Summary
Background and objectives
HIV-associated nephropathy (HIVAN) is well described, but the clinical features of a group of renal pathologies characterized by Ig or immune complex depositions referred to as HIV-associated immune complex kidney disease (HIVICK) have not been well established. The objective of this study is to assess risk factors for HIVICK compared with contemporaneous control participants.
Design, setting, participants, & measurements
A nested case-control study of 751 HIV-infected patients followed from January 1996 to June 2010 was conducted. Groups were compared using the chi-squared test or rank-sum analysis. Conditional logistic regression was used to estimate odds ratios (ORs) for HIVICK. Incidences of overall ESRD and with/without combined antiretroviral therapy (cART) exposure were calculated.
Results
HIVICK patients were predominantly African American (92%). Compared with matched controls, patients with HIVICK were more likely to have HIV RNA >400 copies/ml (OR, 2.5; 95% confidence interval [95% CI], 1.2 to 5.2), diabetes (OR, 2.8; 95% CI, 1.1 to 6.8), and hypertension (OR, 2.3; 95% CI, 1.2 to 4.5). Compared with HIVAN, patients with HIVICK had more antiretroviral therapy exposure, lower HIV viral loads, and higher CD4 and estimated GFR. ESRD was less common in the HIVICK versus the HIVAN group (30% versus 82%; P<0.001), and the use of cART was not associated with ESRD in HIVICK patients (25% versus 26; P=0.39).
Conclusions
HIVICK was predominantly observed in African-American patients and associated with advanced HIV disease. ESRD incidence is lower in HIVICK patients compared with those with HIVAN. Unlike HIVAN, cART use was not associated with the incidence of ESRD in HIVICK.
Introduction
Renal disease is common in HIV-infected patients, with the prevalence of impaired kidney function ranging between 2.4% and 12% (1,2) and proteinuria ranging from 10% to 30% (3–5). Kidney disease in HIV-infected patients encompasses an array of renal pathologies (6). These processes may be directly mediated by HIV, such as in HIV-associated nephropathy (HIVAN); related to HIV coexisting conditions, such as diabetes, hypertension, and intravenous drug use; or associated with drugs toxic to the kidneys. Although the racial predilection of, clinical risk factors for, and pathologic characteristics of HIVAN have been well described (7–11), less is known about a spectrum of pathologies ranging from postinfectious GN (PIGN) to “lupus-like” GN, collectively referred to as HIVICK (12–14).
Prior studies suggest that individuals with HIVICK present with significant but lower degrees of proteinuria and similar immunocompromise as those with HIVAN and with variable degrees of kidney dysfunction (13–17); these studies, however, were primarily conducted either in the era preceding the availability of combination antiretroviral therapy (cART) or were composed of study populations in which few participants were receiving cART. Furthermore, the majority of these studies were conducted in regions outside the United States. Therefore, these findings may not be applicable to those within the United States due to differences in sociodemographic and clinical characteristics. Whether racial predilection exists for HIVICK as it does for HIVAN is also unclear. In an early study of black and white Europeans, only 1 of the 29 black participants presented with immune complex kidney disease, whereas 16 of the 31 white participants had immune complex kidney disease on renal biopsy (13). Similar findings were observed by Boissier et al. in which 80% of HIV-infected patients with immune complex kidney disease were white (14). In contrast, a more recent study of black Africans suggests that these disorders are not uncommon among blacks, with 62% having immune complex kidney disease (17). Moreover, although untreated HIVAN progresses relentlessly to ESRD, data on the natural history of HIVICK and its response to cART are lacking due to the paucity of data on renal outcomes in prior studies (12,13,18–20).
To better characterize HIVICK among HIV-infected persons in the United States, we first conducted a case-control study using the Johns Hopkins HIV Clinical Cohort to assess the risk factors associated with HIVICK or HIVAN renal histopathology. Next, we compared progression to ESRD in participants with HIVICK and HIVAN, and assessed other risk factors for progression to ESRD, including use of cART.
Materials and Methods
Setting
The Johns Hopkins HIV Clinical Cohort is an open cohort that includes data from >6000 participants who have received HIV care in the clinic from 1990 onward (10,21–24). Trained technicians abstracted comprehensive demographic, clinical, laboratory, and pharmaceutical data from clinical records using structured data collection forms. Data were collected at enrollment and at 6-month intervals thereafter. Clinical diagnoses, other selected conditions (including initiation of renal replacement therapy), and deaths were routinely abstracted since cohort inception. National vital statistics provided supplemental information on mortality. Electronic data sources provided laboratory, pathology, and procedural visit data. Participants provided written informed consent, and this study was approved by the Johns Hopkins Medicine Institutional Review Board.
Study Design and Patient Selection
Participants who had a kidney biopsy between January 1, 1995 and June 30, 2011, were identified through linkage with the institutional pathology database. Cases were defined as patients with either HIVAN or HIVICK. We defined HIVAN as glomerular segmental or global collapse plus podocyte hypertrophy (25). We defined HIVICK as pathologic findings consistent with “lupus-like” GN, membranous nephropathy, membranoproliferative GN, IgA nephropathy, PIGN, cryoglobulinemia, and immune complex renal disease not otherwise specified (12,13,18,19,26). Lupus-like GN was determined by the presence of IgA, IgM, IgG, and C3 depositions on immunofluorescence, whereas PIGN was determined based on the presence of subepithelial deposits on electron microscopy and C3 deposition on immunofluorescence.
Risk factors were assessed in patients with HIVAN or HIVICK and in contemporaneous control participants. Because kidney biopsies may have been performed at variable time intervals from the appearance of clinical kidney disease and because clinical care may have been modified in light of clinically apparent kidney disease, we defined a clinical recognition date for each participant with HIVAN or HIVICK through clinical record review. The recognition date was defined as the date before kidney biopsy when a laboratory abnormality indicating kidney disease was identified as a concern by the HIV primary care provider in clinical documents. This date was used to match control participants and to define exposures (e.g., CD4 cell count or use of cART before this date). Using incidence density sampling with replacement, we randomly selected four control participants from the clinical cohort for each HIVICK and HIVAN patient, matching on kidney disease recognition date, age (in six strata), sex, and duration of follow-up in the clinic. We did not match on race because we wanted to assess race as a risk factor for HIVICK.
Data Collection and Definitions
Histopathologic diagnosis was based on the pathology report generated by a renal pathologist at the time of a patient’s renal biopsy. When participants had >1 kidney biopsy, we used results from the earliest biopsy unless that specimen was inadequate for diagnosis. Concurrent findings of both HIVICK and HIVAN (n=19) were categorized as HIVAN. We defined cART as the use of ≥3 antiretroviral drugs within the previous 3 months. We determined the estimated GFR (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration method (27). Diabetes and hypertension were defined according to the presence of a clinical diagnosis or prescription of antidiabetic or antihypertensive medications, respectively. We defined ESRD as the initiation of renal replacement therapy including renal transplantation but excluding temporary dialysis with renal recovery.
Statistical Analyses
We compared continuous and categorical factors using the Wilcoxon rank-sum and chi-squared tests, respectively. In the risk factor analysis, we aimed to assess and compare risk factors for HIVICK and HIVAN with reference to control participants. To assess risk factors for HIVICK and HIVAN, we conducted separate risk factor analyses for each condition (comparing with contemporary control participants) using conditional logistic regression. Potential risk factors included race, injection drug use, cART use in the 3 months before recognition date, CD4 cell count, HIV RNA level, hepatitis C virus antibody (HCV), hepatitis B virus surface antigen, diabetes, and hypertension. For both HIVICK and HIVAN, we constructed parsimonious models, by sequentially removing factors from the full model with a backward stepwise selection procedure (criterion for removal: P>0.10). This analysis was also performed after excluding patients with PIGN.
To compare progression to ESRD from the time of kidney biopsy between HIVAN and HIVICK patients, we utilized the Kaplan–Meier method, log-rank test, and Cox proportional hazards models. Because individuals who reached ESRD <31 days after kidney biopsy were unlikely to fully benefit from cART initiation, they were excluded. Participant follow-up was censored at death, January 1, 2012, or 1 year after the last clinic encounter. We adjusted for age, calendar period, and loge-transformed eGFR before kidney biopsy. In addition, we assessed factors associated with progression to ESRD in participants with HIVICK and HIVAN in separate Cox models. Factors of interest included use of cART in the 30 days after kidney biopsy, calendar period, HIV RNA <400 copies/ml, and CD4 count <200 cells/mm3. Sensitivity analyses were also performed whereby patients with PIGN were excluded from the HIVICK group and those with concurrent HIVAN and HIVICK were evaluated as a separate group from those with only HIVAN.
Results
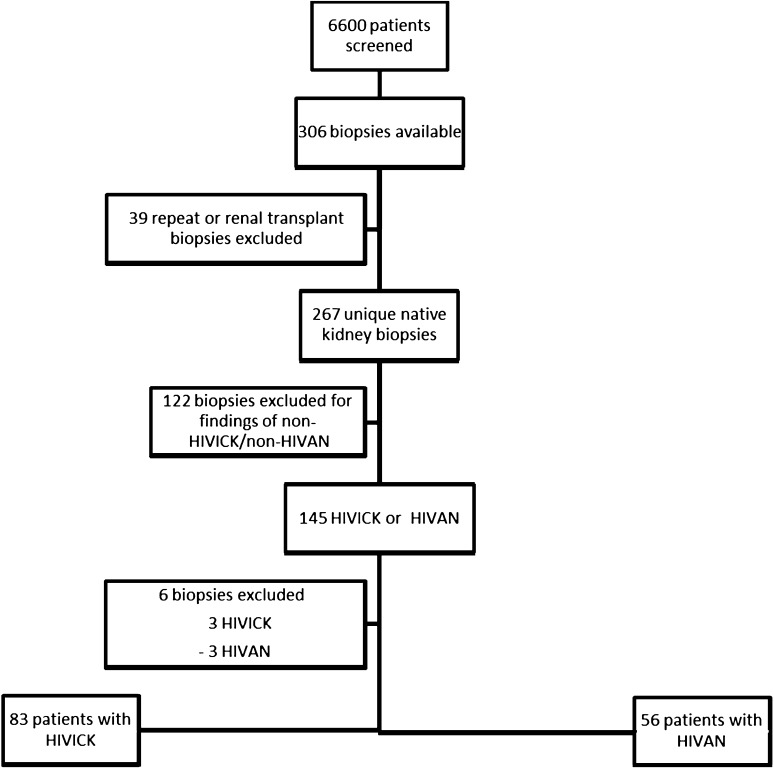
Among 6422 participants followed in the Johns Hopkins HIV Clinical Cohort, there were 308 kidney biopsies performed. After excluding biopsies obtained before January 1, 1995, renal transplant biopsies, and repeat kidney biopsies, there were 267 kidney biopsies from unique participants. Among these individuals, 145 had HIVICK or HIVAN. Six of these participants (three each with HIVAN and HIVICK) were excluded from the analysis due to inadequate clinical information. Of the remaining 139 participants, 83 had HIVICK and 56 had HIVAN and were included in the analysis (Figure 1). The subtypes of HIVICK are shown in Table 1. Of note, four of the individuals with membranoproliferative GN pathology were had positive HCV antibody test results. The majority of the PIGN patients occurred in the post-cART era, with 95% occurring after December 1, 2001.
Figure 1.
Screening and inclusion/exclusion of HIV patients for analysis. HIVAN, HIV-associated nephropathy; HIVICK, HIV-associated immune complex kidney disease.
Table 1.
Renal biopsy findings and number of patients who developed ESRD among 83 patients with HIVICK
| Diagnosis | Patients | ESRDa |
|---|---|---|
| Postinfectious GN | 41 (49) | 11 (27) |
| Lupus-like GNb | 11 (13) | 7 (64) |
| IgA nephropathy | 8 (10) | 1 (12) |
| Membranoproliferative GNb,c | 7 (9) | 1 (14) |
| Membranous nephropathy | 5 (6) | 3 (60) |
| Immune complex GN NOS | 11 (13) | 3 (27) |
Data are presented as n (%). HIVICK, HIV-associated immune complex kidney disease; NOS, not otherwise specified (represented resolving immune complex disease with membranous, membranoproliferative patterns)
Includes patients with ESRD within 31 days of renal biopsy; percentage among those with the given diagnosis.
One patient with lupus-like nephritis and one with membranoproliferative GN each had concurrent postinfectious GN.
Two participants with concurrent cryoglobulinemia.
Clinical Characteristics of Participants with HIVAN and HIVICK
The demographic and clinical characteristics of patients at the time of clinical recognition compared with matched controls are listed in Table 2. Patients with HIVICK were predominantly men (67%) and African American (92%). A large proportion also had a history of injection drug use (64%) and positive hepatitis C antibody test results (63%).
Table 2.
Baseline characteristics of patients with HIVICK and HIVAN and their respective controls
| Characteristic | HIVICK | HIVAN | ||
|---|---|---|---|---|
| Patients (n=83) | Controls (n=332) | Patients (n=56) | Controls (n=224) | |
| Age (yr) | 46 (41–51) | 45 (41–51) | 42 (36–46) | 42 (36–47) |
| Men | 56 (67) | 224 (67) | 33 (59) | 132 (59) |
| African-American race | 77 (92) | 246 (74) | 54 (96) | 165 (77) |
| Injection drug use | 53 (64) | 156 (47) | 28 (50) | 103 (46) |
| Diabetes | 13 (16) | 24 (7) | 6 (11) | 14 (6) |
| Hypertension | 42 (51) | 95 (29) | 18 (32) | 42 (19) |
| Hepatitis C positive | 52 (63) | 152 (48) | 30 (54) | 105 (49) |
| Hepatitis B positive | 4 (5) | 22 (7) | 1 (2) | 12 (6) |
| Any prior antiretroviral therapy | 40 (48) | 200 (60) | 21 (38) | 137 (61) |
| Prior cARTa | 36 (43) | 191 (57) | 10 (18) | 109 (49) |
| HIV1 RNA, log (IQR) | 4.28 (2.06–4.89) | 2.6 (1.7–4.39) | 5 (3.6–5.57) | 2.67 (1.87–4.38) |
| HIV RNA ≥400 copies/ml | 48 (68) | 145 (46) | 35 (90) | 93 (52) |
| CD4+cell count (cells/mm3) | 263 (156–469) | 361 (222–574) | 122 (24–271) | 335 (137–525) |
| CD4+cell count <200 cells/mm3 | 27 (36) | 73 (22) | 28 (61) | 81 (36) |
| Nadir CD4+cell count (cells/mm3) | 122 (61–244) | 163 (46–290) | 46 (15–200) | 160 (59–360) |
| Serum creatinine (mg/dl) | 1.5 (1.2–2.3) | 0.9 (0.8–1.0) | 2.3 (1.7–3.8) | 0.9 (0.7–1.1) |
| eGFR (ml/min per 1.73 m2) | 54 (37–74) | 105 (84–122) | 32 (18–53) | 105 (89–125) |
| Proteinuria (mg/d) | 920 (436–2085) | n/a | 2115 (379–8781) | n/a |
| Time since enrollment (yr) | 4.4 (1.1–9.7) | 41. (1.2–7.9) | 1.5 (0–4.9) | 1.3 (0.4–4.2) |
Data are presented as n (%) or median (interquartile range) unless otherwise specified. HIVIC, HIV-associated immune complex kidney disease; HIVAN, HIV-associated nephropathy; cART, combined antiretroviral therapy; IQR, interquartile range; eGFR, estimated GFR by the Chronic Kidney Disease Epidemiology Collaboration equation; n/a, not applicable.
cART 3 months prior.
Patients with HIVICK were older than those with HIVAN at the time of clinical recognition (P=0.03). HIVICK patients were also more likely to have hypertension (51% versus 32%; P=0.03), were more likely to use cART (43% versus 18%; P=0.002), were less likely to have HIV RNA ≥400 copies/ml (68% versus 90%; P=0.01), had higher median CD4 counts (263 versus 122 cells/mm3; P<0.001), and had higher median eGFR (54 versus 32 ml/min per 1.73 m2; P<0.001) at the clinical recognition date compared with HIVAN patients. Compared with HIVAN patients, HIVICK patients were slightly less likely to have proteinuria of ≥1+ on dipstick testing (97% versus 83%, respectively). Moreover, compared with HIVAN patients, those with HIVICK had milder degrees of proteinuria, with 69% of HIVAN and 35% of HIVICK patients having 3+ proteinuria. Patients with HIVICK were also enrolled in the cohort for a longer period of time before their disease was clinically recognized compared with patients with HIVAN (median 4.4 years versus 1.5 years for the HIVICK and HIVAN groups, respectively; P=0.01).
Determination of Risk Factors for HIVICK and HIVAN
All 139 participants were successfully matched to 4 control participants. Patients and control participants were closely matched on sex, age, calendar date, and duration of follow-up in the cohort (Table 2). Table 3 lists the unadjusted and adjusted analyses of clinical features associated with HIVICK renal pathology compared with matched controls. In unadjusted analysis, African-American race was a risk factor for HIVICK (odds ratio [OR], 4.8; 95% confidence interval [95% CI], 2.0 to 11.5) and for HIVAN (OR, 10.0; 95% CI, 2.3 to 42.9), although African-American race was not statistically significant for HIVICK after adjustment (OR, 2.3; 95% CI, 0.9 to 6.8). Conversely, race remained a strong risk factor for HIVAN (OR, 10.3; 95% CI, 1.3 to 79.5). In adjusted models, HIV RNA >400 cells/ml was significantly associated with both HIVICK and HIVAN (OR, 2.5; 95% CI, 1.2 to 5.1; and OR, 11.7; 95% CI, 2.6 to 52.7, respectively).
Table 3.
Risk factors for HIVICK or HIVAN versus matched controls
| Risk Factor, n (%) | HIVICK Patients and Matched Controls | HIVAN Patients and Matched Controls | ||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Black race | 4.8 (2.0 to 11.5) | 2.3 (0.9 to 6.8) | 10.0 (2.3 to 42.9) | 10.3 (1.3 to 79.5) |
| Injection drug use | 2.0 (1.2 to 3.4) | — | 1.2 (0.6 to 2.4) | — |
| HIV RNA >400 copies/ml | 3.2 (1.7 to 5.9) | 2.5 (1.2 to 5.2) | 17.2 (4.0 to 74.3) | 11.7 (2.6 to 52.7) |
| CD4+cell count <200 cells/mm3 | 1.9 (1.1 to 3.4) | 1.9 (0.95 to 3.7) | 3.6 (1.7 to 7.5) | — |
| Hepatitis C antibody positive | 2.1 (1.2 to 3.7) | 1.8 (0.95 to 3.4) | 1.2 (0.7 to 2.3) | — |
| Hepatitis B surface antigen positive | 0.7 (0.2 to 2.1) | — | 0.3 (0.04 to 2.4) | — |
| Diabetes | 2.5 (1.2 to 5.1) | 2.8 (1.1 to 6.8) | 2.0 (0.7 to 5.8) | — |
| Hypertension | 2.8 (1.7 to 4.8) | 2.3 (1.2 to 4.5) | 2.3 (1.1 to 4.6) | — |
| cART | 0.5 (0.3 to 0.9) | — | 0.2 (0.1 to 0.4) | 0.4 (0.14 to 1.1) |
A dash indicates that the risk factor was dropped from the model by backward stepwise selection for P>0.10. HIVIC, HIV-associated immune complex kidney disease; HIVAN, HIV-associated nephropathy; OR, odds ratio; 95% CI, 95% confidence interval; cART, combined antiretroviral therapy defined as ≥3 antiretroviral drugs >10 days in prior 3 months.
After adjustment for race, HIV RNA >400 cells/ml, CD4 <200 cells/mm3, and HCV coinfection, HIVICK was significantly associated with diabetes (OR, 2.8; 95% CI, 1.1 to 6.8) and hypertension (OR, 2.3; 95% CI, 1.2 to 4.5). The association with diabetes dissipated when individuals with PIGN were excluded from the analysis.
Incidence of ESRD
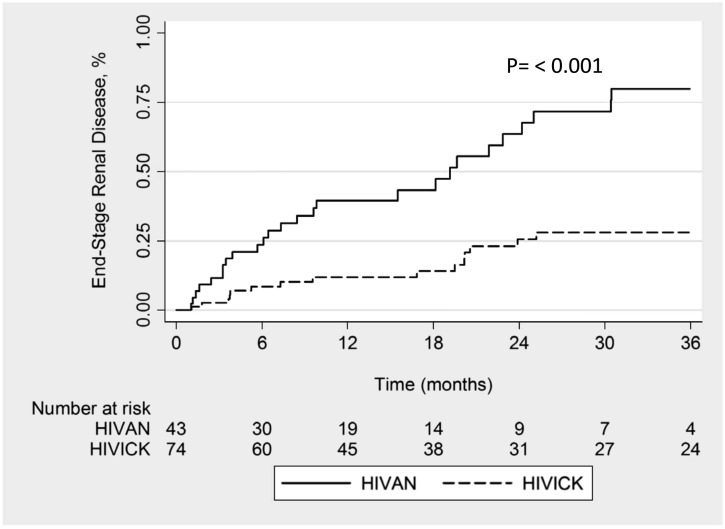
After exclusion of individuals who developed ESRD within 31 days, 74 HIVICK and 43 HIVAN patients were available for analysis. Cumulative progression to ESRD was lower in patients with HIVICK than in those with HIVAN (32% and 70% at 24 months, respectively; P<0.001) (Figure 2). Exclusion of individuals with PIGN revealed similar disparities in cumulative progression to ESRD between those with HIVICK versus HIVAN (37% versus 65% at 24 months, respectively; P=0.01). Similarly, exclusion of participants who had not received highly active antiretroviral therapy before the renal biopsy also yielded similar results, with a cumulative incidence of ESRD of 25% in those with HIVICK and 68% in those with HIVAN at 24 months (P=0.02).
Figure 2.
Kaplan–Meier analysis demonstrating incidence of ESRD for HIVAN and HIVICK. HIVAN, HIV-associated nephropathy; HIVICK, HIV-associated immune complex kidney disease.
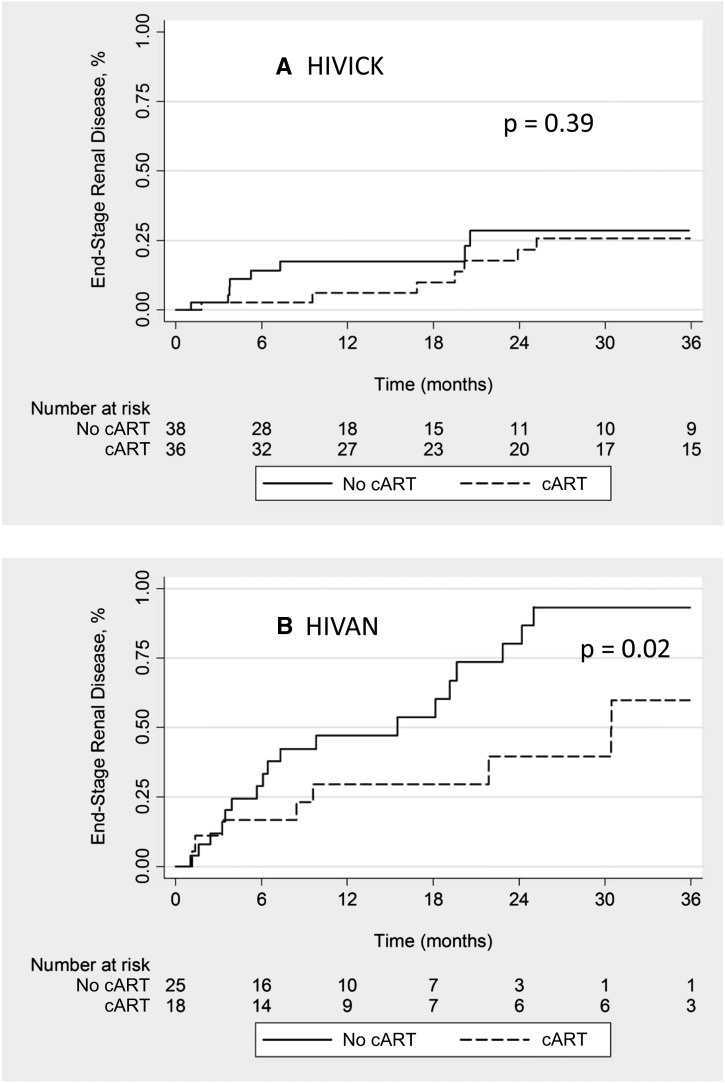
Among participants included in the ESRD progression analysis, 36 HIVICK patients and 18 HIVAN patients had cART exposure in the 30 days after biopsy. Among individuals with HIVICK, there was no difference in the incidence of ESRD between those with cART use versus without (25% versus 26%, respectively; P=0.39) (Figure 3A). Of note, 71% of those with HIVICK who initiated cART after their renal biopsy achieved HIV-1 RNA levels <400 copies/ml. The proportion of HIVICK individuals who attained an HIV-1 RNA level <400 copies/ml was similar between those who did and did not progress to ESRD (31% versus 49%, respectively; P=0.11). Despite exclusion of individuals with PIGN, cART remained unassociated with progression to ESRD among those with HIVICK (hazard ratio [HR], 1.07; 95% CI, 0.28 to 4.03). In contrast, cART use was associated with lower incidence of ESRD compared with no cART use among those with HIVAN (44% versus 72%, respectively; P=0.02) (Figure 3B).
Figure 3.
Incidence of ESRD by cART exposure. HIVAN, HIV-associated nephropathy; HIVICK, HIV-associated immune complex kidney disease; HAART, highly active antiretroviral therapy.
Unadjusted HRs associated with the development of ESRD for patients with HIVICK and HIVAN are shown in Table 4. Significant variables included HIVICK on biopsy (HR, 0.30; 95% CI, 0.18 to 0.51), cohort follow-up in 2002 onward compared with before 2002 (HR, 0.50; 95% CI, 0.30 to 0.83), older age (HR, 0.62 per 10 years; 95% CI, 0.43 to 0.91), and loge-transformed eGFR (HR, 0.32 per 1 loge higher; 95% CI, 0.23 to 0.44). After adjustment for all statistically significant variables from univariate analyses, only loge-transformed eGFR remained significantly associated with the risk of ESRD (HR, 0.35 per 1 loge higher; 95% CI, 0.24 to 0.52).
Table 4.
Unadjusted hazard ratios for developing ESRD in HIVICK and HIVAN patients
| Risk Factor | Hazard Ratio | 95% Confidence Interval |
|---|---|---|
| HIVICK versus HIVAN | 0.30 | 0.18 to 0.51 |
| Cohort follow-up before versus after 2002 | 0.50 | 0.30 to 0.83 |
| Log transformed eGFR | 0.32 | 0.23 to 0.44 |
| Women versus men | 0.98 | 0.58 to 1.64 |
| Black race | 1.73 | 0.42 to 7.1 |
| Injection drug use | 0.82 | 0.50 to 1.35 |
| HCV | 1.10 | 0.50 to 2.42 |
| Diabetes, n (%) | 0.88 | 0.40 to 1.94 |
| Hypertension, n (%) | 0.81 | 0.49 to 1.34 |
| Increase in age 10 yr | 0.62 | 0.43 to 0.91 |
HIVIC, HIV-associated immune complex kidney disease; HIVAN, HIV-associated nephropathy; eGFR, estimated GFR; HCV, hepatitis C virus.
In parallel sensitivity analyses excluding PIGN patients and those who received highly active antiretroviral therapy before their renal biopsy, individuals with HIVICK remained at lower risk of progressing to ESRD compared with those with HIVAN (HR, 0.45; 95% CI, 0.23 to 0.87; and HR, 0.35; 95% CI, 0.14 to 0.90). Finally, unadjusted survival analyses in which participants with concurrent HIVAN and HIVICK were regarded as a third group yielded similar findings as the primary analyses, with HIVICK individuals at lower risk for progressing to ESRD (HR, 0.27; 95% CI, 0.15 to 0.46). Individuals with both HIVAN and HIVICK had similar risk for progressing to ESRD as those with HIVAN (HR, 0.82; 95% CI, 0.56 to 1.21), but higher than those with HIVICK (HR, 2.43; 95% CI, 1.11 to 5.33).
Discussion
In this analysis of predominantly African-American HIV-infected patients, HIVICK was not an uncommon biopsy finding. HIVICK patients generally have milder renal disease and less advanced HIV infection at the time of clinical recognition compared with those with HIVAN consistent with previous studies (6,20). To our knowledge, our study is the first to analyze risk factors for the specific development of HIVICK against matched controls.
Risk factors for the development of HIVICK included HIV RNA levels >400 cells/ml, diabetes, and hypertension. The importance of HIV RNA in the development of HIVICK has been suggested by the failure to generate this entity in experimental models. Contrary to HIVAN, in which many HIV-1 transgenic models exhibit the clinical and pathologic features of HIVAN, none of these animal models exhibit features of HIVICK (28,29). This observation suggests that viral replication or immune responses to viral proteins may be essential to trigger HIVICK (30). The role of hypertension and diabetes in the development of immune complex disease is less clear. The presence of these conditions may have predisposed the patients to receiving a kidney biopsy, although previous studies of non-HIVAN renal disease did not find these to be significant factors in patients receiving a kidney biopsy (6). Up to 43% of patients in this series with HIVICK were receiving cART at the time of clinical recognition. cART use has been associated with an increase of both hypertension and diabetes (31–34), which may explain why HIVICK patients were more likely to have hypertension than HIVAN patients. Alternatively, one may argue that HIVICK is more likely than HIVAN to cause, rather than being triggered by, hypertension. Furthermore, the association with diabetes was entirely driven by PIGN, which is consistent with the previous observation that diabetes was the most predisposing factor for postinfectious GN (35).
Over half of the patients in the HIVICK group were coinfected with HCV, which can predispose to immune complex kidney disease (36,37). Although George et al. recently published findings that showed an increase in immune complex GN among patients coinfected with HIV and HCV compared with those with HIV alone (38), there was no increased HCV coinfection in HIVICK versus HIVAN patients, nor was HCV associated with increased odds of HIVICK compared with matched controls in our adjusted analyses. Combined ART use was not associated with decreased odds of HIVICK despite higher cART use among patients with HIVICK compared with patients with HIVAN. Subsequent immune reconstitution may be associated with increased immune activity (39), thereby theoretically increasing antigen-antibody formation and the development of HIVICK. Recent evidence suggests that genetic factors may play an important role in determining renal histopathology in HIV-infected individuals (40,41). In a cohort of African-American patients with biopsy-proven non-HIVAN renal pathology, Fine et al. found that 76% of patients carrying two APOL1 risk alleles had FSGS. In contrast, immune complex GN was found more often on renal biopsy in patients with only one or no APOL1 risk allele (47% and 40%, respectively) (40,41). Although the findings may represent interplay between the APOL1 genotype and the immune system, it may also be explained by an earlier clinical presentation for patients with FSGS before having the opportunity to develop an immune complex GN (40).
Patients with HIVICK were less likely to progress to ESRD than those with HIVAN; this observation remained robust in sensitivity analyses. These findings are consistent with previous studies that suggest that non-HIVAN renal diseases progress more slowly than HIVAN (6,20,42). The use of cART was not associated with the incidence of ESRD in patients with HIVICK, with the two groups having nearly identical proportions developing ESRD by 24 months. A number of factors may explain our findings including the relatively lower overall incidence of ESRD in this population, small study group size, or the possibility of inducing HIVICK with cART due to immune reconstitution. Furthermore, one may argue that ART is unlikely to affect the vigorous polyclonal antibody responses driven by the high levels of circulating antigens in these patients and is responsible for the immune complex deposition with subsequent complement activation in the kidney. Wearne et al. recently suggested that cART may stabilize immune complex kidney disease (42), although there was no significant effect on ESRD incidence in their analysis. This study, however, had a substantially smaller sample size, with only 16 patients with isolated immune complex GN on kidney biopsy, which may limit the interpretations of those findings (42). Consistent with previous studies, cART use was associated with lower incidence of ESRD in patients with HIVAN (20,42,43).
Compared with previous studies of HIVICK, our study had a larger proportion of individuals with PIGN and immune complex that was not otherwise specified. Uncontrolled HIV infection or different exposures such as injection drug use or HCV coinfection may underlie these differences. Compared with the previous studies by Boissier et al. (14) and Gerntholtz et al. (17), our study population had higher HIV-1 viremia and a larger proportion of participants with injection drug use and HCV antibody seropositivity. Conversely, we had fewer participants with any cART exposure compared with prior studies.
We acknowledge several limitations to this analysis. Our findings should be interpreted cautiously in light of the fact that the spectrum of pathologies considered as HIVICK does not necessarily share the same pathogenic mechanisms, clinical presentations, or natural history. Second, this is a single-center study in a predominantly urban setting, so the findings may not be generalizable to other geographic or clinical settings. The relatively small sample size may have influenced the results, although this is the largest biopsy cohort of HIVICK patients compared with the published literature. This group of patients was also predominantly African American, so the results may not be applicable to other patient populations. Finally, unrecognized modifying factors may have biased the results. Although the control group for this analysis was not a biopsy group, the control participants were closely matched on important clinical characteristics and included a robust sample size of HIV-infected patients with immune complex kidney disease that has not been evaluated in previous studies.
In conclusion, our study offers further understanding of the clinical factors and outcomes associated with HIVICK. The association of higher HIV viremia with findings of HIVICK on biopsy may suggest a pathogenic mechanism for the development of immune complex GN in HIV, either by disruption of normal immune function and regulation, or via direct antigenic stimulation and subsequent antibody response. Combined cART failed to significantly affect the incidence of ESRD in this cohort, and the routine use of cART for HIVICK is not suggested by our findings at this time.
Disclosures
None.
Acknowledgments
We acknowledge the Johns Hopkins HIV Clinical Cohort patients, without whom none of this research would be possible.
This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (P01DK056492 to M.G.A. and 1K23DK081317 to M.M.E.) and the National Institutes of Health (R01 DA026770 to G.M.L.).
These data were presented in abstract form at the 2012 Annual Meeting of the American Society of Nephrology, October 30–November 4, 2012, in San Diego, California.
Footnotes
M.C.F. and M.M.E. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Fernando SK, Finkelstein FO, Moore BA, Weissman S: Prevalence of chronic kidney disease in an urban HIV infected population. Am J Med Sci 335: 89–94, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Jones CY, Jones CA, Wilson IB, Knox TA, Levey AS, Spiegelman D, Gorbach SL, Van Lente F, Stevens LA: Cystatin C and creatinine in an HIV cohort: The nutrition for healthy living study. Am J Kidney Dis 51: 914–924, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szczech LA, Grunfeld C, Scherzer R, Canchola JA, van der Horst C, Sidney S, Wohl D, Shlipak MG: Microalbuminuria in HIV infection. AIDS 21: 1003–1009, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fine DM, Wasser WG, Estrella MM, Atta MG, Kuperman M, Shemer R, Rajasekaran A, Tzur S, Racusen LC, Skorecki K: APOL1 risk variants predict histopathology and progression to ESRD in HIV-related kidney disease. J Am Soc Nephrol 23: 343–350, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Post FA, Wyatt CM, Mocroft A: Biomarkers of impaired renal function. Curr Opin HIV AIDS 5: 524–530, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Berliner AR, Fine DM, Lucas GM, Rahman MH, Racusen LC, Scheel PJ, Atta MG: Observations on a cohort of HIV-infected patients undergoing native renal biopsy. Am J Nephrol 28: 478–486, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Humphreys MH: Human immunodeficiency virus-associated glomerulosclerosis. Kidney Int 48: 311–320, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Klotman PE: HIV-associated nephropathy. Kidney Int 56: 1161–1176, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Ross MJ, Bruggeman LA, Wilson PD, Klotman PE: Microcyst formation and HIV-1 gene expression occur in multiple nephron segments in HIV-associated nephropathy. J Am Soc Nephrol 12: 2645–2651, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Lucas GM, Eustace JA, Sozio S, Mentari EK, Appiah KA, Moore RD: Highly active antiretroviral therapy and the incidence of HIV-1-associated nephropathy: A 12-year cohort study. AIDS 18: 541–546, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Estrella M, Fine DM, Gallant JE, Rahman MH, Nagajothi N, Racusen LC, Scheel PJ, Atta MG: HIV type 1 RNA level as a clinical indicator of renal pathology in HIV-infected patients. Clin Infect Dis 43: 377–380, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Diallo AD, Nochy D, Niamkey E, Yao Beda B: [Etiologic aspects of nephrotic syndrome in Black African adults in a hospital setting in Abidjan]. Bull Soc Pathol Exot 90: 342–345, 1997 [PubMed] [Google Scholar]
- 13.Nochy D, Glotz D, Dosquet P, Pruna A, Guettier C, Weiss L, Hinglais N, Idatte JM, Mery JP, Kazatchkine M, Druet P, Bariéty J: Renal disease associated with HIV infection: A multicentric study of 60 patients from Paris hospitals. Nephrol Dial Transplant 8: 11–19, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Boissier F, Khalil A, Chalumeau-Lemoine L, Lescure FX, Parrot A: Rash diagnosis of blood expectoration. Lancet 379: 1170, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Casanova S, Mazzucco G, Barbiano di Belgiojoso G, Motta M, Boldorini R, Genderini A, Monga G: Pattern of glomerular involvement in human immunodeficiency virus-infected patients: An Italian study. Am J Kidney Dis 26: 446–453, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Haas M, Kaul S, Eustace JA: HIV-associated immune complex glomerulonephritis with “lupus-like” features: A clinicopathologic study of 14 cases. Kidney Int 67: 1381–1390, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Gerntholtz TE, Goetsch SJ, Katz I: HIV-related nephropathy: A South African perspective. Kidney Int 69: 1885–1891, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Méry JP, Delahousse M, Nochy D: Amyloidosis and infection due to human immunodeficiency virus. Clin Infect Dis 16: 733–734, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Nochy D, Glotz D, Dosquet P, Pruna A, Lemoine R, Guettier C, Weiss L, Hinglais N, Idatte JM, Mery JP: Renal lesions associated with human immunodeficiency virus infection: North American vs. European experience. Adv Nephrol Necker Hosp 22: 269–286, 1993 [PubMed] [Google Scholar]
- 20.Szczech LA, Gupta SK, Habash R, Guasch A, Kalayjian R, Appel R, Fields TA, Svetkey LP, Flanagan KH, Klotman PE, Winston JA: The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int 66: 1145–1152, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Gallant JE, Chaisson RE, Moore RD: The effect of adjunctive corticosteroids for the treatment of Pneumocystis carinii pneumonia on mortality and subsequent complications. Chest 114: 1258–1263, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD: Illicit drug use and HIV-1 disease progression: A longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol 163: 412–420, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Moore R: HIV therapy and prevention: economics and cost-effectiveness. Hopkins HIV Rep 10: 2–, 10–11., 1998 [PubMed] [Google Scholar]
- 24.Moore RD: Understanding the clinical and economic outcomes of HIV therapy: The Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol 17[Suppl 1]: S38–S41, 1998 [DOI] [PubMed] [Google Scholar]
- 25.D’Agati V, Suh JI, Carbone L, Cheng JT, Appel G: Pathology of HIV-associated nephropathy: A detailed morphologic and comparative study. Kidney Int 35: 1358–1370, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Gindea S, Schwartzman J, Herlitz LC, Rosenberg M, Abadi J, Putterman C: Proliferative glomerulonephritis in lupus patients with human immunodeficiency virus infection: A difficult clinical challenge. Semin Arthritis Rheum 40: 201–209, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenstiel P, Gharavi A, D’Agati V, Klotman P: Transgenic and infectious animal models of HIV-associated nephropathy. J Am Soc Nephrol 20: 2296–2304, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Papeta N, Chan KT, Prakash S, Martino J, Kiryluk K, Ballard D, Bruggeman LA, Frankel R, Zheng Z, Klotman PE, Zhao H, D’Agati VD, Lifton RP, Gharavi AG: Susceptibility loci for murine HIV-associated nephropathy encode trans-regulators of podocyte gene expression. J Clin Invest 119: 1178–1188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Couser WG: Basic and translational concepts of immune-mediated glomerular diseases. J Am Soc Nephrol 23: 381–399, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Seaberg EC, Muñoz A, Lu M, Detels R, Margolick JB, Riddler SA, Williams CM, Phair JP, Multicenter AIDS Cohort Study : Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 19: 953–960, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Chow DC, Souza SA, Chen R, Richmond-Crum SM, Grandinetti A, Shikuma C: Elevated blood pressure in HIV-infected individuals receiving highly active antiretroviral therapy. HIV Clin Trials 4: 411–416, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Gazzaruso C, Bruno R, Garzaniti A, Giordanetti S, Fratino P, Sacchi P, Filice G: Hypertension among HIV patients: Prevalence and relationships to insulin resistance and metabolic syndrome. J Hypertens 21: 1377–1382, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, Visscher BR, Margolick JB, Dobs AS: Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 165: 1179–1184, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Nasr SH, Markowitz GS, Stokes MB, Said SM, Valeri AM, D’Agati VD: Acute postinfectious glomerulonephritis in the modern era: Experience with 86 adults and review of the literature. Medicine (Baltimore) 87: 21–32, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE, Hartwell P, Couser WG, Corey L, Wener MH, Alpers CE, Willson R: Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med 328: 465–470, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Doutrelepont JM, Adler M, Willems M, Durez P, Yap SH: Hepatitis C infection and membranoproliferative glomerulonephritis. Lancet 341: 317, 1993 [DOI] [PubMed] [Google Scholar]
- 38.George E, Nadkarni GN, Estrella MM, Lucas GM, Sperati CJ, Atta MG, Fine DM: The impact of hepatitis C coinfection on kidney disease related to human immunodeficiency virus (HIV): A biopsy study. Medicine (Baltimore) 90: 289–295, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Martin-Blondel G, Mars LT, Liblau RS: Pathogenesis of the immune reconstitution inflammatory syndrome in HIV-infected patients. Curr Opin Infect Dis 25: 312–320, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Fine DM, Wasser WG, Estrella MM, Atta MG, Kuperman M, Shemer R, Rajasekaran A, Tzur S, Racusen LC, Skorecki K: APOL1 risk variants predict histopathology and progression to ESRD in HIV-related kidney disease. J Am Soc Nephrol 23: 343–350, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atta MG, Estrella MM, Kuperman M, Foy MC, Fine DM, Racusen LC, Lucas GM, Nelson GW, Warner AC, Winkler CA, Kopp JB: HIV-associated nephropathy patients with and without apolipoprotein L1 gene variants have similar clinical and pathological characteristics. Kidney Int 82: 338–343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wearne N, Swanepoel CR, Boulle A, Duffield MS, Rayner BL: The spectrum of renal histologies seen in HIV with outcomes, prognostic indicators and clinical correlations. Nephrol Dial Transplant 27: 4109–4118, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Atta MG, Gallant JE, Rahman MH, Nagajothi N, Racusen LC, Scheel PJ, Fine DM: Antiretroviral therapy in the treatment of HIV-associated nephropathy. Nephrol Dial Transplant 21: 2809–2813, 2006 [DOI] [PubMed] [Google Scholar]