Summary
Dabigatran is an oral direct thrombin inhibitor widely used to prevent and treat various thromboembolic complications. An advantage of this agent over other anticoagulants is that routine laboratory monitoring and related dose adjustments are considered unnecessary. A major disadvantage is the absence of a reliable means of reversing its anticoagulant effect. After U.S. Food and Drug Administration approval, recently emerged data suggest a higher bleeding risk with dabigatran, especially in the elderly. Clinicians are thus faced with caring for patients with serious bleeding events without readily available tests to measure drug levels or the anticoagulant effects of dabigatran and without effective antidotes to rapidly reverse the anticoagulant effect. On the basis of dabigatran's pharmacokinetic profile, hemodialysis and continuous renal replacement therapy have been used to remove dabigatran with the hope, still unproven, that this would rapidly reverse the anticoagulant effect and reduce bleeding in patients with normal and those with reduced kidney function. However, the best clinical approach to the patient with serious bleeding is not known, and the risks of placing a hemodialysis catheter in an anticoagulated patient can be substantial. This article reviews this issue, addressing clinical indications, drug pharmacokinetics, clinical and laboratory monitoring tests, and dialytic and nondialytic approaches to reduce bleeding in dabigatran-treated patients.
Anticoagulant therapy plays a central role in the prevention and treatment of venous and arterial thromboembolic diseases in various clinical settings. Several anticoagulants are approved for this indication, with vitamin K antagonists and heparin and its analogues the most widely used (1). However, the clinical approach to anticoagulant therapy has changed recently. For example, warfarin has a narrow therapeutic window and variable dose-response relations, which necessitate frequent laboratory monitoring. Limitations associated with warfarin and heparin use (2) have led to the exploration of newer anticoagulants with improved efficacy, safety, and ease of clinical monitoring.
An ideal anticoagulant would possess the following characteristics: (1) excellent oral bioavailability, (2) a predictable pharmacokinetic profile, (3) limited need for routine laboratory monitoring, (4) rapid anticoagulant reversibility with an antidote, and (5) an acceptable safety profile (3). Dabigatran etexilate is an oral direct thrombin inhibitor that meets many but not all of these characteristics. It has a rapid onset of action, results in a predictable anticoagulation response, and does not require routine laboratory monitoring. There is not, however, a widely available test to quantitatively define the state of anticoagulation or an antidote to rapidly reverse the anticoagulant effects of dabigatran. Given the significant renal clearance of dabigatran, patients with CKD or those who develop AKI are at increased bleeding risk due to drug accumulation (4). Because no antidote is available, an increasing number of severe and even fatal bleeding complications are being reported (5), and renal replacement therapy (RRT) is being widely recommended in an effort to improve outcomes in dabigatran-treated patients with uncontrolled bleeding. Despite its problems, dabigatran is approved in many countries; it carries the trade names Pradaxa in Europe and the United States and Pradax in Canada (6).
Here, we review clinical indications, pharmacokinetics, laboratory tests available to monitor anticoagulation, and nondialytic and dialytic management of dabigatran-associated bleeding complications.
Clinical Indications
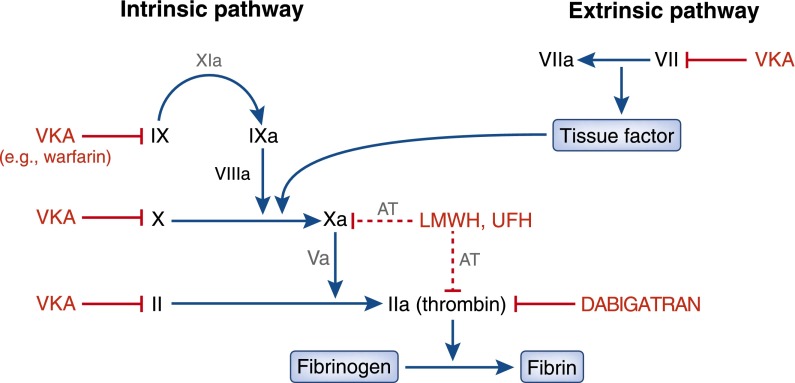
Warfarin exerts its anticoagulant effect by inhibiting the hepatic conversion of vitamin K to vitamin K epoxide, which is required for the subsequent activation of several clotting factors (Figure 1). In contrast, dabigatran directly inhibits thrombin activity by binding the molecule with high affinity and specificity (7). As illustrated in Figure 1, targeting thrombin inhibits the final enzyme in the common coagulation pathway.
Figure 1.
Effects of vitamin K antagonists (VKA), such as warfarin, low-molecular-weight heparin (LMWH), and dabigatran, on the coagulation pathway. VKAs indirectly inhibit both the intrinsic and extrinsic pathways. LMWH indirectly inhibit factor Xa and thrombin via inhibition of antithrombin (AT). Dabigatran directly inhibits factor IIa (thrombin). UFH, unfractionated heparin.
Effective and safe anticoagulant agents are critical for the appropriate care of numerous clinical conditions. Dabigatran has been evaluated in numerous trials for different indications and was approved by the U.S. Food and Drug Administration (FDA) in October 2010. Table 1 summarizes the key studies (8–13). Taken together, these trials suggest that dabigatran therapy at various doses is noninferior to other anticoagulants (warfarin and enoxaparin) for prevention or treatment of various thromboembolic disorders. In addition, with a few exceptions, dabigatran had a similar safety profile with regard to major bleeding events compared with currently approved anticoagulants. The major benefits are the ease of administration and simplicity of clinical monitoring. However, one concern was the observation that within 12 weeks of initial approval, dabigatran was responsible for more serious adverse events than 98.7% of all other medications (14,15). Dabigatran was associated with 307 serious adverse events (severe hemorrhage or thromboembolic events) in elderly patients compared with 202 with warfarin. Notably, the drug was being used extensively for off-label use or nonspecific general anticoagulation. Furthermore, these studies did not include patients with underlying kidney disease, the very elderly, and others at increased risk for AKI or CKD who could receive the drug in clinical practice.
Table 1.
Trials comparing dabigatran with other anticoagulants
| Trial (Reference) | Patients | Dabigatran Dose | Comparison Drug | Major Efficacy Endpoints | Bleeding Complications |
|---|---|---|---|---|---|
| RE-LY (8) | n=18,113 Nonvalvular atrial fibrillation | 110 or 150 mg twice daily | Warfarin | Similar with 110 mg, lower rates of stroke or systemic embolism with 150 mg | Less with 110 mg and similar with 150 mg |
| RE-MODEL (9) | n=2,076 Total knee replacement | 150 or 220 mg daily | Enoxaparin | Total VTE events and all-cause mortality not inferior | Similar rates |
| RE-NOVATE (10) | n=3,494 Total hip replacement | 150 or 220 mg daily | Enoxaparin | Total VTE events and all-cause mortality not inferior | Similar rates |
| RE-NOVATE II (11) | n=2,055 Total hip replacement | 220 mg daily | Enoxaparin | Total VTE events and all-cause mortality not inferior | Similar rates |
| RE-MOBILIZE (12) | n=2,615 Total knee replacement | 150 or 220 mg daily | Enoxaparin | Total VTE events and all-cause mortality inferior for VTE | Similar rates |
| RE-COVER (13) | n=2,564 Acute VTE | 150 mg twice daily | Warfarin | Incidence of recurrent VTE, and death not inferior | Similar rates |
RE-LY, Randomized Evaluation of Long-Term Anticoagulation Therapy; VTE, venous thromboembolism.
Pharmacokinetics
Dabigatran etexilate is the inactive prodrug of dabigatran. After oral ingestion, the drug is rapidly absorbed with an absolute bioavailability of approximately 7% and is completely hydrolyzed into its active form by nonspecific esterases in the gut, plasma, and liver (16). Dabigatran etexilate is only transiently detectable in plasma. Peak dabigatran plasma concentrations are dose dependent and are observed 1.5–3 hours after oral dosing. The mean terminal half-life of dabigatran is about 9 hours in healthy volunteers. In older healthy volunteers, the half-life is 12–16 hours, which is more typical of the patient population receiving the drug. Steady-state plasma dabigatran levels in patients with atrial fibrillation receiving 150 mg twice daily are approximately 180 ng/ml at peak (about 2 hours) and about 90 ng/ml at trough (approximately 12 hours). Approximately 35% of dabigatran is bound to plasma proteins independent of plasma concentrations. It is a small, lipophilic molecule (molecular weight, 471) and has a volume of distribution of 60–70 L (about 0.9–1.0 L/kg), indicating a moderate extravascular distribution.
Dabigatran etexilate is not metabolized by cytochrome P450 enzymes or other oxidoreductases and has no drug-drug interactions with cytochrome P450 substrates, inhibitors, or inducers (17). Dabigatran undergoes conjugation with activated glucuronic acid to yield pharmacologically active conjugates. Drug interactions do exist for this agent. The prodrug, but not dabigatran, acts as a substrate for P-glycoprotein (PGP), thereby limiting potential effects to drug absorption. Substantial increases in dabigatran exposure result from interference of other administered drugs with the PGP efflux transporter (reducing dabigatran efflux back into the intestinal lumen). Clinically important medications, such as amiodarone and verapamil, are examples of agents that interfere with PGP function and raise dabigatran plasma concentrations (18). Because approximately 42% of hospitalized patients with atrial fibrillation take drugs that interact with PGP to some extent, there is potential concern for excessive drug exposure for a large number of patients (19).
Approximately 80%–85% of dabigatran is excreted by the kidneys via glomerular filtration (renal clearance, 90 ml/min), with negligible contributions from tubular secretion or absorption (16). A small amount (6%) is excreted via the biliary system. Urine contains primarily unchanged dabigatran and small amounts of dabigatran glucuronides. As a result, reduced kidney function is associated with increased dabigatran plasma concentrations and a prolonged half-life, increasing patient exposure to the anticoagulant effects of the drug.
Monitoring
Although monitoring of the international normalized ratio (INR) is critical for warfarin use, dabigatran produces a consistent pharmacodynamic effect and monitoring is not considered necessary (20). Plasma drug concentrations and coagulation parameter values have been assessed to determine their correlation with clinical efficacy and safety (20). A correlation was observed between dabigatran plasma levels and the degree of anticoagulant effect, as measured by prolongation of activated partial thromboplastin time (aPTT), INR, ecarin clotting time (ECT) and thrombin time (TT). A linear relationship was observed between ECT, INR, and TT, whereas a nonlinear increase in aPTT occurred with increasing dabigatran plasma concentrations (16,20). Although a linear relationship between dabigatran’s plasma concentration and many of these assays exists, they are insensitive within a range of clinically relevant plasma concentrations and are not able to accurately estimate dabigatran’s anticoagulant effect.
TT measures the direct activity of thrombin and is thus most sensitive to the presence of dabigatran’s anticoagulant effect; however, it is not useful for quantitative monitoring of the drug's anticoagulant effect (20). Bleeding risk can be assessed by ECT, which is a better marker of the anticoagulant effect of dabigatran than TT and aPTT are. This test is not widely available in clinical laboratories, so most hospitals use TT and aPTT to interpret the extent of anticoagulation (20,21). A more specific method to precisely measure dabigatran concentrations, the HEMOCLOT thrombin inhibitor assay (Aniara, Mason, OH) (22), is not widely available.
In summary, dabigatran monitoring represents a challenge in clinical practice because of the poor correlation of available coagulation measures with dabigatran plasma levels and the limited availability and standardization of more specific tests to better assess its anticoagulant effect.
Nondialytic Therapy and Reversal of Coagulopathy
As with all anticoagulant therapies, certain clinical situations will arise in which urgent reversal of coagulopathy is needed. However, unlike most other anticoagulant or antiplatelet therapies, no specific antidote or reversal agent is available for dabigatran. Because of its relatively short half-life in patients with normal kidney function, immediate discontinuation of drug coupled with supportive care and localization of bleeding may be sufficient in cases of mild to moderate bleeding. Published case reports and postmarketing data make it clear that severe or life-threatening bleeding can occur in a variety of clinical settings that require the implementation of urgent strategies aimed at antagonizing the anticoagulant effect of dabigatran.
Supportive care along with administration of various clotting factor replacement agents in the setting of life-threatening bleeding associated with dabigatran have yielded inconsistent results (23–26). Most cases involved patients with mild to moderate CKD or with initially normal kidney function but with AKI, resulting in impaired dabigatran clearance. Evidence for the use and effectiveness of the therapies that are discussed below is mostly based on studies in healthy volunteers or in experimental animals. Much uncertainty remains, so expert consultation from a hematologist or inpatient anticoagulation service should be obtained promptly in dabigatran-treated patients presenting with severe or life-threatening bleeding.
Oral Activated Charcoal
In in vitro models, oral activated charcoal effectively adsorbs >99.9% of administered dabigatran, suggesting that gastric lavage with activated charcoal within 2 hours of drug ingestion is a reasonable initial treatment strategy in bleeding patients who have recently taken a dabigatran dose or in the setting of an overdose (20). No clinical data document effectiveness in clinical practice.
Fresh Frozen Plasma
Fresh frozen plasma (FFP) reduced the severity of intracranial hemorrhage in mice receiving high-dose dabigatran, although it did not reduce mortality (27). More importantly, no clinical trial evidence supports use of FFP in patients taking dabigatran. Case reports in general have indicated little or no effect of FFP in reversing thrombin generation measures or improving bleeding related to dabigatran (24). FFP is not recommended as a primary option for reversal of the anticoagulant effect of dabigatran by the American College of Chest Physicians (28).
Prothrombin Complex Concentrates
Prothrombin complex concentrates (PCCs) contain vitamin K–dependent coagulation factors as well as varying levels of proteins C and S. Two separate three-factor PCCs (containing factors II, IX, and X; Profilnine SD, Grifols Biologicals Inc., Los Angeles, CA, and Bebulin VH, Baxter Healthcare, Westlake Village, CA), are available in the United States. Efficacy data of various preparations of PCC are heterogeneous and conflicting. Animal models using mice and rabbits have shown that PCC can reduce bleeding complications (29,30). In case reports delineating its use in emergent bleeding situations, PCC is often used in conjunction with a variety of other clinical strategies, making it difficult to isolate the clinical effectiveness of PCC alone. A single randomized controlled trial in healthy volunteers showed that four-factor PCC (not currently available in the United States) had no effect on reversing clotting times or conventional coagulation assay abnormalities (31). Despite these findings, one of the currently available three-factor PCC formulations is recommended by the American College of Chest Physicians as a potential reversal agent for dabigatran-associated bleeding (28).
Activated PCCs
The activated form of PCC (commercially available as factor VIII inhibitor bypassing activity [FEIBA], Baxter Bioscience, Vienna, Austria) is a complex containing factors II, VII, IX, and X, which are activated during the manufacturing process (32). FEIBA has been shown in in vitro and other studies to correct thrombin generation measures in samples treated with dabigatran (33). This approach is promising, but only isolated case reports (34) demonstrate its clinical effectiveness in practice.
Recombinant Factor VII
Similar to PCC, recombinant activated factor VII (rFVIIa) has been used as an emergent treatment for dabigatran-associated bleeding (24,25). This potent procoagulant and hemostatic agent (NovoSeven, Novo Nordisk, Bagsvaerd, Denmark) acts by directly activating thrombin on the surface of platelets in the absence of tissue factor (20). Animal data have failed to show a reduction in dabigatran-associated bleeding in mice treated with rFVIIa (27,28); however, a healthy human volunteer study suggests that rFVIIa partially corrects clotting times and coagulation abnormalities (35). Given the lack of clinical trial data, it is uncertain whether rFVIIa has any clinical effectiveness in this setting; however, the American College of Chest Physicians recommends it as a potential reversal agent for dabigatran-associated bleeding (28).
Dabigatran-Specific Antibody Fragments
Finally, van Ryn and colleagues have presented preliminary findings regarding a novel antibody fragment (Fab) that potently binds dabigatran and inhibits its anticoagulant activity (36,37). Although still early in development, this may hold promise that a specific dabigatran antidote will be developed in the future.
Role of Dialytic Therapies
On the basis of dabigatran’s pharmacokinetic characteristics, hemodialysis should be effective in removing it from plasma. In cases of severe life-threatening bleeding, hemodialysis has been used as an efficient method of drug removal in patients with CKD, AKI, and normal kidney function. As with nondialytic therapies to reverse dabigatran’s effect, most of the evidence supporting the role of hemodialysis in dabigatran removal is based on healthy volunteers and animal studies.
In a small, open-label single center study, Stangier et al. (16) demonstrated that a 4-hour hemodialysis session removed an average of 62%–68% of an administered 50-mg oral dose in six patients with ESRD by measuring drug levels in the arterial and venous dialysis tubing at 2 and 4 hours during the treatment. It should be noted that the medication was administered at the start of the dialysis session and that the FDA-approved dosage of dabigatran is between 75 and 150 mg twice daily, higher than the administered study dosage.
Chang and colleagues argued that these clearance estimates are unreliable because they were not obtained during steady-state conditions (34). They reported that a 3-hour hemodialysis treatment using a high-flux dialyzer and blood flow of 350 ml/min in a patient with normal kidney function reduced the plasma concentration of dabigatran by about 10 ng/ml per hour, but there was rebound in dabigatran plasma levels from 29 ng/ml to 43 ng/ml 2 hours after the cessation of hemodialysis (34,38,39).
To address the issue of hemodialysis efficacy in the setting of therapeutic dabigatran doses at steady state, two separate industry-sponsored, open-label studies showed that hemodialysis eliminated 50%–60% of plasma dabigatran using 4-hour treatments with blood flow rates between 200 and 400 ml/min (40,41). The mean fraction cleared from plasma was 48.8% (range, 41%–58%) for a blood flow rate of 200 ml/min and 59.3% (range, 54%–65%) for a blood flow rate of 400 ml/min. Unlike the previous trial by Stangier et al. (38), which used a low, one-time dabigatran dose of 50 mg, these studies used a 3-day dosing protocol (day 1: 150 mg; day 2: 110 mg; day 3: 75 mg) to achieve a higher average drug concentration at steady state. Rebound developed after dialysis was discontinued, representing a 10% increase in plasma concentration.
An interesting, albeit not readily available, treatment modality examined in another industry-sponsored study demonstrated the efficacy of activated charcoal perfusion with hemodialysis for removal of dabigatran in a pig model. Using a variety of in vitro and other experiments, the authors showed that activated charcoal perfusion was effective but not superior to hemodialysis with blood flow rates of 300 ml/min or greater (42).
A participant in the RE-LY (Randomized Evaluation of Long-Term Anticoagulant Therapy) trial with stage III CKD who underwent cardiac surgery with therapeutic levels of dabigatran developed massive postoperative bleeding that required admission to the intensive care unit and infusion of coagulation factors, including three doses of rFVIIa (25). Although dabigatran was stopped 2 days before surgery, the postoperative dabigatran level was 95 ng/ml, a level shown in the trial to be consistent with actively taking the medication. Hemodialysis reduced the plasma dabigatran level from 76 ng/ml to 27 ng/ml after 6 hours of dialysis with a high-flux filter using a dialysate flow of 700 ml/min and an average blood flow of 320 ml/min. Preoperative hemodialysis has also been used to remove dabigatran before emergent cardiac transplantation surgery (43). The authors measured TT before and after dialysis as a qualitative marker of drug effect and found a reduction in TT from 90.6 seconds (4.5 times the upper limit of normal) to 60.2 seconds after 2.5 hours of dialysis at a blood flow rate of 500 ml/min and dialysate flow rate of 800 ml/h using an Optiflux F180 dialyzer.
A single case report describes the use of both continuous venovenous hemodialysis and continuous venovenous hemodiafiltration as part of an emergency treatment strategy for an elderly man with gastrointestinal bleeding (24). Plasma drug concentrations were not measured.
Taken together, these reports provide limited and rather low-quality evidence for a role of hemodialysis in addressing severe bleeding complications in patients with renal impairment taking dabigatran. Hemodialysis does remove dabigatran from the plasma at treatment times and blood flow rates that are routinely used in clinical practice. Because most of the case reports involve concurrent treatment with blood products, plasma, and clotting factor replacement, it becomes difficult to isolate or evaluate the role of hemodialysis in these situations. The presence of a small but potentially clinically significant rebound in plasma drug concentration after dialysis cessation suggests that an extended duration of hemodialysis may be of value, although this requires further study. The role of continuous replacement therapies is uncertain, and thus its use in patients who are hemodynamically stable and could tolerate hemodialysis should probably await studies of dabigatran removal with these treatment modalities. Of note, the issue of dialysis catheter placement in the setting of life-threatening coagulopathy should not be taken lightly. Because life-threatening bleeding situations require a multifaceted approach to treatment, the administration of PCC, activated PCC, or rFVIIa according to current recommendations should be strongly considered before placement of a dialysis catheter to avoid periprocedural bleeding and complications.
Recommended Approach in the Patient with Renal Failure
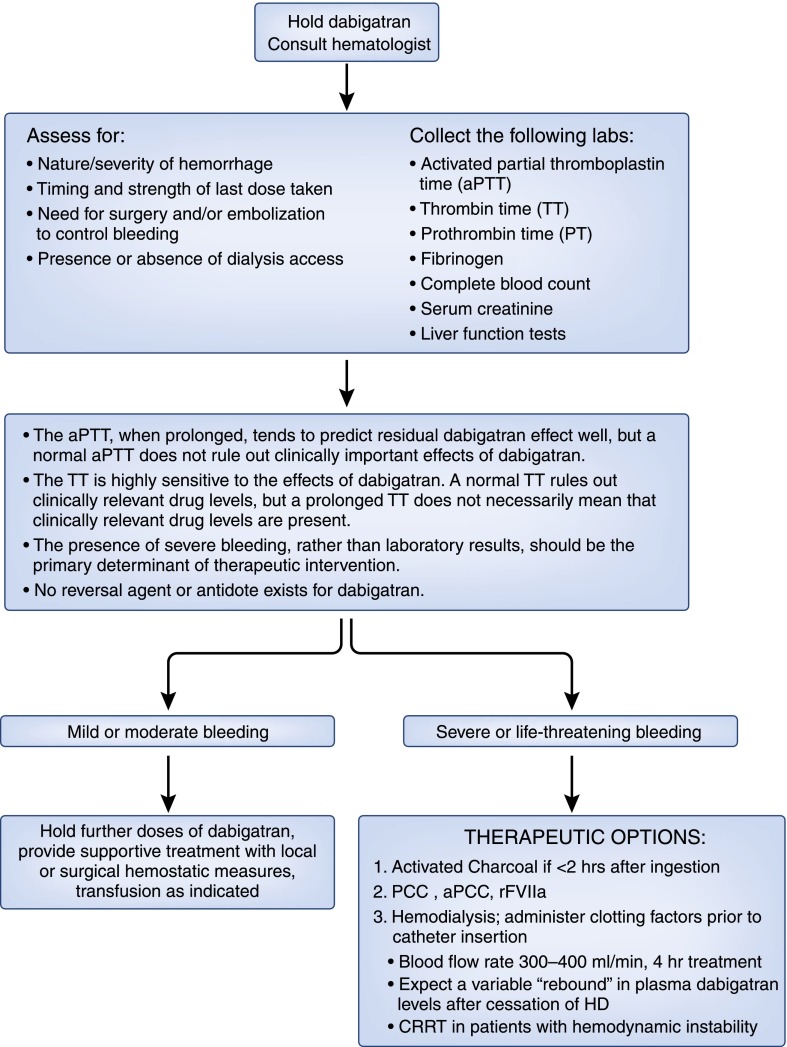
The American Society of Hematology and the American College of Chest Physicians have developed evidence-based clinical practice guidelines for the reversal of dabigatran-associated bleeding. According to their most recent guideline update, there is insufficient clinical experience to firmly guide the management of major bleeding, suspected overdose, urgently needed surgery, or urgent invasive diagnostic or therapeutic procedures in patients who are taking this new drug (31). The issue facing nephrologists will be consideration of use of hemodialysis or continuous renal replacement therapy in dabigatran-related severe or life-threatening bleeding. Consultation with a hematologist expert in use of clotting factors that might reverse the effect of dabigatran should precede initiation of dialysis. Any decision about dialysis should take into account the severity and nature of the bleeding, time since last dose, and level of kidney function, with recognition that the half-life of the drug is 25–30 hours in individuals with creatinine clearance <30 ml/min. There must also be individual consideration of the potential risks and benefits of placing a temporary hemodialysis catheter in an actively bleeding patient; the catheter should be placed with ultrasound guidance whenever possible and by an experienced operator. On the basis of available, albeit limited information, we would recommend hemodialysis over continuous renal replacement therapy unless the latter is indicated because of the hemodynamic status of the patient. Figure 2 outlines our suggested approach to the patient with dabigatran-associated bleeding.
Figure 2.
Suggested approach to patients with dabigatran-associated bleeding. aPCC, activated prothrombin complex concentrate; CRRT, continuous renal replacement therapy; HD, hemodialysis; PCC, prothrombin complex concentrate; rFVIIa, recombinant factor VIIa.
Conclusion
Dabigatran therapy represents a positive advancement in oral anticoagulant therapy. However, its introduction into clinical practice has brought to light potentially serious complications in patients who develop or have underlying kidney disease. The inability to easily measure drug levels or predict the degree of anticoagulation with current assays, the lack of an effective antidote, and delayed drug excretion in patients with kidney disease make management of bleeding difficult. Drug discontinuation, various clotting factor administration, and, at times, hemodialysis are required to manage such patients and reduce or reverse bleeding complications.
Disclosures
None
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hyers TM: Management of venous thromboembolism: past, present, and future. Arch Intern Med 163: 759–768, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS: 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol 57: e101–e198, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Ansell J: Warfarin versus new agents: Interpreting the data. Hematology (Am Soc Hematol Educ Program) 2010: 221–228, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Wartak SA, Bartholomew JR: Dabigatran: Will it change clinical practice? Cleve Clin J Med 78: 657–664, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Radecki RP: Dabigatran: Uncharted waters and potential harms. Ann Intern Med 157: 66–68, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Hankey GJ, Eikelboom JW: Dabigatran etexilate: A new oral thrombin inhibitor. Circulation 123: 1436–1450, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Hauel NH, Nar H, Priepke H, Ries U, Stassen JM, Wienen W: Structure-based design of novel potent nonpeptide thrombin inhibitors. J Med Chem 45: 1757–1766, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, RE-LY Steering Committee and Investigators : Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139–1151, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Kälebo P, Christiansen AV, Hantel S, Hettiarachchi R, Schnee J, Büller HR, RE-MODEL Study Group : Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: The RE-MODEL randomized trial. J Thromb Haemost 5: 2178–2185, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Prins MH, Hettiarachchi R, Hantel S, Schnee J, Büller HR, RE-NOVATE Study Group : Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: A randomised, double-blind, non-inferiority trial. Lancet 370: 949–956, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K, Schnee JM, Friedman RJ, RE-NOVATE II Study Group : Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. Thromb Haemost 105: 721–729, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg JS, Davidson BL, Comp PC, Francis CW, Friedman RJ, Huo MH, Lieberman JR, Muntz JE, Raskob GE, Clements ML, Hantel S, Schnee JM, Caprini JA, RE-MOBILIZE Writing Committee : Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 24: 1–9, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, Baanstra D, Schnee J, Goldhaber SZ, RE-COVER Study Group : Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 361: 2342–2352, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Food and Drug Administration: FDA Drug Safety Podcast for Healthcare Professionals: Safety review of post-market reports of serious bleeding events with the anticoagulant Pradaxa (dabigatran etexilate mesylate) Available at: www.fda.gov/Drugs/DrugSafety/DrugSafetyPodcasts/ucm283151.htm Accessed January 2013
- 15.Radecki RP: Dabigatran: uncharted waters and potential harms. Ann Intern Med 157: 66–68, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Stangier J, Rathgen K, Stähle H, Gansser D, Roth W: The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 64: 292–303, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W: The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 36: 386–399, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Walenga JM, Adiguzel C: Drug and dietary interactions of the new and emerging oral anticoagulants. Int J Clin Pract 64: 956–967, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Jungbauer L, Dobias C, Stöllberger C, Weidinger F: The frequency of prescription of P-glycoprotein-affecting drugs in atrial fibrillation. J Thromb Haemost 8: 2069–2070, 2010 [DOI] [PubMed] [Google Scholar]
- 20.van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M, Clemens A: Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 103: 1116–1127, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Liesenfeld KH, Schäfer HG, Trocóniz IF, Tillmann C, Eriksson BI, Stangier J: Effects of the direct thrombin inhibitor dabigatran on ex vivo coagulation time in orthopaedic surgery patients: A population model analysis. Br J Clin Pharmacol 62: 527–537, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stangier J, Feuring M: Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis 23: 138–143, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Wychowski MK, Kouides PA: Dabigatran-induced gastrointestinal bleeding in an elderly patient with moderate renal impairment. Ann Pharmacother 46: e10, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Harinstein LM, Morgan JW, Russo N: Treatment of dabigatran-associated bleeding: Case report and review of the literature [Published online ahead of print November 16, 2012]. J Pharm Pract doi: 10.1177/0897190012465955 [DOI] [PubMed] [Google Scholar]
- 25.Warkentin TE, Margetts P, Connolly SJ, Lamy A, Ricci C, Eikelboom JW: Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood 119: 2172–2174, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Chen BC, Viny AD, Garlich FM, Basciano P, Howland MA, Smith SW, Hoffman RS, Nelson LS: Hemorrhagic complications associated with dabigatran use. Clin Toxicol (Phila) 50: 854–857, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Zhou W, Schwarting S, Illanes S, Liesz A, Middelhoff M, Zorn M, Bendszus M, Heiland S, van Ryn J, Veltkamp R: Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke 42: 3594–3599, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G: Oral anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141: e44S–88S, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambourne MD, Eltringham-Smith LJ, Gataiance S, Arnold DM, Crowther MA, Sheffield WP: Prothrombin complex concentrates reduce blood loss in murine coagulopathy induced by warfarin, but not in that induced by dabigatran etexilate. J Thromb Haemost 10: 1830–1840, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Pragst I, Zeitler SH, Doerr B, Kaspereit FJ, Herzog E, Dickneite G, van Ryn J: Reversal of dabigatran anticoagulation by prothrombin complex concentrate (Beriplex P/N) in a rabbit model. J Thromb Haemost 10: 1841–1848, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M: Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: A randomized, placebo-controlled, crossover study in healthy subjects. Circulation 124: 1573–1579, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Key NS, Negrier C: Coagulation factor concentrates: past, present, and future. Lancet 370: 439–448, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Khoo TL, Weatherburn C, Kershaw G, Reddel CJ, Curnow J, Dunkley S: The use of FEIBA (R) in the correction of coagulation abnormalities induced by dabigatran. Int J Lab Hematol 35: 222–224, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Chang DN, Dager WE, Chin AI: Removal of dabigatran by hemodialysis. Am J Kidney Dis61: 487–489, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Marlu R, Hodaj E, Paris A, Albaladejo P, Cracowski JL, Pernod G: Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaaroxaban: A randomized crossover ex viv study in healthy volunteers. Thromb Haemost 108: 217–224, 2012 [DOI] [PubMed] [Google Scholar]
- 36.van Ryn, Litzenburger T, Waterman A, Canada K, Hauel N, Sarko C, Kroe-Barrett R, Park J: Dabigatran anticoagulant activity is neutralized by an antibody selective to dabigatran in in-vitro and in-vivo models. Presented at the 60th Annual Scientific Session and Expo of the American College of Cardiology, New Orleans, LA, April 2–5, 2011 [Google Scholar]
- 37.van Ryn JLT, Schurer J: Reversal of anticoagulant activity of dabigatran and dabigatran-induced bleeding in rats by a specific antidote (antibody fragment). Presented at the 85th Scientific Sessions—Resuscitation Science Symposium (ReSS) of the American Heart Association, Los Angeles, CA, November 3–6, 2012 [Google Scholar]
- 38.Stangier J, Rathgen K, Stähle H, Mazur D: Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: An open-label, parallel-group, single-centre study. Clin Pharmacokinet 49: 259–268, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Stangier J, Stähle H, Rathgen K, Fuhr R: Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet 47: 47–59, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Wagner FPH, Formella S, Wiegert E, Moschetti V, Khadzhynov D, Clemens A: Effective elimination of dabigatran with haemodialysis: a phase I single centre study in patients with end-stage renal disease. Presented at the 84th Scientific Sessions 2011 of the American Heart Association, Orlando, FL, November 12–16, 2011 [Google Scholar]
- 41.Härtter STM, Liesenfeld K-H, Nehmiz G, Moschetti V, Formella S, Khadzhynov D, Wagner F, Peters H: Investigation of the elimination of dabigatran by haemodialysisin patients with end stage renal disease. Presented at 113th Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics, National Harbor, MD, March 12–17, 2012 [Google Scholar]
- 42.Lange J, Thiel C, Thiel K, Klingert W, Klingert K, Königsrainer A, Formella S, Clemens A, van Ryn J, Schenk M: Acceleration of dabigatran elimination by activated charcoal perfusion and hemodialysis in a pig model. Presented at the 54th American Society of Hematology Annual Meeting and Exposition, Atlanta, GA, December 8–11, 9, 2012 [Google Scholar]
- 43.Wanek MR, Horn ET, Elapavaluru S, Baroody SC, Sokos G: Safe use of hemodialysis for dabigatran removal before cardiac surgery. Ann Pharmacother 46: e21, 2012 [DOI] [PubMed] [Google Scholar]