Abstract
Objective
To investigate phytochemical screening, antimicrobial activity and qualitative thin layer chromatographic separation of flavonoid components, antioxidant activity and total flavonoid compound of Terminalia arjuna.
Methods
For phytochemical screening, some common and available standard tests were done. Antimicrobial bioassay was done through agar well diffusion method. Detection of antioxidant activity and flavonoid compounds were done through thin layer chromatography. Total antioxidant activity was measured by 2, 2-diphenyl-1-picrylhydrazyl (DPPH) in colorimetric method. Aluminum chloride colorimetric method was used for total flavonoid determination.
Results
Phytochemical screening showed the active compounds presence in high concentration, such as phytosterol, lactones, flavonoids, phenolic compounds and tannins and glycosides. The antimicrobial activity of extract showed that greater inhibition zone against Gram negative bacteria than Gram positive bacteria. This methanolic extract showed a promising antioxidant activity, as absorption of DPPH redicles decreased in DPPH free radical scavenging assay. Flavonoids components having antioxidant property present in the methanol extract at a level of 199.00 mg quercetin equivalent/g of dried methanol extract in colorimetric method.
Conclusions
The Terminalia arjuna bark extract revealed the presence of bio-active constituents which are known to exhibit medicinal as well as physiological activities.
Keywords: Terminalia arjuna, Phytocompound, TLC, Antioxidant, Total flavonoid, Antimicrobial activity, DPPH
1. Introduction
In the ancient India, medicinal plants were used to prevent various critical diseases. The plant kingdom is an important source of herbal drugs. Even in recent years, there has been an increasing awareness about the importance of medicinal plants. Generally, herbal drugs are easily available, safe, less expensive, efficient, and rarely have side effects. According to World Health Organization, medicinal plants would be the best source to obtain variety of drugs[1]. Medicinal plants contain some organic compounds which provide definite physiological action on the human body and these bioactive substances include tannins, alkaloids, carbohydrates, terpenoids, steroids, flavonoids and phenols.
The bio-active phytocompounds are synthesized by primary or rather secondary metabolism of living organisms. Secondary metabolites are chemically and taxonomically extremely diverse compounds with obscure function. They are widely used in the human therapy, veterinary, agriculture, scientific research and countless other areas[2]. Medicinal plants containing active chemical constituents with high antioxidant property play an important role in the prevention of various degenerative diseases[3] and have possible benefits to the humanity. A large number of phytochemicals belonging to several chemical classes have been shown to have inhibitory effects on all types of microorganisms in vitro. Botanical medicines or phytomedicines refer to the use of seeds, berries, leaves, bark, root or flowers of any plant for medicinal purposes by significant number of people. Knowledge of the chemical constituents of plants is desirable because such information will be value for synthesis of complex chemical substances[4]–[6].
Terminalia arjuna (T. arjuna) is a deciduous large-sized fluted tree to 30 m tall and 2-2.5 m diameter at breast height, with an often buttressed trunk. T. arjuna (family Combretaceae), a large tree, is found throughout the South Asian region. This tree is usually an evergreen tree with new leaves appearing in the hot season (February to April) before leaf fall. This tree is an exotic tree in India. It is one of the most versatile medicinal plants having a wide spectrum of biological activity. The bark of T. arjuna is anti-dysentric, antipyretic, astringent, cardiotonic, lithotriptic, anticoagulant, hypolipidemi, antimicrobial[7] and antiuremic[8] agent. Many useful phytoconstituents have been isolated from T. arjuna which included triterpenoids for cardiovascular properties, tannins and flavonoids for its anticancer, antimicrobial properties and so on[9]. The powder of the bark acts as a diuretic in cirrhosis of liver and gives relief in symptomatic hypertension[10]. In studies in mice, its leaves have been shown to have analgesic and anti-inflammatory properties[11]. The purpose of the study was to find out the preliminary phytochemical screening of the extract and to determine the antioxidant and antimicrobial activity of extract of the bark of T. arjuna.
2. Methods and materials
2.1. Plant material collection
The bark of T. arjuna was collected from University Road, Vidyasagar University, Midnapore, Paschim Medinipur, district of West Bengal. The material was identified by the taxonomist of the Botany Department at the Raja N. L. Khan Women's College, Midnapore. The voucher specimens were deposited in the Department of Botany, Raja N. L. Khan Women's College.
2.2. Bacterial strain and culture conditions
Two Gram negative and two Gram positive indicator bacteria used for antimicrobial assay respectively, Escherichia coli (E. coli) (MTCC 443), Klebsiella pneumoniae (K. pneumoniae)(MTCC 109), Staphylococcus aureus (S. aureus) (MTCC 3160) and Streptococcus mutans (S. mutans) (MTCC 890) were provided by microbiological laboratory and clinical detection center Midnapur (West Medinipur, India). They were cultured in tryptone soy broth or agar (TSB or TSA) in aerobic condition at 37 °C.
2.3. Preparation of methanolic extract of bark of T. arjuna
The collected T. arjuna barks were cut into small pieces. The plant parts were dried in an incubator for 7 d at 40 °C, crushed in an electrical grinder and then the powder was separated. A total of 100 g of bark powder of said plant material was washed in 400 mL of petroleum ether for 24 h to remove the greasy pigmented non polar materials. Then the petroleum ether was discarded and residue was dissolved in 500 mL diethyl ether for 2 h in a soxhlet apparatus. The extract was filtered through Whatman No. 1 filter paper and the resulting filtrate was dried in the air. The ether solid extract was dissolved in 300 mL acetone for 1 h in a soxhlet apparatus. Then the extract was filtered through Whatman No. 1 filter paper and the resulting filtrate was dried under reduced pressure at 40 °C on a rotary evaporator. The acetone solid extract was dissolved in 200 mL methanol and was dried in the air. The methanol extract was stored in refrigerator for phytochemical screening, antioxidant and antimicrobial activity study. Percent of yield[12] was calculated as follows:
Extract yield %= (W1/W2)×100
Where, W1 is net weight of powder in grams after extraction and W2 is total weight of wood powder in grams taken for extraction.
2.4. Phytochemicals analysis
Phytochemical analysis of the test sample was carried out according to standard methods[13]–[15].
2.4.1. Salkowski reaction test for phytosterols
To 0.5 mL chloroform extract in a test tube add 1 mL of concentrated (conc.) H2SO4 from the sides of the test tube. Appearance of reddish brown colour in chloroform layer indicates presence of phytosterols.
2.4.2. Liebermann-Burchard's test for triterpenoids
Extract was treated with few drops of acetic anhydride, boil and cool. Conc. sulfuric acid was added from the sides of the test tube which showed a brown ring at the junction of two layers, and formation of deep red color indicated the presence of triterpenoids.
2.4.3. Foam test for saponins
Small amount of extract was taken in a test tube with little quantity of water and shake vigorously. Appearance of foam persisting for 10 min indicated presence of saponins.
2.4.4. Dragendroff's test for alkaloids
Various extracts of the herbal drug were dissolved in chloroform. Chloroform was evaporated and the residue was acidified by adding few drops of Dragendroff's reagent (Potassium bismuth iodide). Appearance of orange red precipitate indicated presence of alkaloids.
2.4.5. Molisch's test for carbohydrates
The extract was mixed with Molisch reagent, and then added conc. H2SO4 along the sides of the test tube to form layers. Appearance of reddish violet ring the interference indicated the presence of carbohydrates.
2.4.6. Lead acetate test for flavanoids
To the alcoholic solution of the extract add few drops of 10% lead acetate solution. Appearance of yellow precipitate indicated presence of flavonoids.
2.4.7. Legal's test for lactones
To the extract mixtures add sodium nitroprusside and pyridine. Then the mixture was treated with NaOH. Appearance of deep red colour indicated the presence of lactones.
2.4.8. Ferric chloride test for phenolic compounds and tannins
Take 2 mL of extract in a test tube and add ferric chloride solution drop by drop. Appearance of bluish black precipitate indicated presence of phenolic compounds and tannins.
2.4.9. Ninhydrin test for proteins
Few drops of ninhydrin added to the extract. Appearance of blue colour indicated presence of amino acid where as proteins may rarely give positive result.
2.4.10. Keller-Killiani test for glycosides
A total of 1 mL of glacial acetic acid, few drops of ferric chloride solution and conc. H2SO4 (Slowly through the sides of the test tube) were add to the extract. Appearance of reddish brown ring at the junction of the liquids indicated the presence of de-oxysugars.
2.5. Thin layer chromatography (TLC) analysis for antioxidant constituents
About 2 µg of extracts of T. arjuna was loaded on TLC plates (Merck, 20 cm×20 cm). The plates were developed in methanol: chloroform: hexane (7:2:1, v/v/v) to separate various constituents of the extracts. The developed plates were air dried. Then the antioxidant constituents were analyzed by DPPH technique[16],[17]. For this 0.05% of DPPH solution in methanol was sprayed on the surface of developed TLC plates and incubated for 10 min at room temperature. The active antioxidant constituents of the T. arjuna extract was detected as yellow spots produced via reduction of DPPH by resolved bands against purple back ground on the TLC plates. Ascorbic acid was used as standard antioxidant[18].
2.6. TLC analysis for flavonoid constituents
About 2 µg of extracts of T. arjuna was loaded on TLC plates (Merck, 20 cm×20 cm). The plates were developed in toluene: chloroform: methanol (4:4:1, v/v/v) to separate flavonoid compounds of the extracts. The developed plate was air dried. Then anisaldehyde sulfuric acid was sprayed on the surface of the plate and incubated for 20 min at 100 °C. The present flavonoid compound of this extracts was detected as blue spot on developed TLC plate. The Rf value of the bands were also determined.
2.7. Antioxidant activity determination by DPPH free radical scavenging assay
DPPH radical scavenging activity of the extract was measured by the method described by Barros et al[19]. For this, different concentrations of extract and ascorbic acid (standard) were prepared with methanol (Sigma-Aldrich) as the test solutions. About 1 mL of each prepared concentrations were placed into test tubes and 0.5 mL of 1 mmol/L DPPH solution in methanol was added. The test tubes were incubated for 15 min and the absorbance was read at 517 nm. A blank solution consisted of DPPH dissolved in same amount of methanol. The DPPH radical scavenging activity percentage was calculated by using the following formula:
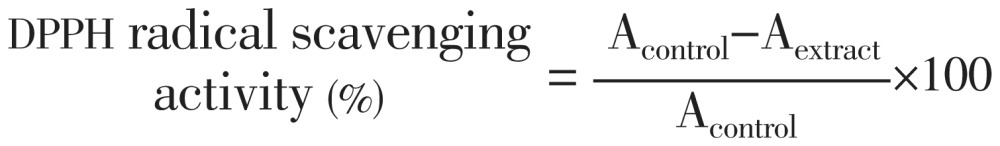 |
Where Acontrol is the absorbance of a DPPH solution without extract; Aextract is the absorbance of the tested extract. All measurements were performed in triplicate.
2.8. Determination of total flavonoid content
Aluminum chloride colorimetric method was used for flavonoids determination[20]. About 1 mL of the plant extracts/standard of different concentration solution was mixed with 3 mL of methanol, 0.2 mL of aluminum chloride, 0.2 mL of 1 mol/L potassium acetate and 5.6 mL of distilled water. It remained at room temperature for 30 min. The absorbance of the reaction mixture was measured at 415 nm with spectrophotometer against blank. Methanol served as blank. The total content of flavonoid compounds in plant methanol extracts in quercetin equivalents was calculated by the following equation:
C=(c×V)/m
Where C is total content of flavonoid compounds, mg/g plant extract, in quercetin equivalent; c is the concentration of quercetin established from the calibration curve in mg/mL, V is the volume of extract in mL, and m is the weight of crude plant extract in g.
2.9. Antimicrobial analysis
The antimicrobial activity was determined in the methanolic T. arjuna bark extract using agar well diffusion method. The antibacterial activities of T. arjuna bark extract (concentration of compound 50%, 100 %) were tested against two Gram-positive S. aureus, S. mutans and two Gram-negative E. coli and K. pneumoniae, human pathogenic bacteria. Zone of inhibition of T. arjuna bark extract were compared with standards like chloramphenicol for antibacterial activity. The results showed that the remarkable inhibition of the bacterial growth was against the tested organisms[9].
3. Result
3.1. Percent of yield determination
The obtained yield (%) of the T. arjuna bark methanolic extract was 0.3%.
3.2. Preliminary phytochemical screening
The preliminary phytochemical analysis in T. arjuna bark methanolic extract showed the active compounds presence in high concentration, such as phytosterol, lactones, flavonoids, phenolic compounds and tannins and glycosides. Also, the active compounds presence in low concentration, such as triterpenoids, saponins, alkaloids, carbohydrates and proteins are shown in Table 1.
Table 1. Preliminary phytochemical analysis of T. arjuna bark extract.
| Phytoconstituents | Tests | Conclusion |
| Phytosterols | Salkowski reaction | ++ |
| Triterpenoids | Liebermann-Burchard's test | + |
| Saponins | Foam test | + |
| Alkaloids | Dragendroff's test | + |
| Carbohydrates | Molisch's test | + |
| Flavanoids | Lead Acetate test | ++ |
| Lactones | Legal's test | ++ |
| Phenolic Compounds and Tannins | 5% Fecl3 Test | ++ |
| Proteins | Ninhydrin test | + |
| Glycosides | Keller-Killiani test | ++ |
+: Present in low concentration; ++: Present in high concentration.
3.3. TLC analysis for antioxidant constituents
The plates TLC were developed in methanol: chloroform: hexane (7:2:1, v/v/v) and sprayed with 0.05% DPPH reagent. Purple colour of DPPH reagent was bleached by yellow spots which was the indication of positive antioxidant activity. The bark extract of T. arjuna in terms of DPPH free radical scavenging activity showed one resolved TLC band with strong antioxidant activity and another spot with weak antioxidant activity as compared to standard antioxidant ascorbic acid (Figure 1).
Figure 1. TLC antioxidant activity analysis of T. arjuna constituents.

Standard: Ascorbic acid; TA: T. arjuna.
3.4. TLC analysis for flavonoid constituents
The plates TLC were developed in chloroform: toluene: methanol (4:4:1) and sprayed with anisaldehyde sulfuric acid reagent. It gives three flavonoid constituents in the TA bark extract with Rf value of 0.25, 0.32, and 0.38. The eluted compounds showed blue color corresponding with flavonoid behavior (Figure 2 and Table 2).
Figure 2. TLC analysis for flavonoid compounds in T. arjuna bark methanolic extract.
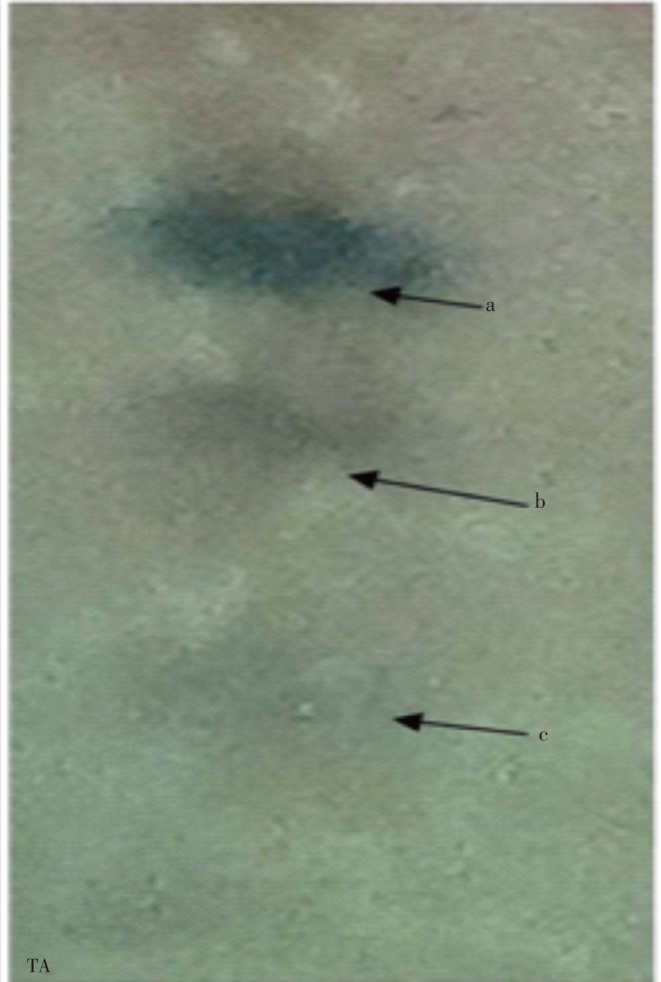
Table 2. Detection of flavonoids through TLC.
| Extract | Solvent system | Revealing reagent | No. of Spot | Rf value |
| Methanol | Chloroform: Toluene: Methanol (4:4:1,v/v/v) | Anisaldehyde sulfuric acid | 3 | a. 0.25 |
| b. 0.32 | ||||
| c. 0.38 |
3.5. Determination of total flavonoid content
The total flavonoid content of the T. arjuna bark was estimated by using aluminium chloride colorimetric technique and found to be 199.00 mg quercetin equivalent/g of dried methanol extract.
3.6. Antimicrobial activity
Antimicrobial activity of T. arjuna bark extract showed greater result against gram negative bacteria than Gram positive bacteria (Figure 3 and Table 3).
Figure 3. Inhibition zone against E. coli (Indicator microbes of Gram negative bacteria).
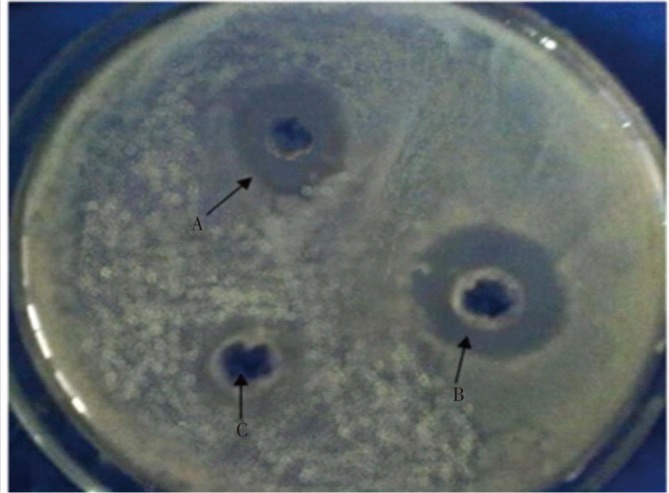
A: Chloramphenicol (Std), B: TA methanolic extract, C: Control (methanol).
Table 3. Antimicrobial activities of T. arjuna bark methanolic extract and zone of inhibition.
| Extract | Diameter of zone of inhibition (mm)* against |
|||||||
|
S. aureus |
S. mutans |
K. pneumoniae |
E. coli |
|||||
| 100% | 50% | 100% | 50% | 100% | 50% | 100% | 50% | |
| Methanolic | 11 | 5.2 | 16 | 5.8 | 21 | 4.5 | 32 | 20 |
| Chloramphenical (std) | 18 | 10 | 20 | 12 | 28 | 15 | 45 | 30 |
*The zone of inhibition (mm) taken as average. Std: Standard Test Dose.
3.7. Antioxidant activity determination by DPPH free radical scavenging assay
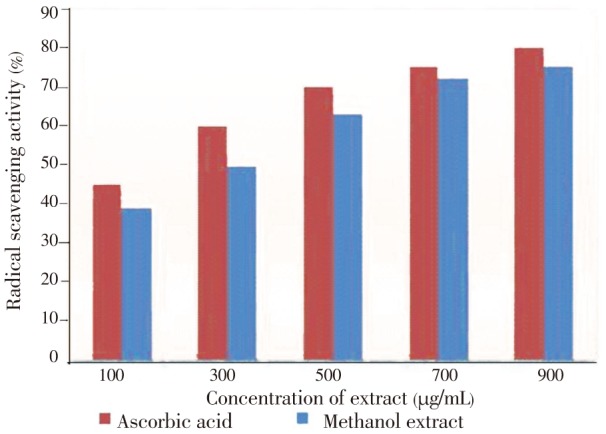
DPPH is a free radical and it gives strong absorption band at 517 nm in the visible region of electromagnetic radiation. As antioxidant compounds donate protons to these radicals, the absorption decreased. The decrease in absorption was taken as a measure of the extent of radical scavenging activity. The results were compared with that of ascorbic acid and the methanolic extract of T. arjuna (Figure 4).
Figure 4. DPPH radical scavenging activity of methanolic extract of T. arjuna bark.
4. Discussion
Medicinal plants were of great importance to the health of individuals and communities[21]. Phytochemical analysis conducted on the plant extracts revealed the presence of constituents which are known to exhibit medicinal as well as physiological activities[22]. Analysis of the plant extracts revealed the presence of phytochemicals, such as proteins, charbohydrates, phenols, tannins, flavonoids, saponins, glycosides, steroids, terpenoids and alkaloids. Several studies have described the antioxidant properties of different parts of various medicinal plants which are rich in phenolic compounds[23],[24]. T. arjuna is a widespread medicinal plant used in the pharmacological system of medicine to care for various degenerative diseases[9]. In this present study, preliminary phytochemical analysis revealed a large amount of phytosterol, lactones, flavonoids, phenolic compounds and tannins and glycosides present in methanol extract of T. arjuna bark. Natural antioxidants mainly come from plants in the form of phenolic compounds, such as flavonoids, phenolic acids, tocopherols etc[25]. The antioxidative properties of flavonoids are due to several different mechanisms, such as scavenging of free radicals, chelation of metal ions, such as iron and copper and inhibition of enzymes responsible for free radical generation[26]. This methanolic extract has great free radical scavenging property and also contains liberal amount of flavonoid components. Flavonoids are hydroxylated phenolic substances known to be synthesized by plants in response to microbial infection and they have been found to be antimicrobial substances against wide array of microorganisms in vitro. Their activity is probably due to their ability to complex with extracellular and soluble proteins and to complex with bacterial cell wall[27]. More than 2 000 flavonoids have been reported among woody and non-woody plants[28]. Activity of methanolic extract of T. arjuna was comparable to that of reference standard drug chloramphenicol disc. T. arjuna bark extract exhibited good antimicrobial activity. The maximum inhibition zone of methanolic extract shows against two gram negative bacteria. Thus, the methanolic extract of T. arjuna has great antioxidant and antimicrobial activities.
It has been shown that T. arjuna bark consists of many useful compounds, such as flavonoids, tannins, phenols, phytosterols, saponins and alkaloids. Its antioxidant activity is largely due to flavonoids. The antioxidant and anti-microbial properties of T. arjuna are responsible on presence of large amount of flavonoid components. So the results further supported the view that the bark of T. arjuna is promising source of natural useful therapeutic agents. The traditional medicine practice is recommended strongly for this plant as well as it is suggested that further work should be carried out to isolate, purify, and characterize the active constituents responsible for the bioactivity study.
Acknowledgments
We are thankful to Raja N. L. Khan Women's College, Paschim Medinipur for the grand of UGC Major Project [F. No. 41-68/2012(SR)] and Dr. Keshab Chandra Mandal, Head of Department Microbiology, Vidyasagar University, Paschim Medinipur, West Bengal, India for providing facilities for this research work.
Comments
Background
Medicinal plants were of great importance to the health of individuals and communities. Medicinal plants would be the best source to obtained variety of drugs. Medicinal plants contain some organic compounds which provide definite physiological action on the human body and these bioactive substances include tannins, alkaloids, carbohydrates, terpenoids, steroids, flavonoids and phenols.
Research frontiers
The present work shows the antioxidant and antimicrobial activity measures of T. arjuna of bark in methanolic extract and also analysis of phytochemical profile.
Related reports
Some lifestyle diseases such as cancer, diabetes, cardiovascular diseases, kidney disease are very common nowaday. T. arjuna has bio-effective phytocompounds which are responsible to cure in those diseases.
Innovations and breakthroughs
T. arjuna is an exotic tree in India. It is one of the most versatile medicinal plants having a wide spectrum of biological activity. In this present study, authors have demonstrated antioxidant, antimicrobial study along with total flavonoid compound measure respectively.
Applications
The purpose of the study was to find out the preliminary phytochemical screening of the extract and to determine the antioxidant and antimicrobial activity of extract of the bark of T. arjuna and in future it may be an agent for treating oxidative stress related disease along with microbial infection.
Peer review
Aim of the study is to investigate phytochemical screening, antimicrobial activity, and qualitative thin layer chromatographic separation of flavonoid components, antioxidant activity and total flavonoid compound of T. arjuna. The T. arjuna bark extract revealed the presence of bio-active constituents which are known to exhibit medicinal as well as physiological activities.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Yadav RN, Agrawala M. Phytochemical analysis of some medicinal plants. J Physiol. 2011;3(12):10–14. [Google Scholar]
- 2.Vasu K, Goud JV, Suryam A, Singara Chary MA. Biomolecular and phytochemical analyses of three aquatic angiosperms. Afr J Microbiol Res. 2009;3(8):418–421. [Google Scholar]
- 3.Lukmanul H, Girija A, Boopathy R. Antioxidant property of selected Ocimum species and their secondary metabolite content. J Med Plants Res. 2008;2(9):250–257. [Google Scholar]
- 4.Mojab F, Kamalinejad M, Ghaderi N, Vanidipour HR. Phytochemicals screening of some species of Iranian plants. Iran J Pharm Res. 2003;3:77–82. [Google Scholar]
- 5.Parekh J, Chanda S. Antibacterial and phytochemical studies on twelve species of Indian medicinal plants. Afr J Biomed Res. 2007;10:175–181. [Google Scholar]
- 6.Parekh J, Chanda S. Phytochemicals screening of some plants from western region of India. Plant Arch. 2008;8:657–662. [Google Scholar]
- 7.Mandal A, Das K, Nandi D K. In vitro bioactivity study of bark extract of Terminalia arjuna on probiotics, commercially available probiotic formulation. Int J Phytopharmacol. 2010;1(2):109–113. [Google Scholar]
- 8.Das K, Chakraborty PP, Ghosh D, Nandi DK. Protective effect of aqueous extract of Terminalia arjuna against dehydrating induced oxidative stress and uremia in male rat. Iran J Pharma Res. 2010;9(2):153–161. [PMC free article] [PubMed] [Google Scholar]
- 9.Nema R, jain P, Khare S, Pradhan A, Gupta A, Singh D. Antibacterial and antifungal activity of Terminalia arjuna leaves extract with special reference to flavanoids. Basic Res J Med Clin Sci. 2012;1(5):63–65. [Google Scholar]
- 10.Chatterjee AS. The treatise on indian medicinal plants: Council of scientific and industrial research. New Delhi: Publication and Information Directorate; 1994. [Google Scholar]
- 11.Biswas M, Biswas K, Karan TK, Bhattacharya S, Ghosh AK, Haldar PK. Evaluation of analgesic and anti-inflammatory activities of Terminalia arjuna leaf. J Phytol. 2011;3(1):33–38. [Google Scholar]
- 12.Patil UH, Gaikwad DK. Phytochemical evaluation and bactericidal potential of Terminalia arjuna stem bark. Int J Pharm Sci Res. 2010;2(3):614–619. [Google Scholar]
- 13.Harbone JB. Phytochemical methods. London: Chapman and Hall; 1998. pp. 117–119. [Google Scholar]
- 14.Fransworth NR. Biological and phytochemical screening of plants. J Pharm Sci. 1996;55:225–227. doi: 10.1002/jps.2600550302. [DOI] [PubMed] [Google Scholar]
- 15.Rangari VD. Pharmacognosy and phytochemistry. Nasik: Carrier Publication; 2002. p. p.132. [Google Scholar]
- 16.Kannan R, Arumugam R, Meenakshi S. Thin layer chromatography analysis of antioxidant constituents from seagrasses of Gulf of Mannar Biosphere Reserve, South India. Int J ChemTech Res. 2010;2:1526–1530. [Google Scholar]
- 17.Raj RS, Radhamany PM. Preliminary phytochemical and in vitro antioxidant properties of Brunfelsia americana L. J Pharm Res. 2010;3:2712–2713. [Google Scholar]
- 18.Dzomba P, Mupa M. Wild Cucumis leaves: phytochemical profile and antioxidant capacity. Asian Pac J Trop Biomed. 2013 forthcoming. [Google Scholar]
- 19.Barros L, Falcao S, Baptista P, Freire C, Vilas-Boas M, Ferreira IC. Antioxidant activity of Agaricus sp. mushrooms by chemical, biochemical and electrochemical assays. Food Chem. 2008;111:61–66. [Google Scholar]
- 20.Wang SY, Jiao H. Correlation of antioxidant capacities to oxygen radical scavenging enzyme activities in blackberry. J Agric Food Chem. 2000;48(11):5672–5676. doi: 10.1021/jf000765q. [DOI] [PubMed] [Google Scholar]
- 21.Pascaline J, Charles M, Lukhoba C, George O. Phytochemical constituents of some medicinal plants used by the Nandis of South Nandi district, Kenya. J Anim Plant Sci. 2011;9:1201–1210. [Google Scholar]
- 22.Sofowora A. New York: John Wiley and Sons; 1993. Medicinal plants and traditional medicine in Africa; pp. 191–289. [Google Scholar]
- 23.Brown JE, Rice-Evans CA. Luteolin rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic Res. 1998;29:247–255. doi: 10.1080/10715769800300281. [DOI] [PubMed] [Google Scholar]
- 24.Krings U, Berger RG. Antioxidant activity of roasted foods. Food Chem. 2001;72:223–229. [Google Scholar]
- 25.Ali SS, Kasoju N, Luthra A, Singh A, Sharanabasava H, Sahuand A, et al. Indian medicinal herbs as source of antioxidants. Food Res Int. 2008;41:1–15. [Google Scholar]
- 26.Benavente-Garcia O, Castillo J, Marin FR, Ortuno A, Del-Rio JA. Uses and properties of Citrus flavonoids. J Agric Food Chem. 1997;45(12):4505–4515. [Google Scholar]
- 27.Marjorie C. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harborne JB. Plant phenolics. In: Bell EA, Charlewood BV, editors. Secondary plant products. Berlin: Verlag Springer; 1980. p. p. 320. [Google Scholar]


