Stem cells hold promise in the treatment of many different types of disease. The generation of induced pluripotent stem cells (iPSCs) by reprogramming somatic cells has provided unprecedented opportunities for studying basic biology and modeling human diseases. In addition, the differentiation of embryonic stem cells or iPSCs into different functional cell types also permits insights into disease mechanism. However, making an organ in a three-dimensional space from a single stem cell has remained challenging. Several successful examples include self-organized intestinal organoids consisting of polarized and columnar villus-like and crypt-like structures1,2 and apicobasally polarized cortical neuronal tissues3 from pluripotent stem cells. The eye and, in particular, the retina have provided an excellent substrate for the exploration of stem-cell approaches to treatment owing to their isolation from systemic exposures and the ease of noninvasive monitoring of their functional regeneration.
For more than a century, researchers have debated how primitive embryonic precursor cells give rise to the optic cup and retina. The retina, which originates from the lateral midbrain, develops through the formation of the optic vesicle and the subsequent formation of the optic cup with its complex two-walled structure with retinal pigment epithelium on the outer wall and the neural retina on the inner wall (Fig. 1A). A prevailing theory is that signals and interactions with surrounding tissues, such as the lens, induce the formation of the optic cup. Others have suggested that this process is a cell-autonomous process and can be self-initiated and formed without extrinsic induction.
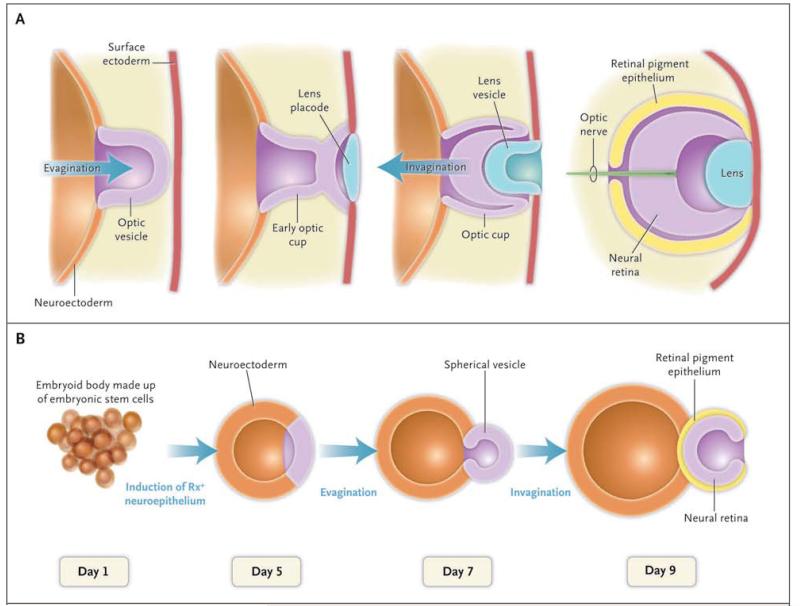
Figure 1. Formation of an Optic Cup in Vivo and in Vitro.
In Panel A, the embryonic neuroectoderm evaginates to form an optic vesicle. The interaction of the apical part of optic vesicle with the lens placode leads to the invagination and subsequent formation of an optic cup with a two-walled structure, consisting of retinal pigment epithelium on the outer wall and neural retina on the inner wall. In Panel B, over a period of 9 days, an optic-cup–like structure is formed in vitro in a three-dimensional culture of aggregates of embryonic stem cells. Self-aggregation of embryonic stem cells forms a spherical structure called an embryoid body. Subsequent invagination of its apical surface forms an optic cup with a two-walled structure consisting of retinal pigment epithelium and neural retina. The initial determination of eye primordium is defined by the expression of eye-specific transcription factor Rx.
A recent study by Eiraku and colleagues4 provides support for the latter hypothesis. Specifically, the investigators found that aggregates of embryoid bodies that were generated from mouse embryonic stem cells with extracellular matrix proteins added to the medium could recapitulate the development of the eye. Seven days after the initiation of the culture, they observed eye-field and retinal precursor cells. On day 8, they observed an optic-vesicle–like structure, and on day 9, a double-walled optic cup expressing transcription factors specific to the retinal pigment epithelium (Fig. 1B). These findings suggest that the optic cup forms by self-organizing. To test for the relevance of the optic-cup structure that is formed in vitro to developmental events in vivo, the authors excised the neuronal layer from the optic cup and placed it in three-dimensional cell culture under conditions optimized for neuronal maturation. They found that the retinal progenitors differentiated into a structure resembling postnatal retina with a laminated structure and with synaptic activities.
It remains to be seen whether the formation of the optic cup is cell autonomous. The evidence described by Eiraku et al. argues against this notion. Vesicles that were excised with a small amount of nonretinal neuroectodermal epithelium developed into spatially ordered retinal pigment epithelium and neural retina epithelium and formed an optic-cup structure. In contrast, vesicles with epithelium isolated without nonretinal tissues did not invaginate to form an optic cup. In addition, the embryoid bodies from which the cups emerge are complex and contain many different cell types that may include those normally surrounding the developing eye.
The investigators took advantage of multiphoton microscopy in their study of the self-assembly process. They observed that the morphology of primitive tissue underwent a four-step developmental rearrangement before becoming an optic-cup–like structure and that a decrease in myosin activity and the cytoskeletal organization in the regions of the epithelium that bend inward can explain an invagination process leading to the formation of a cup shape. Using computer modeling, the investigators proposed a mechanism involving three principal forces in the formation of the optic cup.
Whether this optic-cup structure can be further induced to form an entire eye structure that includes lens, iris, cornea, and sclera, pending additional inductive signals, remains to be seen. However, this work augurs well for the rapid, large-scale establishment of cell-culture models that are amenable to drug screening, characterization of disease phenotype, and mechanistic studies. Such models may be obtained with iPSCs that are derived from persons with specific mutant genotypes associated with diseases, such as macular degeneration, retinitis pigmentosa, and glaucoma. Perhaps this approach will at least partly supplant the use of genetically engineered animal models.
Contributor Information
Kang Zhang, Molecular Medicine Research Center and Department of Ophthalmology, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu, China, and the Shiley Eye Center and Institute for Genomic Medicine, University of California San Diego, La Jolla
Sheng Ding, Glad-stone Institute of Cardiovascular Disease and the Department of Pharmaceutical Chemistry, University of California, San Francisco
References
- 1.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 2.Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eiraku M, Watanabe K, Matsuo-Takasaki M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–32. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–6. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]