Summary
Background and objectives
Dabigatran is an oral direct thrombin inhibitor that is Food and Drug Administration-approved for prevention of stroke in patients with atrial fibrillation. No antidote is available for reversal of dabigatran’s anticoagulant effect. Despite limited clinical data, hemodialysis has been suggested as a strategy to remove dabigatran during acute bleeding. This work presents five cases, in which extracorporeal therapy was performed for dabigatran removal in acutely bleeding patients.
Design, setting, participants, & measurements
The series is comprised of five consecutive cases of patients receiving dabigatran 150 mg per os two times daily who were admitted with life-threatening bleeding between March of 2012 and January of 2013. Dabigatran plasma concentrations ranged from 149 to 1200 ng/ml. Treatment included administration of blood products to all patients and then, high-flux intermittent hemodialysis alone or followed by continuous renal replacement therapy.
Results
Dabigatran concentrations decreased by 52%–77% during intermittent hemodialysis but rebounded up to 87% within 2 hours after completion of dialysis. Initiation of continuous renal replacement therapy after intermittent hemodialysis attenuated the rebound effect in one patient and contributed to a reduction in dabigatran concentrations of 81% over 30 hours.
Conclusions
Extracorporeal therapy lowered dabigatran concentrations, suggesting that it removed the drug and may effectively accelerate total clearance, especially in patients with impaired kidney function. The use of prolonged intermittent hemodialysis or intermittent hemodialysis followed by continuous renal replacement therapy is recommended for the management of life-threatening bleeding in patients receiving dabigatran. The advantage of extracorporeal therapy should be weighed against the risk of bleeding with catheter insertion.
Introduction
Dabigatran etexilate is a prodrug that is administered orally and rapidly metabolized to the active moiety dabigatran, a direct thrombin inhibitor approved by the Food and Drug Administration (FDA) to reduce the risk of stroke and systemic embolism in patients with atrial fibrillation (1,2). The drug has advantages over warfarin that make it appealing; namely, it is as effective as warfarin in preventing stroke in patients with nonvalvular atrial fibrillation with similar or lower bleeding risk (3,4). Also, because it normally exhibits a predictable pharmacokinetic–pharmacodynamic profile, it does not require routine monitoring (5–7). This finding has generated considerable enthusiasm among clinicians, evidenced by approximately 3.7 million dabigatran prescriptions dispensed by outpatient retail pharmacies to roughly 725,000 patients in the United States alone from the time of FDA approval in October of 2010 through August of 2012 (8). However, renal clearance of dabigatran is decreased in patients with impaired kidney function. Thus, as dabigatran use increases, the likelihood of patients presenting with significant hemorrhagic complications will also increase. Unfortunately, to date, there is neither a method to quickly assess the degree of dabigatran-induced anticoagulation nor an antidote for rapid reversal in patients with active bleeding (9–11).
The implications of having no antidote available to reverse the anticoagulant effect of dabigatran have become apparent in the form of postmarketing reports of dabigatran-related adverse events (12), an FDA Drug Safety Communication (1), and several published cases of dabigatran-induced bleeding (9,13–21). The manufacturer suggests intermittent hemodialysis (IHD) as a possible treatment strategy (2), but its precise role in dabigatran-induced bleeding is currently undefined (13). Although IHD has been shown to partially remove dabigatran in stable, nonbleeding ESRD patients (22,23), use of intermittent and continuous renal replacement therapy modalities and plasmapheresis have been reported in the treatment of dabigatran-associated bleeding cases with mixed results (17–20), and to our knowledge, dabigatran plasma concentrations and/or removal have been quantified in only two cases to date (16,21). We present the first case series describing the use of extracorporeal therapy specifically for removal of dabigatran in five consecutive cases of patients admitted with life-threatening bleeding between March of 2012 and January of 2013. Dabigatran plasma concentration monitoring was performed to characterize the disposition of dabigatran before, during, and after treatment. Considerations pertaining to the assessment of dabigatran-induced anticoagulation are also presented.
Case 1
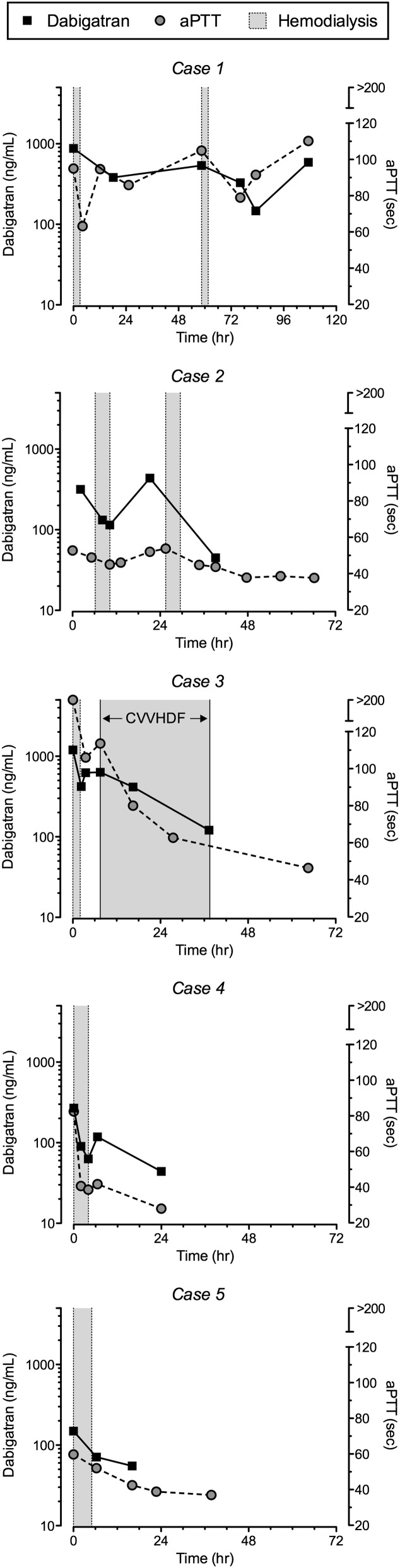
A 77-year-old man receiving dabigatran at 150 mg per os (PO) two times daily, with a past medical history of atrial fibrillation, hypertension, and nonischemic cardiomyopathy and a baseline serum creatinine (SCr) of 0.9 mg/dl, was admitted with a perforated colon. His SCr on admission was 1.6 mg/dl (Table 1). The patient required urgent exploratory laparotomy. He was given multiple blood products (Table 1) followed by IHD treatment for 3 hours preoperatively for removal of dabigatran (Table 2). The dabigatran concentration before IHD was 875 ng/ml, and it decreased to 382 ng/ml 15 hours after IHD. On postoperative day 2, active bleeding continued from the incision, and the patient received 14 units packed red blood cells. The patient’s kidney function acutely declined, and by postoperative day 3, the dabigatran concentration had rebounded to 538 ng/ml. A second IHD session for 3 hours was performed, and 14 hours postdialysis, the dabigatran concentration was found to be 329 ng/ml. However, his activated partial thromboplastin time (aPTT), prothrombin time, international normalized ratio, and thrombin clotting time (TT) continued to be elevated. The patient continued to decompensate with multiorgan failure, developed disseminated intravascular coagulation, and died on admission day 6.
Table 1.
Laboratory parameters and therapeutic interventions
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Vital signsa | |||||
| Pulse (beats/min) | 103 | 126 | 102 | 85 | 118 |
| BP (mmHg) | 122/65 | 210/97 | 95/68 | 122/59 | 124/55 |
| Laboratory parametersa | |||||
| Baseline SCr (mg/dl) | 0.9 | 1.0 | NA | 0.9 | NA |
| SCr (mg/dl) | 1.6 | 3.1 | 1.4 | 1.2 | 2.9 |
| Estimated GFR (ml/min)b | 42 | 20 | 51 | 42 | 16 |
| Hemoglobin (g/dl) | 14 | 11.6 | 12 | 9.8 | 6.9 |
| Platelets (×103/mm3) | 362 | 180 | 243 | 122 | 68 |
| HCO3 (mmol/L) | 24 | 21 | 15 | 22 | 27 |
| aPTT (s) | 95 | 50 | 133 | 82 | 59.7 |
| PT (s) | 66 | 20 | 61 | 21.4 | 21.5 |
| INR | 8.4 | 1.8 | 7.6 | 1.9 | 2 |
| TT (s) | >150 | >150 | >150 | >150 | >150 |
| Therapeutic interventions | |||||
| Vitamin K (mg) | 30 | N/A | N/A | N/A | N/A |
| PRBCs (U) | 14 | N/A | 10 | 2 | 15 |
| Platelets (U) | 4 | 1 | 6 | 1 | 2 |
| FFP (U) | 4 | 9 | 20 | 2 | 20 |
| rFactor VII (mg) | 5 | 4 | 8 | 2 | N/A |
SCr, serum creatinine; NA, not available; aPTT, activated partial thromboplastin time; PT, prothrombin time; INR, international normalization ratio; TT, thrombin clotting time; N/A, not applicable; PRBCs, packed red blood cells; FFP, fresh frozen plasma; rFactor VII, recombinant Factor VII.
Values were obtained on admission, with the exception of baseline SCr.
eGFR was calculated using admission SCr value and the four-variable Modification of Diet in Renal Disease equation.
Table 2.
Hemodialysis parameters, pre- and postdialysis dabigatran concentrations, and corresponding aPTT and TT results
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| IHD duration (h) | 3 | 4 | 2 | 4 | 5 |
| QB/QD (ml/min) | 350/800 | 400/800 | 350/800 | 350/800 | 350/800 |
| Dialyzera | PS | PS | PS | PS | PS |
| DB concentrationb | |||||
| Pre-IHD (ng/ml) | 875 | 318 | 1200 | 269 | 149 |
| Post-IHD (ng/ml) | 382c | 115 | 420 | 63 | 71d |
| Change (%) | ↓56 | ↓64 | ↓65 | ↓77 | ↓52 |
| aPTT (s)b | |||||
| Pre-IHD | 95 | 52.7 | >200 | 82.5 | 59.7 |
| Post-IHD | 63 | 45 | 106 | NA | 53.2 |
| TT (s)b | |||||
| Pre-IHD | >150 | >150 | >150 | >150 | >150 |
| Post-IHD | >150 | 98e | >150 | 77 | >150 |
| Number of IHD sessions | 2 | 2 | 1f | 1 | 1 |
IHD, intermittent hemodialysis; QB, blood flow rate; QD, dialysate flow rate; PS, polysulfone; DB, dabigatran; aPTT, activated partial thromboplastin time; NA, not available; TT, thrombin clotting time.
Rexeed 18 polysulfone dialyzer (Asahi Kasei Medical Co., Tokyo, Japan).
Represents values immediately before and after the first hemodialysis session, unless otherwise specified.
Drawn 15 hours after completion of IHD (likely also reflects rebound and must be interpreted with caution).
Drawn 1 hour after completion of IHD.
After completion of second IHD session.
One IHD session was followed by continuous venovenous hemodiafiltration treatment.
Case 2
An 86-year-old man receiving dabigatran at 150 mg PO two times daily and clopidogrel at 75 mg orally, with a past medical history of atrial fibrillation, coronary artery disease, and hypertension and baseline SCr of 1.0 mg/dl, was admitted with subdural and subarachnoid hemorrhage caused by a fall. His SCr on admission was 3.1 mg/dl (Table 1). The patient received one IHD session for 4 hours on the day of admission for dabigatran removal. Dabigatran concentrations were elevated at 318 ng/ml predialysis and decreased to 132 ng/ml with 2 hours of IHD and 115 ng/ml at the end of 4 hours of IHD (Figure 1 and Table 2). The dabigatran concentration was checked again 12 hours later, and evidence of substantial rebound was observed, because the concentration was 437 ng/ml. He received a second IHD session over 4 hours. The repeat dabigatran concentration 10 hours after completion of IHD was 41 ng/ml. The patient was discharged to an inpatient rehabilitation unit on day 7 after admission.0
Figure 1.
Plasma dabigatran concentrations and activated partial thromboplastin time (aPTT) results over time. Black squares connected by a solid line represent dabigatran. Gray circles connected by a dashed line represent aPTT. Gray shaded areas represent duration of hemodialysis treatment, except for in case 3, where continuous venovenous hemodiafiltration (CVVHDF) treatment is indicated.
Case 3
A 65-year-old man receiving dabigatran at 150 mg PO two times daily, with a past medical history of atrial fibrillation, hypertension, and alcohol abuse, was found on his floor bleeding from a chronic lower-extremity ulcer. His SCr on admission was 1.4 mg/dl (Table 1). He continued to have ongoing bleeding from his ulcer, despite a pressure bandage, and his hemoglobin fell from 12 to 6.5 g/dl within 8 hours of admission. The patient then received IHD for 2 hours to remove dabigatran (Table 2). The concentration decreased from 1200 ng/ml before IHD to 420 ng/ml at the end of dialysis. Six hours after completion of IHD, the dabigatran concentration rebounded to 626 ng/ml. The patient continued to actively bleed, requiring multiple packed red blood cell transfusions, and he became hemodynamically unstable and oliguric; therefore, continuous venovenous hemodiafiltration (CVVHDF) was initiated. The dialysate and blood flow rates were 3000 ml/h and 250 ml/min, respectively, and an AN69 hemofilter (Prismaflex M100; Gambro) was used with no anticoagulation. The dabigatran concentration before initiation of CVVHDF was 626 ng/ml, and it decreased to 416 ng/ml after 8 hours and 121 ng/ml after 30 hours of treatment. However, the patient continued to decompensate with ongoing bleeding, developed disseminated intravascular coagulation, and died on day 4 of admission.
Case 4
An 81-year-old woman from a skilled nursing facility receiving dabigatran at 150 mg PO two times daily, with a past medical history of atrial fibrillation, hypertension, and systemic lupus erythematosis and baseline SCr of 0.9 mg/dl, was admitted after a fall. A computed tomography scan showed subarachnoid hemorrhage and fracture of the fourth cervical vertebrae and left zygomatic maxillae. Her SCr on admission was 1.2 mg/dl. She received IHD for 4 hours on the day of admission for dabigatran removal (Table 2). The dabigatran concentration was 269 ng/ml and declined to 90 ng/ml after 2 hours of IHD and 63 ng/ml at the end of the 4-hour IHD session. Two hours after completion of IHD, the dabigatran concentration rebounded, nearly doubling to 118 ng/ml; then, it declined to 44 ng/ml after 24 hours because of improvement in native kidney function. The patient was discharged to an inpatient rehabilitation unit on day 5 of admission.
Case 5
A 77-year-old woman receiving dabigatran at 150 mg PO two times daily and aspirin at 81 mg daily, with a past medical history of atrial fibrillation, hypertension, and hypothyroidism, presented to an outside hospital with abdominal pain. She was found to have colonic volvulus, underwent abdominal laparotomy, and was then transferred to our institution for ongoing gastrointestinal bleeding. Her last dose of dabigatran was 4 days before admission to our center. Her SCr on admission was 2.9 mg/dl (Table 1). She received IHD for 5 hours on the day of admission for dabigatran removal (Table 2). The concentration was 149 ng/ml on admission and 71 ng/ml 1 hour after completion of IHD. The patient’s native kidney function simultaneously improved, and 12 hours after completion of IHD, the dabigatran concentration had further declined to 55 ng/ml. The patient was discharged to an inpatient rehabilitation unit on day 15 of admission.
Discussion
The FDA-approved dabigatran dosing recommendation is 150 mg PO two times daily for patients with a creatinine clearance (CrCl)>30 ml/min and 75 mg PO two times daily for patients with a CrCl=15–30 ml/min (8). The drug is not approved for use in patients with ESRD (CrCl<15 ml/min or dialysis dependent) (24). In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial, the annual rate of major bleeding was 3.11% in patients receiving dabigatran at 150 mg PO two times daily (3). All of our patients had been on a stable dosage regimen of dabigatran of 150 mg PO two times daily, which produces steady state peak and trough dabigatran plasma concentrations of approximately 180 and 90 ng/ml, respectively, assuming a CrCl>60 ml/min (25,26). However, renal clearance of dabigatran decreases in patients with an acute or chronic decline in kidney function, resulting in a corresponding increase in half-life from about 13 hours in individuals with normal kidney function to 18, 28, and 34 hours in patients with CrCl=30–50 ml/min, CrCl<30 ml/min, and ESRD, respectively (22). Obviously, this increase leads to significantly higher serum concentrations and enhanced anticoagulant effects of dabigatran for a given dose in these individuals compared with individuals with normal kidney function (5,14,15,18). Although baseline kidney function was known only in cases 1, 2, and 4, all patients were presumed to have AKI, which likely explains their elevated dabigatran plasma concentrations on admission. Cases 1–4 had dabigatran concentrations of 875, 318, 1200, and 269 ng/ml, respectively (Figure 1 and Table 2). Although case 5 had a normal dabigatran concentration of 149 ng/ml, the patient’s last dose of dabigatran was 4 days before presentation. This finding suggests that it was dramatically elevated after the last dose, which would also explain the uncontrolled bleeding observed during emergency abdominal surgery. All of our patients were deemed to have life-threatening hemorrhagic complications. Because no specific antidote or validated methodology is currently available to reverse dabigatran-associated bleeding, extracorporeal therapy was performed for dabigatran removal in an effort to achieve adequate hemostasis.
Dabigatran exhibits several physicochemical and pharmacokinetic properties that favor extracorporeal removal. Specifically, it is a small molecule with a molecular weight of approximately 630, it is predominantly (80%) excreted unchanged in the urine, and it displays relatively low protein binding of 26%–28% (2,5,22). Recent reports suggest that IHD clears dabigatran from the blood (16,21–23). An open-label study conducted in six stable, nonbleeding ESRD patients receiving IHD reported extraction ratios (i.e., concentration differences in the dialyzer inlet and outlet lines) of up to 68% (22). The manufacturer reports that approximately 57% of dabigatran can be cleared from plasma over 4 hours using a high-flux dialyzer with a blood flow rate (QB) of 300 ml/min and dialysate flow rate (QD) of 700 ml/min (2). A recent study also performed in stable nonbleeding ESRD patients reported similar findings, with an extraction ratio and dialytic clearance of 61% and 183 ml/min, respectively, observed with 4 hours of high-flux dialysis (QB=400 ml/min, QD=700 ml/min) (23). These findings suggest that IHD may be an effective means of removing dabigatran. To date, however, the effect of extracorporeal treatment on dabigatran removal and bleeding propensity in the setting of toxicity has not been formally studied.
Intermittent and continuous renal replacement therapy modalities (16–19,21) and plasmapheresis (20) have been reported in the treatment of dabigatran-associated bleeding cases with mixed results, but dabigatran plasma concentrations and/or removal have been quantified in only two published cases to date (16,21). Warkentin et al. (16) reported a 64% reduction in dabigatran plasma concentrations after 6 hours of high-flux IHD (QB=320 ml/min, QD=700 ml/min). Chang et al. (21) reported a 41% decrease in dabigatran plasma concentrations during a 2-hour interval of high-flux IHD and noted a substantial rebound in the plasma concentration (48%) 2 hours after completing dialysis (QB=350 ml/min, QD was not reported). We observed similar reductions in dabigatran concentrations of 52%–77% with high-flux IHD treatment (QB=350–400 ml/min, QD=800 ml/min) (Table 2) followed by a rebound that required additional IHD or CVVHDF treatments in three of five cases. The rebound in dabigatran concentrations was mirrored by a corresponding increase in aPTT (Figure 1). Case 3 had an extraordinarily high dabigatran concentration of 1200 ng/ml on admission, which declined 65% with a 2-hour IHD session; 6 hours post-IHD, the concentration rebounded 49%, which was attenuated by initiation of CVVHDF (Figure 1). The concentration decreased 81% after 30 hours of CVVHDF. An even larger rebound effect was observed in the patient in case 4, in whom the dabigatran concentration increased 87% within 2 hours after completion of IHD. Given dabigatran’s large volume of distribution of 50–70 L (2), it is not surprising that the drug exhibits substantial rebound after IHD, which reflects redistribution of the drug from the peripheral (i.e., adipose tissue) to the central compartment. Although the largest rebound was observed in the patient in case 2, in whom the dabigatran concentration increased from 115 to 437 ng/ml in 12 hours, an increase of this magnitude likely reflects ongoing gastrointestinal absorption in addition to redistribution. The manufacturer and others report a rebound effect of approximately 7%–15% after IHD (2,23). However, our findings of much greater rebound effects, which are in agreement with the work by Chang et al. (21), suggest that the steady state volume of distribution observed in the setting of toxicity may not reflect the volume seen in stable patients during normal therapeutic use.
In our five cases, plasma samples for dabigatran analysis were drawn during the course of clinical care and inconsistently timed. Dialysate was not collected. As such, we are unable to perform standard pharmacokinetic calculations used to determine extracorporeal clearance: namely, the recovery method and the arterial-venous (A-V) pair method (27). However, some degree of extracorporeal clearance can be inferred by changes in half-life during extracorporeal therapy (28,29). The first-order elimination rate constant and corresponding half-life of dabigatran were calculated during and after extracorporeal treatment as previously described (29). Insufficient dabigatran concentrations were available to calculate half-life in cases 1 and 2. In case 3, a patient with oliguric kidney failure, the dabigatran half-life was 1.3 hours during IHD and 12.6 hours during CVVHDF. In case 4, the intradialytic half-life was 1.9 hours, and it was 12.3 hours off dialysis, which likely reflects recovery of kidney function. Lastly, an intradialytic half-life of 4.7 hours was calculated for case 5; however, this calculation is an underestimate, because it includes a trough value (71 ng/ml) that was observed 1 hour after completion of IHD. Therefore, it likely also reflects partial rebound. The half-life increased to 26.6 hours off dialysis. Using recently published guidelines to determine the dialyzability of toxins, if the half-life observed during extracorporeal therapy is <25% of the half-life observed off therapy, then the drug may be dialyzable, and extracorporeal therapy may be an effective strategy for removal (27). Therefore, conservatively estimating a dabigatran half-life of 2 hours during high-flux hemodialysis versus 13 hours in normal kidney function or more than 28 hours in patients with CrCl<30 ml/min (22) suggests that IHD may be an effective means of dabigatran removal, particularly in the setting of impaired kidney function.
A diluted thrombin time clotting assay (Hemoclot Thrombin Inhibitor Assay; Hyphen BioMed) is used by our institution for the determination of dabigatran plasma concentrations as described previously (25). The assay is highly reproducible, with intra- and interassay coefficients of variation for dabigatran of 2.2% and 5.3%, respectively (30). In our experience, dabigatran concentrations took up to 24 hours to be reported by the laboratory. When a faster turnaround time for this test or alternatives is available, it will be instrumental in identifying patients who may or may not benefit from extracorporeal therapy. Unfortunately, laboratory measurements that provide a rapid and accurate assessment of the degree of dabigatran-induced anticoagulation are not readily available. At clinically relevant plasma concentrations, dabigatran is capable of prolonging the aPTT, TT, and ecarin clotting time (ECT). However, it has variable effects on the prothrombin time and international normalized ratio, rendering these tests poor surrogates of anticoagulation status in patients receiving dabigatran (26,31,32). TT is the most sensitive test for the detection of dabigatran activity (33); it is so sensitive that it often exceeds the upper limit of detection, which we observed in all five cases. ECT reflects thrombin generation and is another very sensitive test for measuring the anticoagulant effects of dabigatran (33). Unfortunately, its clinical availability is limited in the United States, and we were unable to test ECT in our patients. The aPTT value represents a faster and more feasible option to monitor the anticoagulant effect of dabigatran. The aPTT assay concentration–response curve is curvilinear and flattens at higher concentrations of dabigatran (>200 ng/ml), which decreases its sensitivity and may limit its use in the overdose setting (25,31). Some have concluded that aPTT should be used to provide a qualitative assessment of anticoagulation status instead of a quantitative measure of the degree of anticoagulation (31). We evaluated the relationship between dabigatran plasma concentrations and corresponding aPTT results observed in our patients using Spearman’s correlation coefficient (Prism 5.0b; GraphPad Software). A significant correlation was observed between the two parameters (Spearman’s correlation coefficient=0.89, P<0.001) (Figure 2), consistent with a previous study (34). We cannot rule out an independent effect of blood product administration in our patients on reported aPTT values. However, this finding and our experience using aPTT suggest that an extremely elevated aPTT value may be suggestive of a supratherapeutic dabigatran concentration.
Figure 2.
Relationship (Spearman correlation coefficient [rS]) between plasma dabigatran concentrations and corresponding aPTT results.
In summary, we present a series of five cases of acute bleeding in patients on a stable dose of dabigatran, in whom extracorporeal therapy as either IHD or CVVHDF was performed for dabigatran removal. Our findings of a 52%–77% reduction in plasma concentrations and a corresponding change in half-life suggest that IHD may be an effective strategy for rapid removal of dabigatran in the setting of acute bleeding. Notably, we observed a rebound in dabigatran concentrations of up to 87% within 2 hours after completion of IHD. Initiation of CVVHDF after IHD seemed to attenuate the rebound effect and contributed to a reduction in dabigatran concentrations of 81% over 30 hours. Therefore, we recommend the use of prolonged IHD or IHD followed by continuous renal replacement therapy in the management of life-threatening bleeding in patients receiving dabigatran. Serial monitoring of dabigatran plasma concentrations and/or coagulation parameters may be necessary to determine when therapeutic concentrations have been achieved and hence, extracorporeal therapy should be discontinued. The advantage of extracorporeal therapy should be weighed against the risk of bleeding with catheter insertion in these patients with severely deranged coagulation profiles.
Disclosures
None.
Acknowledgment
This work was presented in part at Kidney Week 2012, the American Society of Nephrology Annual Meeting, held November 3, 2012, in San Diego, CA (Singh et al. J Am Soc Nephrol 23: 892A, 2012).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.US Food and Drug Administration: FDA Drug Safety Communication: Safety Review of Post-Market Reports of Serious Bleeding Events with the Anticoagulant Pradaxa (Dabigatran Etexilate Mesylate), 2011. Available at: www.fda.gov/Drugs/DrugSafety/ucm282724.htm#sa Accessed January 21, 2013
- 2.Boehringer Ingelheim Pharmaceuticals, Inc.: Pradaxa® (Dabigatran Etexilate Mesylate) Prescribing Information, 2012. Available at: www.pradaxa.com Accessed January 21, 2013
- 3.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, RE-LY Steering Committee and Investigators : Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139–1151, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, Yang S, Alings M, Kaatz S, Hohnloser SH, Diener HC, Franzosi MG, Huber K, Reilly P, Varrone J, Yusuf S: Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: An analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 123: 2363–2372, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Stangier J, Stähle H, Rathgen K, Fuhr R: Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet 47: 47–59, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Stangier J: Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet 47: 285–295, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Stangier J, Clemens A: Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 15[Suppl 1]: 9S–16S, 2009 [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration: FDA Drug Safety Communication: Update on the Risk for Serious Bleeding Events with the Anticoagulant Pradaxa (Dabigatran Etexilate Mesylate), 2012. Available at: www.fda.gov/Drugs/DrugSafety/ucm326580.htm Accessed January 21, 2013
- 9.Cotton BA, McCarthy JJ, Holcomb JB: Acutely injured patients on dabigatran. N Engl J Med 365: 2039–2040, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Ganetsky M, Babu KM, Salhanick SD, Brown RS, Boyer EW: Dabigatran: Review of pharmacology and management of bleeding complications of this novel oral anticoagulant. J Med Toxicol 7: 281–287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battinelli EM: Reversal of new oral anticoagulants. Circulation 124: 1508–1510, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Moore TJ, Furberg CD, Cohen MR: Anticoagulants: The Leading Reported Drug Risk in 2011: May 31, 2012: New Data from 2011 Quarters 3–4. QuarterWatch 2012, 2012. Available at: www.ismp.org/quarterwatch/pdfs/2011Q4.pdf Accessed January 21, 2013
- 13.Chen BC, Viny AD, Garlich FM, Basciano P, Howland MA, Smith SW, Hoffman RS, Nelson LS: Hemorrhagic complications associated with dabigatran use. Clin Toxicol (Phila) 50: 854–857, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Legrand M, Mateo J, Aribaud A, Ginisty S, Eftekhari P, Huy PT, Drouet L, Payen D: The use of dabigatran in elderly patients. Arch Intern Med 171: 1285–1286, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Harper P, Young L, Merriman E: Bleeding risk with dabigatran in the frail elderly. N Engl J Med 366: 864–866, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Warkentin TE, Margetts P, Connolly SJ, Lamy A, Ricci C, Eikelboom JW: Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood 119: 2172–2174, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Cano EL, Miyares MA: Clinical challenges in a patient with dabigatran-induced fatal hemorrhage. Am J Geriatr Pharmacother 10: 160–163, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Wychowski MK, Kouides PA: Dabigatran-induced gastrointestinal bleeding in an elderly patient with moderate renal impairment. Ann Pharmacother 46: e10, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Harinstein LM, Morgan JW, Russo N: Treatment of dabigatran-associated bleeding: Case report and review of the literature [published online ahead of print November 16, 2012]. J Pharm Pract [DOI] [PubMed] [Google Scholar]
- 20.Kamboj J, Kottalgi M, Cirra VR, Shah N, Kamboj R: Direct thrombin inhibitors: A case indicating benefit from ‘plasmapheresis’ in toxicity: A call for establishing “guidelines” in overdose and to find an “antidote!” Am J Ther 19: e182–e185, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Chang DN, Dager WE, Chin AI: Removal of dabigatran by hemodialysis. Am J Kidney Dis 61: 487–489, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Stangier J, Rathgen K, Stähle H, Mazur D: Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: An open-label, parallel-group, single-centre study. Clin Pharmacokinet 49: 259–268, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Khadzhynov D, Wagner F, Formella S, Wiegert E, Moschetti V, Slowinski T, Neumayer HH, Liesenfeld KH, Lehr T, Härtter S, Friedman J, Peters H, Clemens A: Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost 109: 596–605, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Hart RG, Eikelboom JW, Ingram AJ, Herzog CA: Anticoagulants in atrial fibrillation patients with chronic kidney disease. Nat Rev Nephrol 8: 569–578, 2012 [DOI] [PubMed] [Google Scholar]
- 25.van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M, Clemens A: Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: Interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 103: 1116–1127, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Hankey GJ, Eikelboom JW: Dabigatran etexilate: A new oral thrombin inhibitor. Circulation 123: 1436–1450, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Lavergne V, Nolin TD, Hoffman RS, Roberts D, Gosselin S, Goldfarb DS, Kielstein JT, Mactier R, Maclaren R, Mowry JB, Bunchman TE, Juurlink D, Megarbane B, Anseeuw K, Winchester JF, Dargan PI, Liu KD, Hoegberg LC, Li Y, Calello DP, Burdmann EA, Yates C, Laliberté M, Decker BS, Mello-Da-Silva CA, Lavonas E, Ghannoum M: The EXTRIP (EXtracorporeal TReatments In Poisoning) workgroup: Guideline methodology. Clin Toxicol (Phila) 50: 403–413, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Takki S, Gambertoglio JG, Honda DH, Tozer TN: Pharmacokinetic evaluation of hemodialysis in acute drug overdose. J Pharmacokinet Biopharm 6: 427–442, 1978 [DOI] [PubMed] [Google Scholar]
- 29.Singh SM, McCormick BB, Mustata S, Thompson M, Prasad GV: Extracorporeal management of valproic acid overdose: A large regional experience. J Nephrol 17: 43–49, 2004 [PubMed] [Google Scholar]
- 30.HYPHEN Biomed: Hemoclot Thrombin Inhibitors Product Information, 2011. Available at: http://www.aniara.com/pdf/INS-ACK002L-RUO.pdf Accessed March 19, 2013
- 31.Liesenfeld KH, Schäfer HG, Trocóniz IF, Tillmann C, Eriksson BI, Stangier J: Effects of the direct thrombin inhibitor dabigatran on ex vivo coagulation time in orthopaedic surgery patients: A population model analysis. Br J Clin Pharmacol 62: 527–537, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halbmayer WM, Weigel G, Quehenberger P, Tomasits J, Haushofer AC, Aspoeck G, Loacker L, Schnapka-Koepf M, Goebel G, Griesmacher A: Interference of the new oral anticoagulant dabigatran with frequently used coagulation tests. Clin Chem Lab Med 50: 1601–1605, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Nowak G: The ecarin clotting time, a universal method to quantify direct thrombin inhibitors. Pathophysiol Haemost Thromb 33: 173–183, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Stangier J, Rathgen K, Stähle H, Gansser D, Roth W: The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 64: 292–303, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]