Abstract
Objective
To investigated into the anti-quorum sensing (QS) activity of Syzygium cumini L. (S. cumini) and Pimenta dioica L. (P. dioica) using Chromobacterium violaceum (C. violaceum) strains.
Methods
In this study, anti-QS activity of ethanol extract of Syzygium cumini L. and Pimenta dioica L. were screened using C. violaceum CV026 biosensor bioassay. By bioassay guided fractionation of S. cumini and P. dioica, ethyl acetate fraction (EAF) with strong anti-QS activity was separated. Inhibition of QS regulated violacein production in C. violaceum ATCC12472 by EAF was assessed at different concentrations. The effect of EAF on the synthesis of autoinducer like N-acyl homoserine lactone (AHL) was studied in C. violaceum ATCC31532 using its mutant C. violaceum CV026 by standard methods.
Results
EAF inhibited violacein production in C. violaceum ATCC12472 in a concentration dependent manner without significant reduction in bacterial growth. Complete inhibition of violacein production was evidenced in 0.75-1.0 mg/mL concentration of EAF without inhibiting the synthesis of the AHL. TLC biosensor overlay profile of EAF revealed two translucent spots in S. cumini and P. dioica that inhibited C6-AHL mediated violacein production in C. violaceum CV026.
Conclusions
This study indicates the anti-QS activity of the tested medicinal plants against C. violaceum.
Keywords: Anti-quorum sensing, Chromobacterium, Medicinal plants, Quorum sensing, Pathogenicity
1. Introduction
Quorum sensing (QS), a population density dependent mechanism present in many bacteria, is mediated through small signal molecules called autoinducers that regulate the target gene expression responsible for the phenotypes essential to pathogenicity/symbiosis[1]. In Gram negative bacteria, QS is mediated through N-acyl homoserine lactones (AHL)[2]. Deeper understanding on QS has led to its role in controlling the production of virulence factors such as exopolysaccharide synthesis, biofilm formation, swarming motility, pigment production and antibiotic production in some pathogenic bacteria[3],[4]. Owing to the importance of QS during bacterial pathogenesis, interfering with this mechanism is being considered as a rational strategy to attenuate their virulence[5]. Increasing emergence of antibiotic resistance in Gram negative bacteria also demands alternative strategies to combat bacterial infections and anti-QS approach is being viewed as an attractive alternative. QS inhibitory compounds, unlike conventional antibiotics, do not kill or inhibit microbial growth and are less likely to impose a selective pressure for the development of drug resistant bacteria[6].
Plant metabolites having the ability to control the growth of microbes have been traditionally used to treat human diseases including microbial infections[7]. However, the mode of action of many phytocompounds against the target organism is not clearly understood. Recent research has revealed that a few natural products including plant extracts have properties for modulating bacterial QS system, thereby reducing the virulence[8],[9]. Syzygium cumini (S. cumini) and Pimenta dioica (P. dioica) are widely used medicinal plants having ability to alleviate bacterial infections and are used in traditional healing in different parts of the world. Due to their use in treating microbial infections, it is possible that these plants may possess anti-QS properties.
Chromobacterium violaceum (C. violaceum), a Gram negative bacterium having a wide geographic distribution, produces the pigment violacein in response to QS regulated gene expression. Further, the whole genome studies on C. violaceum showed that violacein production is regulated by vioD, vioC, vioB and vioA genes arranged in an operon through a QS system mediated by AHL[10]. A biosensor strain, C. violaceum CV026 (mini-Tn5 mutant of wild type strain), deficient in autoinducer synthase which requires exogenous addition of AHL to produce violacein was developed for studying various QS mechanisms, and it offers a convenient tool for the biological assay of screening QS inhibitors[11]. This study investigated into the anti-QS activity of S. cumini and P. dioica using C. violaceum strains.
2. Materials and methods
2.1. Bacterial strains, media and culture conditions
Bacterial strains C. violaceum ATCC12472, C. violaceum ATCC31532 and C. violaceum CV026 were used. All these strains were cultured in Luria-Bertani (LB) medium at 32 °C for 24 h. When required, the medium for C. violaceum CV026 was supplemented with autoinducer, C6-AHL (Sigma). For all the experiments, the inoculum was prepared by growing the bacteria in 10 mL LB broth at 32 °C for 24 h in a shaking incubator (130 r/min).
2.2. Collection of plant materials and extract preparation
Leaves of S. cumini and P. dioica were collected from Mangalore region, Karnataka (India). The leaves were washed in sterile water, shade dried and pulverized to a fine powder in an analytical mill (IKA, Germany). Plant extracts were prepared by mixing 100 g of powdered leaves in 90% ethanol and kept under agitation at room temperature for 48 h. The extract was filtered through sterile filter paper (Whatman No. 1) and concentrated to complete dryness under a vacuum evaporator.
2.3. Biosensor bioassay for detecting anti-QS activity of the plant extracts
Biosensor bioassay for anti-QS activity was carried out according to the methods described previously[11]. Briefly, plant extracts re-suspended in ethanol were loaded onto 6 mm sterile discs (Himedia, India) at different concentrations (100 µg–3.0 mg/disc). LB agar plates were prepared by supplementing 10 µL of 5 µg/mL of C6-AHL and inoculated with C. violaceum CV026. Discs containing plant extracts were placed on the agar plates and incubated for 24 h at 32 °C in upright position. Discs loaded with solvent (ethanol) were included as vehicle only controls. Inhibition of QS was detected by the presence of a zone of colorless, but viable cells around the discs could clearly be differentiated from the zone of growth inhibition (antibacterial activity).
2.4. Bioassay guided purification of anti-QS compounds
The ethanol extract was subjected to bioassay guided purification of active compounds. In the first step, the ethanol extracts of S. cumini and P. dioica were defatted with n-hexane and treated with water, shaken well to resolve into water soluble and water insoluble parts. The water soluble fraction was repeatedly extracted with ethyl acetate and the final ethyl acetate fraction (EAF) was separated. All the fractions were concentrated and subjected to C. violaceum CV026 biosensor bioassay. EAF of S. cumini and P. dioica showed strong anti-QS activity in bioassay.
2.5. Inhibition of violacein production in C. violaceum ATCC12472
Inhibition of violacein production in the presence of plant extracts was tested using C. violaceum ATCC12472 and quantified as previously described method with modification[12]. For this, the dried EAF was re-suspended in dimethyl sulfoxide and added to LB broth (10 mL) at concentrations of 0.25–1.0 mg/mL. Solvent controls were prepared similarly and all the tubes were inoculated with 100 µL of C. violaceum ATCC12472. The inoculated tubes were incubated at 32 °C for 24 h in a shaking incubator. After incubation, 1 mL culture was centrifuged at 8 000 r/min for 10 min to precipitate the insoluble violacein. The culture supernatant was discarded and 1 mL of water saturated n-butanol was added to the pellet. The solution was vortexed vigorously for 30 seconds to completely solubilize violacein and centrifuged at 8 000 r/min for 10 min to remove the cells. The violacein was quantified spectrophotometrically at OD585 (UV-1800, Shimadzu, Japan). To test the effect of plant extracts on bacterial growth, culture grown in the presence of active fraction was serially diluted and plated on LB agar medium. After incubation at 32 °C for 24 h, bacterial number was enumerated using the colony counter.
2.6. Effect on modulation of AHL synthesis and its activity
The effect of plant extracts on AHL synthesis and AHL activity was determined using a C6-AHL overproducing strain, C. violaceum ATCC31532 and its mutant C. violaceum CV026[13]. C. violaceum ATCC31532 was cultured in the presence of EAF at a concentration of 0.25-1.0 mg/mL under conditions as described earlier and violacein produced was quantified spectrophotometrically after 24 h incubation. Culture medium inoculated with dimethyl sulfoxide was included as solvent control. AHL was extracted from the cell free supernatant (8 mL) using dichloromethane (3:1 v/v) and evaporated under a thin stream of nitrogen gas. For determining the AHL activity, the dried AHL fractions were re-suspended in 70% methanol (20 µL) and added to fresh 10 mL LB medium inoculated with biosensor strain C. violaceum CV026 which responded to exogenous AHL by producing violacein. Induction of violacein by the AHL fractions in C. violaceum CV026 was measured spectrophotometrically after incubation at 32 °C for 24 h as described earlier.
2.7. Thin layer chromatography (TLC) with biosensor overlay
The active fraction of plant extracts were analyzed by silica gel TLC using biosensor overlay to detect the migration of anti-QS compounds. EAF (10 µL) was spotted on a silica TLC plate and chromatographed using chloroform:methanol (8:2) solvent system. After elution, the plate was dried and overlaid with sterile LB medium containing exogenous C6-AHL inoculated with an overnight culture of C. violaceum CV026 biosensor strain. The TLC overlay was incubated at 32 °C for 24 h and anti-QS activity was detected by the presence of a turbid zone in a purple background.
2.8. Data analysis
All the experiments were conducted in quadruplicates and one way analysis of variance (ANOVA) was used to analyze the differences between the treatments. P<0.01 was considered as significant unless specified.
3. Results
Biosensor bioassay of ethanol extracts of S. cumini and P. dioica showed turbid zone of violacein inhibition around the discs indicating anti-QS activity of plant extracts. A strong anti-QS activity was evident for the tested plant extracts in the concentration of 3 mg/disc. However, a weak QS inhibition zone was observed at concentrations between 0.5-1.0 mg/disc and no activity was found at lower concentrations of plant extracts.
In an effort to identify anti-QS compounds, the crude ethanol extract was subjected to bioassay guided fractionation. Biosensor bioassay of different fractions (hexane, aqueous and EAF) using C. violaceum CV026 revealed strong anti-QS activity for the EAF. Hexane and aqueous fractions did not show anti-QS activity against C. violaceum CV026. The purified EAF showed strong anti-QS activity at 0.5–1.0 mg/disc concentration with a zone of inhibition of (24.0±1.2) mm.
Violacein production in C. violaceum ATCC12472 was inhibited in a concentration dependent manner by EAF of S. cumini and P. dioica leaves (Figure 1). The purified EAF extracted from S. cumini and P. dioica showed more than 80% inhibition at 0.5 mg/mL and at 1.0 mg/mL, it showed complete inhibition of violacein production. It is interesting to note that, the growth of C. violaceum ATCC12472 in the presence of plant extracts was not inhibited as the cell counts showed no significant difference compared to the control (Figure 1). The violacein production and cell counts in the solvent controls were similar to the control.
Figure 1. Concentration of violacein produced (OD585) and cell counts (log CFU/mL) in C. violaceum ATCC12472 cultures grown in the presence of the active fraction of (a) P. dioica and (b) S. cumini.
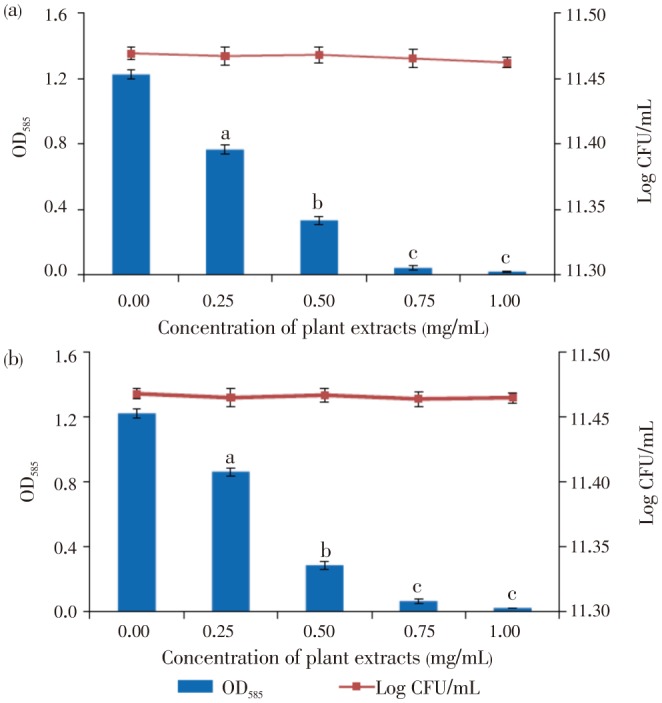
Data points are mean±SD (n=4). aP<0.05; bP<0.01; cP<0.001.
Inhibition of AHL activity was seen by the inhibition of violacein production in C. violaceum ATCC31532, but not in C. violaceum CV026. S. cumini and P. dioica at concentrations between 0.25–1.0 mg/mL of EAF inhibited the QS mediated violacein production in C. violaceum ATCC31532 in a concentration dependent manner (Figure 2). However, AHL extracted from these culture supernatants were able to induce the violacein production in the mutant C. violaceum CV026 without any significant difference between the treatments. The solvent did not interfere with either violacein production or AHL synthesis in C. violaceum ATCC31532 and was similar to the control. Hence, it was clear that AHL synthesis was not inhibited in C. violaceum ATCC31532 by the active fraction, but AHL mediated violacein production was inhibited.
Figure 2. Effect of active fractions of (a) P. dioica and (b) S. cumini on AHL synthesis in C6-AHL overproducing strain C. violaceum ATCC31532 and its activity in C. violaceum CV026.

Data points are mean±SD (n=4). aP<0.05; bP<0.01; cP<0.001.
TLC biosensor overlay profile of EAF of S. cumini and P. dioica revealed two spots with anti-QS zone in both S. cumini (Rf of 0.16 and 0.87) and P. dioica (Rf of 0.16 and 0.81) (Figure 3).
Figure 3. TLC biosensor overlay profile of active fraction of (a) P. dioica and (b) S. cumini.

Spots with anti-QS zone at different Rf values were indicated by arrows. The experiments were repeated twice.
4. Discussion
The present study demonstrates the anti-QS properties of S. cumini and P. dioica plants against C. violaceum CV026 grown in the presence of C6-AHL. Inhibition of QS regulated violacein production in C. violaceum is commonly used for anti-QS screening studies. The AHL synthesized by different strains of C. violaceum vary in their acyl chain length or substitution, and the two wild type strains used in this study though produce two different major AHL molecules[11], the tested plant extracts were able to inhibit the violacein synthesis in both. Other reports have also found natural plant extracts, such as that of Myristica cinnamomea, showing anti-QS activity at 3 mg/mL against C. violaceum CV026[14].
S. cumini is an ancient medicinal plant and has a long tradition in alternative medicine. All parts of the plant have been used for a wide variety of ailments, including cough, diabetes, dysentery, inflammation and ringworm[15]. P. dioica, also known as allspice, is traditionally used as analgesic, antimicrobial, antioxidant, stimulant, carminative and muscle relaxative[16]. Most of the studies on these plants have been conducted using crude preparation of the plant without pointing out their chemical profile.
The mechanism of action of anti-QS compounds on QS system is a complex phenomenon. In this study, all the tested plant extracts inhibited AHL mediated violacein production in C. violaceum. However, synthesis of AHL in C. violaceum was not inhibited by the plant extracts as AHL extracted from the culture could induce the violacein production in the mutant. The anti-QS compounds are known to include molecules that mimic autoinducer structure and/or function and compounds that are antagonistic to the autoinducer molecules[17]. In addition, these compounds could potentially target other components of QS system, such as interfering with the stability and function of the regulator protein or autoinducer synthase[18],[19].
Qualitative phytochemical screening of anti-QS spot showed positive for flavonoids in S. cumini and P. dioica showed positive for phenols. The bioactivity of S. cumini leaves are mainly attributed to flavonoids such as quercetin, myricetin, myricitin and triterpenoids[15]. P. dioica plants are rich in polyphenols such as phenolic acids, flavonoids, catechins, diterpenes and lupeol[16]. Anti-QS activity of some polyphenols (epigallocatechin gallate, ellagic acid, tannic acid), flavonoids (naringin, neohesperidin, quercetin and hesperidin), alkaloids and essential oils (cinnamaldehyde and its derivatives) has earlier been demonstrated against C. violaceum[20],[21]. Further, studies are needed to identify the major compound responsible for anti-QS activity present in the tested plants.
Efficacy of plant compounds in vivo has earlier been described against QS targets in Pseudomonas aeruginosa (P. aeruginosa) infections using animal model systems. Fresh garlic extract having strong anti-QS activity, injected subcutaneously for 7 d [1.5% of the mass of the mouse (20 g)] promoted rapid clearing of pulmonary P. aeruginosa infections[8]. Oral treatment with a fresh garlic extract for 14 d significantly lowered renal tissue destruction by anti-QS activity against P. aeruginosa infections in the mouse urinary tract infection model[22]. Similarly, furanone C-30, a synthetic analogue of a natural furanone derived from the red alga, administered subcutaneously for 3 d (0.7 µg/g body weight) to P. aeruginosa infected mice reduced the pulmonary infection by targeting the QS in P. aeruginosa and promoted their clearance by the mouse immune response[23].
Compounds that can inhibit QS have an enormous scope for developing therapeutics for countering the antibiotic use in some infections. Anti-QS drugs such as anti-QS based antiseptic ointments, drops for ear infections, tablets for stomach ulcers and mouthwash for oral infections have been emerged. Similarly, functional foods for controlling infections in immunocompromized individuals can also be developed from the plant products rich in compounds with anti-QS activity[24].
Due to the clinical, environmental and industrial application of the QS inhibitors, search for potential compounds with anti-QS activity has increased over the last few years[25]. Efforts towards the development of anti-QS compounds should provide a means of treating bacterial infections without the overuse of antibiotics that unavoidably develop resistant organisms. The present study is an effort towards exploring the anti-QS property of some medicinal plants and with further studies, identification of active compounds and understanding the mechanism can be achieved.
Acknowledgments
This study was funded by the Department of Biotechnology, Govt. of India, under Rapid Grant for Young Investigator Scheme (Grant No. BT/PR13242/GBD/27/226/2009).
Comments
Background
QS is a generic phenomenon in Gram negative bacteria and is linked to virulence. Treatment of infections caused by such bacteria poses a challenge as they are often multidrug resistance. Hence anti-QS compounds seem to hold a promise for development of novel non-antibiotic agents against the pathogens.
Research frontiers
The present research work investigated the anti-QS activity of ethanol extract of S. cumini and P. dioica against C. violaceum ATCC12427. The study has also demonstrated the presence of anti-QS activity in different concentrations.
Related reports
The studies on S. cumini and P. dioica have been conducted using crude preparation of the plant without pointing out their chemical profile and anti-QS activity of these herbs has not been reported earlier. Other reports have found natural plant extracts of Myristica cinnamomea, showing anti-QS activity against C. violaceum CV026.
Innovations and breakthroughs
S. cumini is an ancient medicinal plant and all parts of the plant have been used for a wide variety of ailments, including cough, diabetes, dysentery, inflammation and ringworm. P. dioica, also known as allspice, is traditionally used as analgesic, antimicrobial, antioxidant, stimulant, carminative and muscle relaxative. This work has validated the traditional use of tested plants for bacterial infections by inhibiting the QS activity in C. violaceum.
Applications
Compounds that can inhibit QS have an enormous scope for developing novel non-antibiotic, anti-pathogenic therapeutic agents, which interfere with bacterial cell to cell communication and render them less virulent and more susceptible to biocide treatment. The active ingredients of these herbs may be utilized to formulate new antiseptic and anti-infective drugs.
Peer review
This valuable scientific work has demonstrated the anti-QS activity of two medicinal herbs, S. cumini and P. dioica using C. violaceum in which QS-regulated violacein production was inhibited. The presence of anti-QS compound was confirmed on TLC biosensor overlay. The purified compound may be explored further to be a potential anti-infective agent for bacterial infections.
Footnotes
Foundation project: Supported by the Department of Biotechnology, Govt. of India, under Rapid Grant for Young Investigator scheme(Grant no. BT/PR13242/GBD/27/226/2009).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Swift S, Throup JP, Williams P, Salmond GP, Stewart GS. Quorum sensing: a population-density component in the determination of bacterial phenotype. Trends Biochem Sci. 1996;21:214–219. [PubMed] [Google Scholar]
- 2.Bassler BL. Small talk. Cell-to-cell communication in bacteria. Cell. 2002;109:421–424. doi: 10.1016/s0092-8674(02)00749-3. [DOI] [PubMed] [Google Scholar]
- 3.Smith JL, Fratamico PM, Yan X. Eavesdropping by bacteria: the role of SdiA in Escherichia coli and Salmonella enterica serovar Typhimurium quorum sensing. Foodborne Pathog Dis. 2011;8:169–178. doi: 10.1089/fpd.2010.0651. [DOI] [PubMed] [Google Scholar]
- 4.Schonewille E, Nesse LL, Hauck R, Windhorst D, Hafez HM, Vestby LK. Biofilm building capacity of Salmonella enterica strains from the poultry farm environment. FEMS Immunol Med Microbiol. 2012;65:360–365. doi: 10.1111/j.1574-695X.2012.00966.x. [DOI] [PubMed] [Google Scholar]
- 5.Adonizio AL, Downum K, Bennett BC, Mathee K. Anti-quorum sensing activity of medicinal plants in southern Florida. J Ethnopharmacol. 2006;105:427–435. doi: 10.1016/j.jep.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Hong KW, Koh CL, Sam CK, Yin WF, Chan KG. Quorum quenching revisited - From signal decays to signaling confusion. Sensors (Basel) 2012;12:4661–4696. doi: 10.3390/s120404661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahid M, Shahzad A, Sobia F, Sahai A, Tripathi T, Singh A, et al. Plant natural products as a potential source for antibacterial agents: Recent trends. Anti-infect Agents Med Chem. 2009;8:211–225. [Google Scholar]
- 8.Bjornsjolt T, Jensen PØ, Rasmussen TB, Christophersen L, Calum H, Hentzer M, et al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology. 2005;151:3873–3880. doi: 10.1099/mic.0.27955-0. [DOI] [PubMed] [Google Scholar]
- 9.Khan MS, Zahin M, Hasan S, Husain FM, Ahmad I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett Appl Microbiol. 2009;49:354–360. doi: 10.1111/j.1472-765X.2009.02666.x. [DOI] [PubMed] [Google Scholar]
- 10.August PR, Grossman TH, Minor C, Draper MP, MacNeil IA, Pemberton JM, et al. Sequence analyses and functional characterization of the violacein biosynthetic pathway from Chromobacterium violaceum. J Mol Microbiol Biotechnol. 2000;2:513–519. [PubMed] [Google Scholar]
- 11.McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, et al. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997;143:3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 12.Choo JH, Rukayadi Y, Hwang JK. Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbiol. 2006;42:637–641. doi: 10.1111/j.1472-765X.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- 13.Vattem DA, Mihalik K, Crixell SH, McLean RJ. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia. 2007;78:302–310. doi: 10.1016/j.fitote.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Chong YM, Yin WF, Ho CY, Mustafa MR, Hadi HA, Awang K, et al. Malabaricone C from Myristica cinnamomea exhibits anti-quorum sensing activity. J Nat Prod. 2010;74:2261–2264. doi: 10.1021/np100872k. [DOI] [PubMed] [Google Scholar]
- 15.Ayyanar M, Babu PS. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pacific J Trop Biomed. 2012;2:240–246. doi: 10.1016/S2221-1691(12)60050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marzouk MS, Moharram FA, Mohamed MA, Gamal-Eldeen AM, Aboutabl EA. Anticancer and antioxidant tannins from Pimenta dioica leaves. Z Naturforsch C. 2007;62:526–536. doi: 10.1515/znc-2007-7-811. [DOI] [PubMed] [Google Scholar]
- 17.Teplitski M, Robinson JB, Bauer WD. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density dependent behaviours in associated bacteria. Mol Plant Microbe Interact. 2000;13:637–648. doi: 10.1094/MPMI.2000.13.6.637. [DOI] [PubMed] [Google Scholar]
- 18.Manefield M, Rasmussen TB, Henzter M, Andersen JB, Steinberg P, Kjelleberg S, et al. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology. 2002;148:1119–1127. doi: 10.1099/00221287-148-4-1119. [DOI] [PubMed] [Google Scholar]
- 19.Keshavan ND, Chowdary PK, Haines DC, González JE. L-Canavanine made by Medicago sativa interferes with quorum sensing in Sinorhizobium meliloti. J Bacteriol. 2005;187:8427–8436. doi: 10.1128/JB.187.24.8427-8436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber B, Eberl L, Feucht W, Polster J. Influence of polyphenols on bacterial biofilm formation and quorum-sensing. Z Naturforsch. 2003;58:879–884. doi: 10.1515/znc-2003-11-1224. [DOI] [PubMed] [Google Scholar]
- 21.Kociolek MG. Quorum-sensing inhibitors and biofilms. Anti-infective agents Med Chem. 2009;8:315–326. [Google Scholar]
- 22.Harjai K, Kumar R, Singh S. Garlic blocks quorum sensing and attenuates the virulence of Pseudomonas aeruginosa. FEMS Immunol Med Microbiol. 2010;58:161–168. doi: 10.1111/j.1574-695X.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- 23.Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Givskov M. Beyond nutrition: health-promoting foods by quorum sensing inhibition. Future Microbiol. 2012;7:1025–1028. doi: 10.2217/fmb.12.84. [DOI] [PubMed] [Google Scholar]
- 25.Ponnusamy K, Paul D, Kim YS, Kweon JH. 2(5H)-Furanone: a prospective strategy for biofouling-control in membrane biofilm bacteria by quorum sensing inhibition. Braz J Microbiol. 2010;41:227–234. doi: 10.1590/S1517-838220100001000032. [DOI] [PMC free article] [PubMed] [Google Scholar]


