Summary
Background and objectives
Apparent treatment-resistant hypertension is defined as systolic/diastolic BP≥140/90 mmHg with concurrent use of three or more antihypertensive medication classes or use of four or more antihypertensive medication classes regardless of BP level.
Design, setting, participants, & measurements
The prevalence of apparent treatment-resistant hypertension among Reasons for Geographic and Racial Differences in Stroke study participants treated for hypertension (n=10,700) was determined by level of estimated GFR and albumin-to-creatinine ratio, and correlates of apparent treatment-resistant hypertension among those participants with CKD were evaluated. CKD was defined as an albumin-to-creatinine ratio≥30 mg/g or estimated GFR<60 ml/min per 1.73 m2.
Results
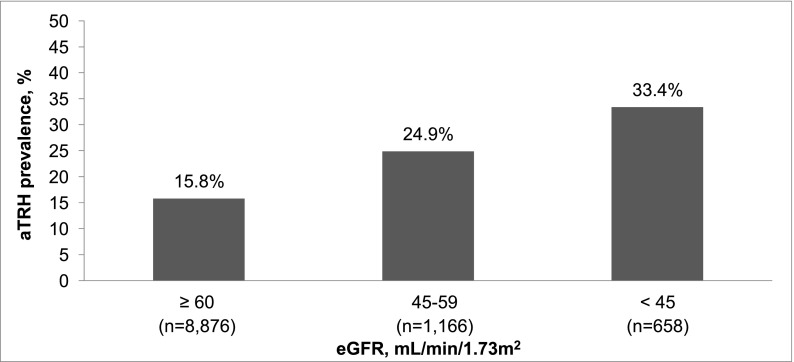
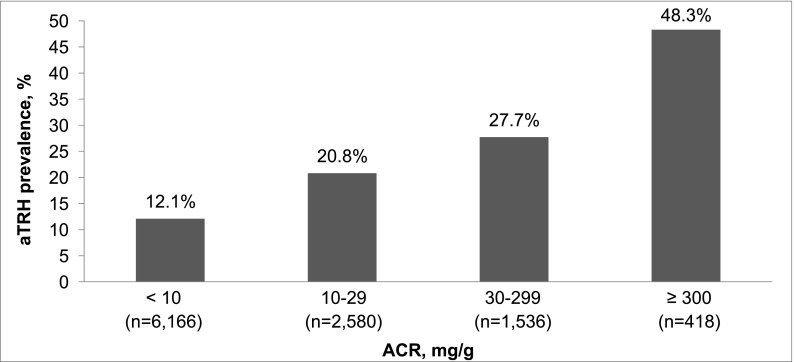
The prevalence of apparent treatment-resistant hypertension was 15.8%, 24.9%, and 33.4% for those participants with estimated GFR≥60, 45–59, and <45 ml/min per 1.73 m2, respectively, and 12.1%, 20.8%, 27.7%, and 48.3% for albumin-to-creatinine ratio<10, 10–29, 30–299, and ≥300 mg/g, respectively. The multivariable-adjusted prevalence ratios (95% confidence intervals) for apparent treatment-resistant hypertension were 1.25 (1.11 to 1.41) and 1.20 (1.04 to 1.37) for estimated GFR levels of 45–59 and <45 ml/min per 1.73 m2, respectively, versus ≥60 ml/min per 1.73 m2 and 1.54 (1.39 to 1.71), 1.76 (1.57 to 1.97), and 2.44 (2.12 to 2.81) for albumin-to-creatinine ratio levels of 10–29, 30–299, and ≥300 mg/g, respectively, versus albumin-to-creatinine ratio<10 mg/g. After multivariable adjustment, men, black race, larger waist circumference, diabetes, history of myocardial infarction or stroke, statin use, and lower estimated GFR and higher albumin-to-creatinine ratio levels were associated with apparent treatment-resistant hypertension among individuals with CKD.
Conclusions
This study highlights the high prevalence of apparent treatment-resistant hypertension among individuals with CKD.
Introduction
Hypertension is common among individuals with CKD, with previous studies suggesting a prevalence exceeding 80% (1–3). Although the majority of people with CKD is treated with multiple classes of antihypertensive medication, a substantial proportion still has uncontrolled hypertension (3). Apparent treatment-resistant hypertension (aTRH) is defined as BP that remains above goal, despite concurrent use of three or more antihypertensive medications from different classes or use of four or more antihypertensive medication classes regardless of BP level (4). Studies suggest that aTRH is common, and its prevalence is increasing among US adults. Based on data from the 2005–2008 National Health and Nutrition Examination Surveys (NHANES), Egan et al. (5) estimated the prevalence of aTRH to be 11.8% among hypertensive adults. Furthermore, in recent studies, aTRH has been associated with an increased risk of cardiovascular disease outcomes and the pooled outcome of dialysis, transplantation, or death (6,7).
Egan et al. (5) previously reported estimated GFR (eGFR)<60 ml/min per 1.73 m2 and albumin-to-creatinine ratio (ACR)>300 mg/g to be associated with an increased odds ratio for aTRH. However, the prevalence of aTRH by level of eGFR and albuminuria was not reported. Identifying an increased prevalence of aTRH with reduced eGFR and increased ACR may help raise awareness of aTRH and define the need for screening and routine clinical evaluation of aTRH among patients with CKD. Therefore, the goal of the current analysis was to determine the association between the level of eGFR and ACR and the prevalence of aTRH. Additionally, we sought to identify clinical and demographic correlates of aTRH in individuals with CKD. To address these aims, we analyzed data from a large, population-based sample of adults participating in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study.
Materials and Methods
Study Participants
The REGARDS study is a population-based cohort study of 30,239 black and white US adults≥45 years of age enrolled between June of 2003 and October of 2007 (8). Participants were recruited from the 48 contiguous US states and the District of Columbia. The present analysis was restricted to 15,227 individuals with hypertension who were taking one or more classes of antihypertensive medication. Those individuals missing serum creatinine, urine albumin or urine creatinine, BP data, or information from the pill bottle review (n=1721) were also excluded. Additionally, participants who reported being on dialysis at baseline or were missing information on dialysis status (n=122) were excluded. Finally, we excluded participants with uncontrolled BP on one or two antihypertensive medication classes (n=2684) from the main analyses, because we were unable to determine whether these participants had aTRH. As described below, these participants were included in secondary analyses. After these exclusion criteria were applied, data from 10,700 participants were analyzed (Supplemental Figure 1). The REGARDS study protocol was approved by the Institutional Review Boards governing research in human subjects at the participating centers, and all participants provided written consent.
Data Collection
Of relevance to the current analysis, information on the following demographic, behavioral, and medical history characteristics were collected during a telephone interview: age, sex, race, education, annual household income, smoking status, alcohol consumption, frequency of physical activity, and history of diabetes, stroke, or myocardial infarction. Medication adherence was assessed using the four-item Morisky Medication Adherence Scale. Scores on this scale can range from zero to four, with higher scores indicating worse adherence. During the in-home examination, standardized protocols were followed to obtain two BP measurements. Also, an electrocardiogram was obtained, waist circumference was measured, and blood and urine samples were collected. A pill bottle review was conducted to record information for all medications that participants reported taking during the 2 weeks preceding the in-home study visit. Medication doses were not recorded. Total and HDL cholesterol were measured by colorimetric reflectance spectrophotometry, and high-sensitivity C-reactive protein was measured using a high-sensitivity particle-enhanced immunonephelometric assay. Diabetes was defined as fasting serum glucose≥126 mg/dl, nonfasting serum glucose≥200 mg/dl, or use of antidiabetes medication.
Definition of eGFR and ACR
Using the blood sample collected during the in-home examination, serum creatinine was measured using an isotope dilution mass spectrometry traceable method. eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration Equation (9) and categorized as ≥60, 45–59, and <45 ml/min per 1.73 m2. Using spot urine samples collected during the in-home examination, urinary albumin was measured with the BN ProSpec Nephelometer from Dade Behring (Marburg, Germany). Urinary creatinine was measured with a rate-blanked Jaffé procedure using the Modular-P Analyzer (Roche/Hitachi, Indianapolis, IN). ACR was categorized as <10, 10–29, 30–299, and ≥300 mg/g. CKD was defined as an ACR≥30 mg/g or an eGFR<60 ml/min per 1.73 m2.
Definition of aTRH
During the in-home examination, BP was measured two times by trained technicians with a standardized protocol using aneroid sphygmomanometers. Participants were asked to sit quietly for 3 minutes with both feet on the floor before the BP measurements. Measurements were taken in the left arm, when possible, using an appropriately sized cuff. The cuff was inflated to 20 mmHg above the pulse obliteration level and slowly deflated. After a 30-second rest period, this process was repeated on the same arm to obtain the second BP measurement. Quality control for BP measurement in REGARDS was monitored by central examination of digit preference, and technicians were retrained as necessary (8,10). The two BP measurements were averaged for analysis. Uncontrolled BP was defined as systolic BP≥140 mmHg and/or diastolic BP≥90 mmHg. aTRH was defined as uncontrolled BP with concurrent use of three or more antihypertensive medication classes or use of four or more antihypertensive medication classes regardless of BP level. Antihypertensive medication classes were defined using the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (11). One-pill combinations were classified into multiple medication classes.
Statistical Analyses
Characteristics of REGARDS participants were calculated by CKD and aTRH status. We calculated the prevalence of aTRH by levels of eGFR (≥60, 45–59, and <45 ml/min per 1.73 m2) and ACR (<10, 10–29, 30–299, and ≥300 mg/g). We used Poisson regression models to obtain crude and multivariable-adjusted prevalence ratios of aTRH associated with eGFR and ACR. Prevalence ratios are recommended instead of odds ratios for cross-sectional studies with common outcomes (12). Initial multivariable adjustment included age, race, and sex. A subsequent model included additional adjustment for geographic region of residence, income, education, physical activity, current smoking, alcohol use, waist circumference, diabetes, total cholesterol, HDL cholesterol, statin use, C-reactive protein, history of myocardial infarction, history of stroke, ACR (in analyses investigating the prevalence ratio for aTRH associated with eGFR), and eGFR (in analyses investigating the prevalence ratio for aTRH associated with ACR). In sensitivity analyses, we conducted the above analyses limited to individuals with perfect medication adherence, defined by appropriate medication-taking behaviors on each item on the Morisky Medication Adherence Scale and use of a secondary definition of aTRH requiring the use of a diuretic. Next, we calculated the prevalence of aTRH by the cross-tabulation of eGFR and ACR levels and multivariable-adjusted prevalence ratios for aTRH associated with levels of eGFR and ACR jointly, using REGARDS participants with eGFR≥60 ml/min per 1.73 m2 and ACR<10 mg/g as the reference category. Finally, for individuals with CKD, we calculated prevalence ratios for aTRH associated with the study covariates included in the full multivariable-adjusted model described above. We repeated the above analyses including 2684 individuals with uncontrolled BP on one or two classes of antihypertensive medication categorized as not having aTRH. All analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
Results
Participant Characteristics
After excluding individuals with uncontrolled BP on one or two classes of antihypertensive medication, 17.9% of REGARDS study participants with hypertension had aTRH. Individuals with both CKD and aTRH were more likely to have diabetes or a history of myocardial infarction or stroke than those individuals with neither CKD nor aTRH or with CKD alone or aTRH alone. Those individuals with aTRH, regardless of CKD status, were more likely to be black, have larger waist circumferences, and use statins (Table 1).
Table 1.
Characteristics of Reasons for Geographic and Racial Differences in Stroke (REGARDS) study participants with and without CKD by apparent treatment-resistant hypertension (aTRH) status
| Characteristic | CKD | No CKD | ||||
|---|---|---|---|---|---|---|
| aTRH (n=880) | No aTRH (n=2254) | P Value | aTRH (n=1032) | No aTRH (n=6534) | P Value | |
| Age, yr | 69.1 (8.9) | 69.6 (9.2) | 0.14 | 66.3 (8.1) | 64.5 (8.5) | <0.001 |
| Women, % | 48.3 | 56.3 | <0.001 | 51.4 | 58.9 | <0.001 |
| Black race, % | 60.3 | 46.4 | <0.001 | 58.9 | 46.0 | <0.001 |
| Region, % | ||||||
| Nonbelt | 45.9 | 45.0 | 0.86 | 43.5 | 42.1 | 0.49 |
| Belt | 33.0 | 33.1 | 0.86 | 36.1 | 35.9 | 0.49 |
| Buckle | 21.1 | 21.9 | 0.86 | 20.4 | 22.0 | 0.49 |
| Income<$20,000, % | 28.3 | 23.5 | 0.10 | 22.5 | 17.5 | <0.001 |
| Less than high school education, % | 21.0 | 17.7 | 0.01 | 18.2 | 12.2 | <0.001 |
| Waist circumference, cm | 104.5 (16.7) | 99.6 (15.9) | <0.001 | 103.7 (16.0) | 97.5 (14.6) | <0.001 |
| Diabetes, % | 52.7 | 38.1 | <0.001 | 36.3 | 22.6 | <0.001 |
| Current smoking, % | 13.2 | 12.9 | 0.69 | 10.4 | 12.9 | 0.05 |
| Current alcohol use, % | 28.1 | 29.9 | 0.34 | 33.8 | 35.4 | 0.31 |
| Physical activity, % | ||||||
| 4+ times/wk | 20.3 | 24.5 | 0.02 | 26.5 | 28.5 | 0.08 |
| 1–3 times/wk | 32.1 | 32.1 | 0.02 | 35.0 | 36.7 | 0.08 |
| None | 47.6 | 43.4 | 0.02 | 38.5 | 34.9 | 0.08 |
| History of myocardial infarction, % | 27.9 | 20.4 | <0.001 | 23.0 | 13.1 | <0.001 |
| History of stroke, % | 16.8 | 12.5 | 0.001 | 9.7 | 6.5 | <0.001 |
| Statin use, % | 58.2 | 45.8 | <0.001 | 47.9 | 41.3 | <0.001 |
| Total cholesterol, mg/dl | 178.9 (39.8) | 185.5 (40.3) | <0.001 | 181.4 (37.5) | 186.7 (38.7) | <0.001 |
| HDL cholesterol, mg/dl | 46.5 (14.8) | 49.5 (15.8) | <0.001 | 49.7 (14.6) | 51.2 (15.7) | 0.004 |
| C-reactive protein, mg/L | 3.1 (1.4, 6.9) | 3.0 (1.3, 6.7) | 0.13 | 2.6 (1.2, 5.7) | 2.4 (1.1, 5.5) | 0.07 |
| Systolic BP, mmHg | 143.7 (19.4) | 124.1 (10.0) | <0.001 | 140.9 (17.1) | 123.0 (9.9) | <0.001 |
| Diastolic BP, mmHg | 79.1 (12.1) | 73.7 (8.2) | <0.001 | 80.8 (11.1) | 75.1 (7.5) | <0.001 |
| Estimated GFR, ml/min per 1.73 m2 | 60.8 (23.5) | 64.7 (24.3) | <0.001 | 87.3 (15.6) | 88.6 (15.3) | 0.01 |
| Albumin-to-creatinine ratio, mg/g | 64.1 (21.0, 256.3) | 36.1 (9.0, 88.9) | <0.001 | 8.3 (5.3, 14.1) | 6.5 (4.5, 10.3) | <0.001 |
Numbers are mean (SD) or percent, except for C-reactive protein and albumin-to-creatinine ratio, which are presented as median (25th percentile, 75th percentile). CKD is defined as an albumin-to-creatinine ratio≥30 mg/g or an estimated GFR<60 ml/min per 1.73 m2.
Prevalence of aTRH by eGFR and ACR
The prevalence of aTRH was 15.8%, 24.9%, and 33.4% for REGARDS participants with an eGFR≥60, 45–59, and <45 ml/min per 1.73 m2, respectively (Figure 1) and 12.1%, 20.8%, 27.7%, and 48.3% for ACR<10, 10–29, 30–299, and ≥300 mg/g, respectively (Figure 2). The prevalence of aTRH was 28.0% and 32.1% among participants with eGFR<60 and ACR≥30 mg/g, respectively. Also, 28.1% of those participants with CKD versus 13.6% of their counterparts without CKD had aTRH. Lower eGFR and higher ACR were associated with higher prevalence ratios for aTRH in crude and multivariable-adjusted models (Table 2). Results were similar when limited to individuals with perfect medication adherence (Supplemental Table 1) and when using a secondary definition of aTRH requiring the use of a diuretic (Supplemental Table 2).
Figure 1.
Prevalence of apparent treatment-resistant hypertension (aTRH) by estimated GFR (eGFR) among Reasons for Geographic and Racial Differences in Stroke (REGARDS) study participants.
Figure 2.
Prevalence of aTRH by albumin-to-creatinine ratio (ACR) among REGARDS study participants.
Table 2.
Prevalence ratios for aTRH associated with estimated GFR (eGFR) and albumin-to-creatinine ratio (ACR) among REGARDS study participants
| Characteristic | n | Crude Prevalence Ratio (95% CI) | Age-, Race-, and Sex-Adjusted Prevalence Ratio (95% CI) | Multivariable-Adjusteda Prevalence Ratio (95% CI) |
|---|---|---|---|---|
| eGFR, ml/min per 1.73 m2 | ||||
| ≥60 | 8876 | 1 (ref) | 1 (ref) | 1 (ref) |
| 45–59 | 1166 | 1.57 (1.41 to 1.76) | 1.49 (1.33 to 1.67) | 1.25 (1.11 to 1.41) |
| <45 | 658 | 2.12 (1.88 to 2.38) | 1.90 (1.68 to 2.15) | 1.20 (1.04 to 1.37) |
| ACR, mg/g | ||||
| <10 | 6166 | 1 (ref) | 1 (ref) | 1 (ref) |
| 10–29 | 2580 | 1.72 (1.55 to 1.90) | 1.68 (1.52 to 1.86) | 1.54 (1.39 to 1.71) |
| 30–299 | 1536 | 2.29 (2.06 to 2.54) | 2.09 (1.88 to 2.33) | 1.76 (1.57 to 1.97) |
| ≥300 | 418 | 3.99 (3.54 to 4.50) | 3.48 (3.08 to 3.93) | 2.44 (2.12 to 2.81) |
CI, confidence interval.
Adjusted for age, race, sex, geographic region, income, education, physical activity, current smoking, alcohol use, waist circumference, diabetes, total cholesterol, HDL cholesterol, statin use, C-reactive protein, history of myocardial infarction, history of stroke, ACR (log transformed in eGFR analyses), and eGFR (in ACR analyses).
Cross-Tabulation of eGFR and ACR on aTRH
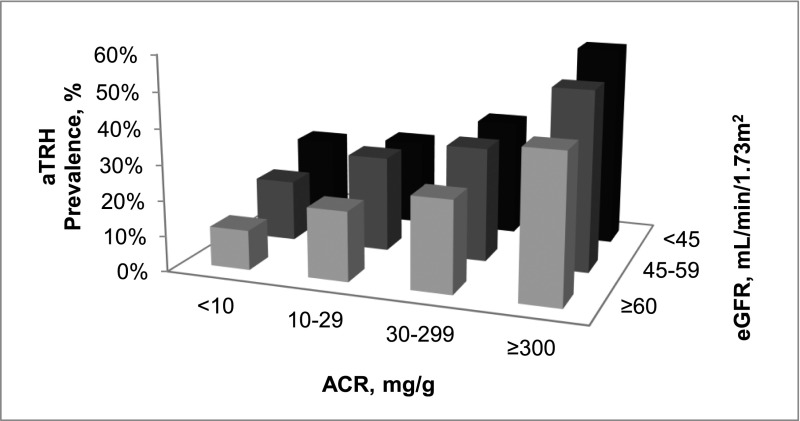
The prevalence of aTRH was 11.2% among individuals with eGFR≥60 ml/min per 1.73 m2 and ACR<10 mg/g. Within each eGFR level, the prevalence of aTRH increased at higher ACR levels. Similarly, for ACR<10 or ≥300 mg/g, there was a higher prevalence of aTRH at lower eGFR levels (Figure 3). However, for ACR of 10–29 or 30–299 mg/g, the prevalence of aTRH was higher for individuals with an eGFR of 45–59 ml/min per 1.73 m2 but not <45 ml/min per 1.73 m2 compared with an eGFR≥ 60 ml/min per 1.73 m2. The prevalence of aTRH among individuals with eGFR<45 ml/min per 1.73 m2 and ACR≥300 mg/g was 56.4%. Similar patterns were present after multivariable adjustment (Table 3).
Figure 3.
Prevalence of aTRH by the cross-tabulation of ACR and eGFR among REGARDS study participants.
Table 3.
Multivariable-adjusted prevalence ratios for aTRH associated with cross-tabulation of ACR level and eGFR level among REGARDS study participants
| ACR, mg/g | eGFR, ml/min per 1.73 m2 | ||
|---|---|---|---|
| ≥60 | 45–59 | <45 | |
| <10 | 1 (ref) | 1.36 (1.11 to 1.67) | 1.73 (1.30 to 2.30) |
| 10–29 | 1.59 (1.41 to 1.79) | 1.99 (1.62 to 2.45) | 1.62 (1.15 to 2.29) |
| 30–299 | 1.88 (1.65 to 2.14) | 2.22 (1.79 to 2.75) | 2.02 (1.59 to 2.57) |
| ≥300 | 2.73 (2.25 to 3.31) | 3.13 (2.44 to 4.03) | 3.44 (2.88 to 4.10) |
Numbers are prevalence ratios (95% confidence intervals). Ratios are adjusted for age, race, sex, geographic region, income, education, physical activity, current smoking, alcohol use, waist circumference, diabetes, total cholesterol, HDL cholesterol, statin use, C-reactive protein, history of myocardial infarction, and history of stroke
Correlates of aTRH among Hypertensive Individuals with CKD
After age and race adjustment, women were associated with a lower prevalence ratio for aTRH, and after age and sex adjustment, black race was associated with a higher prevalence ratio of aTRH (Table 4). After age, race, and sex adjustment, income<$20,000, larger waist circumference, diabetes, history of myocardial infarction or stroke, statin use, eGFR<45 ml/min per 1.73 m2, and higher ACR were associated with a higher prevalence ratio for aTRH. Physical activity four or more times per week versus none and higher total and HDL cholesterol were associated with a lower prevalence ratio for aTRH. After multivariable adjustment, women were associated with a lower prevalence ratio for aTRH. Black race, larger waist circumference, diabetes, history of myocardial infarction or stroke, statin use, lower eGFR, and higher ACR were associated with a higher prevalence ratio for aTRH.
Table 4.
Prevalence ratios for aTRH associated with study covariates among REGARDS study participants with CKD
| Characteristic | Age, Race, and Sex Adjusted | Multivariable Adjusteda |
|---|---|---|
| Age, per 10 yr | 0.99 (0.93 to 1.05) | 1.05 (0.98 to 1.13) |
| Women | 0.75 (0.67 to 0.84) | 0.87 (0.76 to 0.99) |
| Black race | 1.56 (1.38 to 1.75) | 1.49 (1.31 to 1.68) |
| Region | ||
| Nonbelt | 1 (ref) | 1 (ref) |
| Belt | 1.03 (0.91 to 1.17) | 1.04 (0.92 to 1.19) |
| Buckle | 1.04 (0.90 to 1.20) | 1.05 (0.91 to 1.22) |
| Income<$20,000 | 1.18 (1.04 to 1.33) | 1.08 (0.94 to 1.23) |
| Less than high school education | 1.07 (0.93 to 1.23) | 0.99 (0.85 to 1.15) |
| Waist circumference, per 15 cm | 1.19 (1.13 to 1.25) | 1.13 (1.07 to 1.20) |
| Diabetes | 1.44 (1.29 to 1.62) | 1.13 (1.00 to 1.28) |
| Current smoking | 0.98 (0.83 to 1.16) | 1.01 (0.85 to 1.20) |
| Alcohol use | 0.95 (0.83 to 1.07) | 1.02 (0.90 to 1.17) |
| Physical activity | ||
| None | 1 (ref) | 1 (ref) |
| 1–3 times/wk | 0.91 (0.80 to 1.04) | 1.00 (0.88 to 1.14) |
| 4+ times/wk | 0.79 (0.68 to 0.92) | 0.90 (0.77 to 1.05) |
| History of myocardial infarction | 1.34 (1.19 to 1.51) | 1.20 (1.06 to 1.36) |
| History of stroke | 1.23 (1.07 to 1.42) | 1.15 (1.00 to 1.33) |
| Statin use | 1.44 (1.29 to 1.62) | 1.29 (1.14 to 1.46) |
| Total cholesterol, per 40 mg/dl | 0.90 (0.84 to 0.95) | 0.97 (0.91 to 1.04) |
| HDL cholesterol, per 15 mg/dl | 0.86 (0.81 to 0.92) | 0.95 (0.88 to 1.02) |
| C-reactive protein>3 mg/L | 1.07 (0.96 to 1.20) | 0.96 (0.86 to 1.08) |
| eGFR, ml/min per 1.73 m2 | ||
| ≥60 | 1 (ref) | 1 (ref) |
| 45–59 | 0.95 (0.83 to 1.09) | 1.18 (1.00 to 1.40) |
| <45 | 1.25 (1.08 to 1.44) | 1.22 (1.05 to 1.43) |
| ACR, mg/g | ||
| <10 | 1 (ref) | 1 (ref) |
| 10–29 | 1.44 (1.16 to 1.79) | 1.32 (1.06 to 1.65) |
| 30–299 | 1.43 (1.20 to 1.70) | 1.50 (1.22 to 1.83) |
| ≥300 | 2.37 (1.97 to 2.85) | 2.24 (1.83 to 2.76) |
CKD was defined as an ACR≥30 mg/g or an eGFR<60 ml/min per 1.73 m2. Numbers are prevalence ratios (95% confidence intervals).
Adjusted for age, race, sex, geographic region, income, education, physical activity, current smoking, alcohol use, waist circumference, diabetes, total cholesterol, HDL cholesterol, statin use, C-reactive protein, history of myocardial infarction, history of stroke, ACR, and eGFR.
Antihypertensive Medication Use
The median number of antihypertensive medication classes being taken was 2.0 (25th, 75th percentiles=1.0, 2.0) for individuals without aTRH compared with 4.0 (25th, 75th percentiles=3.0, 4.0) for those individuals with aTRH (Supplemental Table 3). Over one half of the study participants with aTRH were taking diuretics (86.8%), β-blockers (73.4%), calcium channel blockers (72.1%), and angiotensin-converting enzyme (ACE) inhibitors (62.0%).
Sensitivity Analyses Including Individuals with Uncontrolled BP on One or Two Classes of Antihypertensive Medication
Among individuals on one or two classes of antihypertensive medication, the prevalence of uncontrolled BP was 28.0% compared with 33.2% among those individuals on three or more classes of antihypertensive medication. When including individuals with uncontrolled BP on one or two classes of antihypertensive medication, 14.3% of study participants with hypertension had aTRH. The prevalence of aTRH was 12.5%, 20.4%, and 28.3% for REGARDS participants with eGFR≥60, 45–59, and <45 ml/min per 1.73 m2, respectively, and 10.1%, 15.9%, 20.8%, and 34.7% for those participants with ACR<10, 10–29, 30–299, and ≥300 mg/g, respectively. The prevalence of aTRH was 22.0% for individuals with CKD and 11.0% for individuals without CKD. Additionally, 23.2% and 12.5% of participants with and without eGFR<60 ml/min per 1.73 m2 and 23.9% and 11.9% with and without ACR≥30 mg/g had aTRH, respectively.
Discussion
Using data from a large, population-based sample of black and white adults, we found a strong, graded association between lower eGFR and higher ACR with a higher prevalence of aTRH. The prevalence of aTRH was high and exceeded 50% among individuals with both eGFR<45 ml/min per 1.73 m2 and ACR≥300 mg/g. Among individuals with CKD, black race, larger waist circumference, diabetes, history of myocardial infarction and stroke, and lower eGFR and higher ACR were associated with a higher prevalence ratio for aTRH.
Hypertension is common among individuals with CKD. Furthermore, most people with CKD and hypertension take multiple classes of antihypertensive medication (3,13). In an analysis of 3612 participants in the Chronic Renal Insufficiency study, 83% of individuals with hypertension were taking at least two classes of antihypertensive medication, with 26% and 32% of participants taking antihypertensive medications from three and four or more classes, respectively (3). Consistent with this finding, 87% of 238 predialysis CKD patients in a recent study were taking at least two antihypertensive medications, with 34% of patients taking three antihypertensive medications and 33% of patients taking four or more antihypertensive medications (13). However, the prevalence of aTRH was not evaluated in these prior studies.
The association between aTRH and CKD has been examined in at least two small clinic-based studies. Abdel-Kader et al. (14) reported a 30% prevalence of aTRH among 88 CKD participants in the Pittsburgh-based Sleep-SCORE (Strategies Concentrating On Risk Evaluation) study. In another clinic-based study of 300 patients with CKD, the prevalence of aTRH was 26% at study enrollment and 38% after 6 months of follow-up. Furthermore, in this latter study, aTRH was associated with increased risk of the pooled outcome of dialysis, transplantation, or death over a median of 37.6 months of follow-up (hazard ratio=1.85; 95% confidence interval=1.13 to 3.03) (7). Also, Egan et al. (5) previously identified an association between aTRH and CKD prevalence using NHANES data. The current analysis extends these findings by investigating the association between level of eGFR and albuminuria (separately and jointly) and the prevalence of aTRH and the correlates of aTRH among individuals with CKD in a large population-based sample of US adults.
Data from the REGARDS study indicate that aTRH is a common condition among individuals with CKD, suggesting the need for greater awareness of this comorbidity among clinicians. Among those individuals with CKD, particularly men, blacks, individuals with large waist circumferences, and individuals with a history of diabetes, stroke, or myocardial infarction had a higher prevalence of aTRH. The identification of individuals at high risk of developing aTRH who may benefit from intensive BP monitoring and early therapeutic interventions (e.g., treatment for secondary hypertension, referral to a hypertension specialist, and cessation of medications that increase BP) should be a high priority. Furthermore, the American Heart Association scientific statement on aTRH diagnosis, evaluation, and treatment recommends diuretics as first-line therapy for patients with hypertension, with the subsequent addition of an ACE inhibitor or angiotensin receptor blocker and then a calcium channel blocker as needed to achieve BP control (4). In the current study, 86.8% of participants with aTRH were taking a diuretic. However, only 7.6% were taking an aldosterone antagonist. Although careful monitoring for hyperkalemia is necessary in CKD patients taking aldosterone antagonists, studies have shown that they provide significant antihypertensive and antiproteinuric benefits when added to existing multidrug treatment regimens (15,16). This finding is especially important, because prior studies suggest that BP control can be achieved and maintained, even in difficult to control populations (17,18). Furthermore, the results of this study emphasize the need for the development and dissemination of appropriate therapeutic regimens for CKD patients with aTRH.
The causal pathway between albuminuria and aTRH is not known and may be bidirectional. aTRH may result in microalbuminuria through prolonged increases in glomerular pressure and subsequent renal damage (19). Furthermore, sodium retention and excessive activation of the renin-angiotensin-aldosterone system have been linked to uncontrolled BP in individuals with CKD (20,21). Also, albuminuria is thought to be preceded by systemic endothelial dysfunction (22). Although endothelial dysfunction is associated with incident hypertension, the presence of uncontrolled BP has also been associated with worsening endothelial function (23,24). Several therapies (e.g., smoking cessation and ACE inhibitor use) that improve endothelial function also reduce albuminuria (25). Given the cross-sectional study design used for the current analysis, we could not assess the direction of the albuminuria–aTRH association. Future studies with longitudinal assessments of albuminuria, endothelial function, and BP are warranted to investigate.
The findings of the current study should be considered in the context of certain limitations. Most importantly, the analysis used a cross-sectional study design. CKD is both a common cause and complication of hypertension, and it is unknown whether aTRH preceded the development of CKD or whether CKD resulted in the incidence of aTRH (26). Also, BP, eGFR, and albuminuria were only assessed at a single time point, making misclassification of CKD and aTRH status possible. An additional limitation is the lack of medication dosing information. Some individuals may have been on an inadequate treatment regimen and not truly treatment-resistant. We do not have data on potential secondary causes of aTRH or whether participants took antihypertensive medication on the day of their study visit before the BP measurement (4). Our study minimizes misclassification of the aTRH phenotype through a pill bottle review to identify the number of antihypertensive medication classes being taken, consideration of medication adherence, and standardized in-home BP measurement, which limits potential white-coat effects. Additional studies may be warranted to determine whether the use of home BP monitoring is a potentially useful strategy for diagnosis and management of aTRH in difficult to control populations. Other strengths include the large population-based sample of blacks and whites and the availability of both albuminuria and eGFR measurements.
In conclusion, data from the current study show a strong, graded association between lower eGFR and higher ACR with higher aTRH prevalence. A very high prevalence of aTRH was present among people with both lower eGFR and higher ACR when they were considered jointly. Strategies are needed to improve BP control and better manage aTRH in people with CKD.
Disclosures
R.M.T., E.K.B., C.B.B., M.R.I., and D.T.L. have no disclosures to report. D.A.C. has received grant support from Medtronic. O.M.G. and P.M. have received grant support from Amgen Inc. S.O. has received grant support from AstraZeneca, Merck and Co., National Heart, Lung, and Blood Institute, Novartis, Takeda, Medtronic/Ardian, Daiichi Sankyo Inc., Vivus Inc., and Medronic and has received honoraria or consulting fees from Daiichi Sankyo Inc., Backbeat, Bayer, Novartis, Pfizer, and Medtronic. D.W. has received grant support from Amgen Inc. and serves on the National Nephrology Advisory Board for Amgen.
Supplementary Material
Acknowledgments
The authors thank the other investigators, staff, and participants of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study for their valuable contributions.
This research project is supported by Cooperative Agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services. Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data.
A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00550113/-/DCSupplemental.
References
- 1.Parikh NI, Hwang SJ, Larson MG, Meigs JB, Levy D, Fox CS: Cardiovascular disease risk factors in chronic kidney disease: Overall burden and rates of treatment and control. Arch Intern Med 166: 1884–1891, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Wei GL, McQuillan G, Brancati FL, Levey AS, Jones C, Klag MJ: Prevalence of high blood pressure and elevated serum creatinine level in the United States: Findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 161: 1207–1216, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O'Connor A, Perumal K, Rahman M, Steigerwalt S, Teal V, Townsend R, Weir M, Wright JT, Jr, Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Hypertension awareness, treatment, and control in adults with CKD: Results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 55: 441–451, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM: Resistant hypertension: Diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 51: 1403–1419, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC: Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation 124: 1046–1058, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, O’Connor PJ, Selby JV, Ho PM: Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 125: 1635–1642, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Nicola L, Borrelli S, Gabbai FB, Chiodini P, Zamboli P, Iodice C, Vitiello S, Conte G, Minutolo R: Burden of resistant hypertension in hypertensive patients with non-dialysis chronic kidney disease. Kidney Blood Press Res 34: 58–67, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G: The reasons for geographic and racial differences in stroke study: Objectives and design. Neuroepidemiology 25: 135–143, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell EK, Gao L, Judd S, Glasser SP, McClellan W, Gutiérrez OM, Safford M, Lackland DT, Warnock DG, Muntner P: Blood pressure indexes and end-stage renal disease risk in adults with chronic kidney disease. Am J Hypertens 25: 789–796, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute. National High Blood Pressure Education Program Coordinating Committee : Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Behrens T, Taeger D, Wellmann J, Keil U: Different methods to calculate effect estimates in cross-sectional studies. A comparison between prevalence odds ratio and prevalence ratio. Methods Inf Med 43: 505–509, 2004 [PubMed] [Google Scholar]
- 13.Sarafidis PA, Sharpe CC, Wood E, Blacklock R, Rumjon A, Al-Yassin A, Ariyanayagam R, Simmonds S, Fletcher-Rogers J, Vinen K: Prevalence, patterns of treatment, and control of hypertension in predialysis patients with chronic kidney disease. Nephron Clin Pract 120: c147–c155, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Kader K, Dohar S, Shah N, Jhamb M, Reis SE, Strollo P, Buysse D, Unruh ML: Resistant hypertension and obstructive sleep apnea in the setting of kidney disease. J Hypertens 30: 960–966, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishizaka MK, Zaman MA, Calhoun DA: Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens 16: 925–930, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Ouzan J, Pérault C, Lincoff AM, Carré E, Mertes M: The role of spironolactone in the treatment of patients with refractory hypertension. Am J Hypertens 15: 333–339, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Wright JT, Jr, Agodoa L, Contreras G, Greene T, Douglas JG, Lash J, Randall O, Rogers N, Smith MC, Massry S, African American Study of Kidney Disease and Hypertension Study Group : Successful blood pressure control in the African American Study of Kidney Disease and Hypertension. Arch Intern Med 162: 1636–1643, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F, ACCORD Study Group : Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 362: 1575–1585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koroshi A: Microalbuminuria, is it so important? Hippokratia 11: 105–107, 2007 [PMC free article] [PubMed] [Google Scholar]
- 20.Campese VM, Mitra N, Sandee D: Hypertension in renal parenchymal disease: Why is it so resistant to treatment? Kidney Int 69: 967–973, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Campese VM: Hypertension in dialysis patients. In: Principles and Practice of Dialysis, edited by Henrich WL, Philadelphia, Lippincott Williams & Wilkins, 2004, pp 227–256 [Google Scholar]
- 22.Nannipieri M, Penno G, Rizzo L, Pucci L, Bandinelli S, Mattei P, Taddei S, Salvetti A, Navalesi R: Transcapillary escape rate of albumin in type II diabetic patients. The relationship with microalbuminuria and hypertension. Diabetes Care 20: 1019–1026, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Shimbo D, Muntner P, Mann D, Viera AJ, Homma S, Polak JF, Barr RG, Herrington D, Shea S: Endothelial dysfunction and the risk of hypertension: The multi-ethnic study of atherosclerosis. Hypertension 55: 1210–1216, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace SM, Yasmin, McEniery CM, Mäki-Petäjä KM, Booth AD, Cockcroft JR, Wilkinson IB: Isolated systolic hypertension is characterized by increased aortic stiffness and endothelial dysfunction. Hypertension 50: 228–233, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Ochodnicky P, Henning RH, van Dokkum RP, de Zeeuw D: Microalbuminuria and endothelial dysfunction: Emerging targets for primary prevention of end-organ damage. J Cardiovasc Pharmacol 47[Suppl 2]: S151–S162, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kestenbaum B, Rudser KD, de Boer IH, Peralta CA, Fried LF, Shlipak MG, Palmas W, Stehman-Breen C, Siscovick DS: Differences in kidney function and incident hypertension: The multi-ethnic study of atherosclerosis. Ann Intern Med 148: 501–508, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.