Abstract
Intercellular communication is essential for the coordination of physiological processes between cells in a variety of organs and tissues, including the brain, liver, retina, cochlea and vasculature. In experimental settings, intercellular Ca2+-waves can be elicited by applying a mechanical stimulus to a single cell. This leads to the release of the intracellular signaling molecules IP3 and Ca2+ that initiate the propagation of the Ca2+-wave concentrically from the mechanically stimulated cell to the neighboring cells. The main molecular pathways that control intercellular Ca2+-wave propagation are provided by gap junction channels through the direct transfer of IP3 and by hemichannels through the release of ATP. Identification and characterization of the properties and regulation of different connexin and pannexin isoforms as gap junction channels and hemichannels are allowed by the quantification of the spread of the intercellular Ca2+-wave, siRNA, and the use of inhibitors of gap junction channels and hemichannels. Here, we describe a method to measure intercellular Ca2+-wave in monolayers of primary corneal endothelial cells loaded with Fluo4-AM in response to a controlled and localized mechanical stimulus provoked by an acute, short-lasting deformation of the cell as a result of touching the cell membrane with a micromanipulator-controlled glass micropipette with a tip diameter of less than 1 μm. We also describe the isolation of primary bovine corneal endothelial cells and its use as model system to assess Cx43-hemichannel activity as the driven force for intercellular Ca2+-waves through the release of ATP. Finally, we discuss the use, advantages, limitations and alternatives of this method in the context of gap junction channel and hemichannel research.
Keywords: Cellular Biology, Issue 77, Molecular Biology, Medicine, Biomedical Engineering, Biophysics, Immunology, Ophthalmology, Gap Junctions, Connexins, Connexin 43, Calcium Signaling, Ca2+, Cell Communication, Paracrine Communication, Intercellular communication, calcium wave propagation, gap junctions, hemichannels, endothelial cells, cell signaling, cell, isolation, cell culture
Introduction
Intercellular communication and signaling are essential for the coordination of physiological processes in response to extracellular agonists at the tissue and whole-organ level 1,2 . The most direct way of intercellular communication is created by the occurrence of gap junctions. Gap junctions are plaques of gap junction channels, which are proteinaceous channels formed by the head-to-head docking of two connexin (Cx) hemichannels of adjacent cells 3,4 (Figure 1). Gap junctions allow the passage of small signaling molecules with a molecular weight of less than 1.5 kDa, including Ca2+ or IP3 5, causing and modulating Ca2+-release from the intracellular stores of the neighboring cells 6 (Figure 2). Gap junction channels are tightly regulated by intra- and intermolecular protein interactions and by cellular signaling processes, like redox modification and phosphorylation7. GJs facilitate the coordinated response of connected cells, thereby acting as a chemical and electrical syncytium. For example, the spreading of cardiac action potential across the atrial and ventricular myocytes is mediated by Cx-based GJ channels 85. Cxs not only have a role as gap junction channels, but also form unpaired hemichannels, thereby functioning as channels in membranes similarly to regular ion channels 8-10 (Figure 1). Hemichannels participate in paracrine signaling between neighboring cells by controlling the exchange of ions and signaling molecules between the intra- and extracellular environment.
In many cell types (like epithelial cells, osteoblastic cells, astrocytes, endothelial cells, etc.) and organs (like brain, liver, retina, cochlea and the vasculature), intercellular Ca2+-waves are fundamental for the coordination of multicellular responses 11. Increases in intracellular Ca2+ levels in a certain cell are not limited to this cell, but propagate to the surrounding neighboring cells, thereby establishing an intercellular Ca2+-wave 12,13 . These intercellular Ca2+-waves are important for normal physiological regulation of cell layers as a syncytium and their dysregulation has been associated with pathophysiological processes 11. In the corneal endothelium and epithelium, different groups 14-24, including our own 25-33, studied the mechanisms and roles of intercellular communication. In non-excitable cells, like corneal endothelial cells, two distinct modes of intercellular communication occur 28,29 , namely gap junctional intercellular communication and paracrine intercellular communication. Gap junctional intercellular communication involves a direct exchange of signaling molecules via gap junctions 7. Gap junctional intercellular communication is critical for maintaining tissue homeostasis, controlling cell proliferation, and establishing a synchronized response to extracellular stress 10,34,35 . In a number of pathologies, gap junction coupling is reduced due to defective Cxs, and hereby affecting gap junctional intercellular communication 36. This emphasizes the importance and influence of gap junctional intercellular communication in multicellular organisms. In contrast to gap junctional intercellular communication, paracrine intercellular communication is not dependent on cell-cell apposition, since it involves the release of diffusible extracellular messengers (Figure 2). Different types of signaling molecules are released in the extracellular space by signaling cells. The molecule is then transported to the target cell where it is detected by a specific receptor protein. Subsequently the receptor-signal complex induces a cellular response, which is terminated by removal of the signal, inactivation or desensitization. Released lipophilic extracellular signaling messengers penetrate the membrane and act on intracellular receptors. In contrast, hydrophilic messengers do not cross the plasma membrane of the responding cell, but act as a ligand that binds to surface-expressed receptor proteins, which then relay the signal to the intracellular environment. Three major families of cell surface receptor proteins participate in this process: ion-channel-linked, enzyme-linked, and G protein-linked. The released messenger molecule can act on receptors of the same cell (autocrine), on target cells in close proximity (paracrine), or on distant target cells that require the circulatory system (endocrine).
In many cell types, including corneal endothelium 28,29, ATP is one of the major hydrophilic, paracrine factors that drive the propagation of intercellular Ca2+-waves 37-40. During mechanical deformation, hypoxia, inflammation or stimulation by various agents, ATP can be released from healthy cells 41-44 in response to shear stress, stretch, or osmotic swelling 44,45. Different ATP-release mechanisms have been postulated, including vesicular exocytosis 44 and a plethora of transport mechanisms, such as ATP-binding cassette (ABC) transporters, plasmalemmal voltage-dependent anion channels 46, P2X7 receptor channels 47,48, as well asconnexin hemichannels 49-52 and pannexin hemichannels 43,49,53. Extracellular ATP can be rapidly hydrolyzed to ADP, AMP and adenosine 54,55 by ectonucleotidases that are present in the extracellular environment. The extracellularly released ATP and its metabolite ADP 56 will spread through diffusion. The subsequent interaction of these nucleotides with purinergic receptors in the neighboring cells has been implicated in the propagation of intercellular Ca2+-waves 28,37,51. Two different classes of purinergic receptors are present: adenosine is the principal natural ligand for P1-purinoceptors, while both purine (ATP, ADP) and pyrimidine (UTP, UDP) nucleotides act on most P2-purinoceptors 57.
Intercellular communication can be investigated by different methods such as scrape loading, dye transfer, local uncaging of agonists like IP3 and Ca2+, mechanical stimulation, etc.. Here we describe the study of Ca2+-wave propagation elicited by mechanical stimulation of a single cell. The advantage of studying Ca2+-wave propagation by mechanical stimulation is that it provides an easy tool to quantify the spread of the Ca2+-wave over time and it allows quantitatively comparing different pretreatments of the cells. In the corneal endothelium, these intercellular Ca2+-waves allow a coordinated response from the monolayer, hereby acting as a possible defense mechanism of the non-regenerative corneal endothelium helping the endothelium to withstand extracellular stresses during intraocular surgery, or upon exposure to inflammatory mediators during immune rejection or uveitis 58,59 .
Protocol
1. Isolation of Corneal Endothelial Cells
Before getting started: Isolate the cells from the fresh eyes, obtained from a local slaughterhouse, as soon as possible after enucleating the eye. Make sure that the eye was enucleated from a cow of maximal 18 months old, five minutes post mortem and preserved in Earle's Balanced Salt Solution - 1% iodine solution at 4 °C for transportation to the laboratory.
Take the eye out of the Earle's Balanced Salt Solution - 1% iodine solution and place it in a Petri dish (100 x 20 mm).
Sterilize the eye with a solution containing 70% ethanol and rinse with Earle's Balanced Salt Solution containing 1% iodine.
From this step on, work in a sterile hood. Carefully dissect the cornea from the eye and place it in a Petri dish (35 x 10 mm) containing Earle's Balanced Salt Solution, with the epithelial cell layer facing upward. Carefully remove any remaining iris tissue still attached to the cornea if needed.
Transfer the cornea to another Earle's Balanced Salt Solution containing Petri dish with the endothelial cell layer upward and rinse twice with Earle's Balanced Salt Solution.
Transfer the cornea with the endothelial layer facing upward to an hourglass, which is a cup-shaped dish, and cover it with growth medium. The growth medium consists of Dulbecco's Modified Eagle's Medium containing 25 mM glucose, 10% fetal bovine serum, 6.6% L-glutamine, 2.5 μg/ml amphotericin-B and 1% antibiotic-antimycotic mixture containing 10,000 units/ml of penicillin, 10,000 μg/ml of streptomycin, and 25 μg/ml amphotericin B.
Remove the medium with a suction pipette.
Apply 300 μl of a trypsin solution (0.5 g/L) to the endothelial layer of the cornea (all the steps that include trypsin are done using the same concentration).
Place the hourglass containing the cornea in a covered Petri dish and put it in the incubator for 30 min at 37 °C and 5% CO2.
Gently scrape the endothelial cells away from the cornea with a fire-polished hook-shaped glass Pasteur pipette in a sterile hood.
Suck off the solution containing the endothelial cells and add it to culture flasks (25 cm2) containing 4 ml of culture medium.
Apply 300 μl of growth medium to the cornea and repeat the scraping and add the solution containing the endothelial cells to the culture flask.
Repeat this final step (1.11) once more.
Place the culture flasks containing the corneal endothelial cells in the growth medium in the incubator at 37 °C and 5% CO2.
After two days, add 6 ml of culture medium.
Refresh the growth medium every second day.
2. Cell Culture
Remove the culture medium and wash the cells twice with Earle's Balanced Salt Solution, when confluency is reached (within about 10 days after isolation).
Add 1.5 ml trypsin solution to the cells to detach them and place the flask in the incubator (37 °C, 5% CO2) for 3 to 4 min.
Add 12 ml of growth medium. Pipette the medium three times in and out to disperse the cells and count the cells.
Seed the cells with a variable fraction depending on the cell density and put them in the incubator. Prepare two well-chambered slides (with an area of 4.2 cm2) with a cell count of 165,000 cells (cell density 39,286 per cm2). Prepare 80 cm2 culture flasks for a new passage at a density of 6,250 per cm2, and add fresh culture medium up to a total volume of 25 ml.
Refresh the medium every two days.
Confluency of the cell layer is reached after 3 to 4 days. Use cells for experiments.
When confluency of the cell layer in the flasks is reached, repeat steps 2.1 to 2.6. Cell cultures up to passage 2 can be used for experiments.
3. Mechanical Stimulation for Inducing Calcium Wave
Load the cells in the chambered slide with 10 μM Fluo-4 AM in phosphate-buffered saline for 30 min at 37 °C while gently shaking.
Remove the Fluo-4 AM solution, wash the cells five times with phosphate-buffered saline, incubate the cells with phosphate-buffered saline and leave the cells for at least 5 min at room temperature before measurement.
Excite at 488 nm with Argon laser and use beam splitter HFT 488, collect the fluorescence emission at 530 nm using a longpass emission filter LP 505, set the pinhole at minimum. Use an oil immersion 40X objective (Air, 1.2 N.A.). In experiments with ARL-67156, use a 10X objective (Air, 0.3 N.A.).
Search for a field in which the cells are confluent on the confocal microscope.
Position the pipette so that it is at 45° in respect to the chambered slide and touching the cell membrane. Provoke a short (≈ 1 sec) mechanical stimulation to a single cell. The mechanical stimulation consists of an acute, short-lasting deformation of the cell by briefly touching less than 1% of the cell membrane with a glass micropipette (tip diameter <1 μm) coupled to a piezoelectric crystal nanopositioner, operated through an amplifier which is mounted on a micro-manipulator. The glass micropipettes are made with a microelectrode puller. Make sure that the nanopositioner is operated by a voltage between 0.2 and 1.5 V during the mechanical stimulation. A voltage higher than 1.5 V can result in cell damage. For each cell type and condition, the optimal voltage for mechanical stimulation without cell damage must be carefully determined by applying a series of voltages starting from low (0.2 V) to high (1.5 V) voltage. The voltage is a measure for the force of the stimulation since this voltage determines the mechanical stress and strain that are applied to the cell membrane. The force of the mechanical stimulation can be calculated by multiplying the mechanical stress with the area. Since both the area (<3.14 μm2) and the mechanical stress are very low, the force of the mechanical stimulation is low. (Note that when a cell is damaged, the fluorescence leaks out of the cell and the cell turns dark.)
Measure spatial changes in [Ca2+]i following mechanical stimulation with the confocal microscope.
Collect and store images.
Draw a polygonal region of interest to define the total surface area of responsive cells (active area, AA) using the software of the confocal microscope.
Representative Results
All experiments are executed in compliance with all relevant guidelines, regulations and regulatory agencies and the protocol being demonstrated is performed under the guidance and approval of the animal care and use committee of the KU Leuven.
In bovine corneal endothelial cells (BCEC), functional gap junctions are expressed and both gap junctional intercellular communication and paracrine intercellular communication contribute significantly to intercellular communication in an interactive way, but the main pathway has been shown to be the paracrine intercellular communication pathway mediated by ATP release through Cx43-based hemichannels 28,29 . ATP and ADP are hydrolyzed to adenosine monophosphate (AMP) by the ectonucleotidase E-NTPD1 (ectonucleoside triphosphate diphosphohydrolase 1, CD39 ATP diphosphohydrolase), and subsequently to adenosine by the 5'-ectonucleotidase CD73 28,29 . ATP and ADP contribute to the Ca2+-wave propagation by binding to P2Y1 and P2Y2 receptors 28,29 . Both receptors couple to PLC via Gq and thereby evoke IP3-induced Ca2+-release (Figure 2). In BCEC, the stimulation of the purinergic P2Y receptors results in a rapid increase in [Ca2+]i, which is insensitive to the removal of [Ca2+]o 60. [Ca2+]i peaks evoked by agonist stimulation are followed by a [Ca2+]i decrease which can lead to a stable, agonist-dependent elevation, [Ca2+]o-dependent oscillatory fluctuations, or a return to baseline 60-62. In BCEC, there has been evidence that Ca2+-release occurs via a pathway involving PLC and IP3 29. Emptying of the IP3-sensitive stores leads to an initial peak in [Ca2+]i, subsequently followed by a capacitative Ca2+-influx leading to the onset of the plateau phase 63.
Mechanical stimulation leads to a rapid initial rise in Ca2+ that originates from the point of the stimulation and then spreads throughout the mechanically stimulated cell. Finally, the intracellular Ca2+-levels slowly diminish back to the baseline level. Upon reaching the cell boundaries, the intercellular Ca2+-wave propagates to the surrounding neighboring cells (NB) in a wave-like manner as a Ca2+-transient, which decays to basal level (Figure 3). In control conditions, Ca2+-transients were observed up to approximately 4 to 8 cell layers away from the mechanically stimulated cell (Figure 3). The line graph (at the right side of the panels in Figure 3) shows the time course of the Ca2+-transients (represented as normalized fluorescence (NF) values) in the mechanically stimulated cell and in the neighboring cell layers one to five (NB1, NB2, NB3, NB4 and NB5). From Figure 3, it is clear that the normalized fluorescence decreases, while the time delay for the onset of [Ca2+]i-rise increases with increasing distance from the mechanically stimulated cell. The maximal normalized fluorescence in the mechanically stimulated cell was reached in 0.95±0.04 sec. After reaching a maximal normalized fluorescence value, the normalized fluorescence showed a very gradual and slow decline, returning to the basal value 152 ± 6 sec after the application of the stimulus 25.
Inhibition of the paracrine intercellular communication pathway by using a combination of exogenous apyrase VI (5 U/ml for 30 min) and apyrase VII (5 U/ml for 30 min) caused a 7.5-fold decrease in the area covered by the Ca2+-wave, the so-called active area (AA, P < 0.001; N = 7, n = 35) (Figure 4A). Apyrase is known to hydrolyze ATP and ADP. Apyrase VI has a high ATPase/ADPase ratio and apyrase VII preferentially hydrolyzes ADP 56.
Since paracrine intercellular communication in the corneal endothelium largely occurs through ATP release,28,29 and ATP is hydrolyzed in the extracellular space by ectonucleotidases, known to be expressed in the corneal endothelium,29,64,65 we investigated the effect on AA in conditions where ATP hydrolysis is inhibited using ectonucleotidase inhibitors. Inhibition of ectonucleotidases with ARL-67156 (ARL; 100 μM for 30 min) resulted in strong enhancement of the Ca2+-wave propagation, as has been demonstrated previously in BCEC 25,26,28,29 . The exposure of BCEC to ARL caused a 3.5-fold increase of the AA compared to control conditions (P < 0.001; N = 12, n = 60) (Figure 4B).
In previous studies in our laboratory, connexin mimetic peptides (Gap26 and Gap27, Table 2) were used to distinguish the relative contributions of gap junctional intercellular communication and paracrine intercellular communication to intercellular Ca2+-wave propagation following mechanical stimulation, with an inactive peptide (Table 2) as a control 28,29 .
Inhibition of gap junction channels with Gap27 significantly decreased the propagation of the Ca2+-wave in BCEC 30. The AA was significantly reduced upon pretreatment with Gap27 (300 μM for 30 min) (P < 0.001; N = 8, n = 40) 25 (Table 1). Inhibition of connexin hemichannels with the connexin-mimetic peptide Gap26 28,30 significantly reduced the propagation of the Ca2+-wave in BCEC 28. The AA was significantly reduced upon pretreatment with Gap26 (300 μM for 30 min) (P < 0.001; N = 8, n = 40) 25 (Table 1).
We also showed that the 43-kDa Cx isoform was a main component underlying the hemichannel-mediated ATP release that provokes intercellular Ca2+-waves. Using two independently designed siRNA molecules targeting Cx43, we found that the AA was reduced by about 65% 33 (Table 1). This was further underpinned by experiments using TAT-L2 (100 μM, 30 min), a cell-permeable peptide corresponding to the second half of the intracellular loop of Cx43 (Table 2), which provoked a major reduction in AA (P < 0.001; N = 3, n = 30) (Table 1). Importantly, this reduction in AA by TAT-L2 incubation was not observed in the absence of paracrine signaling, supporting the concept that TAT-L2 selectively inhibits Cx43-based hemichannels but not gap junction channels 33. Furthermore, an inactive TAT-L2 mutant (TAT-L2H126K/I130N) can be used as a control (Table 2).
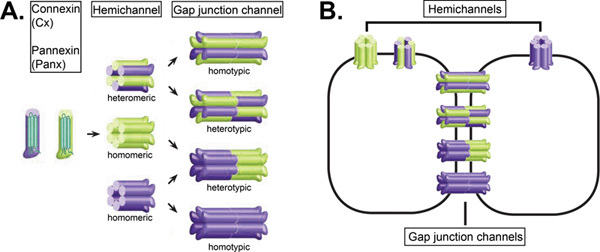 Figure 1. Formation of gap junction channels and hemichannels: schematic representation. A. A connexin or pannexin hemichannel is formed when six connexins or pannexins, which are four-transmembrane-domain-containing proteins, are radially arranged around a central pore. Hemichannels are located in the plasma membrane. They can consist of identical protein subtypes (homomeric hemichannels) or they can consist of different protein subtypes, when two or more isoforms are expressed in the same cell (heteromeric hemichannels). A homotypic gap junction channel results from the docking of two identical homomeric or heteromeric hemichannels. A heterotypic gap junction channel results from the docking of two different homomeric or heteromeric channels. B. Schematic structure of connexin and pannexin gap junction channels connecting two adjacent cells and hemichannels.
Figure 1. Formation of gap junction channels and hemichannels: schematic representation. A. A connexin or pannexin hemichannel is formed when six connexins or pannexins, which are four-transmembrane-domain-containing proteins, are radially arranged around a central pore. Hemichannels are located in the plasma membrane. They can consist of identical protein subtypes (homomeric hemichannels) or they can consist of different protein subtypes, when two or more isoforms are expressed in the same cell (heteromeric hemichannels). A homotypic gap junction channel results from the docking of two identical homomeric or heteromeric hemichannels. A heterotypic gap junction channel results from the docking of two different homomeric or heteromeric channels. B. Schematic structure of connexin and pannexin gap junction channels connecting two adjacent cells and hemichannels.
(Partially modified from 66)
This figure was originally published in BioEssays. Catheleyne D'hondt, Raf Ponsaerts, Humbert De Smedt, Geert Bultynck, and Bernard Himpens. Pannexins, distant relatives of the connexin family with specific cellular functions. BioEssays. 2009, 31, 953-974 (2009). BioEssays. Click here to view larger figure.
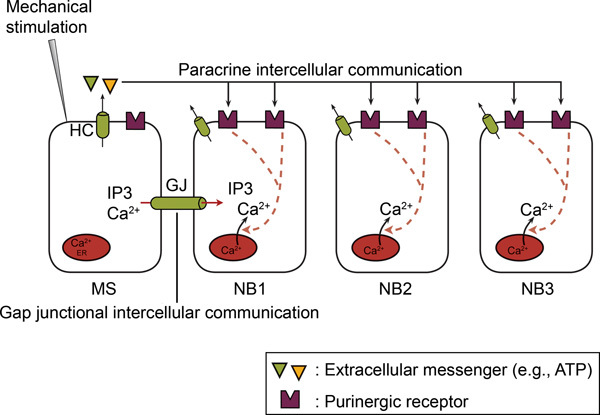 Figure 2. In non-excitable cells, the intercellular Ca2+-wave propagation involves both gap junctional intercellular communication and paracrine intercellular communication. Upon mechanical stimulation of a single cell, a Ca2+-rise occurs in the mechanically stimulated cell (MS) via Ca2+-influx and/or Ca2+-release. Subsequently, the Ca2+-rise propagates from the mechanically stimulated to the neighboring cells (NB) as an intercellular Ca2+-wave. The intercellular propagation involves two mechanisms, namely gap junctional intercellular communication and paracrine intercellular communication. In gap junctional intercellular communication, a direct exchange of a mediator (IP3 and/or Ca2+) occurs between the cytoplasms of adjacent cells via gap junctions (GJs). In paracrine intercellular communication, a messenger (e.g. ATP) is released into the extracellular space, thereby acting on receptors located on the surface of neighboring cells. Hemichannels (HCs) or other mechanisms mediate this ATP release (see text). Ectonucleotidases hydrolyze ATP to ADP and AMP. ATP and ADP act on P2Y and/or P2X receptors on neighboring cells. (Taken from 66.)
Figure 2. In non-excitable cells, the intercellular Ca2+-wave propagation involves both gap junctional intercellular communication and paracrine intercellular communication. Upon mechanical stimulation of a single cell, a Ca2+-rise occurs in the mechanically stimulated cell (MS) via Ca2+-influx and/or Ca2+-release. Subsequently, the Ca2+-rise propagates from the mechanically stimulated to the neighboring cells (NB) as an intercellular Ca2+-wave. The intercellular propagation involves two mechanisms, namely gap junctional intercellular communication and paracrine intercellular communication. In gap junctional intercellular communication, a direct exchange of a mediator (IP3 and/or Ca2+) occurs between the cytoplasms of adjacent cells via gap junctions (GJs). In paracrine intercellular communication, a messenger (e.g. ATP) is released into the extracellular space, thereby acting on receptors located on the surface of neighboring cells. Hemichannels (HCs) or other mechanisms mediate this ATP release (see text). Ectonucleotidases hydrolyze ATP to ADP and AMP. ATP and ADP act on P2Y and/or P2X receptors on neighboring cells. (Taken from 66.)
This figure was originally published in BioEssays. Catheleyne D'hondt, Raf Ponsaerts, Humbert De Smedt, Geert Bultynck, and Bernard Himpens. Pannexins, distant relatives of the connexin family with specific cellular functions. BioEssays. 2009. 31, 953-974 (2009). BioEssays.
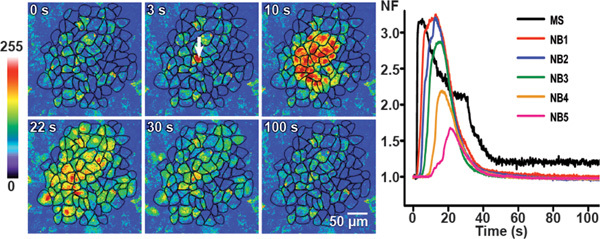 Figure 3. Calcium wave propagation in control conditions in BCEC. Mechanical stimulation induced calcium transients are shown at different time-points in control conditions in BCEC by representative pseudo-colored fluorescence images. The fluorescence intensities before stimulation are shown in the first image. The white arrow in the second image identifies the mechanically stimulated cell. The calcium wave propagates to six neighboring cell layers with a total area of cells reached by the wave (active area: AA) of 62,870 μm2.
Figure 3. Calcium wave propagation in control conditions in BCEC. Mechanical stimulation induced calcium transients are shown at different time-points in control conditions in BCEC by representative pseudo-colored fluorescence images. The fluorescence intensities before stimulation are shown in the first image. The white arrow in the second image identifies the mechanically stimulated cell. The calcium wave propagates to six neighboring cell layers with a total area of cells reached by the wave (active area: AA) of 62,870 μm2.
The right panel with the line graphs shows the time course of the normalized fluorescence value (NF) in the mechanically stimulated cell (MS) and the average value of NF in the neighboring cells (NB) layers 1 to 5 (NB1 to NB5). (Partially modified from 25.)
This figure was originally published in Investigative Ophthalmology and Visual Science. Catheleyne D'hondt, Raf Ponsaerts, Sangly P Srinivas, Johan Vereecke, and Bernard Himpens. Thrombin inhibits intercellular calcium wave propagation in corneal endothelial cells by modulation of hemichannels and gap junctions. Invest. Ophthalmol. Vis. Sci. 2007. 48 (1), 120-33 (2007). Investigative Ophthalmology and Visual Science.
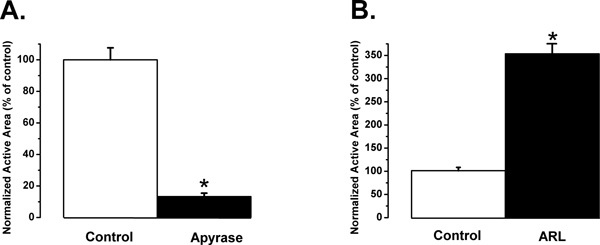 Figure 4. Significant changes in the spread of intercellular Ca2+-waves after treatment with exogenous nucleotidases (left) and with ectonucleotidase inhibitors (right) in BCEC. A. Significant decrease in AA after treatment of BCEC with exogenous apyrases (apyrase VI (5 U/ml) and apyrase VII (5 U/ml) for 30 min) which hydrolyze ATP and ADP, hereby inhibiting the paracrine intercellular communication pathway (N = 7, n = 35). * signifies P < 0.001 in the presence vs. absence of apyrase. B. Significant increase in AA after treatment of BCEC with a selective ectonucleotidase inhibitor ARL-67156 (ARL; 100 μM for 30 min), herby enhancing the paracrine intercellular communication pathway (N = 12, n = 60). * signifies P < 0.001 in the presence vs. absence of ARL.
Figure 4. Significant changes in the spread of intercellular Ca2+-waves after treatment with exogenous nucleotidases (left) and with ectonucleotidase inhibitors (right) in BCEC. A. Significant decrease in AA after treatment of BCEC with exogenous apyrases (apyrase VI (5 U/ml) and apyrase VII (5 U/ml) for 30 min) which hydrolyze ATP and ADP, hereby inhibiting the paracrine intercellular communication pathway (N = 7, n = 35). * signifies P < 0.001 in the presence vs. absence of apyrase. B. Significant increase in AA after treatment of BCEC with a selective ectonucleotidase inhibitor ARL-67156 (ARL; 100 μM for 30 min), herby enhancing the paracrine intercellular communication pathway (N = 12, n = 60). * signifies P < 0.001 in the presence vs. absence of ARL.
| Normalized AA | St. error | N | n | Statistics | |
| control | 100 | 8.31 | 3 | 30 | |
| siScramble | 87.97 | 9.3 | 3 | 30 | |
| siCx43-1 | 32.45 | 8.23 | 3 | 30 | * |
| siCx43-2 | 35.49 | 7.06 | 3 | 30 | * |
| Control peptide | 91.68 | 6.5 | 8 | 40 | |
| Gap 26 | 46.67 | 4.24 | 8 | 40 | * |
| Gap 27 | 53 | 4.76 | 8 | 40 | * |
| TAT-L2 | 6.99 | 0.71 | 3 | 30 | * |
Table 1. Effect of siRNA molecules targeting Cx43, connexin mimetic peptides and the cell-permeable peptide TAT-L2 on the normalized active area (AA) in BCEC. N represents the number of days of experiments, n represents the number of mechanical stimulations. * signifies P < 0.001 compared to control conditions.
| AA sequence | |
| Control peptide | SRGGEKNVFIV |
| Gap26 | VCYDKSFPISHVR |
| Gap27 | SRPTEKTIFII |
| TAT-L2 | YGRKKRRQRRR-DGANVDMHLKQIEIKKFKYGIEEHGK |
| TAT-L2H126K/I130N | YGRKKRRQRRR-DGANVDMKLKQNEIKKFKYGIEEHGK |
| 10Panx1 | WRQAAFVDSY |
Table 2. Amino acid sequences of the used peptides.
Discussion
In this manuscript, we describe a simple method to measure intercellular Ca2+-wave propagation in monolayers of primary bovine corneal endothelial cells by providing a localized and controlled mechanical stimulation using a micropipette. Mechanically stimulated cells respond with a local increase in intracellular IP3 and Ca2+, both of which are essential intracellular signaling molecules that drive intercellular Ca2+-wave propagation 11,67 . IP3 is directly transferred to neighboring cells via gap junction channels 5, while Ca2+ provokes the opening of hemichannels and the release of ATP 68,69 , which triggers Ca2+ signals in neighboring cells through the activation of G-protein coupled P2 receptors 37-40. The relative contribution of gap junction channels and hemichannels in this process can be characterized by the use of Cx-mimetic peptides, ATP-degrading enzymes (ectonucleotidases) and inhibitors of ectonucleotisdases. The properties of mechanical stimulation-induced intercellular Ca2+-wave propagation in bovine corneal endothelial cells have been thoroughly characterized in our lab 25-33. Our results indicate that the intercellular Ca2+-wave propagation is mainly driven by a Cx-hemichannel-mediated release of ATP, with Cx43 playing a prominent role. As such, this method applied to primary bovine corneal endothelial cells is particularly suitable to identify or characterize the regulation of Cx43 hemichannels at endogenous levels in native cells. Using this method, we have found that the activity of Cx43 hemichannels is critically controlled by the actomyosin cytoskeleton, which may serve as an endogenous brake preventing excessive, and thus deleterious, Cx43 hemichannel opening 25,31,32. We further elucidate the molecular mechanisms underlying this regulation and find an important role of intramolecular loop/tail interactions that are essential for the opening of Cx43 hemichannels 33.
Clearly, this system is very suitable for studying the function of Cx and Panx-based gap junction channels and hemichannels and is definitely not limited to bovine corneal endothelial cells but is adaptable to virtually any cell type and also more complex tissues, as has been shown in the brain where mechanical stimulation triggered large intercellular Ca2+-waves encompassing the entire hemisphere 70. Different tools are present to assess the contribution of gap junction channels and hemichannels, including Cx-mimetic peptides, ATP-degrading enzymes, inhibitors of ectonucleotidases and pharmacological compounds like carbenoxolone and 10Panx1 (Table 2) 49,50 . To determine the contribution of a certain Cx or Panx isoform in this process, carefully designed siRNA probes targeting two independent regions of the mRNA and a scrambled control should be used. The extent of knockdown should be determined at the total protein level using Western-blotting assays and the single cell level using fluorescent microscopy. The transfection efficiency of the siRNA probes in the experimental cell monolayers should be assessed by fluorescent microscopy. For this, one can develop a duplex siRNA in which a fluorescent label has been incorporated at the 3' end of the sense strand. It is important to note that for proper analysis, a prominent reduction of the Cx or Panx isoform (>90% reduction), as well as a homogenous transfection of the siRNA duplexes in the cell monolayer (>90% of the cells transfected with siRNA probe) should be obtained. The selectivity of the designed siRNA probes towards the target should be assessed 71. In short, these tools should be validated so that they do not affect the expression of other Cx or Panx isoforms or other key components driving intercellular Ca2+-waves, like P2X or P2Y receptors. To assess the contribution of Cx43 hemichannels, we have collaborated with the lab of Dr. Leybaert in developing a cell-permeable peptide corresponding to the second half of the intracellular loop of Cx43 (TAT-L2) that acts as a selective, potent inhibitor of Cx43 hemichannels while maintaining Cx43-gap junction channel activity 33. In our studies, we use 100 μM TAT-L2 to obtain a complete inhibition of Cx43 hemichannels, but lower concentrations may be sufficient 72. TAT-L2H126K/I130N is recommended as a negative control 73. To assess the contribution of Panx1 channels, the 10Panx1 peptide can be applied. These tools are important not only for excluding plasma membrane disruption (see below), but also for demonstrating that paracrine ATP signaling mediates the mechanisms of intercellular Ca2+-wave propagation by hemichannels and not by other mechanisms (like maxi-anion channels or the release of ATP-containing vesicles). Finally, as for all studies using primary cells, the cell culture conditions and the number of passages must be standardized, as the biological properties of the cells and thus the expression profile of the different Cx and Panx isoforms may change over time 26.
Nevertheless, there are a number of drawbacks to this method. A major disadvantage to the method is that mechanical stimulation may trigger plasma membrane disruption, leading to the entry of extracellular Ca2+ and the release of signaling molecules like ATP, both of which underlie bona fide intercellular Ca2+-wave propagation 11. This definitely complicates the (quantitative) analysis of the intercellular Ca2+-wave. Therefore, it is highly recommended that one should i) use proper controls, i.e. cell lines which lack the expression of Cx or Panx isoforms, and tools that interfere with the function of Cx and Panx as gap junction channels and/or hemichannels and ii) standardize the mechanical stimulation procedure (make sure that during the mechanical stimulus the nanopositioner is operated by a voltage between 0.5 and 2 V).
Furthermore, it is important to note that this method does not stand by itself, but should be underpinned by additional experimental approaches to study Cx and Panx channels. This can be achieved by using a local increase of intracellular signaling molecules that participate in mechanical-stimulation-mediated intercellular Ca2+-wave propagation, like the uncaging of intracellular IP3 or Ca2+ 11,74. To initiate intercellular Ca2+-wave independent of mechanical stimulation, one can use micro-injection, such as recombinant pro-apoptotic Bax 11,75 and photo-activatable Ca2+-buffers like diazo-2 that causes a local drop in extracellular [Ca2+] 76, a known trigger for hemichannel opening. Alternatively, one can use in situ electroporation of cell-impermeable signaling molecules that trigger intracellular Ca2+-release and intercellular Ca2+-waves, such as IP3 67,68. The latter technique is also used to investigate the spreading of cell death 5,77. These non-mechanical stimuli provide a better assessment of the stimulus intensity-response. In addition to these Ca2+-wave propagation methods, it is important to determine the activity of the Cx or Panx channels using other approaches, including the determination of hydrophilic dye uptake (like Lucifer yellow) 78 and the release of ATP not only in response to mechanical stimulation but also in response to extracellular Ca2+-buffers (like EGTA) and intracellular Ca2+-release molecules (like the Ca2+-ionophore A23187) 11,74. In addition, the best proof for the regulation at the channel level is provided by electrophysiological experiments, either dual voltage clamp systems or whole-cell path clamp of Xenopus oocytes injected with Cx or Panx mRNA or of HeLa cells ectopically expressing Cx isoforms together with a marker like GFP 79-84.
To conclude, the use of mechanical stimulation for inducing intercellular Ca2+-waves provides a simple and reliable method to investigate intracellular communication and examine the contribution and properties of Cx and Panx channels.
Disclosures
Authors have nothing to disclose.
Acknowledgments
Research work performed in the laboratory was supported by grants from the Research Foundation - Flanders (FWO; grant numbers G.0545.08 and G.0298.11), the Interuniversity Attraction Poles Program (Belgian Science Policy; grant number P6/28 and P7/13) and is embedded in an FWO-supported research community. CDH is a post-doctoral fellow of the Research Foundation - Flanders (FWO). The authors are very grateful to all current and former members of the Laboratory of Molecular and Cellular Signaling (KU Leuven), Dr. SP Srinivas (Indiana University School of Optometry, USA), the laboratory of Dr. Leybaert (Ghent University) and of Dr. Vinken (VUB) who provided helpful discussions, optimized procedures or were involved in the development of tools for the study of connexin hemichannels.
References
- Vinken M, et al. Connexins and their channels in cell growth and cell death. Cell Signal. 2006;18:592–600. doi: 10.1016/j.cellsig.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Mese G, Richard G, White TW. Gap junctions: basic structure and function. J. Invest. Dermatol. 2007;127:2516–2524. doi: 10.1038/sj.jid.5700770. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur. J. Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- White TW, Bruzzone R, Paul DL. The connexin family of intercellular channel forming proteins. Kidney Int. 1995;48:1148–1157. doi: 10.1038/ki.1995.398. [DOI] [PubMed] [Google Scholar]
- Decrock E, et al. Connexin-related signaling in cell death: to live or let die? Cell Death Differ. 2009;16:524–536. doi: 10.1038/cdd.2008.196. [DOI] [PubMed] [Google Scholar]
- Herve JC. Gap junctional complexes: from partners to functions. Prog. Biophys. Mol. Biol. 2007;94:1–4. doi: 10.1016/j.pbiomolbio.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Herve JC, Bourmeyster N, Sarrouilhe D, Duffy HS. Gap junctional complexes: from partners to functions. Prog. Biophys. Mol. Biol. 2007;94:29–65. doi: 10.1016/j.pbiomolbio.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J. Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- Ebihara L, Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J. Gen. Physiol. 1993;102:59–74. doi: 10.1085/jgp.102.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem. J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leybaert L, Sanderson MJ. Intercellular Ca2+ waves: mechanisms and function. Physiol. Rev. 2012;92:1359–1392. doi: 10.1152/physrev.00029.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson MJ, Charles AC, Dirksen ER. Mechanical stimulation and intercellular communication increases intracellular Ca2+ in epithelial cells. Cell Regul. 1990;1:585–596. doi: 10.1091/mbc.1.8.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himpens B, Stalmans P, Gomez P, Malfait M, Vereecke J. Intra- and intercellular Ca2+ signaling in retinal pigment epithelial cells during mechanical stimulation. Faseb J. 1999;13:63–68. doi: 10.1096/fasebj.13.9001.s63. [DOI] [PubMed] [Google Scholar]
- Williams KK, Watsky MA. Bicarbonate promotes dye coupling in the epithelium and endothelium of the rabbit cornea. Curr. Eye Res. 2004;28:109–120. doi: 10.1076/ceyr.28.2.109.26234. [DOI] [PubMed] [Google Scholar]
- Hernandez Galindo EE, Theiss C, Steuhl KP, Meller D. Gap junctional communication in microinjected human limbal and peripheral corneal epithelial cells cultured on intact amniotic membrane. Exp Eye Res. 2003;76:303–314. doi: 10.1016/s0014-4835(02)00314-7. [DOI] [PubMed] [Google Scholar]
- Williams K, Watsky M. Gap junctional communication in the human corneal endothelium and epithelium. Curr. Eye Res. 2002;25:29–36. doi: 10.1076/ceyr.25.1.29.9964. [DOI] [PubMed] [Google Scholar]
- Anderson SC, Stone C, Tkach L, SundarRaj N. Rho and Rho-kinase (ROCK) signaling in adherens and gap junction assembly in corneal epithelium. Invest. Ophthalmol. Vis. Sci. 2002;43:978–986. [PubMed] [Google Scholar]
- Joyce NC, Harris DL, Zieske JD. Mitotic inhibition of corneal endothelium in neonatal rats. Invest. Ophthalmol. Vis. Sci. 1998;39:2572–2583. [PubMed] [Google Scholar]
- Klepeis VE, Weinger I, Kaczmarek E, Trinkaus-Randall V. P2Y receptors play a critical role in epithelial cell communication and migration. J. Cell Biochem. 2004;93:1115–1133. doi: 10.1002/jcb.20258. [DOI] [PubMed] [Google Scholar]
- Klepeis VE, Cornell-Bell A, Trinkaus-Randall V. Growth factors but not gap junctions play a role in injury-induced Ca2+ waves in epithelial cells. J. Cell Sci. 2001;114:4185–4195. doi: 10.1242/jcs.114.23.4185. [DOI] [PubMed] [Google Scholar]
- Laux-Fenton WT, Donaldson PJ, Kistler J, Green CR. Connexin expression patterns in the rat cornea: molecular evidence for communication compartments. Cornea. 2003;22:457–464. doi: 10.1097/00003226-200307000-00012. [DOI] [PubMed] [Google Scholar]
- Rae JL, Lewno AW, Cooper K, Gates P. Dye and electrical coupling between cells of the rabbit corneal endothelium. Curr. Eye Res. 1989;8:859–869. doi: 10.3109/02713688909000876. [DOI] [PubMed] [Google Scholar]
- Watsky MA, Rae JL. Dye coupling in the corneal endothelium: effects of ouabain and extracellular calcium removal. Cell Tissue Res. 1992;269:57–63. doi: 10.1007/BF00384726. [DOI] [PubMed] [Google Scholar]
- Williams KK, Watsky MA. Dye spread through gap junctions in the corneal epithelium of the rabbit. Curr. Eye Res. 1997;16:445–452. doi: 10.1076/ceyr.16.5.445.7039. [DOI] [PubMed] [Google Scholar]
- D'hondt C, Ponsaerts R, Srinivas SP, Vereecke J, Himpens B. Thrombin inhibits intercellular calcium wave propagation in corneal endothelial cells by modulation of hemichannels and gap junctions. Invest. Ophthalmol. Vis. Sci. 2007;48:120–133. doi: 10.1167/iovs.06-0770. [DOI] [PubMed] [Google Scholar]
- D'hondt C, Ponsaerts R, Srinivas SP, Vereecke J, Himpens B. Reduced intercellular communication and altered morphology of bovine corneal endothelial cells with prolonged time in cell culture. Curr. Eye Res. 2009;34:454–465. doi: 10.1080/02713680902913022. [DOI] [PubMed] [Google Scholar]
- D'hondt C, Srinivas SP, Vereecke J, Himpens B. Adenosine Opposes Thrombin-Induced Inhibition of Intercellular Calcium Wave in Corneal Endothelial Cells. Invest Ophthalmol. Vis. Sci. 2007;48:1518–1527. doi: 10.1167/iovs.06-1062. [DOI] [PubMed] [Google Scholar]
- Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2005;46:1208–1218. doi: 10.1167/iovs.04-1181. [DOI] [PubMed] [Google Scholar]
- Gomes P, Srinivas SP, Vereecke J, Himpens B. ATP-dependent paracrine intercellular communication in cultured bovine corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2005;46:104–113. doi: 10.1167/iovs.04-0846. [DOI] [PubMed] [Google Scholar]
- Gomes P, Srinivas SP, Vereecke J, Himpens B. Gap junctional intercellular communication in bovine corneal endothelial cells. Exp Eye Res. 2006. [DOI] [PubMed]
- Ponsaerts R, et al. The myosin II ATPase inhibitor blebbistatin prevents thrombin-induced inhibition of intercellular calcium wave propagation in corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2008;49:4816–4827. doi: 10.1167/iovs.07-1533. [DOI] [PubMed] [Google Scholar]
- Ponsaerts R, et al. RhoA GTPase Switch Controls Cx43-Hemichannel Activity through the Contractile System. PLoS ONE. 2012;7:e42074. doi: 10.1371/journal.pone.0042074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsaerts R, et al. Intramolecular loop/tail interactions are essential for connexin 43-hemichannel activity. Faseb J. 2010;24:4378–4395. doi: 10.1096/fj.09-153007. [DOI] [PubMed] [Google Scholar]
- Charles A. Reaching out beyond the synapse: glial intercellular waves coordinate metabolism. Sci STKE. 2005;2005:pe6. doi: 10.1126/stke.2702005pe6. [DOI] [PubMed] [Google Scholar]
- Laird DW. Life cycle of connexins in health and disease. Biochem. J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsell DP, Dunlop J, Hodgins MB. Human diseases: clues to cracking the connexin code. Trends Cell Biol. 2001;11:2–6. doi: 10.1016/s0962-8924(00)01866-3. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Klepeis VE, Weinger I, Kaczmarek E, Randall VT. P2Y receptors play a critical role in epithelial cell communication and migration. J. Cell Biochem. 2004;93:1115–1133. doi: 10.1002/jcb.20258. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Lopez-Garcia JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. J. Neurosci. 2000;20:2835–2844. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Williams M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J. Pharmacol. Exp. Ther. 2000;295:862–869. [PubMed] [Google Scholar]
- Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim. Biophys Acta. 2003;1615:7–32. doi: 10.1016/s0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol. Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- Dubyak GR, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab. Invest. 2006;86:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- Boudreault F, Grygorczyk R. Cell swelling-induced ATP release and gadolinium-sensitive channels. Am. J. Physiol. Cell Physiol. 2002;282:C219–C226. doi: 10.1152/ajpcell.00317.2001. [DOI] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Khokhlov AA, Kolesnikov SS. Voltage dependence of ATP secretion in mammalian taste cells. J. Gen. Physiol. 2008;132:731–744. doi: 10.1085/jgp.200810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. Embo J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- D'hondt C, et al. Pannexin channels in ATP release and beyond: an unexpected rendezvous at the endoplasmic reticulum. Cell Signal. 2011;23:305–316. doi: 10.1016/j.cellsig.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Leybaert L, et al. Connexin channels, connexin mimetic peptides and ATP release. Cell Commun. Adhes. 2003;10:251–257. doi: 10.1080/cac.10.4-6.251.257. [DOI] [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- Verma V, Hallett MB, Leybaert L, Martin PE, Howard Evans W. Perturbing plasma membrane hemichannels attenuates calcium signalling in cardiac cells and HeLa cells expressing connexins. Eur. J. Cell Biol. 2008. [DOI] [PubMed]
- Pharmacol BrJ. 2006;147:S172–S181. [Google Scholar]
- Slakey LL, Gordon EL, Pearson JD. A comparison of ectonucleotidase activities on vascular endothelial and smooth muscle cells. Ann. N.Y. Acad. Sci. 1990;603:366–378. doi: 10.1111/j.1749-6632.1990.tb37686.x. [DOI] [PubMed] [Google Scholar]
- Gordon EL, Pearson JD, Slakey LL. The hydrolysis of extracellular adenine nucleotides by cultured endothelial cells from pig aorta. Feed-forward inhibition of adenosine production at the cell surface. J. Biol. Chem. 1986;261:15496–15507. [PubMed] [Google Scholar]
- Moerenhout M, Himpens B, Vereecke J. Intercellular communication upon mechanical stimulation of CPAE- endothelial cells is mediated by nucleotides. Cell Calcium. 2001;29:125–136. doi: 10.1054/ceca.2000.0165. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Edelhauser HF. The resiliency of the corneal endothelium to refractive and intraocular surgery. Cornea. 2000;19:263–273. doi: 10.1097/00003226-200005000-00002. [DOI] [PubMed] [Google Scholar]
- George AJ, Larkin DF. Corneal transplantation: the forgotten graft. Am. J. Transplant. 2004;4:678–685. doi: 10.1111/j.1600-6143.2004.00417.x. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Wu KY, Wang HZ, Fong JC. Change of cytosolic Ca2+ mobility in cultured bovine corneal endothelial cells by endothelin-1. J. Ocul. Pharmacol. Ther. 2003;19:1–9. doi: 10.1089/108076803762718060. [DOI] [PubMed] [Google Scholar]
- Crawford KM, MacCallum DK, Ernst SA. Histamine H1 receptor-mediated Ca2+ signaling in cultured bovine corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 1992;33:3041–3049. [PubMed] [Google Scholar]
- Crawford KM, MacCallum DK, Ernst SA. Agonist-induced Ca2+ mobilization in cultured bovine and human corneal endothelial cells. Curr. Eye Res. 1993;12:303–311. doi: 10.3109/02713689308999454. [DOI] [PubMed] [Google Scholar]
- Srinivas SP, Yeh JC, Ong A, Bonanno JA. Ca2+ mobilization in bovine corneal endothelial cells by P2 purinergic receptors. Curr. Eye Res. 1998;17:994–1004. doi: 10.1076/ceyr.17.10.994.5242. [DOI] [PubMed] [Google Scholar]
- Satpathy M, Gallagher P, Jin Y, Srinivas SP. Extracellular ATP opposes thrombin-induced myosin light chain phosphorylation and loss of barrier integrity in corneal endothelial cells. Exp Eye Res. 2005;81:183–192. doi: 10.1016/j.exer.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Srinivas SP, et al. Cell volume response to hyposmotic shock and elevated cAMP in bovine trabecular meshwork cells. Exp. Eye Res. 2004;78:15–26. doi: 10.1016/j.exer.2003.10.001. [DOI] [PubMed] [Google Scholar]
- D'hondt C, Ponsaerts R, De Smedt H, Bultynck G, Himpens B. Pannexins, distant relatives of the connexin family with specific cellular functions. Bioessays. 2009;31:953–974. doi: 10.1002/bies.200800236. [DOI] [PubMed] [Google Scholar]
- Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- De Vuyst E, et al. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006;25:34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vuyst E, et al. Ca2+ regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium. 2009;46:176–187. doi: 10.1016/j.ceca.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Iyer S, Deutsch K, Yan X, Lin B. Batch RNAi selector: a standalone program to predict specific siRNA candidates in batches with enhanced sensitivity. Computer Methods and Programs in Biomedicine. 2007;85:203–209. doi: 10.1016/j.cmpb.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Stehberg J, et al. Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. Faseb J. 2012;26:3649–3657. doi: 10.1096/fj.11-198416. [DOI] [PubMed] [Google Scholar]
- Evans WH, Bultynck G, Leybaert L. Erratum to: Manipulating Connexin Communication Channels: Use of Peptidomimetics and the Translational Outputs. J. Membr. Biol. 2012;245:451. doi: 10.1007/s00232-012-9488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder P, et al. ATP-mediated cell-cell signaling in the organ of Corti: the role of connexin channels. Purinergic Signal. 2010;6:167–187. doi: 10.1007/s11302-010-9192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AC, et al. affects intracellular Ca2+ stores and induces Ca2+ wave propagation. Cell Death Differ. 2004;11:1265–1276. doi: 10.1038/sj.cdd.4401508. [DOI] [PubMed] [Google Scholar]
- Torres A, et al. Extracellular Ca2+ acts as a mediator of communication from neurons to glia. Sci. Signal. 2012;5:ra8. doi: 10.1126/scisignal.2002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decrock E, et al. Transfer of IP(3) through gap junctions is critical, but not sufficient, for the spread of apoptosis. Cell Death Differ. 2012;19(3):947–957. doi: 10.1038/cdd.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramello M, Piazza V, Bukauskas FF, Pozzan T, Mammano F. Impaired permeability to Ins(1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nat. Cell Biol. 2005;7(1,4,5):63–69. doi: 10.1038/ncb1205. [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Bukauskiene A, Verselis VK. Conductance and permeability of the residual state of connexin43 gap junction channels. J. Gen. Physiol. 2002;119:171–186. doi: 10.1085/jgp.119.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Verselis VK. Gap junction channel gating. Biochim. Biophys. Acta. 2004;1662:42–60. doi: 10.1016/j.bbamem.2004.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G. Where are the gates in gap junction channels? Clin. Exp. Pharmacol. Physiol. 1996;23:1047–1052. doi: 10.1111/j.1440-1681.1996.tb01167.x. [DOI] [PubMed] [Google Scholar]
- Retamal MA, Schalper KA, Shoji KF, Bennett MV, Saez JC. Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc. Natl. Acad. Sci. U.S.A. 2007;104:8322–8327. doi: 10.1073/pnas.0702456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama J, et al. Effect of charge substitutions at residue his-142 on voltage gating of connexin43 channels. Biophys. J. 2006;91:4054–4063. doi: 10.1529/biophysj.106.085787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplantez T, Verma V, Leybaert L, Evans WH, Weingart R. Gap26, a connexin mimetic peptide, inhibits currents carried by connexin43 hemichannels and gap junction channels. Pharmacological Research: The Official Journal of the Italian Pharmacological Society. 2012;65:546–552. doi: 10.1016/j.phrs.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Delmar M. Gap junctions as active signaling molecules for synchronous cardiac function. J. Cardiovasc. Electrophysiol. 2000;11:118–120. doi: 10.1111/j.1540-8167.2000.tb00747.x. [DOI] [PubMed] [Google Scholar]


