Summary
Background and objectives
Nocturnal hypoxemia is highly prevalent among patients with CKD. Nocturnal hypoxemia contributes to systemic inflammation, oxidative stress, endothelial cell dysfunction, and activation of the renin-angiotensin system, which are common pathologic mechanisms of CKD progression. This study investigated whether nocturnal hypoxemia is independently associated with CKD progression.
Design, setting, participants, & measurements
This two-center retrospective cohort study included 161 patients with stages 3–4 CKD enrolled from January of 2009 to July of 2011 with a body mass index less than 25.0 kg/m2. The 4% oxygen desaturation index, the number of events per hour in which oxygen saturation decreases by >4% during sleep, was measured, and the declining rate of the estimated GFR was followed over 1 year. The severity of nocturnal hypoxemia was categorized as none (oxygen desaturation index<5.0), mild (5.0≤oxygen desaturation index<15.0), or moderate to severe (15.0≤oxygen desaturation index).
Results
The mean estimated GFR of the total cohort at baseline was 31 ml/min per 1.73 m2. Eighty patients (49.7%) were diagnosed with nocturnal hypoxemia; 64 patients were diagnosed with mild nocturnal hypoxemia, and 16 patients were diagnosed with moderate-to-severe nocturnal hypoxemia. The estimated GFR declined three- to fourfold faster in patients with moderate-to-severe nocturnal hypoxemia than patients with no or mild nocturnal hypoxemia (the mean values [95% confidence intervals] were −2.14 [−1.06 to −3.21], −3.02 [−1.31 to −4.74], and −8.59 [−2.00 to −15.2] ml/min per 1.73 m2 per year in the no, mild, and moderate-to-severe nocturnal hypoxemia groups, respectively; P=0.003). Nocturnal hypoxemia remained a significant predictor of decline in estimated GFR after adjustment for various baseline clinical factors.
Conclusions
In nonobese patients with CKD, nocturnal hypoxemia is an independent risk factor of a rapid decline in kidney function.
Introduction
Sleep disordered breathing (SDB) is a common clinical condition among patients with CKD; its prevalence has been reported to be as high as 65% (1–4) compared with approximately 20% in the general population (5,6). The hallmark of SDB is its recurrent episodes of hypoxia–reoxygenation sequence coupled with the majority of apnea events throughout the night. This intermittent nocturnal hypoxemia (NH) causes oxidative stress by increasing the generation of reactive oxygen species through activation of hypoxia-inducible factor-1 and NADPH oxidase-2 (7–11). In addition, NH can directly activate the sympathetic nervous system (12) and the renin-angiotensin system (13) and promote inflammation (14,15) and endothelial cell dysfunction (16), contributing to the development of atherosclerosis. In fact, one of the most deleterious aspects of NH is its independent risk for cardiovascular mortality in patients with end stage kidney disease (ESKD) (17–20) as well as the general population (21–24).
In addition to its harmful effects on the cardiovascular system, NH may contribute to the progression of CKD based on the accumulating evidence that hypoxia plays a significant role in tubulointerstitial injury in the kidney as part of the final common pathway to ESKD (25). We and others have performed cross-sectional studies showing a significant negative association between the prevalence and/or severity of SDB and kidney function in the CKD population (1–4,26). To date, there is only a single cohort study of the longitudinal relationship between SDB and kidney function, which showed that NH was a significant predictor of accelerated loss of kidney function (27). However, the majority of the participants in that study had maintained baseline kidney function (mean estimated GFR [eGFR]=70.8 ml/min per 1.73 m2) and severe obesity, which hampered the generalization of the finding to the general CKD population.
Both CKD and SDB are now becoming major public health problems that affect mortality and quality of life in a large proportion of the adult population worldwide (28–30). In this circumstance, it is especially of importance to elucidate their interrelationship. In the current cohort study, we aimed to study the clinical impact of NH on kidney function among patients with stages 3 and 4 CKD. In particular, we enrolled patients whose body mass index (BMI) was less than 25.0 kg/m2, the normal range for body weight as defined by the World Health Organization (31,32), to assess the association between NH and kidney function independently from obesity.
Materials and Methods
Participants
A retrospective two-center cohort study was conducted in the nephrology units of two tertiary care hospitals in Japan: Osaka General Medical Center and Ohmihachiman Community Medical Center. Both of these centers have conducted CKD educational programs covering approximately 1 week of hospitalization. This program, targeted at stable CKD patients regardless of the presence or absence of sleep complaints, was intended to educate patients about their kidney disease and provide individualized nutritional therapy. No specific comorbidities influenced or hindered participation in this program. As a routine screening examination for NH, 4% oxygen desaturation index (ODI) was measured in essentially all participants in this program, regardless of their clinical characteristics. The participants in this study were recruited at Osaka General Medical Center from May of 2009 to June of 2011 and Ohmihachiman Community Medical Center from January of 2009 to July of 2011.
In light of the study object, we included only patients with stages 3–4 CKD (15<eGFR≤60 ml/min per 1.73 m2) whose BMI was less than 25.0 kg/m2. The eGFR was calculated from the equation for estimating GFR for Japanese individuals (33).
There were four exclusion criteria: patients were (1) followed up for less than 6 months, (2) diagnosed with chronic obstructive pulmonary disease, (3) receiving oxygen supplementation or continuous positive airway pressure (CPAP) therapy with good adherence (defined as use for an average of 4 hours a night on at least 70% of nights), and (4) hospitalized for acute complications, such as AKI, acute heart failure, stroke, or infection, but did not participate in the CKD educational program.
The study protocol was approved by the Faculty of Medicine Ethics Committees of both Osaka General Medical Center and Ohmihachiman Community Medical Center.
Outcome
All participants were followed by a nephrologist for at least 1 year after the measurement of 4% ODI, and serum creatinine (SCr) levels were measured after 3, 6, and 12 months. The outcome was the slope of the eGFR versus time plot (eGFR slope; milliliters per minute per 1.73 m2 per year). Regression lines for eGFR over time were created from the four eGFR data points (measured at baseline and after 3, 6, and 12 months) (least-squares method) to obtain a regression coefficient for each subject.
The eGFR data for one of three follow-up points were missing for nine participants; in these cases, the regression coefficients were calculated from the eGFR at baseline and the available two follow-up points.
Pulse Oximetry
The arterial oxygen saturation (SpO2) during sleep of each subject was monitored transcutaneously using a pulse oximeter (PULSOX; Teijin Pharma, Ltd., Japan) in an unattended manner. The sampling frequency of this monitor was 1 Hz. Desaturation was defined as a >4% drop in the SpO2 level from the baseline level; the 4% ODI was the number of desaturation events per hour of recording time, which was calculated using computer software. The participants were divided into three groups according to commonly used conventional cutoff values of 4% ODI: the no (ODI<5), mild (5≤ODI<15), and moderate-to-severe (15≤ODI) NH groups (34). In addition, we also calculated the duration of SpO2<90%.
To examine the relationship between 4% ODI and apnea–hypopnea index (AHI) in this cohort, AHI was simultaneously measured using a type 3 portable monitor (Morpheus; Teijin Pharma Ltd., Japan) in the participants of one of two nephrology centers (Osaka General Medical Center; n=41). The definitions of apnea and hypopnea were according to The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (35). There was a strong correlation between 4% ODI and AHI as determined by Spearman’s rank correlation coefficient (P<0.001, r=0.86). The sensitivity and specificity of the ODI≥5 for the AHI≥5 were 77.2% and 89.5%, respectively, and the sensitivity and specificity of the ODI≥15 for the AHI≥15 were 80.0% and 100%, respectively.
Baseline Characteristics
The participant demographics and laboratory data, including age, sex, BMI, CKD etiology, medications used (angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aldosterone receptor antagonists, and statins), SCr, serum albumin, and 24-hour urine protein (UP), were obtained from their medical records.
At Osaka General Medical Center, BP was measured using a fully automatic device with the patient in a sitting position after awaking in the morning. At Ohmihachiman Community Medical Center, the patients underwent ambulatory BP monitoring, and the BP average over 24 hours was used for the analysis. Mean BP was defined as the diastolic pressure plus one third of the pulse pressure.
Statistical Analyses
Data were presented as the number (percent) for categorical variables and the mean ± SD for continuous variables with normal distribution or median (interquartile range) for data with skewed distribution. A normal quantile-quantile plot was used to assess the normality of each variable. The baseline characteristics and eGFR slope were compared across the three NH groups using the chi-squared test, ANOVA with the Tukey–Kramer posthoc test, and the Kruskal–Wallis test as appropriate. The correlation between 4% ODI as a continuous variable and the eGFR slope was assessed using Pearson’s product–moment correlation coefficient. To investigate an independent association between the NH groups and the eGFR slope, a mixed effect multiple linear regression model was constructed, in which all baseline characteristics (age, sex, eGFR, BMI, mean BP, UP, serum albumin, type 2 diabetic nephropathy, and medications [angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, aldosterone receptor antagonists, and statins]) were included as fixed effects and center as a random effect. In addition, we performed the same analyses to estimate the correlation between the duration of SpO2<90% (as a continuous variable) and eGFR slope.
Data were missing for BP (n=1), serum albumin (n=3), and UP (n=2); because of the small number of missing data, all results were based on the available data without using any imputation method. All reported P values were two-sided, and values of P<0.05 were considered to indicate statistical significance. Statistical analyses were performed using StataIC 12 Statistical Software (StataCorp LP, College Station, TX).
Results
During the enrollment period, a total of 190 patients with stages 3–4 CKD whose BMI was less than 25.0 kg/m2 underwent the screening examination for NH; of these patients, 29 patients were excluded from the study (2 patients were diagnosed with chronic obstructive pulmonary disease, 16 patients were hospitalized for AKI and systemic infection, and 11 patients were followed up for less than 6 months). No participants received oxygen supplementation or CPAP therapy with good adherence. The remaining 161 participants were eligible for additional analysis. The baseline characteristics according to three NH groups are summarized in Table 1. The mean BMI was slightly but significantly higher in the mild and moderate-to-severe NH groups than the non-NH group. The etiologies of CKD were hypertensive nephrosclerosis (n=59), type 2 diabetic nephropathy (n=39), chronic GN (n=35), polycystic kidney disease (n=3), hydronephrosis (n=2), other (n=3), and unknown (n=20). No patients with chronic GN were on active immunosuppressive therapy, including oral steroids. The prevalence of type 2 diabetic nephropathy was approximately two times as high in the moderate-to-severe NH group as the other two groups, although this difference was not statistically significant.
Table 1.
Baseline characteristics of 161 patients with CKD stratified by the severity of nocturnal hypoxemia
| Characteristic | Total (n=161) | Severity of Nocturnal Hypoxia | P Value | ||
|---|---|---|---|---|---|
| None (n=81) | Mild (n=64) | Moderate to Severe (n=16) | |||
| Age, yr | 68.8±11.4 | 66.8±12.9 | 71.0±9.8 | 69.8±7.9 | 0.08 |
| Man, n (%) | 122 (75.8) | 57 (70.4) | 52 (81.3) | 13 (81.3) | 0.28 |
| Body mass index, kg/m2 | 21.8 (20.1, 23.1) | 21.1 (19.6, 22.6) | 22.1 (20.8, 23.7) | 23.0 (22.2, 24.1)a | <0.001 |
| Type 2 diabetes mellitus, n (%) | 39 (24.2) | 15 (18.5) | 17 (26.6) | 7 (43.8) | 0.08 |
| Systolic BP, mmHg | 129.5±17.9 | 127.0±17.6 | 131.8±17.9 | 133.3±18.7 | 0.20 |
| Diastolic BP, mmHg | 77.0±17.2 | 76.8±18.8 | 78.4±16.4 | 72.5±10.0 | 0.47 |
| Serum creatinine, mg/dl | 1.85±0.59 | 1.87±0.65 | 1.88±0.53 | 1.58±0.47 | 0.17 |
| Estimated GFR, ml/min per 1.73 m2 | 31±11 | 31±11 | 30±10 | 36±10 | 0.12 |
| Urine protein, g/d | 0.22 (0.06, 0.79) | 0.22 (0.06, 0.79) | 0.24 (0.07, 0.88) | 0.19 (0.04, 3.18) | 0.38 |
| Serum albumin, g/dl | 3.68±0.62 | 3.62±0.74 | 3.82±0.38 | 3.42±0.66 | 0.03 |
| ACEI/ARB use, n (%) | 115 (71.4) | 51 (63.0) | 51 (79.7) | 13 (81.3) | 0.06 |
| Aldosterone receptor antagonists, n (%) | 7 (4.4) | 4 (4.9) | 3 (4.7) | 0 (0) | 0.67 |
| Statin use, n (%) | 44 (27.5) | 20 (24.7) | 19 (30.2) | 5 (31.3) | 0.72 |
Values are the mean ± SD, median (interquartile range), or number (%). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin type 2 receptor blocker.
P<0.05 versus the non-NH group (posthoc Tukey–Kramer test).
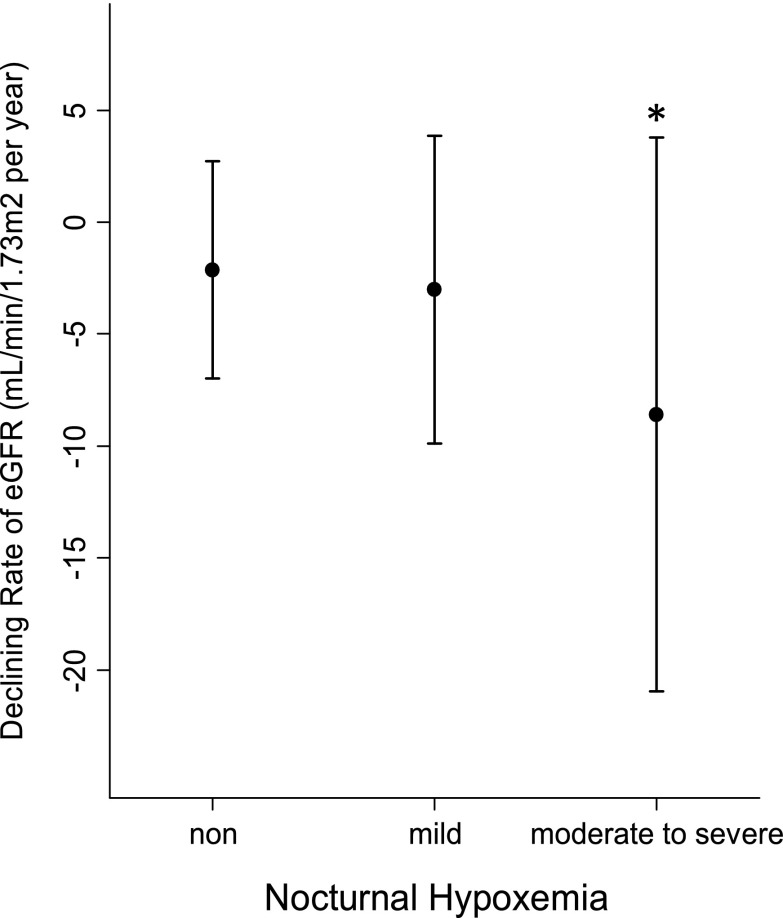
Figure 1 shows the eGFR slope for each NH group. The eGFR declined three- to fourfold faster in the moderate-to-severe NH group than the non- and mild NH groups (mean levels ± SD were −2.14±4.86, −3.02±6.86, and −8.59±12.37 ml/min per 1.73 m2 per year in the non-, mild, and moderate-to-severe NH groups, respectively; P=0.003, ANOVA). This significant association was maintained after excluding those patients with missing follow-up data on SCr (P=0.02, ANOVA). A subcohort analysis including only patients with type 2 diabetic nephropathy found a similar result (data not shown). A significant negative correlation was observed between eGFR slope and 4% ODI treated as a continuous variable (Pearson’s r=−0.17, P=0.02). Moreover, NH remained a significant predictor of a rapid decline in kidney function in the mixed effect linear regression model adjusting for center and all kinds of baseline characteristics (P=0.03) (Table 2). Additional analyses of the relationship between duration of SpO2<90% and eGFR slope were performed in 120 participants whose data on duration of SpO2<90% were available. A significant negative correlation was found in the Pearson’s product–moment correlation coefficient (r=−0.30, P=0.001); its significance was maintained in the mixed effect multiple linear regression model (P=0.001).
Figure 1.
Univariate association between declining rate of estimated GFR and nocturnal hypoxemia group. The symbols represent the mean, and the bars represent the SD of declining rate of estimated GFR. *P<0.05 versus the non- and mild nocturnal hypoxemia groups (posthoc Tukey–Kramer test).
Table 2.
Mixed effects multivariate linear regression model for declining rate of estimated GFR
| Variable | Standardized Partial Regression Coefficient | P Value |
|---|---|---|
| Age, yr | −0.02 | 0.80 |
| Sex, man | 0.06 | 0.42 |
| Body mass index, kg/m2 | 0.02 | 0.77 |
| Type 2 diabetes mellitus | −0.09 | 0.23 |
| Mean BP, mmHg | 0.06 | 0.40 |
| Nocturnal hypoxemia group | −0.16 | 0.03 |
| Estimated GFR, ml/min per 1.73 m2 | −0.19 | 0.008 |
| Urine protein, g/d | −0.40 | <0.001 |
| Serum albumin, g/dl | 0.05 | 0.46 |
| ACEI/ARB use | 0.05 | 0.51 |
| Aldosterone receptor antagonist use | 0.03 | 0.66 |
| Statin use | −0.08 | 0.26 |
Model adjusted for center, age, sex, estimated GFR, body mass index, mean BP, urine protein, serum albumin level, type 2 diabetic nephropathy, and medications used (ACEIs/ARBs, aldosterone receptor antagonists, and statins). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin type 2 receptor blocker.
Discussion
The major finding of this cohort study was that NH was significantly associated with a faster decline in eGFR among patients with CKD whose BMI was less than 25.0 kg/m2. Although high prevalence of NH and its risk for cardiovascular mortality has been shown among ESKD patients (17–20), little is known about NH in the nondialysis CKD population. To the best of our knowledge, this study is the first longitudinal study of nonobese patients with CKD to show a significant influence of NH on the progression of kidney failure. The several previous cross-sectional studies showing a significant association between SDB and kidney function in patients with CKD (1–4,26) support our findings. As mentioned above, the single previous longitudinal study (27) showing a faster declining rate of eGFR in patients with NH than in patients without NH is difficult to extrapolate to the CKD population because of their maintained baseline kidney function. In addition, the participants in that study were severely obese (mean BMI=35.7 kg/m2 in patients with NH versus mean BMI=30.6 kg/m2 in patients without NH). Because obesity is both a risk factor for the progression of kidney failure (36–38) and a major cause of NH (39), it could confound the relationship between NH and deterioration of kidney function. To address this concern, we included only patients with CKD whose BMI was less than 25.0 kg/m2; although BMI still differed significantly between the NH groups in our cohort, this small difference seems insufficient to explain the remarkably steeper eGFR slope observed in the moderate-to-severe NH group. In addition, we showed in our multivariate analysis that the significant relationship between NH and CKD progression was independent of BMI.
Several mechanisms by which NH could accelerate CKD progression can be hypothesized. NH causes hypertension through activation of the renin-angiotensin system and the sympathetic nervous system (12,13). Therefore, NH could contribute to CKD progression by increasing BP. In our study, however, BP level did not differ significantly among the NH groups. The reason for this finding is unclear, but it may be partly because CKD itself is a strong cause of hypertension, thus masking the influence of NH on BP in this population. In addition, our patients were participating in the CKD educational programs and therefore, received intensive pharmaceutical treatment for hypertension and instruction in dietary sodium restriction. Indeed, the BP level in all NH groups was close to the optimal level for the prevention of CKD progression (40). Taken together, in the current study, hypertension did not likely mediate the association between NH and CKD progression.
Rather, our finding of an independent relationship between the eGFR slope and NH suggests the presence of a direct NH-related kidney injury pathway. NH is known to induce systemic inflammation, oxidative stress, endothelial cell dysfunction, and activation of the renin-angiotensin system (7–11,13–16), all of which are also intimately involved in CKD progression (25,41). NH can, therefore, be assumed to impede kidney function through these pathologic factors, possibly through ischemic tubulointerstitial and vascular damage. Additional studies should investigate the precise mechanisms underlying the promotion of CKD progression by NH.
Our results have an important clinical implication. Kidney failure and nephrotic proteinuria have been reported to cause obstructive sleep apnea, mainly because of airway edema resulting from fluid overload and low osmolality (42–44). In turn, this study has shown the possible adverse effects of NH on kidney function in patients with CKD. Therefore, CKD and NH seem to aggravate each other. This reciprocal relationship and their common background of metabolic syndrome are anticipated to form a vicious cycle, resulting in a remarkable clustering of cardiovascular risk within an individual CKD patient. Additional clinical studies are warranted to verify whether NH is truly a risk factor for cardiovascular disease in the CKD population and whether CPAP treatment could modify this risk.
Several limitations to the interpretation of the results of this study should be noted. First, this study cannot prove a causal relationship between NH and CKD progression because of its observational nature. Although our multivariate analysis was controlled for several major clinical factors related to both CKD progression and NH, the possibility of residual confounding by unmeasured factors, such as the presence or absence of cardiovascular complications, cannot be excluded. Second, because of the retrospective data collection from two independent nephrology centers, the examination procedures and laboratory measurements methods, especially BP monitoring, were not unified and standardized across the centers. We, therefore, used a mixed effect model including center as a random effect to incorporate center-to-center variation and within-center clustering into the multivariate analysis. We also confirmed that BP level did not differ significantly among the three NH groups when analyzed separately within each center (data not shown), which was also true of the total cohort. Third, given the short follow-up duration of 1 year, the slope of eGFR assessed in this study might be a less accurate surrogate for hard renal end points, such as ESKD and doubling of SCr. Although large community-based cohort studies have reported the change in eGFR over a 1-year period to be a significant predictor of the subsequent risk for ESKD and death (45,46), the findings of our study should be confirmed by a longer observational study. Fourth, the exclusion of patients with obesity from our study left a small proportion of participants in the moderate-to-severe NH group. This disproportion might have influenced the results, although a significant association between eGFR slope and severity of NH was shown even when ODI was treated as a continuous variable. A larger clinical study is required to overcome this shortcoming. Fifth, the study participants had attended CKD educational programs, which might have created a selection bias to adherence to the treatment. Our findings, therefore, require additional external validation. Sixth, the participants of this study were relatively elderly because of the inclusion of only those patients with low BMI. In Japan, obesity is less prevalent, and craniofacial bone structure differences may be a more important factor in NH than in non-Asian populations (47). Therefore, the generalizability of our findings to the younger, obese, and non-Asian population is uncertain, although the study by Ahmed et al. (27), which targeted younger and much more obese participants than those patients in our study, showed that NH was a significant predictor of the rapid decline of kidney function.
In conclusion, NH, a frequent comorbidity of CKD, was an independent risk factor of a rapid decline in kidney function in nonobese patients with CKD. The precise mechanisms underlying kidney injury by NH should be further investigated. The close relationship between NH and CKD and their association with metabolic syndrome are anticipated to pose a currently unrecognized but serious cardiovascular risk in the CKD population. Additional studies are needed to determine whether CPAP therapy improves renal prognosis and reduces cardiovascular risk in this population.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Markou N, Kanakaki M, Myrianthefs P, Hadjiyanakos D, Vlassopoulos D, Damianos A, Siamopoulos K, Vasiliou M, Konstantopoulos S: Sleep-disordered breathing in nondialyzed patients with chronic renal failure. Lung 184: 43–49, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi Y, Shoji T, Kawabata H, Niihata K, Suzuki A, Kaneko T, Okada N, Isaka Y, Rakugi H, Tsubakihara Y: High prevalence of obstructive sleep apnea and its association with renal function among nondialysis chronic kidney disease patients in Japan: A cross-sectional study. Clin J Am Soc Nephrol 6: 995–1000, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roumelioti ME, Buysse DJ, Sanders MH, Strollo P, Newman AB, Unruh ML: Sleep-disordered breathing and excessive daytime sleepiness in chronic kidney disease and hemodialysis. Clin J Am Soc Nephrol 6: 986–994, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholl DD, Ahmed SB, Loewen AH, Hemmelgarn BR, Sola DY, Beecroft JM, Turin TC, Hanly PJ: Declining kidney function increases the prevalence of sleep apnea and nocturnal hypoxia. Chest 141: 1422–1430, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S: The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Young T, Peppard PE, Taheri S: Excess weight and sleep-disordered breathing. J Appl Physiol 99: 1592–1599, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR: Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: Implications for recurrent apneas. Proc Natl Acad Sci U S A 100: 10073–10078, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR: Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR: Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol 226: 2925–2933, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamauchi M, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Suzuki T, Kimura H: Oxidative stress in obstructive sleep apnea. Chest 127: 1674–1679, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Pialoux V, Hanly PJ, Foster GE, Brugniaux JV, Beaudin AE, Hartmann SE, Pun M, Duggan CT, Poulin MJ: Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am J Respir Crit Care Med 180: 1002–1009, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Gilmartin GS, Lynch M, Tamisier R, Weiss JW: Chronic intermittent hypoxia in humans during 28 nights results in blood pressure elevation and increased muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 299: H925–H931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster GE, Hanly PJ, Ahmed SB, Beaudin AE, Pialoux V, Poulin MJ: Intermittent hypoxia increases arterial blood pressure in humans through a Renin-Angiotensin system-dependent mechanism. Hypertension 56: 369–377, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Tanaka A, Oda N, Okada S, Ohta S, Naito H, Adachi M: Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med 172: 625–630, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Garvey JF, Taylor CT, McNicholas WT: Cardiovascular disease in obstructive sleep apnoea syndrome: The role of intermittent hypoxia and inflammation. Eur Respir J 33: 1195–1205, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK: Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med 169: 348–353, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Zoccali C, Mallamaci F, Tripepi G: Nocturnal hypoxemia predicts incident cardiovascular complications in dialysis patients. J Am Soc Nephrol 13: 729–733, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Masuda T, Murata M, Honma S, Iwazu Y, Sasaki N, Ogura M, Onishi A, Ando Y, Muto S, Shimada K, Kario K, Kusano E, Asano Y: Sleep-disordered breathing predicts cardiovascular events and mortality in hemodialysis patients. Nephrol Dial Transplant 26: 2289–2295, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Tang SC, Lam B, Yao TJ, Leung WS, Chu CM, Ho YW, Ip MS, Lai KN: Sleep apnea is a novel risk predictor of cardiovascular morbidity and death in patients receiving peritoneal dialysis. Kidney Int 77: 1031–1038, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Benz RL, Pressman MR, Hovick ET, Peterson DD: Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am J Kidney Dis 35: 1052–1060, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Marin JM, Carrizo SJ, Vicente E, Agusti AG: Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 365: 1046–1053, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V: Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353: 2034–2041, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Valham F, Mooe T, Rabben T, Stenlund H, Wiklund U, Franklin KA: Increased risk of stroke in patients with coronary artery disease and sleep apnea: A 10-year follow-up. Circulation 118: 955–960, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O’Connor GT, Boland LL, Schwartz JE, Samet JM: Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 163: 19–25, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kanbay A, Buyukoglan H, Ozdogan N, Kaya E, Oymak FS, Gulmez I, Demir R, Kokturk O, Covic A: Obstructive sleep apnea syndrome is related to the progression of chronic kidney disease. Int Urol Nephrol 44: 535–539, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Ahmed SB, Ronksley PE, Hemmelgarn BR, Tsai WH, Manns BJ, Tonelli M, Klarenbach SW, Chin R, Clement FM, Hanly PJ: Nocturnal hypoxia and loss of kidney function. PLoS One 6: e19029, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Coresh J: Chronic kidney disease. Lancet 379: 165–180, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM: Sleep-disordered breathing and mortality: A prospective cohort study. PLoS Med 6: e1000132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldwin CM, Griffith KA, Nieto FJ, O’Connor GT, Walsleben JA, Redline S: The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep 24: 96–105, 2001 [DOI] [PubMed] [Google Scholar]
- 31.WHO Expert Consultation : Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Zheng W, McLerran DF, Rolland B, Zhang X, Inoue M, Matsuo K, He J, Gupta PC, Ramadas K, Tsugane S, Irie F, Tamakoshi A, Gao YT, Wang R, Shu XO, Tsuji I, Kuriyama S, Tanaka H, Satoh H, Chen CJ, Yuan JM, Yoo KY, Ahsan H, Pan WH, Gu D, Pednekar MS, Sauvaget C, Sasazuki S, Sairenchi T, Yang G, Xiang YB, Nagai M, Suzuki T, Nishino Y, You SL, Koh WP, Park SK, Chen Y, Shen CY, Thornquist M, Feng Z, Kang D, Boffetta P, Potter JD: Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med 364: 719–729, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Chung F, Liao P, Elsaid H, Islam S, Shapiro CM, Sun Y: Oxygen desaturation index from nocturnal oximetry: A sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg 114: 993–1000, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Iber C, Ancoli-Israel S, Chesson A, Quan S for the American Academy of Sleep Medicine: The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, 1st Ed., Westchester, IL, American Academy of Sleep Medicine, 2007 [Google Scholar]
- 36.Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S: Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int 65: 1870–1876, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS: Body mass index and risk for end-stage renal disease. Ann Intern Med 144: 21–28, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Reynolds K, Gu D, Muntner P, Chen J, Wu X, Yau CL, Duan X, Chen CS, Hamm LL, He J: Body mass index and risk of ESRD in China. Am J Kidney Dis 50: 754–764, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T: Progression and regression of sleep-disordered breathing with changes in weight: The Sleep Heart Health Study. Arch Intern Med 165: 2408–2413, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, Tuttle K, Douglas J, Hsueh W, Sowers J, National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group : Preserving renal function in adults with hypertension and diabetes: A consensus approach. Am J Kidney Dis 36: 646–661, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Agarwal R: Proinflammatory effects of oxidative stress in chronic kidney disease: Role of additional angiotensin II blockade. Am J Physiol Renal Physiol 284: F863–F869, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Beecroft JM, Hoffstein V, Pierratos A, Chan CT, McFarlane PA, Hanly PJ: Pharyngeal narrowing in end-stage renal disease: Implications for obstructive sleep apnoea. Eur Respir J 30: 965–971, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Tang SC, Lam B, Lai AS, Pang CB, Tso WK, Khong PL, Ip MS, Lai KN: Improvement in sleep apnea during nocturnal peritoneal dialysis is associated with reduced airway congestion and better uremic clearance. Clin J Am Soc Nephrol 4: 410–418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang SC, Lam B, Lam JC, Chan CK, Chow CC, Ho YW, Ip MS, Lai KN: Impact of nephrotic edema of the lower limbs on obstructive sleep apnea: Gathering a unifying concept for the pathogenetic role of nocturnal rostral fluid shift. Nephrol Dial Transplant 27: 2788–2794, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Turin TC, Coresh J, Tonelli M, Stevens PE, de Jong PE, Farmer CK, Matsushita K, Hemmelgarn BR: Short-term change in kidney function and risk of end-stage renal disease. Nephrol Dial Transplant 27: 3835–3843, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Turin TC, Coresh J, Tonelli M, Stevens PE, de Jong PE, Farmer CK, Matsushita K, Hemmelgarn BR: One-year change in kidney function is associated with an increased mortality risk. Am J Nephrol 36: 41–49, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Sakakibara H, Tong M, Matsushita K, Hirata M, Konishi Y, Suetsugu S: Cephalometric abnormalities in non-obese and obese patients with obstructive sleep apnoea. Eur Respir J 13: 403–410, 1999 [DOI] [PubMed] [Google Scholar]