Abstract
Objective
To determine the bionomics and susceptibility status of the malarial vector Anopheles superpictus (An. superpictus) to different insecticides in the Sistan-Baluchestan province which has the highest malarial prevalence in Iran.
Methods
Different sampling methods, in addition to scoring abdominal conditions, were used to define the seasonal activity and endo/exophilic behavior of this species. In addition, the standard WHO susceptibility tests were applied on adult field strains.
Results
Most adult mosquitoes were collected from outdoor shelters. The peak of seasonal activity of An. superpictus occurred at the end of autumn. Most larvae were collected from natural and permanent breeding places with full sunlight and no vegetation. Blood feeding activities occurred around midnight. Compared with the abdominal conditions of adult mosquitoes collected indoors, the abdominal conditions of adult mosquitoes collected outdoors were gravid and semigravid. This species was suspected to be resistant to DDT, but was susceptible to other insecticides.
Conclusions
An. superpictus was present in almost all outdoor shelters, and the ratios of gravid, semigravid/unfed, and freshly fed confirmed that this species had a higher tendency to rest outdoors than indoors. This behavior can protect An. superpictus from indoor residual spraying in this malarious area. To the best of our knowledge, this is the first report on the susceptibility status of An. superpictus in Southeastern Iran. We do not suggest the use of DDT for indoor residual spraying in southeast Iran.
Keywords: Anopheles superpictus, Malaria, Iran
1. Introduction
To date, malaria is a major endemic infectious disease in southern and southeastern foci of Iran, including the provinces of Sistan-Baluchestan (Sistan va Baluchestan), Hormozgan, and Kerman[1]. Sistan-Baluchestan province, with an unstable malaria pattern[2], has reported to account 42%-60% of the total malaria cases in the country[3], with two seasonal peaks, mostly in the spring and autumn[2]. Indoor residual spraying and long-lasting insecticide-treated mosquito nets have been applied as mosquito control strategies in the national malaria elimination program in this part of the country[4],[5]. Six anopheline species have been identified as malarial vectors in this area: Anopheles culicifacies, Anopheles stephensi, Anopheles dthali, Anopheles fluviatilis, Anopheles pulcherrimus, and Anopheles superpictus (An. superpictus)[2].
An. superpictus Grassi, 1899 (Diptera: Culicidae), has been recognized as the human malarial vector in the southern Palearctic region, central and southern Europe[6]–[12], middle eastern countries, India, northern Africa, and Russia as well as new republic countries separated from the old Soviet Union[13],[14]. Moreover, it is the main malarial vector widely found in Iran[14],[15]–[18], and previous studies have indicated its both exophagic and endophilic behavior[19]. Although some studies in western Iran have shown that An. superpictus was susceptible to DDT, malathion, and lambda-cyhalothrin[20], the susceptibility status of this species in Southeastern Iran has not been reported.
Therefore, this study was performed to determine the ecology and susceptibility of An. superpictus to the WHO-recommended insecticides for achieving the appropriate malarial control in southeast Iran.
2. Material and methods
2.1. Study area
The investigation was conducted in the Sarbaz district of the Sistan-Baluchestan province, where malaria is still endemic (Figure 1)[2],[21],[22].
Figure 1. Map of Sistan-Baluchestan province, location of Sarbaz district, Southeastern Iran[23].
Sarbaz is geographically located in the southeast of the Sistan-Baluchestan province near the Pakistan border and covers an area of 11 500 km2 between 60°45′-63°20′ E longitude and 26°-27°N latitude. Its total population was approximately 144 442 in 2012[22]. Sarbaz district has one of the highest malarial incidences in the province, which are affected by two major factors; foreign disease reservoirs (several patients identified in this district were Afghan refugees and Pakistani immigrants)[3],[22], and subtropical climate (suitable for vector activity and seasonal malaria transmission)[2].
Fieldwork was conducted in the Pishin region that has been a major malarious zone in the Sarbaz district in recent years. This area is located 20 km away from the Pakistan border and has been reported to have many imported malaria cases. Pishin is a plateau, with most of the land being used for agricultural purposes. Irrigation of farms is through water from rivers, deep wells, cement pools, and the Pishin dam; all these water bodies provide suitable mosquito breeding sites (Sarbaz Health Center, unpublished data, 2012). Three villages (Lad, Laksar, and Soudan) were randomly selected for the monthly entomological survey based on the occurrence of malaria transmission and the presence of An. Superpictus, as reported previously.
2.2. Entomological survey
Entomological evaluations were performed monthly from January to December 2012 (over a 12-month period), according to the methods suggested by the World Health Organization (WHO)[23]. Adult mosquitoes were collected by indoor pyrethrum space-pray collections (spray sheet collection or total catch), night biting catch on human and cow, inlet and outlet window trap, and shelter pit trap. Mosquitoes were caught early in the morning in eight fixed shelters: four human dwellings and four sheep or cattle sheds in each village. Hand catching (manual aspirators) was performed in outdoor resting places: three pit shelters and one well. The number of female mosquitoes per dwelling/shelter was evaluated as the adult density[2]. In addition, the shelters details, including temperature, humidity, date, and time of collection were recorded on related forms.
The collected female mosquitoes were classified based on abdominal conditions in each sampling technique, according to the WHO procedure[23]. This process was performed to evaluate the endo/exophilic behavior. Gravid (G) and/or semigravid (SG) condition of the female abdomen were determine as resting stages, and females with unfed (U) and freshly fed (F) guts were demonstrative of seeking stages. The ratio G+CG/U+F was used to determine the tendency to rest in or outdoors[24].
The dipping method was used for larvae collection from water sources as breeding places. Density of larvae was calculated using the mean number of larvae per 10 dippers. Next, the larvae were preserved in lactophenol and mounted on permanent microscope slides using de Faure's medium[18]. Adult mosquitoes as well as the third and fourth instar larvae were identified using morphological characters as standard keys[25]. In addition, physical breeding site characteristics, including habitat temperature, depth, type (permanent or temporary, natural or artificial), water situation (stagnant or running, clear or turbid), vegetation (with or without vegetation), sunlight situation (full or partial sunlight or shade), and substrate type (mud, sand, rock, or cement) were visually recorded on related forms or by using handheld field equipment.
2.3. Adult susceptibility tests
Susceptibility tests were performed according to the WHO standard methods[26]. The insecticide impregnated papers provided by WHO were used as follows: DDT 4%, malathion 5%, propoxur 0.1%, lambdacyhalothrin 0.05%, permethrin 0.75%, and deltamethrin 0.05%.
An. superpictus larvae were collected from different breeding places. They were transferred to an insectarium with 25°C-29°C temperature and 65%-80% relative humidity. The emerging adults (2 to 3-day old and sugar fed) were tested. Female mosquitoes were exposed to insecticides for 1 h (exposure time). At each exposure time, 20-25 adults were tested. Their mortality rate was recorded after a 24-h recovery period using cotton pads soaked with a 10%-glucose solution. Insecticide exposure as well as recovery period occurred in the insectarium[1],[2].
The results of study were considered acceptable if mortality in the control group was less than 5% and rejected if the mortality in the control group was more than 20%; mortality rate between 5%-20% was corrected using Abbott's formula[2].
3. Results
3.1. Entomological survey
Table 1 shows the relative densities of An. superpictus female mosquitoes collected using the four methods. Most mosquitoes were collected by hand catching (using an aspirator and flashlight) from artificial outdoor places (shelter pits). Pyrethrum space spray in animal sheds was the second commonly used sampling method. A few quantities of mosquitoes were collected using the inlet window-trap method.
Table 1. Relative density of An. superpictus sampled by 4 methods, southeastern Iran, 2012.
| Month | Pyrethrum space-spray |
Outdoor shelters |
Inlet window trap | Outlet window trap | ||
| Human dwelling | Animal shed | Artificial(Pit shelters) | Natural (Well) | |||
| Jan | 1.00 | 1.00 | 0.33 | 1.00 | 0.00 | 0.00 |
| Feb | 1.00 | 1.00 | 0.00 | 1.00 | 0.00 | 0.00 |
| Mar | 1.00 | 2.00 | 0.33 | 0.00 | 1.00 | 4.00 |
| Apr | 1.00 | 2.00 | 0.66 | 0.00 | 1.00 | 2.00 |
| May | 1.00 | 1.00 | 0.66 | 1.00 | 0.00 | 1.00 |
| Jun | 0.25 | 0.50 | 2.00 | 0.00 | 0.00 | 0.00 |
| Jul | 0.00 | 0.25 | 1.00 | 0.00 | 0.00 | 0.00 |
| Aug | 0.00 | 0.25 | 2.00 | 0.00 | 0.00 | 0.00 |
| Sep | 0.25 | 0.25 | 3.00 | 0.00 | 0.00 | 0.00 |
| Oct | 0.25 | 1.00 | 1.00 | 1.00 | 0.00 | 0.00 |
| Nov | 1.00 | 1.00 | 2.00 | 1.00 | 0.00 | 1.00 |
| Dec | 1.00 | 2.00 | 3.00 | 1.00 | 1.00 | 3.00 |
| Total | 7.75 | 12.25 | 15.98 | 6.00 | 3.00 | 11.00 |
3.1.1. Seasonal and monthly pattern
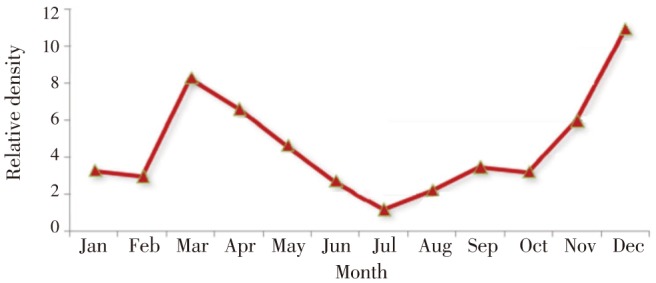
An. superpictus showed maximum abundance at the end of autumn. It had low activity during the hot summer. The results showed two activity peaks in March and December. Since January, it's remained active throughout the 12 months, peaking in March and then slowly decreasing. Its relative density again increased from September, with a second peak in December (Figure 2).
Figure 2. Monthly prevalence of An. superpictus, southeastern Iran, 2012.
3.1.2. Resting behavior
The resting behavior of female mosquitoes was evaluated according to the abdominal conditions. The ratio of resting stages to seeking stages (G, SG/U, and F) for An. superpictus revealed that it possessed behavior more exophilic (1.34) than endophilic (0.33) (Table 2). Furthermore, abdominal conditions of An. superpictus determined using window trap methods showed that most female mosquitoes had a greater tendency to rest outdoors than indoors.
Table 2. Abdominal condition of An. superpictus based on collecting sites, southeastern Iran, 2012.
| Collection method |
Indoor collection |
Outdoor collection |
Inlet window trap |
Outlet window trap |
||||||||
| Abdominal condition | U, F | G, SG | G, SG/F, U | U, F | G, SG | G, SG/F, U | U, F | G, SG | G, SG/F, U | U, F | G, SG | G, SG/F, U |
| % | 75.00 | 25.00 | 0.33 | 42.60 | 57.40 | 1.34 | 100.00 | 0.00 | - | 63.60 | 36.40 | 0.57 |
3.1.3. Blood feeding activity
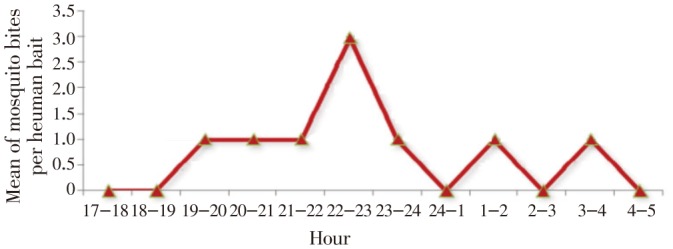
This research was performed in November when the second malaria peak occurred. The biting activity of An. superpictus on humans started from sunset to sunrise but did not show continuous activity during the entire night (Figure 3). On an average, seven An. superpictus were collected on human bait. The peak of landing occurred approximately at midnight, followed by short peaks after sunset.
Figure 3. Biting activities of An. superpictus during night on human, southeastern Iran, November, 2012.
3.1.4. Larval habitat characteristics
During monthly sampling, An. superpictus were collected from 19 larval breeding sites throughout the year, except in July when the larvae could not be found in breeding places. Larvae were found in different natural and artificial habitats; for instance, water leakages from farms irrigation cannels, stream margins, river beds and with less density in marshes, cement pools, water leaks from pipeline fractures, and ponds due to rain (Figure 4). Temperature of breeding places during larval sampling ranged from 10 °C to 29 °C and between 10 and 50-cm depth, with a pH of 7.2-7.8. This species were mostly caught in a habitat without vegetation, having clear and stagnant water, full sunlight, and mud beds (Table 3).
Figure 4. Some anopheline breeding places in Pishin region, Sarbaz district, southestern Iran, 2012.

a: Ponds due to rain; b: Cement pools; c: Leaks from water pipe line fracture; d: Marshes.
Table 3. Physical habitat characteristics of An. superpictus larvae, southeastern Iran, 2012.
| Characters |
Type |
Water situation |
Vegetation |
sunlight situation |
Substrate type |
|||||||||||
| Clases | Per | Tem | Nat | Art | Sta | Run | Cle | Tur | veg | No veg | Ful | Par | Sha | Mud | San | Roc |
| Percentage* | 68.4 | 32.6 | 89.6 | 10.4 | 71.7 | 29.3 | 91.6 | 8.4 | 35.5 | 64.5 | 56.3 | 28.3 | 15.4 | 60.5 | 5.5 | 33.0 |
*:Percent of collected larvae.
Per: Permanent; Tem: Temporary; Nat: Natural; Art: Artificial; Sta: Stagnant; Run: Running; Cle: Clear; Tur: Turbid; veg: With vegetation; No veg: Without vegetation; Ful: Full sunlight; Par: Partial sunlight; Sha: Shaded; Mud: Mud; San: Sand; Roc: Rock or cement.
3.2. Adult susceptibility tests
Susceptibility status of adult mosquitoes was determined using the WHO standard criteria. At least 80 mosquitoes were tested per bioassay. According to the criteria, 98%-100% mortality indicated susceptibility, 80%-97% mortality indicated suspected resistance, <80% indicated resistance[1],[2]; for 20-79 mosquitoes tested, 98%-100% mortality indicated susceptibility, 95%-97% indicated suspected resistance (requires verification of resistance with other methods), and <95% indicated resistance[27].
The results of susceptibility tests showed that the field strains of An. superpictus were resistant to DDT. Mortality rate with this insecticide was 56.00±4.54. This species was susceptible to malathion, propoxur, lambdacyhalothrin, deltamethrin, and permethrin (Table 4).
Table 4. The mortality rate of An. superpictus females exposed to diagnostic dose of insecticides southeastern Iran, 2012.
| Insecticide | Motality (%) | Replicates | No. of mosquitoes tested | No. of dead | Mortality (%)± Error bar |
| DDT | 4.00 | 4 | 84 | 47 | 56.00±4.54 |
| Malathion | 5.00 | 4 | 84 | 84 | 100.00 |
| Propoxor | 0.10 | 4 | 84 | 81 | 98.7±0.99 |
| Deltamethrin | 0.05 | 4 | 84 | 84 | 100.00 |
| Lambdacyhalothrin | 0.05 | 4 | 84 | 84 | 100.00 |
| Permethrin | 0.75 | 4 | 84 | 84 | 100.00 |
| Control | 6 | 132 | 130 | 1.50±1.06 |
4. Discussion
An. superpictus Grassi has been confirmed as the malarial vector in Asia, Europe, and northern Africa. This species is present in approximately all parts of Iran and has already been recorded as the main malarial vector in southeast Iran[1]. To the best of our knowledge, this is the first formal entomological study on the ecology of An. superpictus and its susceptibility to different insecticides in the Sarbaz district, southeastern Iran, near the Pakistan border.
Although An. superpictus were collected from all the places by using different methods, most mosquitoes were collected from shelter pits. Our data agree with those of previous studies on the ecology of malaria vectors in south Iran. These studies have reported that this species is mainly found in pit shelters and can be collected using different techniques[1]; However, the results of this study differ from those of studies performed in northwest Iran, which have described this species being collected only by the hand catching method from indoor places[28].
The resting behavior of female mosquitoes showed that this species had a greater tendency to rest outdoors than indoors. This behavior is representative of an effective vector. These results are consistent with those of some studies that have described both the exophagic and exophilic behavior for this species[14].
The results showed that the relative density of An. superpictus increased from September and peaked in December and then in March. In the other words, the maximum density was reported at the end of autumn and then at the end of winter. These findings are inconsistent with those of a previous study, which described that the peak of An. superpictus activity is from July to September[14]. Perhaps this variation is because of the climate difference in the study area. The weather in the Sarbaz district is very hot during July to August, which is not suitable for mosquito reproduction.
Although the biting activity of An. superpictus was observed from sunset to sunrise, it did not show continuous activity during the entire night. The landing peak was observed at about midnight, followed by short peaks after sunset. This result is relatively similar to that reported by studies, which mentioned that the landing peak happened at early night (unpublished data, Iranshahr Health Research Station, 1990).
The larvae were collected from different natural and artificial habitats, especially water leakages. The temperature of breeding places was in the range of 10-29 °C and depth between 10-50 cm, with a pH of 7.2-7.8. Larvae were mostly caught from a permanent habitat lacking vegetation, having clear and stagnant water, full sunlight, and mud beds. These results are generally consistent with those of previous studies on larval habitats in southern and western Iran that have reported similar details with the characteristics explained above[18],[29],[30]. However, in southern Iran, researchers have reported that this species prefers breeding places with sandy beds, having a temperature of 17-30 °C[29]. Moreover, a dissimilar result was reported by a study conducted in western Iran, in which larvae were frequently collected from flowing water[30], with the temperature of larval habitats being 19-32 °C[18].
By applying the WHO criteria, the results of susceptibility tests demonstrated that the field samples of An. superpictus were susceptible to malathion, propoxur, deltamethrin, lambdacyhalothrin, and permethrin. Some studies have shown the susceptibility of this species to dieldrin, lambdacyhalothrin, and malathion[20]. Despite high coverage of IRS application in southeast Iran, there are still no reports on resistance or tolerance of An. superpictus to malathion, propoxur, lambdacyhalothrin, permethrin, and deltamethrin, but An. stephensi was reported to be tolerant to deltamethrin in this part of the country[31]. Our study showed that An. superpictus was resistant to DDT. In contrast, an investigation in western Iran showed that this species was susceptible to DDT[20]. Our result confirms what is reported in the literature that An. superpictus was resistant to DDT in Uzbekistan[32]. Thus far, three genotypes X, Y, and Z within An. superpictus were identified in Iran. The sympatric Y and Z genotypes are limited to the populations' resident in southeast Iran, whereas the X genotype has high geographical distribution and is found in most parts of Iran[14]. Difference in susceptibility to DDT between western and southeast Iran may be because of different genotypes of An. superpictus. Therefore, further studies are needed to identify the relationship between species composition and insecticide susceptibility in the study area.
Because of IRS application, at least twice per year, in southeast Iran, careful survey is necessary to assess insecticides, particularly pyrethroids. Furthermore, authors do not suggest DDT application; moreover, periodic use of pyrethroids and carbamates in this part of the country has been recommended.
Acknowledgments
This article is a part of the results of the first author's thesis for achievement of MSPH degree in Medical Entomology and Vector Control from Tehran University of Medical Sciences, Iran. Great appreciation goes to Dr. M. Kiyani, Dr. M. Divband, and Mr. A. Hasanzehi from the Centre of Disease Control & Prevention in Province Health Centre, Zahedan University of Medical Sciences. Special thanks to Mr. E. Traz, A. Birnur, Mr. Y. Sharifzadeh, and Mr. N. Nabatzehi from Sarbaz Health Center for their help during fieldwork. This study was financially supported by the Tehran University of Medical Sciences, with the grant number 91-03-27-18800.
Comments
Background
Southeastern Iran with subtropical climate and various breeding places is still an endemic foci of malaria. An. superpictus Grassi as a main malaria vector had been identified in this area. This exophilus species is thought to be very important for maintenance of malaria in the area. This research has an updated information about the ecology and susceptibility status of this species.
Research frontiers
This research focuses on the evaluation of the seasonal activity, blood feeding activity, resting behavior as well as the larval habitats of An. superpictus and describes some attributes of its ecology. Also its susceptibility status to different insecticides is another important result.
Related reports
Several studies have been done about the malaria vectors in the world and Iran but it seems that the ecology and insecticide resistance of An. superpictus and similar species which are not more emphasized in literature should be considered.
Innovations and breakthroughs
In addition to updated information about the ecology of this species as far as I know this is the first formal report about the resistance of An. superpictus to DDT in southeastern Iran.
Applications
Data presented in the current research indicates the resistance of An. superpictus to DDT as well as its seasonal and blood feeding activity that can be used in the vector control program.
Peer review
New and updated information about ecology and insecticides resistance status of An. superpictus as one of the main six malaria vectors in Iran is valuable. The results can be useful for the vector control policy in the national malaria elimination program. It is more cleared all new data should be mentioned for improving the status of malaria control in Iran.
Footnotes
Foundation Project: Supported by the Tehran University of Medical Sciences, with the grant number 91-03-27-18800.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Soleimani-Ahmadi M, Vatandoost H, Shaeghi M, Raeisi A, Abedi F, Eshraghian MR, et al. Vector ecology and susceptibility in a malaria endemic focus in southern Islamic Republic of Iran. East Mediterr Health J. 2012;18(10):1034–1041. doi: 10.26719/2012.18.10.1034. [DOI] [PubMed] [Google Scholar]
- 2.Vatandoost H, Emami SN, Oshaghi MA, Abai MR, Raeisi A, Piazzak N, et al. Ecology of malaria vector Anopheles culicifacies in a malarious area of Sistan va Baluchestan province, south-east Islamic Republic of Iran. East Mediterr Health J. 2011;17(5):439–445. [PubMed] [Google Scholar]
- 3.Nejati J, Ansari Moghadam AR, Keyhani A, Tabatabai SM. Effects of immigration on malaria incidence and its foci classification. Hormozgan Med J. 2012;16(4):281–291. [Google Scholar]
- 4.Enayati A, Hemingway J. Malaria management: Past, present, and future. Annu Rev Entomol. 2010;55:569–591. doi: 10.1146/annurev-ento-112408-085423. [DOI] [PubMed] [Google Scholar]
- 5.Nejati J, Mahjoob M, Kiyani M, Keyhani A, Hasanzehi A. Status of indoor residual spraying by eltamethrin in malaria elimination program, Southeastern Iran. Iran J Toxicol. 2012;6(16):600–604. [Google Scholar]
- 6.Sinka ME, Bangs MJ, Manguin S, Rubio-Palis Y, Chareonviriyaphap T, Coetzee M, et al. A global map of dominant malaria vectors. Parasit Vectors. 2012;5:69. doi: 10.1186/1756-3305-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aytekin S, Aytekin AM, Alten B. Effect of different larval rearing temperatures on the productivity (Ro) and morphology of the malaria vector Anopheles superpictus Grassi (Diptera: Culicidae) using geometric morphometrics. J Vector Ecol. 2009;34(1):32–42. doi: 10.1111/j.1948-7134.2009.00005.x. [DOI] [PubMed] [Google Scholar]
- 8.Romi R, Boccolini D, Menegon M, Rezza G. Probable autochthonous introduced malaria cases in Italy in 2009-2011 and the risk of local vector-borne transmission. Euro Surveill. 2012;17(48):1–4. Availableonline: http://www.eurosurveillance.org. [PubMed] [Google Scholar]
- 9.Sudre B, Rossi M, Bortel WV, Danis K, Baka A, Vakalis N, et al. Mapping environmental suitability for malaria transmission,Greece. Emerg Infect Dis. 2013;19(5):784–786. doi: 10.3201/eid1905.120811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odolini S, Gautret P, Parola P. Epidemiology of imported malaria in the mediterranean region. Mediterr J Hematol Infect Dis. 2012;4(1) doi: 10.4084/MJHID.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bueno-Mari R, Bernues-Baneres A, Jimenez-Peydro R. Updated checklist and distribution maps of mosquitoes (Diptera: Culicidae) of Spain. J Eur Mosquito Control Assoc. 2012;30:91–126. [Google Scholar]
- 12.Vakali A, Patsoula E, Spanakos G, Danis K, Vassalou E, Tegos N, et al. Malaria in Greece, 1975 to 2010. Euro Surveill. 2012;17(47):1–8. doi: 10.2807/ese.17.47.20322-en. [DOI] [PubMed] [Google Scholar]
- 13.Hantosh HA, Hassan HM, Ahma B, Al-fatlawy A. Mosquito species geographical distribution in Iraq 2009. J Vector Borne Dis. 2012;49:33–35. [PubMed] [Google Scholar]
- 14.Oshaghi MA, Yaghobi-Ershadi MR, Shemshad Kh, Pedram M, Amani H. The Anopheles superpictus complex: introduction of a new malaria vector complex in Iran. Bull Soc Pathol Exot. 2008;101(5):429–434. [PubMed] [Google Scholar]
- 15.Hanafi-Bojd AA, Azari-Hamidian S, Vatandoost H, Charrahy Z. Spatio-temporal distribution of malaria vectors (Diptera: Culicidae) across different climatic zones of Iran. Asian Pac J Trop Med. 2011;4(6):498–504. doi: 10.1016/S1995-7645(11)60134-X. [DOI] [PubMed] [Google Scholar]
- 16.Gholizadeha S, Dinparast Djadid N, Nouroozi B, Bekmohammadi M. Molecular phylogenetic analysis of Anopheles and Cellia subgenus anophelines (Diptera: Culicidae) in temperate and tropical regions of Iran. Acta Trop. 2013;126:63–74. doi: 10.1016/j.actatropica.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Mehravaran A, Vatandoost H, Oshaghi MA, Abai MR. Anopheline mosquitoes and their role for malaria transmission in an endemic area, southern Iran. Asian Pac J Trop Med. 2011;1(3):209–211. [Google Scholar]
- 18.Banafshi O, Abai MR, Ladonni H, Bakhshi H, Karami H, Azari-Hamidian S. The fauna and ecology of mosquito larvae (Diptera: Culicidae) in western Iran. Turk J Zool. 2013;37:298–307. [Google Scholar]
- 19.Hanafi-Bojd AA, Vatandoost H, Oshaghia MA, Haghdoost AA, Shahi M, Sedaghat MM, et al. Entomological and epidemiological attributes for malaria transmission and implementation of vector control in southern Iran. Acta Trop. 2011;121:85–92. doi: 10.1016/j.actatropica.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Jalalian M, Mussavi Ivanaki O, Aiwazi A, Jalali A. Susceptibility level of Anopheles superpictus to Malathion, DDT and Lambdacyhalothrin insecticidesin Ilam Province. Sci J Ilam Med Univ. 2001;9:25–29. [Google Scholar]
- 21.Salehi M, Amirmajdi MM, Mashhadi IE, Hakemi Y, Mashhadi AE, Mirinezhad A. Analysis of malaria epidemic features in Sistan and Baluchistan province, southeast of Iran, 2005-2008. Iran Red Crescent Med J. 2010;12(3):247–253. [Google Scholar]
- 22.Youssefi MR, Rahimi MT. Prevalence of malaria infection in Sarbaz, Sistan and Bluchistan Province. Asian Pac J Trop Biomed. 2011;1(6):491–492. doi: 10.1016/S2221-1691(11)60107-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization . Entomological field techniques for malaria control, Part I, II Learner and Tutor's UIDC. Geneva: World Health Organization; 1992. [Google Scholar]
- 24.Basseri HR, Raeisi A, Ranjbar Khakha M, Pakarai A, Hassanzehi A. Seasonal abundance and host-feeding patterns of anopheline vectors in malaria endemic area of Iran. J Parasitol Res. 2010;2010:1–8. doi: 10.1155/2010/671291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azari-Hamidian S, Harbach RE. Keys to the adult females and fourth-instar larvae of the mosquitoes of Iran (Diptera: Culicidae) Zootaxa. 2009;2078:1–33. [Google Scholar]
- 26.World Health Organization . Geneva, Switzerland: World Health Organization; 1981. Instructions for determining the susceptibility or resistance of adult mosquitoes to organochlorine, organophosphate and carbamate insecticides: Establishment of baseline. [Online] Available from: http://whqlibdoc.who.int/temp/WHO_VBC_81.806_cor.1.pdf. [Accessed on 12 August, 2013]. [Google Scholar]
- 27.World Health Organization . Harare: World Health Organization; 2005. Atlas of insecticide resistance in malaria vectors of the WHO African region. African Network for Vector Resistance. [Online] Available from: http://www.afro.who.int/index.php?option=com_docman&task=doc_download&gid=2141. [Accessed on 12 August, 2013]. [Google Scholar]
- 28.Abai MR, Azari-Hamidian S, Ladonni H, Hakimi M, Mashhadi-Esmail K, Sheikhzadeh K, et al. Fauna and checklist of mosquitoes (Diptera: Culicidae) of East Azerbaijan Province, Northwestern Iran. Iran J Arthropod-Borne Dis. 2007;1(2):27–33. [Google Scholar]
- 29.Hanafi-Bojd AA, Vatandoost H, Oshaghi MA, Charrahy Z, Haghdoost AA, Sedaghat MM, et al. Larval habitats and biodiversity of anopheline mosquitoes (Diptera: Culicidae) in a malarious area of southern Iran. J Vector Borne Dis. 2012;49:91–100. [PubMed] [Google Scholar]
- 30.Kassiri H, Amani H. Bionomics and breeding places of the Genus Anopheles (Diptera: Culicidae) in Mahroo and Sepid-Dasht Districts, Luristan Province, Western Iran. Zahedan J Res Med Sci. 2012;14(8):11–17. [Google Scholar]
- 31.Vatandoost H, Hanafi-Bojd AA. Indication of pyrethroid resistance in the main malaria vector, Anopheles stephensi from Iran. Asian Pac J Trop Med. 2012;5(9):722–726. doi: 10.1016/S1995-7645(12)60114-X. [DOI] [PubMed] [Google Scholar]
- 32.Zhakhongirov SM, Abdullaev IT, Ponomarev IM, Muminov MS. Monitoring of the insecticidal resistance of main malaria vectors in Uzbekistan. Med Parazitol (Mosk) 2004;(1):29–33. [PubMed] [Google Scholar]


