Abstract
Objective
To determine the possible bioactive components of the ethanolic extract of leaves of Elaeocarpus serratus (E. serratus).
Methods
The present research was carried out by using GC-MS analysis, while mass spectra of the compounds found in the extract was matched with the National Institute of Standards and Technology and Wiley library.
Results
Thirty components from leaves of the above said plant were identified. The active principles with their retention time, molecular formula, molecular weight and concentration (%) in the ethanol extracts of leaf of E. serratus are obtained.
Conclusions
This is the first report of documentation of active constituents from leaves of E. serratus. The research reveals the potential of E. serratus leaves as a good source of bioactive compounds such as fatty acid esters, alcohols, hydrocarbons, aldehydes, alkenes, fatty acids and amides that justify the use of this plant for its various ailments by traditional practitioners.
Keywords: GC-MS, Elaeocarpus serratus, Hexadecanoic acid methyl ester, Anti-inflammatory
1. Introduction
Medicinal plants are an expensive gift from nature to human as they are the sources of important therapeutic aids for alleviating human ailments. The medicinal actions of plants unique to particular plant species are consistent with the concept that the combination of secondary products in a particular plant is taxonomically distinct[1],[2]. Plants remain a vital source of drugs, and nowadays much emphasis has been given to the development of novel drug used for the treatment and prevention of diseases[3]–[5]. Standardization is an essential measure of quality, purity and authenticity. The standardization of crude drug is an integral part for establishing its correct identity. Also, WHO has emphasized the need to ensure the quality of medicinal plants products using modern controlled technique and applying suitable standards[6]. Gas chromatography-mass spectrometry (GC-MS) is the best technique to identify the bioactive constituents of long chain hydrocarbons, alcohols, acids, esters, alkaloids, steroids, amino acid and nitro compounds[7]–[9]. Therefore, characterization of extracts of medicinal plants is necessary due to its numerous benefits to science and society.
Review of literature divulges that information on the GC-MS analysis of Elaeocarpus serratus L. (E. serratus) belonging to the family Elaeocarpaceae is totally lacking. Hence, the objective of the present study is to identify the phytochemical constituents with the aid of GC-MS technique. This work will help to identify the compounds of therapeutic value.
2. Materials and methods
The leaves of E. serratus were collected from Upper Palani Hills of Western Ghats (Kodaikanal Forest Division), India and were authenticated at Botanical Survey of India (BSI), Southern Circle, Coimbatore, India and the herbarium of voucher specimen number BSI/SRC/5/23/2011-12/Tech. 454 has been deposited at the PG and Research Department of Botany, Vellalar College for Women, Erode (T.N), India. Fresh leaves were collected and air-dried at room temperature. The dried material was then homogenized to obtain coarse powder and stored in air-tight bottles for further analysis. The shade dried, powdered leaf were extracted with ethanol solvent by hot extraction using soxhlet apparatus collected and stored in a vial for further analysis[10].
2.1. GC-MS analysis
Ethanolic extract of leaf of E. serratus were analyzed for the presence of different compounds by GC-MS. GC-MS analysis of some of the potent volatile constituents present in the extracts was performed at The South India Textile Research Association (SITRA), Coimbatore (Tamil Nadu), India. GC analysis of the extract was performed using a GC-MS (Model; Thermo Trace GC Ultra) equipped with a DB-5MS fused silica capillary column (30 m length×0.25 mm outside diameter×0.25 µm internal diameter) and GC interfaced to a Mass Selective Detector (MS-DSQ-II) with XCALIBUR software. For GC-MS detection, an electron ionization system with ionization energy of -70eV was used. Helium gas was used as a carrier gas at a constant flow rate of 1 mL/min and the sample injected was 2 µL; Injector temperature was 250 °C; Ion source temperature was 200 °C. The oven temperature was programmed from 80 °C to 200 °C at the rate of 10 °C/min, held isothermal for 1 min and finally raised to 260 °C at 10 °C/min. Interface temperature was kept at 250 °C. Total GC run time was 46.16 min. The relative percentage of the extract constituents was expressed as percentage with peak area normalization.
2.2. Identification of components
The identity of the components in the extract was assigned by the comparison of their retention indices and mass spectra fragmentation patterns with those stored on the computer library and also with published literatures. National Institute of Standards and Technology library sources were also used for matching the identified components from the plant material[11],[12].
3. Result
The bioactive compounds present in the ethanolic extract of leaf of E. serratus were identified by GC-MS analysis (Figure 1).
Figure 1. GC-MS chromatogram of ethanolic extract of the leaf of E. serratus.
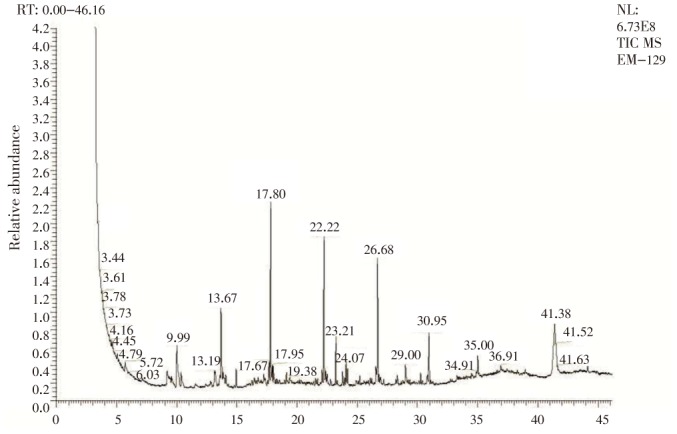
Thirty compounds were detected in the ethanolic extract of E. serratus leaf. The active principles with their retention time, molecular formula, molecular weight and concentration (%) in the ethanol extracts of leaf of E. serratus are presented in Table 1, and the total running time was 46.16 min. The spectra of the compounds were matched with Wiley 9.0 and National Institute of Standards and Technology libraries. The most prevailing major compounds were methanol (20.57%), n-dotriacontanol (10.70%), n-octadecanol (10.08%), docosanoic acid, 1,2,3-propanetriyl ester (9.07%), n-hexadecene (8.52%), bis-(3,5,5-trimethylhexyl) ether (6.30%), ethanone, 1-cyclopentyl- (4.81%), cyclohexane, ethyl- (4.05%) etc., and minor components were hexadecanoic acid methyl ester (0.80%), ricinoleic acid (0.77%), citronellyl isobutyrate (0.69%) and farnesol (0.51%).
Table 1. Phytocompounds identified in the ethanolic extract of the leaf of E. serratus by GC-MS.
| RT | Name of the compound | Molecular formula | Molecular weight | Peak area (%) |
| 3.04 | Methanol | CH4O | 32 | 20.57 |
| 5.13 | 2,2,9,9-Tetramethyl-5,6-diferrocenyldeca-3,7-diene | C34H42Fe2 | 562 | 0.83 |
| 9.23 | 8-Aminocaffeine | C8H11N5O2 | 209 | 1.54 |
| 10.03 | Ethanone, 1-cyclopentyl- | C7H12O | 112 | 4.81 |
| 10.39 | N-(o-Biphenylyl)-5-hydroxypentanamide | C17H19NO2 | 269 | 1.21 |
| 11.57 | 2-Methoxy-4-methylbenzamide | C9H11NO2 | 165 | 0.53 |
| 12.83 | (-)-Menthyl p-(2-Trifluoroacetoxyethyl) benzenesulfinate | C20H27F3O4S | 420 | 0.64 |
| 13.17 | 1,4-Butandial | C6H10O2 | 114 | 2.38 |
| 13.69 | Bis-(3,5,5-trimethylhexyl) ether | C18H38O | 270 | 6.30 |
| 14.94 | 1-(Pent-1-enyl)cyclopropanol | C8H14O | 126 | 1.62 |
| 16.45 | p-Menthane-1-thiol | C10H20S | 172 | 0.54 |
| 17.25 | 1-Benzylthio-4-methyl-1,3-pentadiene | C13H16S | 204 | 1.11 |
| 17.80 | 1-Octadecanol | C18H38O | 270 | 10.08 |
| 19.12 | Citronellyl isobutyrate | C14H26O2 | 226 | 0.69 |
| 22.22 | Docosanoic acid, 1,2,3-propanetriyl ester | C69H98O6 | 1 022 | 9.07 |
| 23.21 | 2-Octyldodecan-1-ol | C20H42O | 298 | 2.81 |
| 23.78 | Ricinoleic acid | C18H34O3 | 298 | 0.77 |
| 24.07 | Dotriacontane | C32H66 | 450 | 2.38 |
| 25.21 | Hexadecanoic acid, methyl ester | C17H34O2 | 270 | 0.80 |
| 26.68 | 1-Hexadecene | C16H32 | 224 | 8.52 |
| 28.32 | Pent-4-enal | C5H8O | 84 | 0.96 |
| 29.02 | 9-Nitro-1-nonene | C9H17NO2 | 171 | 1.52 |
| 30.26 | 1-(Chloromethyl)-3-methylene-1-cyclobutanol | C6H9ClO | 132 | 1.26 |
| 30.95 | Cyclohexane, ethyl- | C8H16 | 112 | 4.05 |
| 35.00 | 5,6-Epoxy-6-methyl-1,9-decadien-4-one | C11H16O2 | 180 | 2.03 |
| 36.93 | 2-Methylsulfonyl-3,3-diethyloxirane | C7H14O3S | 178 | 0.60 |
| 38.95 | 8-Ethenyloxocan-2-one | C9H14O2 | 154 | 0.54 |
| 41.40 | 1-Dotriacontanol | C32H66O | 466 | 10.70 |
| 44.13 | Farnesol | C15H26O | 222 | 0.51 |
| 44.91 | N-Benzyl-N-(1-phenylethylidene)amine | C15H15N | 209 | 0.62 |
RT: retention time.
4. Discussion
Among the identified phytochemicals, the fatty acid esters namely, docosanoic acid, 1,2,3-propanetriyl ester, hexadecanoic acid methyl ester have the property of antioxidant, hypocholesterolemic, nematicide, pesticide, flavouring agent, lubricant and anti-androgenic activities. The biological activities listed are based on Dr. Duke's phytochemical and ethanobotanical databases by Dr. Jim Duke of the Agricultural Research Service/USDA. Analogous to the present study, hexadecanoic acid methyl ester was identified in the methanol extract of Spirulina platensis[13]. The ethanolic extract of Mussaenda frondosa was subjected to GC-MS analysis and 20 chemical constituents namely (-)-quinic acid, 4-((1e)-3-hydroxy-1-propenyl)-2-methoxyphenol, naphthalene,decahydro-2-methoxy,1, 2, 3-benzenetriol, hexadecanoic acid ethyl ester, linoleic acid ethyl ester, oleic acid, etc. were identified[14]. The compounds farnesol and citronellyl isobutyrate are present in the ethanolic leaf extract of E. serratus. The compound farnesol is an acyclic sesquiterpene alcohol, and it has been suggested to function as a chemopreservative and anti-tumor agent[15]. It is also used as a deodorant in cosmetic products because of its anti-bacterial activity[16]. The anti-fungal activity of farnesol has also been reported[17]. Citronellyl isobutyrate is an ester of propanoic acid which is widely used as flavoring agent and is known to possess insect repellent and antimicrobial properties[18]–[20].
Our systematic investigation reveals the potential of E. serratus leaves as a good source of bioactive compounds such as fatty acid esters, alcohols, hydrocarbons, aldehydes, alkenes, fatty acids and amides that justify the use of this plant for its various ailments by traditional practitioners. Further research interest in the study of these active bio compounds may yield nature friendly strong antioxidant, anti-microbial, anti-inflammatory agents and analgesic agents.
Acknowledgments
The authors are grateful to the South India Textile Research Association (SITRA), Coimbatore (Tamil Nadu), India for GC-MS analysis.
Comments
Background
The plant E. serratus, belonging to the family Elaeocarpaceae, is used as diuretic and as a cardiovascular stimulant. The leaves are used in the treatment of rheumatism and as antidote to poison, while the fruits are locally prescribed for the treatment of diarrhea and dysentery.
Research frontiers
The aim of this study is to identify the phytochemical constituents with the aid of GC-MS technique.
Related reports
Review of literature divulges that information on the GC-MS analysis of E. serratus belonging to the family Elaeocarpaceae is totally lacking. The other parameters of this plant has been done by other researchers.
Applications
This plant is used worldwide as a medicine. According to the paper, this work will help to identify the compounds of therapeutic value.
Peer review
This is an interesting study in which the authors identified the phytochemical constituents with the aid of GC-MS technique. The work is useful for the researchers to do the further research on the plant.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Wink D, Vodovotz Y, Grisham M, DeGraff W, Cook J, Pacelli R, et al. Antioxidant effects of nitric oxide. Methods Enzymol. 1999;301:413–424. doi: 10.1016/s0076-6879(99)01105-2. [DOI] [PubMed] [Google Scholar]
- 2.Janakiraman N, Johnson M, Sahaya SS. GC-MS analysis of bioactive constituents of Peristrophe bicalyculata (Retz.) Nees (Acanthaceae) Asian Pac J Trop Biomed. 2012;2(Suppl 1):S46–S49. [Google Scholar]
- 3.Evans WC. Trease and Evans pharmacognosy. 14th ed. London: W.B. Saunders Company Ltd; 2000. pp. 19–20. [Google Scholar]
- 4.Tagboto S, Townson S. Antiparasitic properties of medicinal plants and other naturally occurring products. Adv Parasitol. 2001;50:199–295. doi: 10.1016/s0065-308x(01)50032-9. [DOI] [PubMed] [Google Scholar]
- 5.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the Newman period 1981–2002. J Nat Prod. 2003;66(7):1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 6.Kaushik S, Sharma P, Jain A, Sikarwar SM. Preliminary phytochemical screening and HPTLC fingerprinting of Nicotiana tabacum leaf. J Pharm Res. 2010;3:1144–1145. [Google Scholar]
- 7.Subramanian S, Ramakrishnan N. Chromatographic fingerprint analysis of Naringi crenulata by HPTLC technique. Asian Pac J Trop Biomed. 2011;1(Suppl 2):S195–S198. [Google Scholar]
- 8.Muthulakshmi A, Joshibhi-Margret R, Mohan VR. GC-MS analysis of bioactive components of Feronia elephantum Correa (Rutaceae) J Appl Pharm Sci. 2012;2:69–74. [Google Scholar]
- 9.Yamunadevi M, Wesely EG, Johnson M. Chromatographic finger print analysis of steroids in Aerva lanasa L. by HPTLC technique. Asian Pac J Trop Biomed. 2011;1(6):428–433. doi: 10.1016/S2221-1691(11)60094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukherjee PK. Quality control of herbal drugs. An approaches to evaluation of botanicals. 1st ed. New Delhi: Business Horizons; 2002. pp. 390–403. [Google Scholar]
- 11.Mc Lafferly FW. Registry of mass spectral data. 5 ed. New York: John Wiley & Sons Inc; 1989. [Google Scholar]
- 12.Stein SE. Gaithersburg, USA: 1990. National Institute of Standards and Technology (NIST) Mass Spectral Database and Software. Version 3.02. [Google Scholar]
- 13.Kumar V, Bhatnagar AK, Srivastava JN. Antibacterial activity of crude extracts of Spirulina platensis and its structural elucidation of bioactive compound. J Med Plants Res. 2011;5(32):7043–7048. [Google Scholar]
- 14.Gopalakrishnan S, Vadivel E. GC-MS Analysis of some bioactive constituents of Mussaenda frondosa Linn. Int J Pharm Bio Sci. 2011;2(1):313–320. [Google Scholar]
- 15.Joo JH, Jetten AM. Molecular mechanisms involved in farnesol-induced apoptosis. Cancer Lett. 2009;287(2):123–135. doi: 10.1016/j.canlet.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kromidas L, Perrier E, Flanagan J, Rivero R, Bonnet I. Release of antimicrobial actives from microcapsules by the action of axillary bacteria. Int J Cosmet Sci. 2006;28(2):103–108. doi: 10.1111/j.1467-2494.2006.00283.x. [DOI] [PubMed] [Google Scholar]
- 17.Ramage G, Saville SP, Wickes BL, López-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002;68(11):5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt E, Jirovetz L, Buchbauer G, Denkova Z, Stoyanova A, Murgov I, et al. Antimicrobial testings and gas chromatographic analyses of aroma chemicals. J Essent Oil Bear Plants. 2005;8(1):99–106. [Google Scholar]
- 19.Jirovetz L, Eller G, Buchbauer G, Schmidt E, Denkova Z, Stoyanova A, et al. Chemical composition, antimicrobial activities and odor descriptions of some essential oils with characteristic floral-rosy scent and of their principal aroma compounds. Recent Res Dev Agronomy & Horticulture. 2006;2:1–12. [Google Scholar]
- 20.Azeez S. UK: CAB International; 2008. Chemistry of spices; pp. 287–311. [Online] Available from: http://bookshop.cabi.org/Uploads/Books/PDF/9781845934057/9781845934057.pdf. [Accessed on 17th September, 2013] [Google Scholar]


