Abstract
Aims
We have previously reported alterations in cardiolipin content and inner mitochondrial membrane (IMM) proteomic make-up specifically in interfibrillar mitochondria (IFM) in the type 1 diabetic heart; however, the mechanism underlying this alteration is unknown. The goal of this study was to determine how the cardiolipin biosynthetic pathway and cardiolipin-IMM protein interactions are impacted by type 1 diabetes mellitus.
Main methods
Male FVB mice were made diabetic by multiple low-dose streptozotocin injections and sacrificed five weeks post-diabetic onset. Messenger RNA was measured and cardiac mitochondrial subpopulations were isolated. Further mitochondrial functional experimentation included evaluating the protein expression of the enzymes directly responsible for cardiolipin biosynthesis, as well as ATP synthase activity. Interactions between cardiolipin and ATP synthase subunits were also examined.
Key findings
Western blot analysis revealed a significant decrease in cardiolipin synthase (CRLS) protein content in diabetic IFM, with a concomitant decrease in its activity. ATP synthase activity was also significantly decreased. We identified two novel direct interactions between two subunits of the ATP synthase F0 complex (ATP5F1 and ATP5H), both of which were significantly decreased in diabetic IFM.
Significance
Overall, these results indicate that type 1 diabetes mellitus negatively impacts the cardiolipin biosynthetic pathway specifically at CRLS, contributing to decreased cardiolipin content and loss of interactions with key ATP synthase F0 complex constituents in the IFM.
Keywords: diabetes mellitus, mitochondria, inner mitochondria membrane, cardiolipin, ATP synthase
INTRODUCTION
Cardiovascular complications, including diabetic cardiomyopathy are the primary cause of morbidity in diabetic patients. Diabetic cardiomyopathy has been associated with mitochondrial dysfunction (Devereux et al. 2000; Severson 2004; Tomita et al. 1996). The inner mitochondria membrane (IMM) houses vital processes in a specific lipid environment. Alteration in the IMM lipid environment has been associated with dysfunction to mitochondrial processes (Acehan et al. 2011a; Chicco and Sparagna 2007; Han et al. 2005; Jiang et al. 2000). Proteomic analyses of type 1 diabetic mitochondria indicates that IMM proteins may be particularly prone to damage and loss (Baseler et al. 2011).
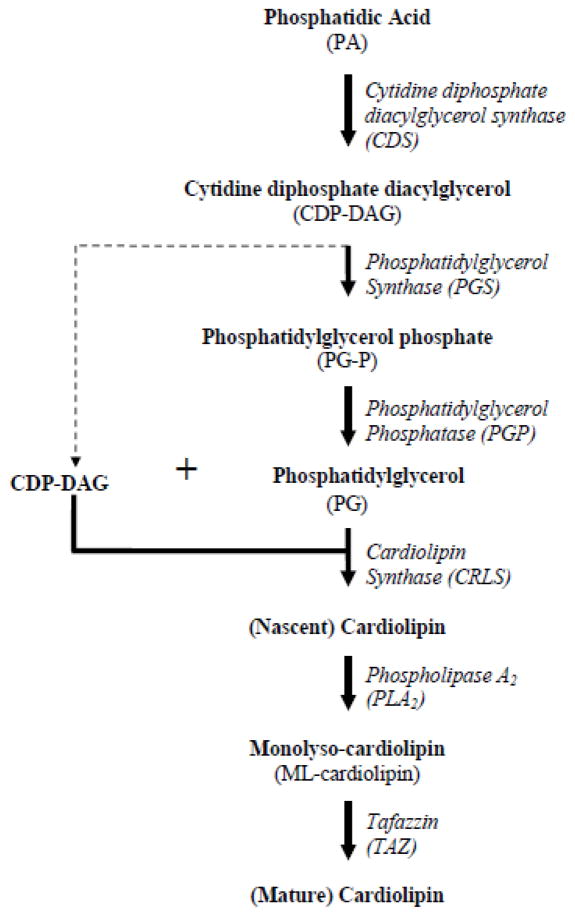
Impaired mitochondrial function, particularly in those processes situated in the IMM, may be associated with disruption to the phospholipid environment contained within. Most abundant in the mammalian heart, cardiolipin is a unique phospholipid, primarily located in the IMM (Schlame et al. 2000) and is thought to play a critical role in mitochondrial structure and bioenergetics (Acehan et al. 2011a). The biosynthetic pathway (Figure 1) begins in the outer mitochondrial membrane where phosphatidic acid is converted into cytidine diphosphate diacylglycerol (CDP-DAG) by cytidine diphosphate diacylglycerol synthase (CDS) (Houtkooper and Vaz 2008). Phosphatidylglycerol synthase (PGS) then converts CDP-DAG to phosphatidylglycerol phosphate (PG-P), where the phosphate is removed by phosphatidylglycerol phosphatase (PGP), resulting in the formation of phosphatidylglycerol (PG). Cardiolipin synthase (CRLS) then catalyzes the condensation of CDP-DAG and PG, resulting in a nascent form of cardiolipin (Chicco and Sparagna 2007; Lu et al. 2006). Because the nascent cardiolipin does not contain the precise fatty acyl side chains for the specific tissue, a remodeling process takes place, where tafazzin (TAZ) or monolysocardiolipin acyltransferase, converts the nascent cardiolipin to mature cardiolipin containing specific fatty acyl side chains (Chicco and Sparagna 2007), which is dependent upon the tissue and environment. Linoleic acid, the most abundant fatty acid found in cardiac mitochondria, constitutes approximately 80% of all side chains (Acehan et al. 2011b; Schlame et al. 2000). The properties of cardiolipin allow it to interact with IMM proteins and facilitate proper mitochondrial function.
Figure 1. Cardiolipin biosynthetic pathway.
Schematic diagram illustrating cardiolipin biosynthetic pathway.
Literature suggests an interaction between cardiolipin and the electron transport chain (ETC) complexes involved in oxidative phosphorylation (Schlame et al. 2000), including complexes I, III, IV, and ATP synthase (Fry and Green 1981; Sedlak and Robinson 1999). Cardiolipin plays a critical role in the organization of mammalian mitochondrial ATP synthase and it has been suggested that the F0 complex of ATP synthase contains high affinity binding sites for cardiolipin (Acehan et al. 2011a); however, the specific binding sites remain undefined. Cardiolipin also serves as a proton reservoir for maintenance of IMM potential thus, contributing to ATP synthesis (Haines and Dencher 2002). Shotgun lipidomics revealed decreased tetralinoleic cardiolipin content in diabetic hearts suggesting a possible mechanistic reliance on the interaction between IMM protein activity and cardiolipin (Han et al. 2005). We have previously reported decreased ETC protein content and function in IFM following a type 1 diabetic insult (Baseler, #2371;Dabkowski, 2009 #2381;Baseler, 2011 #2371), correlating with decreased tetralinoleic cardiolipin content, with no effect on subsarcolemmal mitochondria (SSM) situated beneath the plasma membrane (Dabkowski et al. 2009).
To date, the impact of type 1 diabetes mellitus on the cardiolipin biosynthetic pathway in mitochondrial subpopulations and on the interactions between cardiolipin and IMM proteins has not been studied. We hypothesized that the decreased cardiolipin content associated with type 1 diabetes mellitus results from defective cardiolipin biosynthesis influencing its association with IMM proteins, with the effects being most pronounced in IFM.
MATERIALS AND METHODS
Experimental Animals and Induction of Diabetes
The animal experiments in this study conformed to the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals and were approved by the West Virginia University Animal Care and Use Committee. Male FVB mice were housed in the West Virginia University Health Sciences Center animal facility on a 12-hr light/dark cycle in a temperature controlled room. Mice were given unlimited access to a standard rodent diet and water. Type 1 diabetes mellitus was induced in 6-wk-old mice following the protocol of the Animal Models of Diabetic Complications Consortium using multiple low-dose streptozotocin (STZ; Sigma, St. Louis, MO) intraperitoneal injections. The STZ-induced type 1 diabetic model was chosen because it is the most widely utilized model and the multiple low dose administration is not associated with many of the deleterious side effects observed with the single high-dose approach (Wu and Huan 2001). Injections of 50 mg/kg body weight STZ dissolved in sodium citrate buffer (pH 4.5) were performed daily for 5 consecutive days after 6 hours of fasting. Mice serving as vehicle controls were given the same volume per body weight of sodium citrate buffer. One week post-injection, hyperglycemia was confirmed by measuring blood glucose (Contour Blood Glucose test strips; Bayer, Mishawaka, IN), where >250 mg/dL was considered diabetic. Five weeks after the onset of hyperglycemia, animals were sacrificed for experimentation. Blood glucose levels were again tested at this time and all remained >250 mg/dL.
Mitochondrial Subpopulation Isolation
Five weeks post-diabetic onset, FVB mice and their littermate controls were sacrificed and hearts excised. Hearts were rinsed in phosphate buffered saline (PBS, pH 7.4), then blotted dry. SSM and IFM were isolated as previously described by Palmer et al. (Palmer et al. 1977) with modifications by our laboratory (Dabkowski et al. 2009; Dabkowski et al. 2008; Williamson et al. 2010). Mitochondrial pellets were resuspended in either KME buffer (100 mM KCl, 50 mM MOPS, and 0.5 mM EDTA) for Western blot analysis and activity measurements, or in 1.0% NP-40 wash/binding buffer (10 mM HEPES (pH 7.4), 1.0% NP-40, 150 mM NaCl) for cardiolipin/protein interactions. Protein content was determined by the Bradford method using bovine serum albumin as a standard (Bradford 1976).
Cardiolipin Analyses
Isolated mitochondrial subpopulations from control and diabetic hearts were pooled, and sent off to Avanti Polar Lipids (Alabaster, AL) for cardiolipin analysis. Extraction and analysis of cardiolipin species was based on the protocols of Sparagna et al. (Sparagna et al. 2005) and Minkler et al. (Minkler and Hoppel 2010). In brief, cardiolipin detection was performed by liquid chromatography (Dionex U-3000 binary HPLC) / hybrid tandem mass spectrometry using a quadrapole-linear ion trap (API 4000 Qtrap) operated in the negative ion mode. Mass spectrometer instrument conditions included a spray voltage of −4.5 kV, capillary voltage of −150 V, heated capillary temperature of 400°C, and a sheath gas flow rate of 10 arbitrary units. Mitochondrial cardiolipin and internal standard spectra were identified by multiple reaction monitoring. All experiments were performed in triplicate.
mRNA Isolation and Analysis
mRNA levels were examined as previously described (Baseler et al. 2011). Briefly, hearts were rinsed in phosphate buffered saline (PBS, pH 7.4) then snap frozen in liquid nitrogen. Thirty mg of tissue was excised from control and diabetic hearts from which the mRNA was extracted using an RNeasy mini kit, per manufacturer’s instructions (Qiagen, Valencia, CA) and reverse transcribed. Equal amounts of cDNA from the hearts of control and diabetic mice were subjected to real-time PCR. Custom primers were designed for target genes CDS, PGS, CRLS, and TAZ. SYBR Green I was used for quantification of respective cDNA replicates (Qiagen, Valencia, CA). Data were normalized by gene expression relative to the levels of GAPDH.
Western Blot Analyses
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was run on a 4–12% gel as previously described (Laemmli 1970; Williamson et al. 2010) with equal amounts of protein loaded for each study treatment. Relative amounts of subpopulation-specific CDS, PGS, CRLS, and TAZ, were assessed using the following primary antibodies; anti-CDS rabbit antibody (product No. ab84019, Abcam, Cambridge, MA), anti-PGS rabbit antibody (product No. ab104815, Abcam, Cambridge, MA), CRLS rabbit antibody (product No. 49444_P050, Aviva Systems Biology, San Diego, CA), and anti-TAZ rabbit antibody (product No. ab84927, Abcam, Cambridge, MA). The secondary antibody used was a goat anti-rabbit IgG horseradish peroxidase conjugate (product No. 10004301, Cayman Chemical Company, Ann Arbor, MI). Detection of signal was performed according to the Pierce ECL Western Blotting Substrate detection system manufacturer’s directions (Thermo Fisher Scientific, Rockford, IL). Cytochrome c oxidase (COX IV) was used to control for protein loading (product No. ab16056, Abcam, Cambridge, MA). Quantification of chemiluminescent signals were assessed using a G:Box Bioimaging System (Syngene, Frederick, MD), and the data captured using GeneSnap software (Syngene, Frederick, MD). Densitometry was measured using Image J Software (National Institutes of Health, Bethesda, MD).
Cardiolipin Synthase (CRLS) Activity
CRLS activity was assessed as previously described (Chen et al. 2006) with minor modifications. Briefly, a reaction mixture containing 20 μM 14C-oleoyl-CoA (50 mCi/mmol; American Radiolabeled Chemicals, MO), 2.0 mM LPG [1-oleoyl-2-hydroxy-sn-glycero-3-[phospho-rac-(1-glycerol)] and 2.0 mM CDP-DAG (Avanti Polar Lipids, Alabaster, AL) were incubated with 50 μg of lysed mitochondrial protein for 30 min at 37°C. The reactions were terminated by adding chloroform/methanol, followed by a quick vortex, then chloroform, followed by a quick vortex and finally, 0.1 M KCl to facilitate phase separation. A brief centrifugation was performed to extract lipids. The organic phase was collected, dried under an argon stream and separated by high performance thin layer chromatography (Whatman, GE Healthcare, U.S.A) with chloroform/hexane/methanol/acetic acid (25:15:5:2.5 by volume) as the developing solvent. The high performance thin layer chromatography plates are then stained with iodine vapor. The appropriate lipid spots are scraped off the plate and dissolved in scintillation fluid for scintillation counting.
ATP Synthase Activity
ATP synthase activity was measured in control and diabetic mitochondrial subpopulations as oligomycin-sensitive ATPase activity using an assay coupled with pyruvate kinase which converts the ADP to ATP and produces pyruvate from phosphoenolpyruvate as previously described (Dabkowski et al. 2010). Protein content was assessed as described above (Bradford 1976) with final values expressed as nanomoles of NADH per minute per milligram of protein, equal to the nanomoles of NADH oxidized per minute per milligram of protein.
Lipid-Protein Interaction
Cardiolipin-coated beads (Product No. P-BCLP, Echleon Biosciences Incorporated, Salt Lake City, UT) were used to determine protein interactions with cardiolipin per the manufacturer’s instructions. In brief, 100 μl of cardiolipin-coated beads were incubated with 600 μg of mitochondrial protein diluted in 1.0% NP-40 wash/binding buffer overnight at 4°C. The beads were spun down at 800 × g. The supernatant containing the unbound proteins was collected and the beads were washed four times with 10X excess of wash/binding buffer. The proteins bound to the beads were eluted with 4X Laemmli sample buffer and heated to 95°C. A Western blot was then performed on the eluted protein from the beads and the unbound protein located in the supernatant using the following specific antibodies: anti-ATP5F1 mouse antibody (product No. ab117991, Abcam, Cambridge, MA), anti-ATP5H mouse antibody (product No. ab110275, Abcam, Cambridge, MA), anti-ATPB mouse antibody (product No. ab14730, Abcam, Cambridge, MA), anti-ATP5A (product No. ab110273, Abcam, Cambridge, MA), and anti-GRP75 rabbit antibody (product No. 2816S, Cell Signaling Technology, Danvers, MA). The secondary antibodies used were either a goat anti-rabbit IgG horseradish peroxidase conjugate (product No. 10004301, Cayman Chemical Company, Ann Arbor, MI) or a goat anti-mouse IgG horseradish peroxidase conjugate (product No. 31430, Thermo Scientific, Rockford, IL). Detection of signal was performed according to the Pierce ECL Western Blotting Substrate detection system manufacturer’s directions (Thermo Fisher Scientific, Rockford, IL). Quantification of chemiluminescent signals were assessed using a G:Box Bioimaging System (Syngene, Frederick, MD), and the data captured using GeneSnap software (Syngene, Frederick, MD) as above. Densitometry was measured using Image J Software (National Institutes of Health, Bethesda, MD) as above. The association between cardiolipin and the mitochondrial protein of interest was determined by using the densitometric ratio of the protein bound to native cardiolipin/protein bound to native cardiolipin + protein bound to the cardiolipin-coated beads.
Statistics
Means and SEMs were calculated for all data sets. Data were analyzed using a Student’s paired t-test (GraphPad Software Inc., La Jolla, CA). Differences between control and diabetic groups were considered statistically significant when a P < 0.05 was observed.
RESULTS
Cardiolipin Content in the Diabetic Heart
Previous reports (Han et al. 2005) have indicated an overall decrease in cardiolipin content in the diabetic heart and our laboratory has previously reported decreased tetralinoleic cardiolipin content in diabetic IFM (Dabkowski et al. 2009). Because CRLS synthesizes the nascent form of cardiolipin, total cardiolipin content was measured. The cardiolipin content in the IFM significantly decreased from 84.46 ± 1.93 nmol/mg of protein in the control heart to 48.75 ± 2.83 nmol/mg of protein in the diabetic heart, with no difference in the SSM (44.99 ± 1.75 nmol/mg of protein in control heart compared to 37.87 ± 0.11 nmol/mg of protein in diabetic heart). Therefore, we hypothesized that the decrease in cardiolipin may be caused by an alteration in the cardiolipin biosynthetic pathway during diabetic insult.
mRNA Analyses of Cardiolipin Biosynthesis Pathway Constituents
To gain insight into the impact of diabetes mellitus on transcriptional regulation of the enzymatic components of the cardiolipin biosynthetic pathway, the mRNA levels of three constituent enzymes, CDS, PGS, and CRLS, as well as the remodeling enzyme TAZ, were analyzed. Results indicated no significant differences between control and diabetic heart for any of the constituents responsible for cardiolipin biosynthesis or remodeling (Table 1). These results suggest that the observed decreased in IFM cardiolipin content resulting from type 1 diabetic insult does not occur at the transcriptional level.
Table 1. mRNA analysis of inner mitochondrial membrane proteins.
mRNA was isolated from control and diabetic hearts. The mRNA content levels for inner mitochondrial membrane proteins; were assessed. Values are expressed as fold change in diabetic heart as compared to control heart; n=4 for each group
| mRNA | Diabetic vs. Control | p-value |
|---|---|---|
| Cardiolipin biosynthesis | ||
| Cytidine diphosphate-diacylglycerol synthase 1 | 1.18 | 0.17 |
| Phosphatidylglycerol synthase 1 | 0.92 | 0.47 |
| Cardiolipin synthase | 0.98 | 0.91 |
| Tafazzin | 0.86 | 0.24 |
| Protein import | ||
| Mitochondrial import inner membrane translocase subunit TIM16 | 0.98 | 0.89 |
| Mitochondrial import inner membrane translocase subunit TIM14 | 0.97 | 0.98 |
| Oxidative phosphorylation | ||
| NADH dehydrogenase [ubiquinone] flavoprotein 1, mitochondrial | 0.91 | 0.20 |
| NADH dehydrogenase [ubiquinone] flavoprotein 2, mitochondrial | 1.05 | 0.60 |
| NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 1 | 0.97 | 0.84 |
| NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 2 | 0.98 | 0.92 |
| NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 3 | 0.94 | 0.45 |
| NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 4 | 0.95 | 0.63 |
| NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 12 | 0.92 | 0.62 |
| Succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial | 1.02 | 0.88 |
| Electron transfer flavoprotein-ubiquinone oxidoreductase, mitochondrial | 0.62 | 0.000049 |
| Cytochrome b-c1 complex subunit 1, mitochondrial | 1.06 | 0.49 |
| Cytochrome b-c1 complex subunit Rieske, mitochondrial | 0.96 | 0.69 |
| Cytochrome c1, heme protein, mitochondrial | 0.99 | 0.98 |
| ATP synthase sub-unit b, mitochondrial | 0.97 | 0.75 |
| Miscellaneous | ||
| Prohibitin-2 | 0.88 | 0.40 |
Because cardiolipin is important for IMM structure and function, we assessed the mRNA levels of other IMM proteins (Table 1). None of the mRNA levels of proteins involved in protein import were altered in the diabetic heart. Of the transcripts encoding oxidative phosphorylation proteins, only electron transfer flavoprotein-ubiquinone oxidoreductase was significantly decreased in the diabetic heart. This decrease suggests a reduction in the electron flux through the respiratory chain, which could potentially cause decreased oxidation phosphorylation. This finding is supported by decreased mitochondrial respiration in the presence of type 1 diabetes mellitus, previously reported by our laboratory (Dabkowski et al. 2009).
Protein Analyses of Cardiolipin Biosynthesis Pathway Constituents
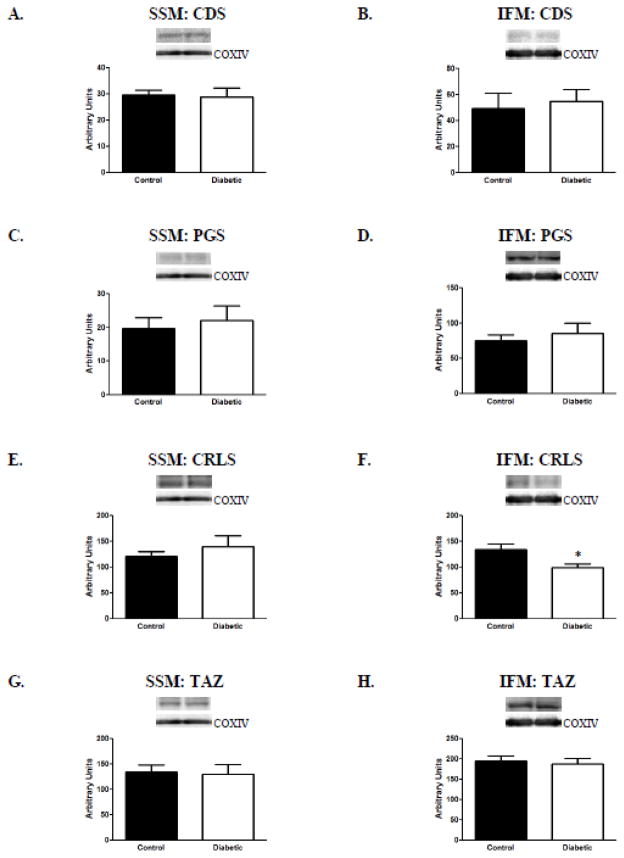
To determine the impact of diabetes mellitus on translational regulation of the enzymatic components of the cardiolipin biosynthetic pathway, the protein levels of three constituent enzymes, CDS, PGS, and CRLS, as well as the remodeling enzyme TAZ, were analyzed. Western blot analyses revealed a significant decrease only in CRLS protein content in diabetic IFM as compared to control, with no impact on CRLS protein content in the SSM (Figures 2E and 2F). No other significant differences were observed in the protein levels of the other cardiolipin biosynthetic enzymes assessed, regardless of subpopulation (Figure 2). It is important to note that CDS-2 is the major isoform in the heart and when the protein expression was measured, there was no difference between the two treatment groups for either subpopulation (data not shown). These results indicate that the observed decreased in IFM cardiolipin content resulting from type 1 diabetic insult is associated with a decrease in the protein content of CRLS, and the biosynthetic pathway.
Figure 2. Protein content of cardiolipin biosynthetic pathway constituents.
Assessment of cardiolipin biosynthetic pathway constituents was performed by Western blot using specific antibodies. (A) and (B): representative CDS content from SSM and IFM control (n=7) and diabetic (n=8), respectively; (C) and (D): representative PGS content from SSM (n=7 for each group) and IFM (n=8 for each group) control and diabetic, respectively; (E) and (F): representative CRLS content from SSM and IFM control (n=8) and diabetic (n=7), respectively; (G) and (H): representative TAZ content from SSM and IFM control (n=7) and diabetic (n=7), respectively. Control for loading was confirmed using COX IV probing. Values are expressed as means ± SEM; *P< 0.05 for control vs. diabetic groups. Abbreviations: CDS = cytidine diphosphate diacylglycerol synthase; PGS = phosphatidylglycerol synthase; CRLS = cardiolipin synthase; TAZ = tafazzin; IFM = interfibrillar mitochondria; SSM = subsarcolemmal mitochondria.
Enzymatic Activity Analyses of CRLS
Because CRLS protein content was the only biosynthetic enzymatic change observed following diabetic insult, we investigated the impact of diabetes mellitus on CRLS enzymatic activity using an assay that utilizes 14C-oleoyl-coenzyme A as an acyl donor. 14C-oleoyl-coenzyme A lipid was incubated with additional compounds and mitochondrial protein then subjected to high performance thin layer chromatography. Our results revealed a decrease in CRLS activity in diabetic IFM as compared to control (Figure 3B), with no change in the SSM (Figure 3A). During high performance thin layer chromatography, PG runs below cardiolipin, so as a control to ensure that the assay was only affecting the radioactivity incorporated into cardiolipin, the amount of radioactivity incorporated into PG was also measured through scintillation counting. The results indicated no change in 14C-PG in diabetic mitochondria relative to control for either subpopulation (Figures 3C and 3D). These results complement the CRLS proteomic data and suggest that loss of CRLS protein in the IFM results in a decrease in CRLS enzymatic activity.
Figure 3. Cardiolipin synthase (CRLS) activity.
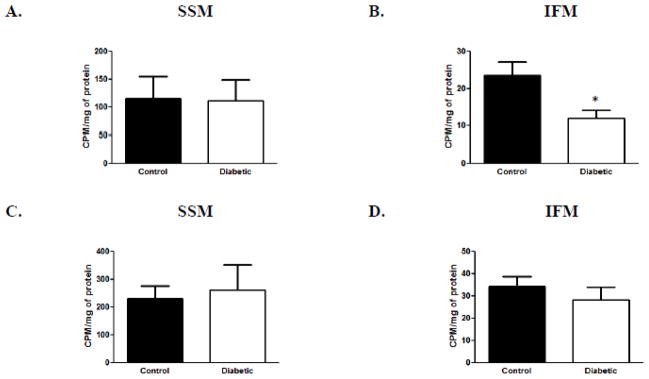
CRLS activity was assessed using a 14C-acyl donor. (A): CRLS activity measured in SSM diabetic and control samples. (B): CRLS activity measured in IFM diabetic samples compared to control samples. (C): PG radioactive counts/minute/mg of protein measured in SSM diabetic samples compared to control samples. (D): PG radioactive counts/minute/mg of protein measured in IFM diabetic samples compared to control samples. Values are expressed as means± SEM; n=8 for each group. *P < 0.05 for control vs. diabetic groups. Abbreviations: CDS = cytidine diphosphate diacylglycerol synthase; PG = phosphatidylglycerol; IFM = interfibrillar mitochondria; SSM = subsarcolemmal mitochondria.
ATP Synthase Activity Analyses
Literature suggests that cardiolipin interacts with components of the ATP synthase complex (Acehan et al. 2011a; Eble et al. 1990); however, these interaction sites are unknown. Further, previous proteomic data from our laboratory indicates that ATP synthase components are decreased in diabetic IFM (Baseler et al. 2011). As a result, we determined whether the activity of the ATP synthase complex was impacted in diabetic mitochondria. Our analyses indicated that ATP synthase activity was significantly decreased in the diabetic IFM as compared to control (Figure 4B) with no differences observed in the SSM subpopulation (Figure 4A). These results suggest that the decrease in ATP synthase activity observed in diabetic IFM is associated with a decrease in cardiolipin content which may be influencing its functional properties.
Figure 4. ATP synthase activity.
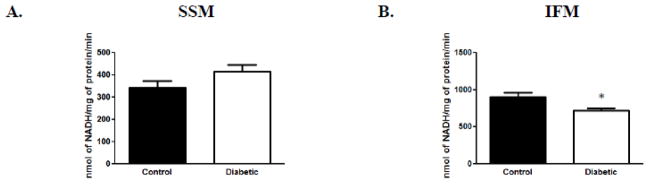
Spectrophotometric analysis of ATP synthase activities in control and diabetic samples. (A): ATP synthase activity from SSM control and diabetic groups. (B): ATP synthase activity from IFM control and diabetic groups. Values are expressed as means ± SEM; n=4 for each group. *P< 0.05 for control vs. diabetic groups. Abbreviations: IFM = interfibrillar mitochondria; SSM = subsarcolemmal mitochondria.
Cardiolipin and ATP Synthase Interaction
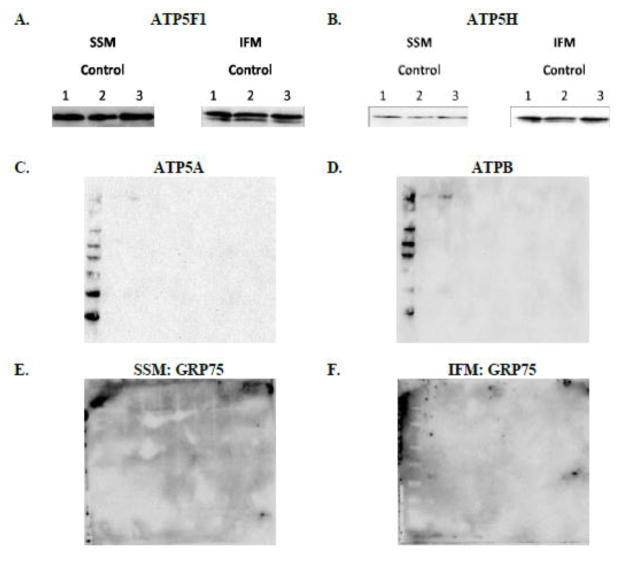
To gain insight into which ATP synthase constituent proteins may be interacting with cardiolipin, we developed an approach in which we incubated cardiolipin-coated beads with mitochondrial proteins and probed for the interaction. Figure 5A and 5B demonstrate direct association of cardiolipin with ATP synthase subunit b (ATP5F1) and ATP synthase subunit d (ATP5H), both of which are subunits of the F0 complex, or proton pore, of the ATP synthase. To validate that the cardiolipin-coated beads were specifically binding to proteins that interact with cardiolipin, interactions of proteins not associated with cardiolipin were examined. First, ATP synthase alpha (ATP5A) and ATP synthase beta (ATPB) were measured. Results indicated that neither protein was interacting with the cardiolipin-coated beads (Figures 5C and 5D, respectively). Next, glucose regulated protein 75 (GRP75), a protein located in the mitochondrial matrix, was examined. Results indicated the absence of GRP75 bound to the cardiolipin-coated beads in both subpopulations (Figures 5E and 5F, respectively).
Figure 5. Cardiolipin – ATP synthase association.
Mitochondrial protein was incubated with cardiolipin-coated beads. Western blots were performed to determine associations between cardiolipin and (A): ATP synthase subunit b (ATP5F1); n=3 for each group and (B): ATP synthase subunit d (ATPH); n=3 for each group (C): ATP synthase alpha (ATP5A); n=2 for each group and (D): ATP synthase beta (ATPB); n=2 for each group and (E): Glucose regulated protein (GRP75) in the SSM; n=3 for each group and (F): Glucose regulated protein (GRP75) in the IFM; n=3 for each group using specific antibodies (Representative blots shown). Abbreviations: IFM = interfibrillar mitochondria; SSM = subsarcolemmal mitochondria.
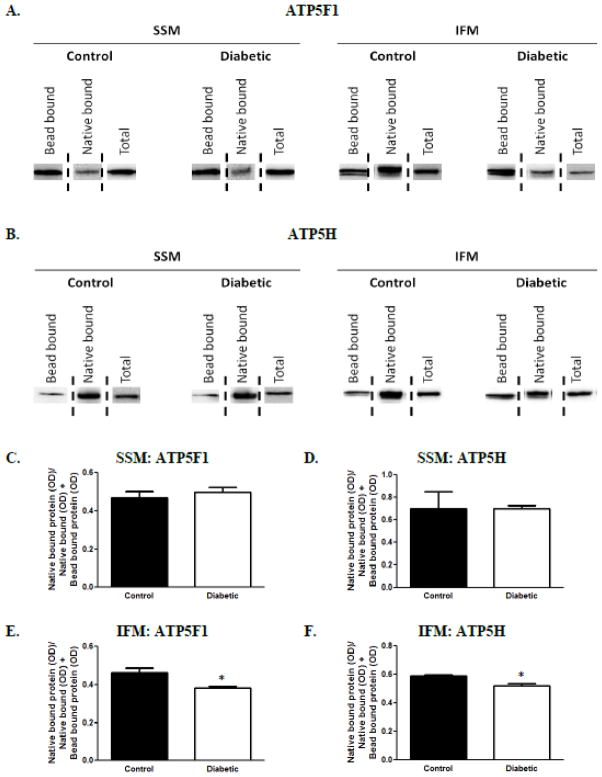
Once the interactions between the aforementioned ATP synthase subunits and cardiolipin were detected, we quantified the association between the lipid and protein that occurs in the mitochondrion during diabetic insult. By assessing proteins that are associated with native cardiolipin which are incapable of binding to the coated beads (native bound) as well as those proteins that can bind to the beads (bead bound) we were able to determine how diabetes mellitus impacts cardiolipin association with these two proteins. Figure 6A represents the protein content of ATP5F1 in the different mitochondrial subpopulations of control and diabetic, respectively, while Figure 6B represents the protein content of ATP5H. In order to quantify the associations of ATP synthase subunits and native cardiolipin, the optical density of the band that resulted from the protein interacting with native cardiolipin (native bound protein) was divided by the optical density of the bands that resulted from both the protein bound to native cardiolipin (native bound) and the protein bound to the cardiolipin-coated beads (bead bound). This ratio was used to quantify the interactions of cardiolipin with the protein in control and diabetic mitochondrial subpopulations. Results indicated no difference in the associations between cardiolipin and ATP5F1 or ATP5H in the diabetic SSM compared to controls (Figures 6C and 6D, respectively). Interestingly, a significant decrease in the interactions between native cardiolipin and both ATP5F1 and ATP5H in diabetic IFM, as compared to control, was observed (Figures 6E and 6F, respectively).
Figure 6. Native cardiolipin – ATP synthase association.
The association between cardiolipin and the detected interactions were measured in control and diabetic mitochondrial subpopulations. (A): Representation of ATP5F1 protein content in control and diabetic SSM and IFM, respectively. The “Bead bound” column represents ATP5F1 that was eluted off of the cardiolipin-coated beads, whereas the “Native bound” column represents ATP5F1 associated with native cardiolipin that was present in the supernatant. The “Total” band represents the total ATP5F1 protein content in isolated mitochondria. (B): Representation of ATP5H in the same manner as ATP5F1 (Figure 6A). Quantification of the association between native cardiolipin and ATP5F1 in control and diabetic hearts in (C): SSM and (E): IFM. Quantification of the association between native cardiolipin and ATP5H in control and diabetic hearts in (D): SSM and (F): IFM. Values are expressed as means ± SEM; n=3 for each group. *P< 0.05 for control vs. diabetic groups. Abbreviations: IFM = interfibrillar mitochondria; SSM = subsarcolemmal mitochondria.
DISCUSSION
Mitochondrial dysfunction is an underlying contributor to diabetic cardiomyopathy (Devereux et al. 2000; Severson 2004; Tomita et al. 1996). The IMM is an important submitochondrial locale which houses the machinery required for a number of mitochondrial functional processes. When the lipid environment of the IMM is altered, the processes contained within can become impaired (Acehan et al. 2011a; Chicco and Sparagna 2007; Han et al. 2005; Jiang et al. 2000). Cardiolipin, makes up approximately 20% of the total IMM phospholipid membrane (Waring et al. 1981) and is required for proper functioning of a number of mitochondrial processes (Chicco and Sparagna 2007; Fry and Green 1981; Jiang et al. 2000). In this study, we observed a decrease in total cardiolipin content in the diabetic IFM. Our laboratory has previously reported significant decreases in tetralinoleic cardiolipin content of diabetic IFM, as well as loss of a number of proteins that are constituents for crucial functional processes (Baseler et al. 2011; Dabkowski et al. 2009). These results are further supported by others who have reported decreased total cardiolipin content 28 days after the induction of diabetes mellitus (Han et al. 2005), as well as decreased tetralinoleic cardiolipin in diabetic mouse hearts compared to control days after the induction of diabetes mellitus (Han et al. 2007). Thus, our hypothesis for the current study centered on understanding whether proteomic loss of IMM constituents and cardiolipin content was the result of dysfunction to the pathway responsible for cardiolipin synthesis. If our hypothesis were to be supported, it would suggest a mechanism contributing to mitochondrial dysfunction in the diabetic heart.
The biosynthesis of cardiolipin occurs in the IMM and is a multistep process (Figure 1). To determine whether alterations in the cardiolipin biosynthetic pathway contribute to loss of cardiolipin, mRNA and protein levels for a number of key constituents were measured. Results indicated no difference in mRNA levels between control and diabetic hearts for any of the enzymes directly responsible for cardiolipin biosynthesis; however, a significant decrease in CRLS protein content was observed in the diabetic IFM. It is unclear why CRLS protein content was decreased specifically in the IFM though the observation may be the result of decreased protein import specifically in the diabetic IFM, which has been reported previously (Baseler et al. 2011).
Because CRLS was the only enzyme affected by diabetic insult, we determined whether the response influenced the activity of the CRLS enzyme. Using 14C-oleoyl-coenzyme A as an acyl donor, we measured incorporation of 14C to assess CRLS activity. One limitation to this method is that remodeling of cardiolipin is not taken into account and other methods do not possess this limitation. Nie et al. (Nie et al. 2010) reported that lysophosphatidylglycerol acyltransferase also acts as a CRLS, converting lyso-PG to PG; therefore, to ensure the CRLS activity assay was only measuring the incorporation of 14C into cardiolipin, we measured the incorporation of 14C into PG. Results indicated no change in 14C- PG levels between diabetic and control samples for either subpopulation, and a significant decrease in 14C-cardiolipin levels in diabetic IFM compared to control, with no change in the SSM. These results instill confidence that the methods utilized in our studies are reliable.
Though our data indicated decreased CRLS protein content and activity in the diabetic IFM relative to control with no change observed in the SSM, the protein content of CRLS was not different between the SSM and IFM subpopulations; therefore, this eliminated the possibility that absolute CRLS protein levels underlie the decreased CRLS activity observed in the IFM. Hatch et al. (Hatch et al. 1995) reported no change in PG or cardiolipin biosynthesis during a 4 day or 28 day diabetic insult in rats and Taylor et al. (Taylor et al. 2002) suggested that neither cardiolipin content nor CRLS activity was altered during STZ-induced diabetes. These results were obtained by examining total mitochondria as opposed to our current study examining mitochondrial subpopulations, which may explain the different results between studies. In either case, the results support the importance of examining the two distinct pools of mitochondria.
Previous research has indicated an association between cardiolipin and ETC complexes I, III and IV, from which the contact points with cardiolipin have been identified (Fry and Green 1981; Sedlak and Robinson 1999). Literature has also shown an association between cardiolipin and ATP synthase (Acehan et al. 2011a; Eble et al. 1990). One study demonstrated that cardiolipin is central for the assembly of ATP synthase dimers, affecting the organization and ultimately the structure of the IMM (Acehan et al. 2011a). Other studies have suggested that the interaction between cardiolipin and the ATP synthase complex occurs on subunits composing the F0 complex of the ATP synthase due to its location within the IMM (Eble et al. 1990). Although an association between cardiolipin and ATP synthase has been reported, the direct contact points have not been identified. For this reason, we used a novel cardiolipin-coated bead assay to separate out the two pools of specific ATP synthase subunits – those already bound to native cardiolipin and those available to bind to the cardiolipin-coated beads. The ATP synthase subunits predicted to interact with cardiolipin were identified using previous proteomic data performed in our laboratory (Baseler et al. 2011). Western blot analysis revealed two direct associations between cardiolipin and ATP synthase.
It is important to note that although the cardiolipin-bead pull-down assay is a quick and accessible method to gather cardiolipin-protein interaction data, the assay requires a large amount of protein. This is a limitation when measuring associations within mitochondrial subpopulations, due to limited yields of mitochondrial subpopulation protein content as compared to the analysis of total mitochondria. Nevertheless, we are the first to detect direct associations between cardiolipin and two specific ATP synthase subunits.
To gain insight into whether cardiolipin association with the identified ATP synthase subunits was compromised in diabetic mitochondria, we performed analyses in control and diabetic mitochondria. Western blot analysis revealed a lighter band when measuring the association between specific ATP synthase subunits and native cardiolipin (represented by the native bound lane) in the diabetic IFM compared to control. This finding suggests that the association between the native form of cardiolipin and the ATP synthase subunits is less in the diabetic IFM compared to control. Western blot analysis also revealed a denser band when measuring the association between the subunit and cardiolipin-coated beads (represented by the bead bound lane) in the diabetic IFM compared to control. The denser band suggests that more ATP synthase subunits are not in association with cardiolipin, but are free to bind to the cardiolipin-coated beads in the diabetic IFM. Taken together, these results indicates that the decrease in interaction between native cardiolipin and ATP synthase could be an underlying mechanism causing decreased ATP synthase activity in the diabetic IFM.
A number of studies have utilized cells lines to examine the physiological role of cardiolipin. When the structural gene encoding CRLS was disrupted resulting in depletion of cardiolipin in the mitochondrial membranes of S. cerevisiae cells, ATPase activities, cytochrome oxidase activities, membrane potential and protein import were all decreased (Jiang et al. 2000). This study concluded that cardiolipin is required for maintaining mitochondrial function. Chen et al. (Chen et al. 2006) identified and characterized the cDNA encoding human CRLS. The human CRLS was expressed in COS-7 cells from which cardiolipin was not only efficiently synthesized in vitro using CDP-DAG and PG as substrates, but was also synthesized at an increased rate. Kiebish et al. (Kiebish et al. 2012) determined that CRLS overexpression in a STZ-induced diabetic model attenuated mitochondrial dysfunction associated with diabetes mellitus. Although these authors did not find a difference in total cardiolipin content between diabetic and control hearts, the overexpression of CRLS increased cardiac cardiolipin remodeling and lipidomic flux, which in turn, increased tetralinoleic cardiolipin. The novel finding of CRLS-mediated regulatory control was central in the preservation of mitochondrial function during diabetic insult. This study further supports the importance of IMM preservation.
Though our data suggest that changes in essential constituents in the cardiolipin biosynthetic pathway (i.e. CRLS) are associated with a decrease in cardiolpin, it is important to note that diabetes mellitus is a multifactorial disease that influences a number of processes, specifically in mitochondria. Increased content of the oxidized form of cardiolipin in the diabetic IFM has been reported by our laboratory (Dabkowski et al. 2009), thus one must consider that an oxidative stress pathway may also be contributing to the results reported in this study. Increased oxidation of cardiolipin would likely predispose the phospholipid to loss, influencing mitochondrial functionality (Ma et al. 2012). Future studies in which manipulation of mitochondrial oxidative milieu specifically at the IMM may lend insight into the contribution of oxidative stress.
CONCLUSION
In conclusion, our results indicate the importance of preserving the IMM during type 1 diabetes mellitus. We show dysregulation of the cardiolipin biosynthetic pathway during diabetic insult, as seen by a decrease in CRLS protein content and activity in the diabetic IFM. These studies are the first to demonstrate a direct association between cardiolipin and ATP synthase F0-complex subunit b and d in both mitochondrial subpopulations. CRLS and IMM conservation represents a potential therapeutic target that may be manipulated for treatment against development of cardiomyopathy associated with the type 1 diabetic heart.
Acknowledgments
We would like acknowledge Dr. Vazhaikkurichi Rajendran in the Biochemistry Department at West Virginia University for the assistance and expertise during the CRLS activity assay experimentation.
This work was supported by the National Institutes of Health from the National Institutes of Diabetes and Digestive and Kidney Diseases [DP2DK083095]. This work was also supported by a Grant-In-Aid from the American Heart Association [0855484D]. Tara Croston is a recipient of an NIH Predoctoral Fellowship [T32HL090610]. Danielle Shepherd is a recipient of an NIH Predoctoral Fellowship [T32HL090610]. Erinne Dabkowski is a recipient of an American Heart Association Predoctoral Fellowship [0815406D]. Cody Nichols is a recipient of an Integrative Graduate Education and Research Traineeship Program [DGE-1144676].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acehan D, Malhotra A, Xu Y, Ren M, Stokes DL, Schlame M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys J. 2011a;100:2184–92. doi: 10.1016/j.bpj.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acehan D, Vaz F, Houtkooper RH, James J, Moore V, Tokunaga C, Kulik W, Wansapura J, Toth MJ, Strauss A, Khuchua Z. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem. 2011b;286:899–908. doi: 10.1074/jbc.M110.171439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseler WA, Dabkowski ER, Williamson CL, Croston TL, Thapa D, Powell MJ, Razunguzwa TT, Hollander JM. Proteomic alterations of distinct mitochondrial subpopulations in the type 1 diabetic heart: contribution of protein import dysfunction. Am J Physiol Regul Integr Comp Physiol. 2011;300:R186–200. doi: 10.1152/ajpregu.00423.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhang XY, Shi Y. Identification and functional characterization of hCLS1, a human cardiolipin synthase localized in mitochondria. Biochem J. 2006;398:169–76. doi: 10.1042/BJ20060303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- Dabkowski ER, Baseler WA, Williamson CL, Powell M, Razunguzwa TT, Frisbee JC, Hollander JM. Mitochondrial dysfunction in the type 2 diabetic heart is associated with alterations in spatially distinct mitochondrial proteomes. Am J Physiol Heart Circ Physiol. 2010;299:H529–40. doi: 10.1152/ajpheart.00267.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabkowski ER, Williamson CL, Bukowski VC, Chapman RS, Leonard SS, Peer CJ, Callery PS, Hollander JM. Diabetic cardiomyopathy-associated dysfunction in spatially distinct mitochondrial subpopulations. Am J Physiol Heart Circ Physiol. 2009;296:H359–69. doi: 10.1152/ajpheart.00467.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabkowski ER, Williamson CL, Hollander JM. Mitochondria-specific transgenic overexpression of phospholipid hydroperoxide glutathione peroxidase (GPx4) attenuates ischemia/reperfusion-associated cardiac dysfunction. Free Radic Biol Med. 2008;45:855–65. doi: 10.1016/j.freeradbiomed.2008.06.021. [DOI] [PubMed] [Google Scholar]
- Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of Diabetes on Cardiac Structure and Function : The Strong Heart Study. Circulation. 2000;101:2271–6. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- Eble KS, Coleman WB, Hantgan RR, Cunningham CC. Tightly associated cardiolipin in the bovine heart mitochondrial ATP synthase as analyzed by 31P nuclear magnetic resonance spectroscopy. J Biol Chem. 1990;265:19434–40. [PubMed] [Google Scholar]
- Fry M, Green DE. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. Journal of Biological Chemistry. 1981;256:1874–80. [PubMed] [Google Scholar]
- Haines TH, Dencher NA. Cardiolipin: a proton trap for oxidative phosphorylation. FEBS Letters. 2002;528:35–9. doi: 10.1016/s0014-5793(02)03292-1. [DOI] [PubMed] [Google Scholar]
- Han X, Yang J, Cheng H, Yang K, Abendschein DR, Gross RW. Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry. 2005;44:16684–94. doi: 10.1021/bi051908a. [DOI] [PubMed] [Google Scholar]
- Han X, Yang J, Yang K, Zhao Z, Abendschein DR, Gross RW. Alterations in Myocardial Cardiolipin Content and Composition Occur at the Very Earliest Stages of Diabetes: A Shotgun Lipidomics Study. Biochemistry. 2007;46:6417–28. doi: 10.1021/bi7004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch GM, Cao SG, Angel A. Decrease in cardiac phosphatidylglycerol in streptozotocin-induced diabetic rats does not affect cardiolipin biosynthesis: evidence for distinct pools of phosphatidylglycerol in the heart. Biochem J. 1995;306 ( Pt 3):759–64. doi: 10.1042/bj3060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper R, Vaz F. Cardiolipin, the heart of mitochondrial metabolism. Cellular and Molecular Life Sciences. 2008;65:2493–506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Ryan MT, Schlame M, Zhao M, Gu Z, Klingenberg M, Pfanner N, Greenberg ML. Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J Biol Chem. 2000;275:22387–94. doi: 10.1074/jbc.M909868199. [DOI] [PubMed] [Google Scholar]
- Kiebish MA, Yang K, Sims HF, Jenkins CM, Liu X, Mancuso DJ, Zhao Z, Guan S, Abendschein DR, Han X, Gross RW. Myocardial Regulation of Lipidomic Flux by Cardiolipin Synthase: SETTING THE BEAT FOR BIOENERGETIC EFFICIENCY. J Biol Chem. 2012;287:25086–97. doi: 10.1074/jbc.M112.340521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lu B, Xu FY, Jiang YJ, Choy PC, Hatch GM, Grunfeld C, Feingold KR. Cloning and characterization of a cDNA encoding human cardiolipin synthase (hCLS1) J Lipid Res. 2006;47:1140–5. doi: 10.1194/jlr.C600004-JLR200. [DOI] [PubMed] [Google Scholar]
- Ma ZA, Zhao Z, Turk J. Mitochondrial dysfunction and beta-cell failure in type 2 diabetes mellitus. Exp Diabetes Res. 2012;2012:703538. doi: 10.1155/2012/703538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkler PE, Hoppel CL. Separation and characterization of cardiolipin molecular species by reverse–phase ion pair high-performance liquid chromatography-mass spectrometry. Journal of Lipid Research. 2010;51:856–65. doi: 10.1194/jlr.D002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J, Hao X, Chen D, Han X, Chang Z, Shi Y. A novel function of the human CLS1 in phosphatidylglycerol synthesis and remodeling. Biochim Biophys Acta. 2010;1801:438–45. doi: 10.1016/j.bbalip.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. Journal of Biological Chemistry. 1977;252:8731–9. [PubMed] [Google Scholar]
- Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res. 2000;39:257–88. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- Sedlak E, Robinson NC. Phospholipase A(2) digestion of cardiolipin bound to bovine cytochrome c oxidase alters both activity and quaternary structure. Biochemistry. 1999;38:14966–72. doi: 10.1021/bi9914053. [DOI] [PubMed] [Google Scholar]
- Severson DL. Diabetic cardiomyopathy: recent evidence from mouse models of type 1 and type 2 diabetes. Can J Physiol Pharmacol. 2004;82:813–23. doi: 10.1139/y04-065. [DOI] [PubMed] [Google Scholar]
- Sparagna GC, Johnson CA, McCune SA, Moore RL, Murphy RC. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. Journal of Lipid Research. 2005;46:1196–204. doi: 10.1194/jlr.M500031-JLR200. [DOI] [PubMed] [Google Scholar]
- Taylor WA, Xu FY, Ma BJ, Mutter TC, Dolinsky VW, Hatch GM. Expression of monolysocardiolipin acyltransferase activity is regulated in concert with the level of cardiolipin and cardiolipin biosynthesis in the mammalian heart. BMC Biochem. 2002;3:9. doi: 10.1186/1471-2091-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M, Mukae S, Geshi E, Umetsu K, Nakatani M, Katagiri T. Mitochondrial respiratory impairment in streptozotocin-induced diabetic rat heart. Jpn Circ J. 1996;60:673–82. doi: 10.1253/jcj.60.673. [DOI] [PubMed] [Google Scholar]
- Waring AJ, Rottenberg H, Ohnishi T, Rubin E. Membranes and phospholipids of liver mitochondria from chronic alcoholic rats are resistant to membrane disordering by alcohol. Proc Natl Acad Sci U S A. 1981;78:2582–6. doi: 10.1073/pnas.78.4.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson CL, Dabkowski ER, Baseler WA, Croston TL, Alway SE, Hollander JM. Enhanced apoptotic propensity in diabetic cardiac mitochondria: influence of subcellular spatial location. Am J Physiol Heart Circ Physiol. 2010;298:H633–42. doi: 10.1152/ajpheart.00668.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KK, Huan Y. Current Protocols in: Pharmacology. John Wiley & Sons, Inc; 2001. Streptozotocin-Induced Diabetic Models in Mice and Rats. [DOI] [PubMed] [Google Scholar]