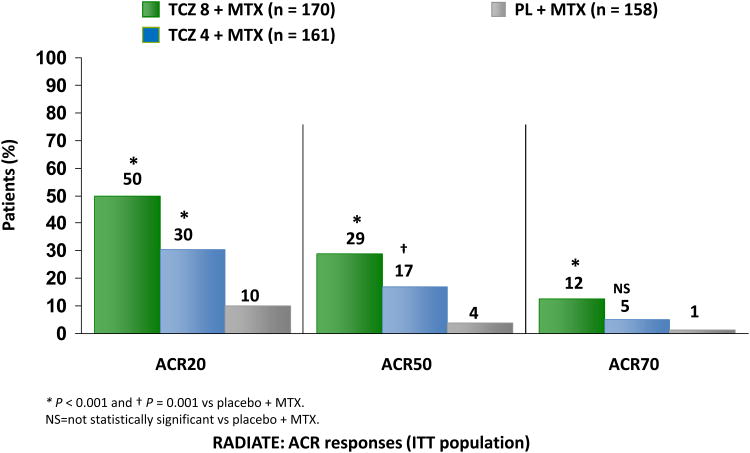
The RA study in anti-TNF-failures (RADIATE) (31) examined the efficacy and safety of TCZ in 499 patients with RA that failed to respond to anti-TNF therapy; most failed for lack of efficacy (Figure 4). Overall, patients who received TCZ 8mg/Kg and 4mg/Kg IV achieved an ACR20 response of 50% and 30% respectively compared to 10% in the oral MTX 10-25 mg every week + placebo group (p<0.001). The authors also analyzed the differences in the rate of response to TCZ based on the type and number of anti-TNF therapies that the patients failed to respond to. The clinical response was comparable irrespective of the type or number of failed anti-TNF (failed only 1, or failed more than 1), although the study was not powered to explicitly examine this subgroup analysis. Overall, significant improvement was observed in the various components of clinical response including SJC, and TJC (p-value < 0.001 for both TCZ dosage groups versus control) as well as physical function (p-value < 0.001 for TCZ 8 mg/Kg IV and p = 0.003 for TCZ 4 mg/Kg IV versus control).
Figure 4.
Efficacy and response rate of tocilizumab in patients exhibiting incomplete response (IR) to anti.tumor necrosis factor (TNF) therapy. *P < 0.001 and †P = 0.001 versus methotrexate (MTX) plus placebo. NS = not statistically significant versus MTX plus placebo. ACR = American College of Rheumatology; RADIATE = Research on Actemra Determining efficacy after Anti-TNF failurEs. The ACR response is defined by a decrease of ≥20%, 50%, or70% in a formula that includes tender and swollen joint counts, the patient's and the physician's global assessments of disease activity, patient's assessment of pain, HAQ-DI score, and acute-phase reactants (CRP or ESR).
Radiographic progression with tocilizumab
The study of active controlled monotherapy used for RA, an interleukin-6 inhibitor (SAMURAI) (34) was a single blinded 52 week study performed in Japan which evaluated the radiographic and clinical benefits that TCZ 8 mg/Kg IV monotherapy versus conventional DMARDs therapy provided to patients with active RA. Readers were blinded to treatment allocation. At 52 weeks, 56% of TCZ-treated patients had no radiographic progression compared with 39% of those receiving conventional DMARDs (p < 0.01). The ACR20, ACR50 and ACR70 response achieved statistical significance for TCZ 8 mg/Kg IV monotherapy compared to conventional oral DMARDs therapy (p<0.001, for each comparison). This indicated superiority of TCZ 8 mg/Kg IV monotherapy; however, these clinical endpoints were assessed unblinded.
The LITHE study was the only multinational phase 3 study that measured radiographic progression of RA (32, 40). This was a 3-arm trial of TCZ 4 mg, 8 mg IV or placebo in combination with MTX 10-25 mg a week. The length of the controlled phase of the study was 2 years; however, only results from year 1 are published (32). LITHE included patients with RA that had an incomplete response to MTX and that did not fail anti-TNF treatment. At 52 weeks, progression of structural damage from baseline was reduced by 74% and 70% with TCZ 8 mg/Kg and 4 mg/Kg respectively as compared to controls (p<0.0001), with mean change in Genant-modified Sharp score of 0.29, 0.34 and 1.39 for TCZ 8 mg/Kg, 4mg/Kg and placebo respectively (p<0.0001). At 2 years, patients in the TCZ 8 mg/Kg + MTX 10-25 mg a week group had significantly less radiographic progression, 85% vs 67% compared to placebo (p<0.001) (32, 40).
Safety profile and laboratory parameters
TCZ, at the two recommended doses 4mg/Kg and 8mg/Kg IV, is currently approved in the U.S. as monotherapy or in combination with MTX or other DMARDs. The rates of treatment-emergent serious adverse events were generally low but increased slightly when TCZ was given in combination with MTX or DMARDs. The rate of serious infections in the 6-month control studies with TCZ 4 and 8 mg/kg IV plus DMARDs was 4.4 and 5.3 events per 100 patient years exposure compared to 3.9 events per 100 patient years exposure in the placebo plus DMARD group. In the monotherapy study, the rate of serious infections was 3.6 events per 100 patient years of exposure in the TCZ 8 mg/Kg IV group and 1.5 events per 100 patient years of exposure in the oral MTX group. In the all-exposure population, the overall rate of serious infections was 4.7 events per 100 patient-years of which the most commonly reported included pneumonia, cellulitis, herpes zoster, gastroenteritis, diverticulitis, sepsis and bacterial arthritis. Serious infections were rarely fatal (rate 0.13 per 100 patients years) (25-26).
Reports of gastrointestinal perforation on TCZ were rare with an overall rate of gastrointestinal perforation of 0.28 events per 100 patient-years primarily reported as complications of diverticulitis (25-26). TCZ may suppress inflammatory and systemic symptoms like fever and delay the detection of diverticulitis. Most cases of GI perforations (31) occurred in patients who were using systemic steroids, non-steroidal anti-inflammatory drugs (NSAIDs) or had a history of diverticulitis. Long-term follow up is needed to evaluate whether there is a causal relationship between TCZ and GI perforation (25-26, 31).
Serious TCZ infusion reactions (occurring during or within 24 hours of the start of infusion) were reported in 8%, 7%, 5% of patients in the 4 mg/kg TCZ plus DMARDs, 8 mg/kg TCZ IV plus DMARDs and placebo plus DMARDs groups, respectively. The most frequently reported event on the TCZ 4 mg/kg and 8 mg/kg IV dose during the infusion was hypertension (1% for both doses). The most frequently reported events occurring within 24 hours of completing an infusion were headache (1% for both doses) and skin reactions (1% for both doses), including rash, pruritus and urticaria. Clinically significant hypersensitivity reactions requiring treatment discontinuation were reported in 0.2% (9/4009) in the all-exposure population (26).
In the five multinational phase III trials, the incidence of TCZ antibodies was rare. Patients who developed them were mostly receiving concomitant MTX (28). One Japanese phase II trial had 2 patients with anti-tocilizumab antibodies (37). Maini et al (35) reported 25 patients with anti-TCZ antibodies while they were on low dose TCZ monotherapy at 2 mg/Kg or 4 mg/Kg IV. He also reported that anaphylaxis and anaphylactoid reactions occurred at low doses of TCZ (1 patient receiving TCZ monotherapy 4mg/Kg and 1 receiving combination therapy of TZC 2mg/Kg with MTX). In that small study, no patients receiving TCZ 8mg/Kg as either monotherapy or combination therapy developed anti-tocilizumab antibodies (35)
No cases of tuberculosis (TB) were reported in any of the five phase 3 trials; however, there was one case of Mycobacterium avium complex (29) and one case of Pneumocystis jiroveci infection reported (28). In a five year open label extension of the STREAM (36) study, there were no reported cases of TB. Since the risk for active TB associated with TCZ has not been well established, the usual protocol for screening and surveillance for TB in anti-TNF therapy is also recommended for TCZ (26). One patient died of reactivation of Epstein-Barr virus (EBV) infection and consequent hemophagocytosis syndrome in a Japanese phase II trial (37).
Changes in the following lab parameters were observed with both doses: increase in hemoglobin and decreases in rheumatoid factor, CRP, ESR and serum amyloid A. The most profound was a sustained decrease in CRP levels observed and maintained through week 24 in patients receiving 8 mg/kg IV of TCZ monthly plus oral MTX 7.5 mg – 25 mg every week, many patients achieved normal CRP levels at the 8mg/kg dose (26, 28-29, 31-32, 35, 40-42); however, the decrease in CRP seen with the TCZ 4 mg/kg IV + plus oral MTX group was not sustained but rather oscillated between normal and elevated.
IL-6 stimulates the production of hepcidin, a liver peptide that modulates hemoglobin production by restricting iron availability and plays an important role in the pathogenesis of the anemia of chronic disease. Effective blockade of IL-6R can decrease hepcidin levels and result in an elevation in hemoglobin production, generally in the range of <13 g/dL (29, 42-44). This effect was only observed in people who started with a baseline anemia and did not occur for patients who had a normal hemoglobin at baseline.
Tocilizumab, tended to elevate liver enzymes (Table 2) and lipid levels. The incidence of elevation of liver enzymes was higher in those patients treated with TCZ in these clinical trials, especially when combined with potentially hepatotoxic medications like MTX. TCZ had an increased risk for elevating liver enzymes. In phase III trials, 34-41% of patients had at least once occurrence of liver enzymes elevation 3X the upper limit of normal when TCZ was given with either MTX or DMARDs versus 17% in the placebo group (Table 2). This elevation was not sustained, and in most patients values normalized while either continuing TCZ or after temporary interruption of study treatment and/or lowering MTX dosage (28-29).
Table 2. Frequency of Liver Associated Enzymes Abnormalities in TCZ Phase III Clinical Trialsⱡ.
| Liver Function Tests | Monotherapy | CombinationTherapy | |||
|---|---|---|---|---|---|
| TCZ 8mg (n=288) | MTX (n= 284) | TCZ 8mg +DMARDs‡ (n=1582) | TCZ 4mg + MTX (n=744) | Placebo + DMARDs‡(n=1170) | |
| Aspartate Aminotransferase* (AST) | 22% | 26% | 41% | 34% | 17% |
| >ULN to 3× ULN | |||||
| >3× ULN to 5× ULN | 0.3% | 2% | 2% | 1% | 0.3% |
| >5× ULN | 0.7% | 0.4% | 0.2% | 0.1% | <0.1% |
| Alanine Aminotransferase* (ALT) | 36% | 33% | 48% | 45% | 23% |
| >ULN to 3× ULN | |||||
| >3× ULN to 5× ULN | 1% | 4% | 5% | 5% | 1% |
| >5× ULN | 0.7% | 1% | 1.5% | 1.3% | 0.3% |
Disease-Modifying Anti-Rheumatic Drugs
Aspartate aminotransferase (AST) ULN = 40 U/L; Alanine aminotransferase (ALT) ULN = 55 U/L ULN= Upper Limit of Normal.
Source: GENETECH. Prescibing Information. http://wwwgenecom/gene/products/information/actemra/pdf/pipdf. 2010 Accessed 2010 May 18.
Increases in mean fasting plasma lipid levels were seen in TCZ trials (26, 28-31, 33, 35, 37). Elevations from baseline in lipid parameters were observed at the first assessment (6 weeks) following initiation of TCZ but remained stable thereafter. Seven patients in the OPTION (28), 16 in TOWARD (35) and 31 in the LITHE (32) studies initiated lipid lowering therapy. This treatment decreased lipid levels back or close to normal level. The package insert recommends that lipid levels be checked 4-8 weeks after initiating TCZ, and every 6 months thereafter (26, 31, 33-36). There were no ischemic cardiac disorders or events associated with TCZ treatment during these trials. One myocardial infarction occurred in the RADIATE study in the control group (31). Increases in triglycerides to levels >500 mg/dL were rarely observed, without evidence of pancreatitis (29). Patients with elevation in plasma cholesterol should be treated based on the guidelines for lipid management recommended by the Adult Treatment Panel III (45). In phase III trials, hyperlipidemia responded to lipid-lowering treatment.
In the 6-month controlled studies, authors reported “no clear relationship between decreases in neutrophils <1000/mm3 and the occurrence of serious infections or neutropenic fever” [24-26, 28]. The neutropenia was transient, and in the majority of cases there was no need for discontinuation of TCZ treatment. (Table 3) Nevertheless, patients that had grade 4 neutropenia or absolute neutrophil count <500 were withdrawn from these studies per protocol. Thrombocytopenia was also observed in these trials (1-2% of patients with platelet counts < 100,000; < 1% with platelet counts < 50,000) without associated clinically significant bleeding events (Table 3).
Table 3. Frequency of Neutropenia and Thrombocytopenia in TCZ Phase III Clinical Trials.
| Decrease in Neutrophil and Platelet Count | TCZ 8mg + DMARDs‡ (n=1582) | TCZ 4mg + MTX (n=774) | Placebo + DMARDs‡ (n=1170) |
|---|---|---|---|
| Neutrophils <1000/mm3 (%) | 3.4% | 1.8% | 0.1% |
| Neutrophils <500/mm3 (%) | 0.3% | 0.4% | 0.1% |
| Platelet count <100,000 | 1.7% | 1.3% | 0.5% |
Disease-Modifying Anti-Rheumatic Drugs
Source: GENETECH. Prescibing Information. http://wwwgenecom/gene/products/information/actemra/pdf/pipdf. 2010 Accessed 2010 May 18.
Dosing, Administration and Precautions
TCZ may be used as monotherapy or concomitantly with MTX or other DMARDs. In the U.S., the recommended starting dose of TCZ for adult patients is 4mg/kg IV given once every 4 weeks as a 60-minute single intravenous infusion in patients with RA that had an inadequate response to one or more TNF antagonist. An increase from 4 mg/kg IV to 8 mg/Kg IV is based on clinical response, and the optimal time for dose escalation is left to the discretion of the clinician. Dose reduction from 8 mg/Kg IV to 4 mg/Kg IV is recommended for the management of certain dose-related laboratory changes including elevation of liver enzymes, neutropenia and thrombocytopenia. Detailed recommendations regarding dose adjustment associated with certain laboratory parameters are available in the package insert. TCZ should not be administered as an IV push, bolus or with other medications through the same IV line (26).
No dose adjustment is indicated for minimal renal impairment (creatinine clearance < 80 mL/min and ≥ 50 mL/min based on Cockcroft-Gault); however for moderate-to-severe renal impairment (creatinine clearance < 50 mL/min based on Cockcroft-Gault), there is limited experience and no specific recommendations for TCZ dose adjustment. It is not recommended for use in patients with active liver disease or hepatic impairment. Regardless of the patient's weight, the maximum recommended dose for TCZ is 800mg per IV infusion.
Regular laboratory monitoring in patients receiving TCZ includes complete blood count with differential and liver function tests prior to initiation and during the course of therapy.
TCZ is listed as pregnancy category C. It has not been adequately studied in pregnant women. Efforts have been made to establish the risk of TCZ during pregnancy. A pregnancy registry exists to monitor outcomes exposed to this medication; results are not yet available. Excretion of this medication in breast milk is unknown, so its use is not recommended while nursing.
Drug Interactions
Cytochrome P450 enzymes (CYP450) in the liver are down-regulated by infection and inflammation mediated by cytokines such as IL-6. Inhibition of IL-6 signaling in patients with RA treated with TCZ may restore CYP450 activities to higher levels than those in the absence of TCZ, leading to increased metabolism of drugs that are CYP450 substrates. It is therefore recommended to monitor drugs that are metabolized by CYP450 subclasess, especially those with narrow therapeutic indices (i.e. warfarin and theophylline) or when the dose is individually adjusted (25-26). Medications that could have a decrease in their therapeutic effect include oral contraceptives, lovastatin, simvastatin and omeprazole. The dose of cyclosporine should be adjusted in the case of co-administration with TCZ, since cyclosporin concentration is decreased by this medication. Decreased concentration of the aforementioned medications can last up to a week after TCZ discontinuation (26).
Pharmacoeconomic Considerations
TCZ is dispensed in 80 mg/4 mL, 200 mg/10 mL, 400 mg/20mL vials and the acquisition cost is $295.00, $699.00 and $1,299.00 USD respectively (46). The estimated acquisition cost for a 75 Kg (165 lb) person for the first 6 months of treatment with 4 mg/Kg IV of TCZ is $5,964.00 USD and for 8 mg/Kg IV of TCZ is $11,988.00 approximately. By way of comparison, the acquisition cost for 3 mg/Kg IV of infliximab would be around $11,090.00 USD when this agent is administered at weeks 0, 2, 6 and then q 8 weeks (47) for a 6 months period. This makes the acquisition cost of these 2 agents comparable to each other, however since many patients treated with infliximab experience either dose escalation or increased frequency of the infusions to achieve efficacy (48-51), the acquisition cost of infliximab might be higher than tocilizumab.
The United Kingdom National Institute for Health and Clinical Excellence (NICE) (52) technology appraisal, in their TCZ report, concluded that TCZ with MTX is cost effective as a second-line treatment only. They also agreed that for individuals that are intolerant to rituximab or for whom rituximab is contraindicated, adding tocilizumab to the current standard of care is cost effective.
Discussion
TCZ is a humanized monoclonal antibody against the IL-6 receptor that provides a promising treatment for the management of RA. Clinical trials results show that TCZ 8mg/Kg IV monotherapy has superior efficacy to oral MTX monotherapy. In combination with MTX, TCZ is comparable to the clinical efficacy seen with MTX + anti-TNF therapy (53-55). TCZ is currently indicated as monotherapy or combination therapy with MTX or DMARDS for patients with RA with refractory disease that have failed at least one anti-TNF agent and the NICE technology appraisal concluded that TCZ with MTX is cos-effective only as second-line treatment. Its efficacy as monotherapy makes it a reasonable alternative for those patients with MTX intolerance and/or who could not tolerate anti-TNF therapy. Rates of response were found to be as early as 2 weeks at both 4 mg/Kg and 8 mg/Kg doses. TCZ has been predominantly tested and efficacious in the RA population that had an incomplete response to MTX and that has not failed an anti-TNF medication (28-30, 32). An important aspect to consider regarding phase III trials performed in Japan (33-34), is that these studies used as comparator group placebo (no treatment) or fixed low doses of MTX (8 mg every week). These aspects could lead to an overestimation of the effect of TCZ, even after taking into consideration physiologic differences in the Japanese population compared to that of other countries. Even though not discussed in this review, TCZ is also effective in systemic onset juvenile idiopathic arthritis (soJIA) (56-57) and has been approved by the FDA for this condition.
The risk of bias in the clinical trials selected for this review was low overall. Major points for concern were that some trials had some efficacy outcomes (secondary) unblinded, others failed to report management of missing data and that these studies where sponsored by pharmaceutical companies and most the investigators reported conflict of interest related to honoraria received from the sponsoring pharmaceutical company. One investigator reported holding a patent for TCZ in one of the trials (33).
As discussed earlier, TCZ had significant effects on laboratory parameters of inflammation including ESR and CRP. The decrease in CRP is rapid and profound. It has been postulated that this may be partially due to a direct effect of blocking IL-6R on CRP besides the actual decrease in systemic inflammation (58), but more studies are needed to confirm this hypothesis. Elevations in lipids were observed, but the clinical significance of this is unclear (59-61). It has been postulated that when lipids levels increase secondary to response to RA treatment and control of systemic inflammation, the accompanying rise in cholesterol may not confer an increased risk for cardiovascular events (59); more long term CV data is needed to test this hypothesis.
Several strengths of this study worth mentioning include the systematic review of the literature which is for the most part in agreement with the PRISMA statement and that most of the evidence based used in this review was derived from double blind RCTs. Although our literature search procedures were extensive this study has several limitations. First, this study excluded non-English studies that could have relevant information regarding TCZ. Second, publication bias is another limitation of this and other reviews, which can lead to overestimation of the actual effect and benefit of TCZ. Third, the risk of selection bias remains possible, but this was minimized in this review by the systematic way that the articles were selected.
Short-term efficacy data are adequate, but longer-term safety surveillance studies are needed, since the TCZ trials were not designed to assess long term safety risk (infections, cardiovascular, GI perforation) in real-world settings. Comparative effectiveness and phamacoeconomic data for TCZ and other biologics is also needed to help with more informed decision-making and to select among the many available treatment options for RA patients.
Conclusion
TCZ is a new therapeutic option for patients with RA with refractory disease that have failed at least one anti-TNF therapy. It appears to have a similar safety profile to other biologics based on current data available from published trials. While short term efficacy and safety profile are promising, additional long term safety data is needed to better characterize the risk-benefit profile of this agent.
Supplementary Material
Appendix 1.
A. PubMed Search Strategy for tocilizumab articles from before 2007
We used the systematic review approach from a previous search done for DMARDs and six biologics (etanercept, infliximab, adalimumab, anakinra, abatacept and rituximab) for the treatment of RA that was performed in PUBMED from January 1989-December, 2006. An updated search was done in PUBMED until August 2011, which not only included the previous DMARDs and six biologics, but also included three new biologics (golimumab, certolizumab, tocilizumab) approved for RA between 2007 and 2011. For efficacy, studies had to be “clinical trials”, comparator group should be placebo or other therapies and clinical outcomes should include ACR 20, 50, 70 among others; see appendix 3 for more details. For safety, specific searches were done for toxicity monitoring and screening for TB using the following terms. For TB, the entry term ‘tuberculosis’ was combined with the intervention terms. For monitoring of side-effects, the entry terms ‘contraindications’, ‘adverse effects’, ‘drug monitoring’, and ‘complications’ were combined with the intervention terms. Details of this search are provided in the previous publication, also summarized in appendix 2a. This library was then search for relevant studies of tocilizumab using the terms “rheumatoid arthritis” and one of the following terms—“tocilizumab”, “interleukin-6 receptor inhibitor”, “myeloma receptor antibody”, “clinical trials”, “joint damage” and radiographic joint damage”
B. Cochrane library Search Strategy for tocilizumab articles
We searched the Cochrane library until August 2011 using he terms ‘rheumatoid arthritis’, “clinical trials” and any of the terms for tocilizumab – “tocilizumab”, “interleukin-6 receptor inhibitor” and “myeloma receptor antibody”.
Appendix 2.
Assessment of Risk of Bias* on Selected Studies for the Systematic Review of Tocilizumab for Rheumatoid Arthritis.
| Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias | |
|---|---|---|---|---|---|---|---|
| OPTION | Low | Low | Low | Low | Low | Low | High |
| TOWARD | Unclear | Unclear | Low | Low | Low | Low | High |
| RADIATE | Unclear | Unclear | Low | Low | Low | Low | High |
| AMBITION | Unclear | Unclear | Low | Low | Unclear | Low | High |
| LITHE | Unclear | Unclear | High | High | Low | Low | High |
| CHARISMA | Low | Low | Low | Low | High | Low | High |
| SAMURAI | Low | Unclear | High | High | Low | Low | High |
| STREAM | Unclear | Unclear | Low | Low | Low | Low | High |
| SATORI | Low | Low | Low | Low | Low | Low | High |
| Nishimoto et al 2004 | Unclear | Unclear | Low | Low | Low | Low | High |
Risk of bias legend: Low, High, Unclear
A. Random Sequence Generation: The investigators provided a random component in the sequence generation process = Low; Non-random component in the sequence generation was provided = High; Insufficient information to permit judgment of low risk or high risk = Unclear
B. Allocation Concealment: Participants and investigators could not foresee assignment = Low; Participants and investigators could foresee assignment and thus introduce bias = High; Insufficient information to permit judgment of low risk or high risk = Unclear
C. Blinding of Participants and Personnel: Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken = Low; No blinding or incomplete blinding of personnel and participants. Blinding attempted but likely this one could have been broken = High; Insufficient information to permit judgment of low risk or high risk = Unclear
D. Blinding of Outcome Assessment: Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken = Low; No blinding or incomplete blinding of outcome. Blinding attempted but likely this one could have been broken = High; Insufficient information to permit judgment of low risk or high risk = Unclear
E. Incomplete Outcome Data: No missing data or data have been imputed using appropriate methods = Low; Reason for missing data related to true outcome. Potentially inappropriate application of simple imputation = High; Insufficient information to permit judgment of low risk or high risk = Unclear
F. Selective Reporting: Study protocol available and all primary and secondary outcomes have been well reported. If protocol not available, this information is clear in the published reports = Low; Not all of the study's pre-specified primary outcomes have been reported = High; Insufficient information to permit judgment of low risk or high risk = Unclear
G. Other Bias: The study appears to be free of other sources of bias = Low; There is at least one important risk of bias (i.e. conflict of interest between sponsors and investigators) = High; Insufficient information to permit judgment of low risk or high risk = Unclear.
Source: Review Manager (RevMan) version 5.1 for Cochrane systematic reviews.
Appendix 3.
Inclusion Criteria of Studies for Efficacy/Indications and Adverse Events/Safety.
| Efficacy/Indications | Adverse Events/Safety | |
|---|---|---|
| Study design | Per ACR RA guidelines, Randomized Clinical Trials (RCT), Controlled Clinical Trials (CCT), Quasi-experimental designs, Cohort studies (prospective or retrospective) and Case-control studies. For the tocilizumab review, Randomized Clinical Trials (RCT), Controlled Clinical Trials (CCT) were considered. Priority given to phase III RCT. Quasi-experimental designs, Cohort studies (prospective or retrospective) and Case-control studies were not selected for final review in this article even though they were initially selected during the main search. | Per ACR RA guidelines, RCTs, CCTs, Quasi-experimental designs, Cohort studies (prospective or retrospective), case-control studies, uncontrolled open-label extension trials, and case series (n > 30). For the tocilizumab review, RCTs, CCTs and open label studies as long as they were extensions of initial RCTs to asses safety. Quasi-experimental designs, Cohort studies (prospective or retrospective) and Case-control studies were not selected for final review in this article even though they were initially selected during the main search. |
| Study Population | RA as specified by the authors | RA as specified by the authors, cohorts who contain other rheumatic diseases in addition to RA were included for the ACR guidelines search. |
| Intervention | Per ACR RA treatment guidelines search
- articles must had at least one intervention arm for any of the
9 biologics or DMARDs at FDA approved doses and routes of
administration: abatacept 10mg/kg every 4 weeks; adalimumab 40mg
every 2 weeks; anakinra 100mg/day; certolizumab 400 mg SQ
initially, then 200-mg every other week or 400 mg monthly;
etanercept 25mg twice a week or 50 mg/week; golimumab 50 mg SQ
every 4 weeks; infliximab 3-10 mg/kg 0, 2, 6 weeks and then
every 8 weeks; rituximab two 1000 mg doses, 2 weeks apart; and
tocilizumab 4 mg/kg IV every 4 weeks (may increase to 8mg/kg).
Methotrexate up to 25 mg/wk; leflunomide 100 mg qd × 3
then 20 mg qd or 20 qd; sulfasalazine, 1.0- 3.0 gm qd;
hydroxychloroquine, 200-400 mg qd (up to 6.4 mg/kg/d);
minocycline 100-200 mg qd. Other concomitant therapies did not
influence the selection. However, publications providing results
for combinations of biologics were not abstracted. Within this search we did a specific search looking for tocilizumab articles with at least one intervention arm for tocilizumab 4 mg/kg IV every 4 weeks (may increase to 8mg/kg) monotherapy or combined with other concomitant therapies. Only clinical trials of tocilizumab were considered for this review. Study designs besides RCTs were excluded. Pediatric RCTs were excluded. |
Per ACR RA treatment guidelines
search-articles must had at least one intervention arm for the 9
biologics or 5 DMARDs was at FDA recommended dosages and routes:
abatacept 10mg/kg every 4 weeks; adalimumab 40mg every 2 weeks;
anakinra 100mg/day; certolizumab 400 mg SQ initially, then
200-mg every other week or 400 mg monthly; etanercept 25mg twice
a week or 50 mg/week; golimumab 50 mg SQ every 4 weeks;
infliximab 3-10 mg/kg 0, 2, 6 weeks and then every 8 weeks;
rituximab two 1000 mg doses, 2 weeks apart; and tocilizumab 4
mg/kg IV every 4 weeks (may increase to 8mg/kg). Methotrexate up
to 25 mg/wk; leflunomide 100 mg qd × 3 then 20 mg qd or
20 qd; sulfasalazine, 1.0- 3.0 gm qd; hydroxychloroquine,
200-400 mg qd (up to 6.4 mg/kg/d); minocycline,100-200 mg qd.
Other concomitant therapies did not influence the selection.
Publications providing combined results for biologics were
included. Within this search we did a specific search looking for tocilizumab articles with at least one intervention arm for tocilizumab 4 mg/kg IV every 4 weeks (may increase to 8mg/kg). Other concomitant therapies did not influence the selection. Only clinical trials of tocilizumab were considered for this review and other study designs besides RCTs were not included in this review. Open label extensions of clinical trials were included for tocilizumab as long as they were for at least 5 years. |
| Comparator Study outcomes | Placebo and/or other therapies ≥ 1 of the following: Tender Joints, Swollen Joints, Physician Global Assessment, Patient Global Assessment, pain, Disease Activity Scale, ACR response criteria 20, ACR50, ACR70, EULAR improvement criteria, Health Assessment Questionnaire, Short Form-36, radiographic outcomes, drug survival/terminations. If more than one time points were available for the outcome assessments, data was abstracted at the time of the primary endpoint or the last data available. | Placebo and/or other therapies ≥ 1 of the following: drug terminations, adverse events, serious adverse events, infections, serious infections (defined by need for intravenous antibiotics or hospitalization) and selected specific morbidities (e.g., infusion or injection site reaction, cancer, heart failure, autoantibodies production) and mortality. |
| Sample size | No restrictions | No restrictions |
ACR – The American College of Rheumatology; EULAR – European League Against Rheumatism; FDA – Food and Drug Administration; DMARDs.
References
- 1.Nishimoto N, Yoshizaki K, Maeda K, Kuritani T, Deguchi H, Sato B, et al. Toxicity, pharmacokinetics, and dose-finding study of repetitive treatment with the humanized anti-interleukin 6 receptor antibody MRA in rheumatoid arthritis. Phase I/II clinical study. J Rheumatol. 2003 Jul;30(7):1426–35. [PubMed] [Google Scholar]
- 2.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998 Jan 15;128(2):127–37. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007 Dec 1;370(9602):1861–74. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- 4.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003 May 15;423(6937):356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 5.Nishimoto N. Interleukin-6 as a therapeutic target in candidate inflammatory diseases. Clin Pharmacol Ther. 2010 Apr;87(4):483–7. doi: 10.1038/clpt.2009.313. [DOI] [PubMed] [Google Scholar]
- 6.Rasch EK, Hirsch R, Paulose-Ram R, Hochberg MC. Prevalence of rheumatoid arthritis in persons 60 years of age and older in the United States: effect of different methods of case classification. Arthritis Rheum. 2003 Apr;48(4):917–26. doi: 10.1002/art.10897. [DOI] [PubMed] [Google Scholar]
- 7.Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am. 2001 May;27(2):269–81. doi: 10.1016/s0889-857x(05)70201-5. [DOI] [PubMed] [Google Scholar]
- 8.Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006 Dec;36(3):182–8. doi: 10.1016/j.semarthrit.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IT, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010 Jan;62(1):22–32. doi: 10.1002/art.27227. [DOI] [PubMed] [Google Scholar]
- 10.Kishimoto T. Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 11.Yamasaki K, Taga T, Hirata Y, Yawata H, Kawanishi Y, Seed B, et al. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988 Aug 12;241(4867):825–8. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- 12.Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990 Dec 21;63(6):1149–57. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- 13.Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, et al. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989 Aug 11;58(3):573–81. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 14.Scheller J, Rose-John S. Interleukin-6 and its receptor: from bench to bedside. Med Microbiol Immunol. 2006 Dec;195(4):173–83. doi: 10.1007/s00430-006-0019-9. [DOI] [PubMed] [Google Scholar]
- 15.Mima T, Nishimoto N. Clinical value of blocking IL-6 receptor. Curr Opin Rheumatol. 2009 May;21(3):224–30. doi: 10.1097/BOR.0b013e3283295fec. [DOI] [PubMed] [Google Scholar]
- 16.Lally F, Smith E, Filer A, Stone MA, Shaw JS, Nash GB, et al. A novel mechanism of neutrophil recruitment in a coculture model of the rheumatoid synovium. Arthritis Rheum. 2005 Nov;52(11):3460–9. doi: 10.1002/art.21394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dayer JM, Choy E. Rheumatology. 1. Vol. 49. Oxford: Jan, Therapeutic targets in rheumatoid arthritis: the interleukin-6 receptor; pp. 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimoto N, Kishimoto T. Humanized antihuman IL-6 receptor antibody, tocilizumab. Handb Exp Pharmacol. 2008;(181):151–60. doi: 10.1007/978-3-540-73259-4_7. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008 Jun 15;59(6):762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Green Sally, editors. Centre TNC. Review Manager (RevMan) [Computer program] Copenhagen: The Cochrane Collaboration; 2011. [Google Scholar]
- 22.Yoshio-Hoshino N, Adachi Y, Aoki C, Pereboev A, Curiel DT, Nishimoto N. Establishment of a new interleukin-6 (IL-6) receptor inhibitor applicable to the gene therapy for IL-6-dependent tumor. Cancer Res. 2007 Feb 1;67(3):871–5. doi: 10.1158/0008-5472.CAN-06-3641. [DOI] [PubMed] [Google Scholar]
- 23.Mihara M, Kasutani K, Okazaki M, Nakamura A, Kawai S, Sugimoto M, et al. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol. 2005 Nov;5(12):1731–40. doi: 10.1016/j.intimp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Frey N, Grange S, Woodworth T. Population Pharmacokinetic Analysis of Tocilizumab in Patients With Rheumatoid Arthritis. J Clin Pharmacol. 2010 Jan 23; doi: 10.1177/0091270009350623. [DOI] [PubMed] [Google Scholar]
- 25.Agency. EM. [Accessed 2010 May 18];RoActemra (tocilizumab):summary of produc characteristics (online) Available from URL: http://wwwemaeuropaeu/humandocs/PDFs/EPAR/RoActemra/emea-combined-h955enpdf.
- 26.GENETECH. [Accessed 2010 May 18];Prescibing Information. 2010 http://wwwgenecom/gene/products/information/actemra/pdf/pipdf.
- 27.Leff J. Tocilizumab Introduction. In: H LR, editor. Tocilizumab Arthritis Advisory Committee Meeting; July 29, 2008. [Google Scholar]
- 28.Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008 Mar 22;371(9617):987–97. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 29.Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008 Oct;58(10):2968–80. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 30.Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010 Jan;69(1):88–96. doi: 10.1136/ard.2008.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008 Nov;67(11):1516–23. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kremer JM, Blanco R, Brzosko M, Burgos-Vargas R, Halland AM, Vernon E, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 2011 Mar;63(3):609–21. doi: 10.1002/art.30158. [DOI] [PubMed] [Google Scholar]
- 33.Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009;19(1):12–9. doi: 10.1007/s10165-008-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007 Sep;66(9):1162–7. doi: 10.1136/ard.2006.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006 Sep;54(9):2817–29. doi: 10.1002/art.22033. [DOI] [PubMed] [Google Scholar]
- 36.Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis. 2009 Oct;68(10):1580–4. doi: 10.1136/ard.2008.092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishimoto N, Yoshizaki K, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody - A multicenter, double-blind, placebo-controlled trial. Arthritis and Rheumatism. 2004 Jun;50(6):1761–9. doi: 10.1002/art.20303. Article. [DOI] [PubMed] [Google Scholar]
- 38.Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993 Jun;36(6):729–40. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 39.Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995 Jun;38(6):727–35. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- 40.Kremer LITHE: Tocilizumab Inhibits Radiographic Progression and Improves Physical Function in Rheumatoid Arthritis Patients at 2 Years with Increasing Clinical Eficacy Over Time. Arhtitis and Rheumatism. 2009;60(10):S238–S239. Abstract. Supplement. [Google Scholar]
- 41.(online). EMARtsopc. RoActemra (tocilizumab):summary of produc characteristics (online)
- 42.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003 Aug 1;102(3):783–8. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 43.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001 Mar 22;344(12):907–16. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 44.Andrews NC. Anemia of inflammation: the cytokine-hepcidin link. J Clin Invest. 2004 May;113(9):1251–3. doi: 10.1172/JCI21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Symmons D, Turner G, Webb R, Asten P, Barrett E, Lunt M, et al. Rheumatology. 7. Vol. 41. Oxford: 2002. Jul, The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century; pp. 793–800. [DOI] [PubMed] [Google Scholar]
- 46.World PR. Pharmacy Rx World; http://www.pharmacyrxworld.com/buy-Actemra.html. [Google Scholar]
- 47.Drugstore. Infliximab prices. 2011 [cited 2011 12/20/2011]; Website drug store]. Available from: http://www.drugstore.com/remicade/vial-100mg-solution/qxn57894003001.
- 48.Ariza-Ariza R, Navarro-Sarabia F, Hernandez-Cruz B, Rodriguez-Arboleya L, Navarro-Compan V, Toyos J. Rheumatology. 3. Vol. 46. Oxford: 2007. Mar, Dose escalation of the anti-TNF-alpha agents in patients with rheumatoid arthritis A systematic review; pp. 529–32. [DOI] [PubMed] [Google Scholar]
- 49.van Vollenhoven RF, Brannemark S, Klareskog L. Dose escalation of infliximab in clinical practice: improvements seen may be explained by a regression-like effect. Ann Rheum Dis. 2004 Apr;63(4):426–30. doi: 10.1136/ard.2003.010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahman MU, Strusberg I, Geusens P, Berman A, Yocum D, Baker D, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007 Sep;66(9):1233–8. doi: 10.1136/ard.2006.065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pavelka K, Jarosova K, Suchy D, Senolt L, Chroust K, Dusek L, et al. Increasing the infliximab dose in rheumatoid arthritis patients: a randomised, double blind study failed to confirm its efficacy. Ann Rheum Dis. 2009 Aug;68(8):1285–9. doi: 10.1136/ard.2008.090860. [DOI] [PubMed] [Google Scholar]
- 52.NICE. Rheumatoid arthritis - tocilizumab: final appraisal determination United Kingdom: National Institute for Health and Clinical Excellence (NICE) 2010 [cited 2011 July 21]; Available from: http://www.nice.org.uk/nicemedia/live/12033/49584/49584.pdf.
- 53.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005 Nov;52(11):3381–90. doi: 10.1002/art.21405. [DOI] [PubMed] [Google Scholar]
- 54.Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006 Jan;54(1):26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 55.Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004 Feb 28;363(9410):675–81. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 56.Yokota S, Miyamae T, Imagawa T, Iwata N, Katakura S, Mori M, et al. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2005 Mar;52(3):818–25. doi: 10.1002/art.20944. [DOI] [PubMed] [Google Scholar]
- 57.Yokota S, Imagawa T, Mori M, Miyamae T, Aihara Y, Takei S, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008 Mar 22;371(9617):998–1006. doi: 10.1016/S0140-6736(08)60454-7. [DOI] [PubMed] [Google Scholar]
- 58.Smolen JS, Aletaha D. Interleukin-6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: the role of acute-phase reactants. Arthritis Rheum. 2011 Jan;63(1):43–52. doi: 10.1002/art.27740. [DOI] [PubMed] [Google Scholar]
- 59.Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis. 2009 Apr;68(4):460–9. doi: 10.1136/ard.2008.101964. [DOI] [PubMed] [Google Scholar]
- 60.Jamnitski A, Visman IM, Peters MJ, Dijkmans BA, Voskuyl AE, Nurmohamed MT. Beneficial effect of 1-year etanercept treatment on the lipid profile in responding patients with rheumatoid arthritis: the ETRA study. Ann Rheum Dis. 2010 Nov;69(11):1929–33. doi: 10.1136/ard.2009.127597. [DOI] [PubMed] [Google Scholar]
- 61.Park YB, Choi HK, Kim MY, Lee WK, Song J, Kim DK, et al. Effects of antirheumatic therapy on serum lipid levels in patients with rheumatoid arthritis: a prospective study. Am J Med. 2002 Aug 15;113(3):188–93. doi: 10.1016/s0002-9343(02)01186-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.