Abstract
Background & Aims
Hepatocellular carcinoma (HCC) develops in response to chronic hepatic injury. Although induced cell death is regarded as the major component of p53 tumor-suppressive activity, we recently found that sustained p53 activation subsequent to DNA damage promotes inflammation-associated hepatocarcinogenesis. Here we aim to explore the mechanism linking p53 activation and hepatic inflammation during hepatocarcinogenesis.
Methods
p53−/− hepatocytes expressing inducible p53 and primary wild type hepatocytes were treated to induce p53 expression. The supernatants were collected and analyzed for the presence of released inflammatory cytokines. Ethyl pyruvate was used in a rat model of carcinogen-induced hepatocarcinogenesis to examine its effect on p53-dependent chronic hepatic injury, inflammation and tumorigenesis.
Results
Here we show that cytoplasmic translocation and circulating levels of potent inflammatory molecule high-mobility group protein 1 (HMGB1) were greater in wild-type rats than in p53+/− rats following carcinogen administration. Restoration of p53 expression in p53-null hepatocytes or induction of endogenous p53 in wild-type hepatocytes gives rise to the release of HMGB1. Administration of the HMGB1 release inhibitor ethyl pyruvate, which does not affect p53-mediated hepatic apoptosis, substantially prevented carcinogen-induced cirrhosis and tumorigenesis in rat livers.
Conclusions
These results suggest that although p53 is usually regarded as a tumor suppressor, its constant activation can promote pro-tumorigenic inflammation, at least in part, via inducing HMGB1 release. Application of HMGB1 inhibitors when restoring p53 in cancer therapy might protect against pro-tumorigenic effects while leaving p53-mediated clearance of malignant cells intact.
Introduction
A strong association between chronic tissue injury and tumorigenesis has been established by many clinical data [1–3]. The mammalian liver is vulnerable to damage induced by toxic chemicals and hepatitis viruses, all of which increase the risk of hepatocellular carcinoma (HCC). Chronic hepatic inflammation, as a consequence of chronic injury, frequently precedes the development of HCC[4]. However, the molecular mechanisms responsible for chronic hepatic injury and inflammation remain elusive.
The tumor suppressor p53 is activated by a wide variety of stress signals that a cell might encounter during malignant progression—genotoxic damage, oncogene activation, and hypoxia. Induction of apoptosis or senescence is the key mechanism by which p53 eliminates cancer cells. p53 can also prevent cancer development through a number of other mechanisms. p53 has been shown to promote autophagy through negative regulation of mTOR signaling and act as an antioxidant to prevent DNA damage and genome instability [5, 6]. When the liver is poisoned by genotoxic chemicals, p53 induces hepatocytes to commit suicide or go into growth arrest. It is therefore conceivable that p53 contributes to chronic tissue injury especially in livers exposed to agents that inflict mutagenic DNA damage. Consistent with this notion, we recently found that in a rat model of carcinogen-induced hepatocarcinogenesis, heterozygous deficiency of p53 results in attenuated hepatic injury, inflammatory responses and tumorigenesis [7]. However, it is still unknown how p53 links hepatocyte death to the activation of tissue inflammation.
High-mobility group box 1 (HMGB1) is a nuclear constituent loosely bound to chromatin that signals tissue damage when released into the extracellular medium, and thus acts as a damage-associated molecular pattern (DAMP) [8]. Extracellular HMGB1 is responsible for the inflammatory response to hepatic injury, as shown in models of acute liver toxicity and liver ischemia-reperfusion [9]. Here we show that sustained p53 activation causes chronic liver injury and gives rise to the release of HMGB1, which may contribute to inflammation-associated hepatocarcinogenesis.
Materials and methods
Animals
Generation of rats lacking one or both alleles of p53 by gene targeting of DAc8 rat ES cells has been described previously [10]. Rats that were p53 heterozygous were intercrossed to produce rats with no, one, or two p53-null alleles. These rats were designated wild-type, heterozygotes (p53+/−) or homozygotes (p53−/−), respectively, and were monitored for DEN-induced hepatocarcinogenesis.
HCC induction by DEN
HCCs in rats were chemically induced by weekly intraperitoneal (i.p.) administration of DEN (70 mg/kg body weight; Sigma-Aldrich) for 10 weeks as described previously [11, 12]. At the indicated times, rats were sacrificed and the livers immediately removed, weighed, and placed in ice-cold phosphate-buffered saline (PBS). The externally visible tumors (≥ 2 mm) were counted and measured by stereomicroscopy. Tumor size was measured using a vernier caliper. Parts of the livers were fixed in 4% paraformaldehyde and paraffin-embedded for histological evaluation. p53−/− rats were excluded from the study due to early spontaneous tumor development. p53+/− rats that developed hemangiosarcoma after DEN treatment were also excluded from analysis.
Animal treatment
For ethyl pyruvate (EP) treatment, pathogen-free male Sprague-Dawley (SD) rats (weighing 160–180 g) were grouped randomly and treated with saline or 40 mg/kg ethyl pyruvate (Sigma-Aldrich) i.p. every other day. The EP regimen started 1 week prior to DEN injection and continued until six weeks after the 10-week DEN treatment had ended. For in vivo adenoviral delivery of HMGB1, 200µl of saline or 3×109 pfu of Ad5-CMV-GFP or Ad5-CMV-HMGB1 were administered via tail vein injection into male SD rats (5 animals per group). All procedures were performed according to investigator’s protocols approved by the USC Institutional Animal Care and Use Committee (IACUC) or the Second Military Medical University Animal Ethics Committee.
Cell culture
Hepatic Kupffer cells and stellate cells were isolated according to the methods of Mello et. al. [13]. Primary hepatocytes were isolated from 250–300 g WT, p53+/−, and p53−/− (p53-null) rats by a two-step perfusion method. The animals were anesthetized by injecting sodium pentobarbital solution (0.1 mL/100 g of body wt) intraperitoneally and their livers were removed intact. The liver was first perfused in vitro via the portal vein with warmed (37°C) Ca2+ and Mg2+ free Hanks balanced salt solution at a flow rate of 5–8 ml/min for 15 min, and then perfused with 0.05% collagenase (Sigma, Type IV) in the same solution supplemented with CaCl2 to a final concentration of 5 mM and HEPES to a final concentration of 50 mM. The reperfusion with collagenase solution lasted 25 min at a rate of 5 ml/min at 37°C. After 10 min of incubation (37°C) with gentle shaking, the suspension was filtered and hepatocytes were sedimented at 70 g for 1 min. All harvests yielded hepatocytes with viability exceeding 90% as indicated by trypan-blue dye exclusion. Isolated hepatocytes were seeded on rat tail collagen I-coated tissue culture dishes and cultured in DMEM/F12 with ITS (Sigma) plus growth factors EGF and HGF (20 ng/ml of each, Peprotech) as the standard medium. Propagation of the p53-null hepatocyte line was performed by passaging cells at a ratio of 1:3 twice per week in the standard medium.
Generation of ip53-hepatocytes
To achieve post-translational regulation of p53 in these cells, rat p53 was fused to an FK506- and rapamycin-binding protein (FKBP)-based destabilizing domain and a transgene for expressing this fusion protein was stably integrated into the p53-null hepatocyte cell line. Expression of this p53 fusion protein can be reversibly modulated by adding or removing the synthetic stabilizing ligand Shield1 (1:2000, Clontech).
ELISArray and ELISA assay
Amounts of inflammatory cytokines in serum of rat and supernatants of macrophages or hepatocytes were measured by a commercially available EILSA kit (Peprotech). To survey p53-induced pro-inflammatory cytokines, we treated the ip53-hepatocytes or primary WT hepatocytes with Shield1 (S1) or Nutlin-3 for 6 or 24 hours. The conditioned media were filtered and qualitatively assayed for various cytokines using Rat Inflammatory Cytokines Multi-Analyte ELISArray Kit (Qiagen).
Detection of HMGB1 release and immunodepletion of HMGB1 from supernatants
For detection of secreted HMGB1 by Western blotting, serum or cell culture-conditioned medium was first filtered through a Centrifugal Filter Unit with Ultracel-50 membrane (Millipore) to clear the samples of cell debris and macromolecular complexes. Samples then were concentrated 15-fold with a Centrifugal Filter Unit with Ultracel-10 membrane and subjected to Western blotting. Amounts of HMGB1 in serum of rats were also measured by a commercially available EILSA kit (IBL international). HMGB1 depletion was performed in two rounds by incubating 0.5 ml of cell supernatant with 20 µg anti-HMGB1 for 16 h at 4°C. Immune complexes were removed by incubation in the presence of 75µl protein G sepharose for 1 h at 4°C under agitation.
LDH (lactate dehydrogenase) release assay
Cell viability was measured by calculating the ratio of released LDH activity to total activity as described by the manufacturer (CytoTox 96® Non-Radioactive Cytotoxicity Assay Kit, Promega).
Histological analysis
Immunohistochemistry staining was performed [on formalin-fixed 5-µm paraffin-embedded tissue sections using UltraVision Quanto Detection System AP (Alkaline Phosphatase) or HRP. Quantification was done using Image J (National Institutes of Health, Bethesda, MD, USA) and Photoshop software (Adobe Corporation, San Jose, CA, USA) using at least 50 images per rat at ×20 magnification for cytoplamic HMGB1 staining.
Antibodis
The antibodies used and the dilution factors were as follows: anti-p53 (1C12) (Cell Signaling; 1:1000 for WB), anti-cleaved caspase-3 (5A1E) (Cell Signaling; 1:1000 for WB), anti-HMGB1 (ab18256) (Abcam; 1:1000 for WB, 1:500 for IHC). IHC, immunohistochemistry; WB, Western blot.
ROS detection
The generation of hydrogen peroxide and superoxide in cell culture was detected using CellROX™ Deep Red dye and MitoSOX™ Red mitochondrial superoxide indicator (Invitrogen), respectively, following the manufacturer's instructions.
Statistical analysis
All data are expressed as means with error bars representing the standard deviation. Statistical calculations were performed using Prism (Graphpad). For comparisons of two groups, two-tailed unpaired Student’s t-tests were performed. For multiple group comparisons with normal distribution, ANOVA with Tukey’s analysis was performed. A p value < 0.05 was considered statistically significant.
Results
p53 deficiency does not affect the innate inflammatory response of non-parenchymal cells in liver
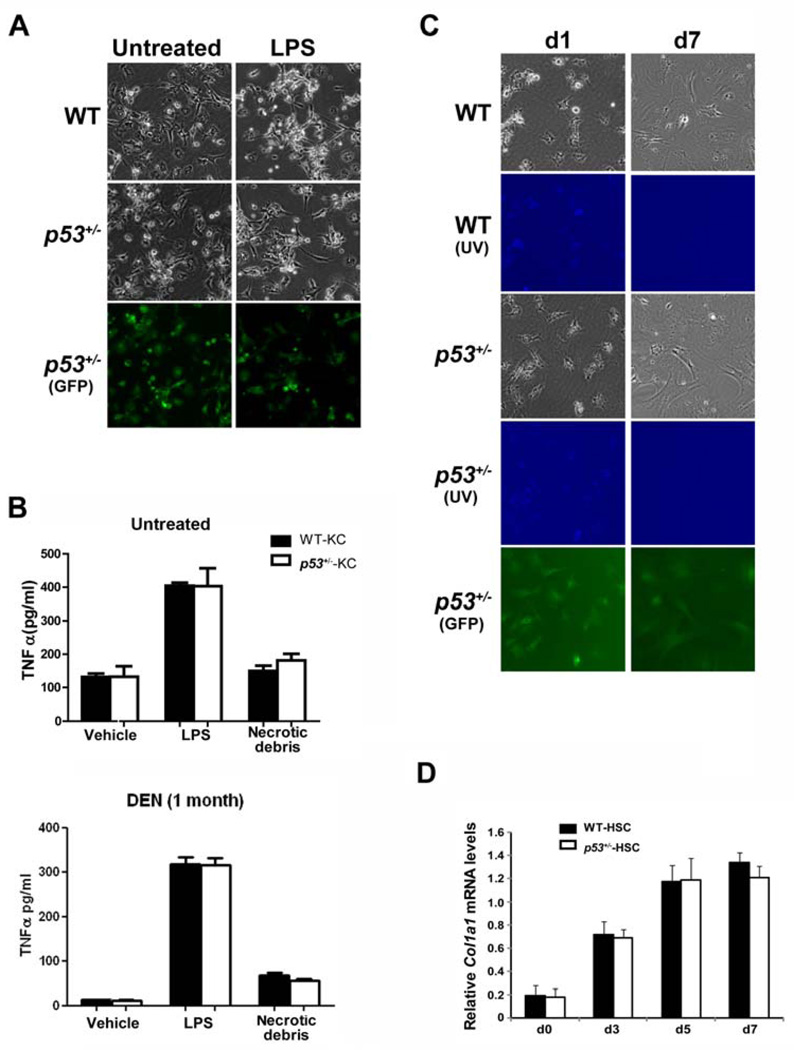
We recently found that p53 deficiency mitigates carcinogen-induced hepatic inflammation, cirrhosis and tumorigenesis [14]. Kupffer cells and hepatic stellate cells (HSCs) are key mediators of hepatic inflammation and fibrogenesis. They have critical roles in both HCC initiation and various key steps in tumor promotion and show a remarkable degree of plasticity in phenotypes during the course of tumor progression [15, 16]. To explore whether they mediate p53-dependent inflammatory effects in the DEN model, we isolated Kupffer cells resident in the unmanipulated or DEN-treated wild-type or p53+/− rats to compare their inflammatory responses to either the exogenous ligand lipopolysaccharide (LPS) or the endogenous stimuli, like necrotic hepatocyte debris. Kupffer cells of both genotypes produced comparable levels of TNFα, a major mediator of stellate cell collagen synthesis, when incubated with either LPS or cellular debris from necrotic hepatocytes (Fig.1A,B). The activation of hepatic stellate cells (HSCs) was also similar between both genotypes as indicated by loss of the characteristic autofluorescence under ultraviolet excitation and induction of the bona-fide HSC activation marker collagen-α1 (Fig. 1C,D). These results indicate that p53-dependent profibrogenic effects in the DEN model might not be due to innate differences between Kupffer cell- and stellate cell-mediated inflammatory reactions. Given DEN is metabolically inactive in these non-parenchymal liver cells, we postulated that parenchymal cells, such as hepatocytes, secrete factors responsible for eliciting the inflammatory response that sustains liver damage and promotes tumor progression. We reasoned that constant p53 activation in hepatocytes triggers the secretion of such inflammatory factors.
Figure 1. p53 deficiency does not affect the innate inflammatory response of non-parenchymal cells in liver.
(A–B) Kupffer cells were isolated from untreated or DEN-treated (i.p. injection with 70 mg/kg DEN weekly for one month) WT and p53+/− rats of the same littermate and treated with lipopolysaccharide (LPS, 10 ng/ml) or necrotic cellular debris prepared by cycles of freeze-thawing of primary hepatocytes for 16 h. Following treatment, cultures were analyzed for morphological evidence of Kupffer cell activation (A) and TNFα production in the media using ELISA (B).
(C–D) Quiescent hepatic stellate cells from WT and p53+/− rats were cultured on plastic for 7 days. Activation of stellate cells is characterized by the development of fibroblastic morphology as well as loss of intracellular vitamin A content as assessed by UV-excited autofluorescence (UV) (C), concomitant with induction of the HSC activation marker Col1a1 (D). Data are presented as mean ± s.d. Cells isolated from heterozygous p53tm1(EGFP-pac) rats (p53+/− rats) express GFP as a conceptual proof.
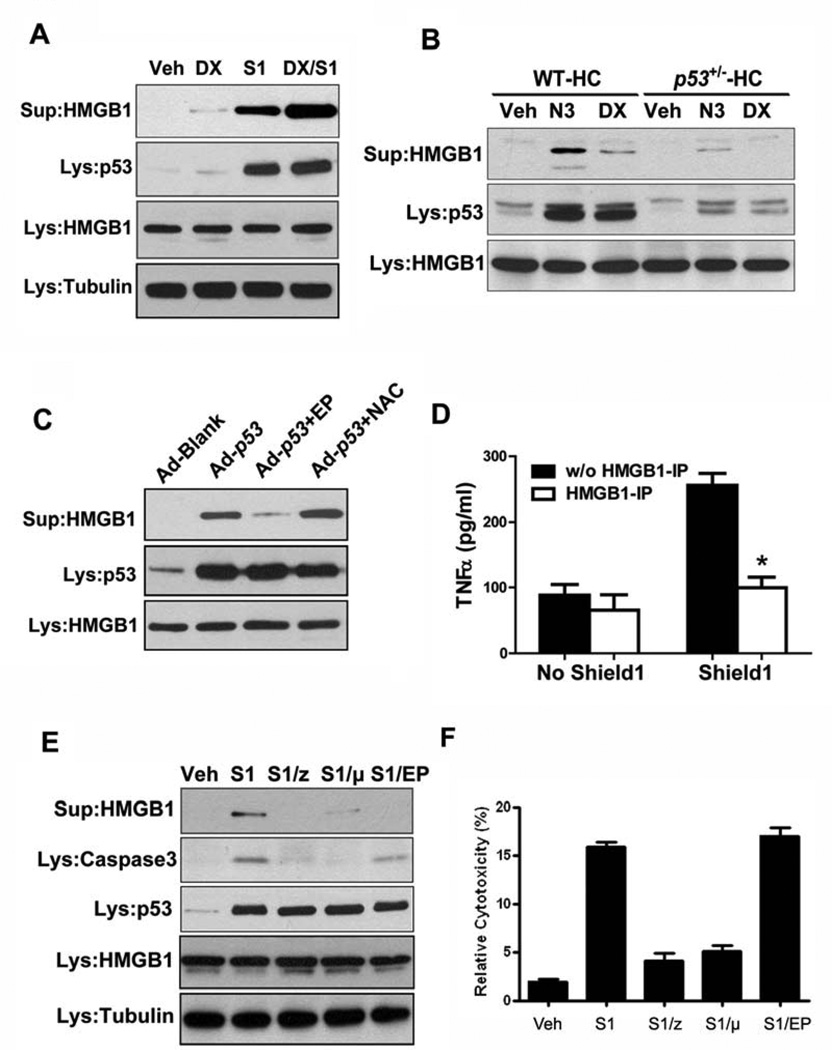
p53 activation leads to HMGB1 release in hepatocytes
To evaluate that hypothesis, we established a hepatocyte cell line isolated from p53-null rats, based on the rationale that loss of p53 bypasses cell-cycle arrest and cellular senescence (Supplementary Fig.1A,B). The cells were engineered to express a post-translationally inducible version of p53 that can mimic the inherent instability of the wild-type protein (ip53-hepatocytes, Supplementary Fig. 1C)[17]. Induction of p53 led to hepatocyte apoptosis as indicated by cleavage of Caspase-3. No obvious senescence could be detected based on the expression of p16INK4, a bona-fide marker of cellular senescence (Supplementary Fig. 2A). Furthermore, a cytokine array revealed that production of senescence-associated inflammatory cytokines such as IL-1 and IL-6 [18, 19] was unaffected by p53 induction (Supplementary Fig. 2B).
Interestingly, the release of HMGB1, a key alarmin that triggers inflammatory responses, was markedly increased following p53 induction and further enhanced in the presence of doxorubicin, a DNA damaging agent. In the absence of p53, however, doxorubicin induced relatively little HMGB1 release (Fig.2A), suggesting that p53 activation is mainly responsible for HMGB1 release. Consistent with these findings, activation of p53 by Mdm2 inhibitor Nutlin-3, which was used to specifically stabilize p53 protein, or doxorubicin elicited much more HMGB1 release from primary wild-type hepatocytes than from p53+/− hepatocytes (Fig.2B). Nutlin-3 seemed to have more potent apoptotic effects than doxorubicin did at the doses used as indicated by the increased cell death in Nutlin-3 treated cells (Supplementary Fig. 2C). Furthermore, the exogenous expression of p53 by adenoviral vector also led to substantial HMGB1 release from wild-type primary hepatocytes (Fig.2C).
Figure 2. p53 activation leads to HMGB1 release in hepatocytes.
(A) The ip53-hepatocytes were treated with Doxorubicin (DX), Shield1 (S1) or both (DX/S1) for 6 h. Concentrated supernatants and total cell lysates were sampled and subjected to Western blot analysis for HMGB1 and p53. Veh, vehicle only; Lys, total cell lysate; Sup, concentrated supernatant.
(B) Primary WT and p53+/− hepatocytes were treated with Nutlin-3 (N3) or doxorubicin (DX) for 6h and the HMGB1 release in the concentrated supernatants was measured by immunoblotting.
(C) Primary WT hepatocytes were infected with blank control adenovirus (Ad-blank) or p53-expressing adenovirus (Ad-p53) for 12 hours. EP (5 mM) or NAC (N-acetylcysteine, 10 mM) were added to some Ad-p53-infected cells for the last 6 hours of incubation. The released HMGB1 in the concentrated supernatants was measured by immunoblotting.
(D) Primary WT rat macrophages were stimulated with the conditioned medium from Shield1-treated ip53-hepatocytes with or without HMGB1 immunodepletion. Supernatants were collected after 16 hours and TNFα levels were quantified using a commercial ELISA kit. w/o, without; IP, immunodepletion. Results are means ± s.d., n = 3 in each group, p = 0.0231.
(E) The ip53-hepatocytes were treated with Shield1 in the presence of Z-VAD-FMK (50 µM, z), Pifithrin-µ (10 µM, µ) or ethyl pyruvate (5 mM, EP) for 6h, and the HMGB1 release was measured by immunoblotting. Note that EP does not inhibit p53-induced apoptosis (Caspase-3 cleavage) but efficiently prevented HMGB1 release.
(F) The ip53-hepatocytes were treated as above in (E) and the cell viability was measured by calculating the ratio of released LDH activity to total activity.
Although ROS have been shown to activate HMGB1 release from cultured hepatocytes [20], intracellular concentrations of hydrogen peroxide and superoxide were not elevated upon p53 induction in hepatocytes (Supplementary Fig. 2D). ROS production was markedly induced by doxorubicin but not by p53 (Supplementary Fig. 2D), while considerable HMGB1 release was elicited by p53 but not doxorubicin (Fig.2A), suggesting that HMGB1 release from hepatocytes is mainly dependent on p53 rather than ROS production. In accordance, treatment with the antioxidant N-acetylcysteine (NAC) exerted little effect on p53-induced HMGB1 release (Fig.2C). By contrast, ethyl pyruvate (EP), a stable lipophilic pyruvate derivative recently found to be a potent inhibitor of HMGB1 release from endotoxin-stimulated macrophages [21], efficiently prevented the HMGB1 release induced by exogenous p53 expression driven by adenoviral vector (Fig.2C).
As secreted HMGB1 is known to be a cytokine mediator of inflammation [22], we then analyzed the functional requirement for HMGB1 in the p53-induced inflammatory response. Exposing rat peritoneal macrophages to conditioned supernatants from ip53-hepatocytes with p53 induction stimulated TNFα release, whereas immunodepletion of HMGB1 from the supernatants eliminated this effect (Fig.2D). These data suggest a key role for HMGB1 in eliciting inflammatory responses in the context of p53-mediated hepatic injury.
When ip53-hepatocytes were treated with pan-caspase inhibitor Z-VAD-fmk and p53 inhibitor pifithrin-µ, both of which were able to inhibit p53-induced apoptosis, HMGB1 release was largely abrogated (Fig.2E). We then tested whether EP has an inhibitory effect on p53-induced cell death and HMGB1 release. Incubation with 5 mM EP did not affect p53-induced apoptosis, as indicated by Caspase-3 cleavage, but efficiently prevented HMGB1 release from ip53-hepatocytes with p53 induction (Fig.2E). Analysis of cell viability revealed that p53 activation led to the increased cell death and loss of membrane integrity, indicating possible leakage of cytoplasmic content to the outside (Fig. 2F). Taken together, these results suggest that p53-induced cell death is mainly responsible for HMGB1 release in hepatocytes.
Inhibition of HMGB1 release mitigates DEN-induced hepatic injury and tumorigenesis
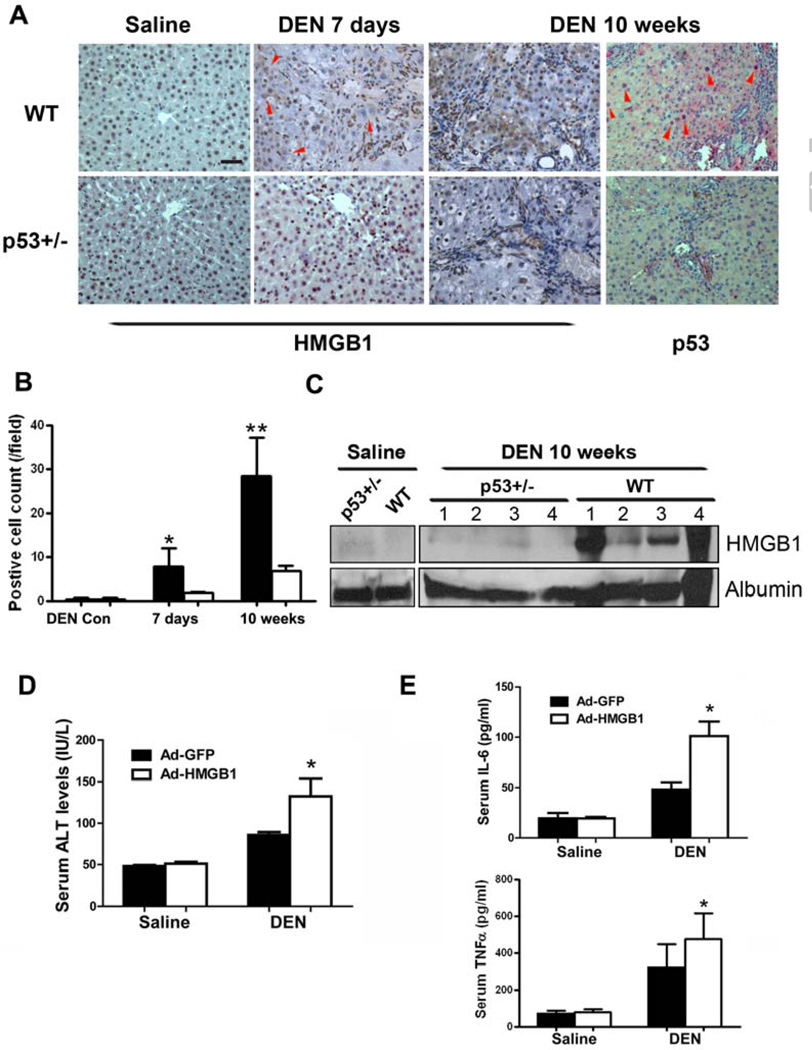
We have recently shown that DEN treatment results in sustained DNA damage and p53 activation in rat livers [14]. To investigate whether DEN treatment could also lead to HMGB1 release in vivo, the cellular localization of HMGB1 in DEN-treated livers was examined as HMGB1 must transit from the nucleus, through the cytoplasm, to the outside of the cell, where it can act as an inflammatory cytokine. This translocation could represent the passive release of HMGB1 during liver cell death [23]. Immunohistochemistry analysis revealed that the cytoplasmic translocation of HMGB1 was more frequent in wild-type livers than in p53+/− livers at day 7 as well as 10 weeks post DEN administration (Fig.3A,B). Nuclear accumulation of p53 in wild-type liver provided direct evidence of sustained p53 activation after 10-week DEN treatment (Fig.3A). Furthermore, after long-term DEN treatment, the circulating levels of HMGB1 were also substantially elevated in wild-type rats compared with p53+/− rats (Fig.3C). To test the in vivo effects of secreted HMGB1 in DEN-induced hepatic injury, we generated adenoviruses that target expression of HMGB1 or GFP to the liver. Adenovirally expressed HMGB1 in liver without DEN injection did not cause observable hepatic injury inflammatory response (Fig.3D,E). However, liver toxicity and inflammatory cytokine production were significantly greater in DEN-treated rats intravenously injected with Ad-HMGB1 than in equivalently treated GFP control rats (Fig.3D,E), suggesting that the increased HMGB1 expression and release exacerbated DEN-induced hepatic injury, possibly via stimulating the inflammatory reaction.
Figure 3. Secreted HMGB1 contributes to DEN-induced chronic hepatic injury and tumorigenesis.
(A) Immunohistochemistry analysis of HMGB1 and p53 in liver sections of WT rats compared with p53+/− rats 7 days or 10 weeks after DEN treatment. Red arrowheads indicate areas showing cytoplasmic translocation of HMGB1 or nuclear accumulation of p53. Scale bars, 50 µm.
(B) Quantification of cells positive for cytoplasmic HMGB1 in liver sections of (A). Results are mean±s.d., *P<0.05, **P<0.01.
(C) Circulating levels of HMGB1 were measured by immunoblotting in WT and p53+/− rats on week 10 of DEN treatment. Albumin in serum was blotted as loading control. Numbers indicate individual rats.
(D) Wild-type rats were i.v. injected with recombinant adenoviruses expressing GFP or HMGB1 and, 24h later, were treated with saline or DEN. Forty eight hours later, serum levels of ALT were measured. Results are means ± s.d., n = 5 in each group. p = 0.0345 for ALT
(E) Serum levels of IL-6 and TNFα were measured in rats treated in (D). Results are means ± s.d., n = 5 in each group. and p= 0.0163 for IL-6 levels and p = 0.0333 for TNFα levels, respectively.
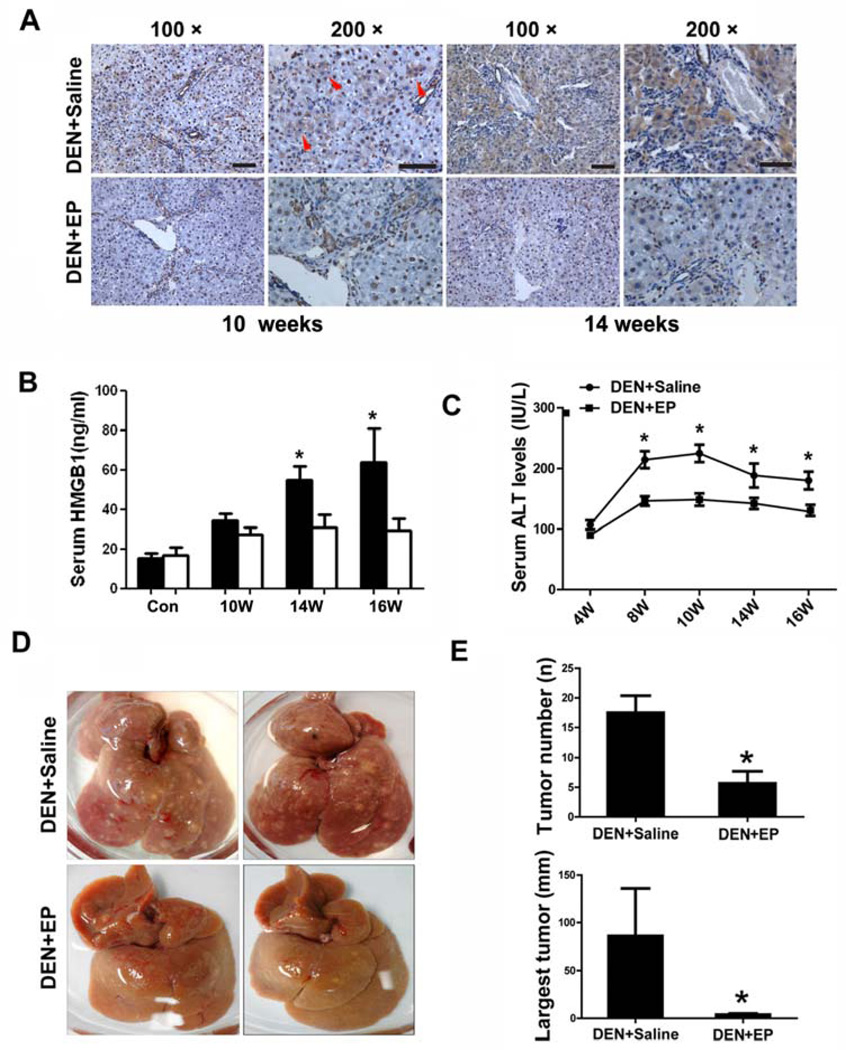
We next attempted to examine the therapeutic potential of the HMGB1-targeting agent EP in DEN-induced hepatocarcinogenesis. EP was administered to rats starting one week prior to DEN injection and continued until six weeks after the 10-week DEN treatment. Examination of ALT levels after one week EP treatment showed minimal change in the serum enzyme level, suggesting the safety of the EP application (data not shown). EP was used until the end of the experiment because the hepatic injury persisted due to the inflammatory responses following DEN-induced liver damage. Immunohistochemical analysis revealed that HMGB1 cytoplasmic translocation was substantially reduced in EP-treated rats after DEN administration (Fig.4A). Furthermore, the circulating levels of HMGB1 were also significantly inhibited in EP-treated rats after long-term DEN treatment (Fig.4B). Most importantly, long-term administration of EP substantially inhibited DEN-induced chronic hepatic injury and tumorigenesis in rat livers (Fig.4C–E).
Figure 4. Eythl pyruvate mitigates chronic hepatic injury and tumorigenesis after DEN treatment.
(A) Wild-type rats were administered with DEN together with saline or EP for 10 or 14 weeks. Immunohistochemistry analysis of HMGB1 shows the preventive effects of EP on cytoplasmic translocation of HMGB1. Red arrowheads indicate areas showing cytoplasmic translocation of HMGB1. 100× and 200× magnifications are shown as indicated. Scale bars, 50 µm.
(B) The circulating levels of HMGB1 were measured by ELISA analysis in saline- or EP-treated groups at the indicated times during DEN treatment. Results are means ± s.d., n = 5. p < 0.05.
(C) The serum levels of ALT were measured in saline- or EP-treated groups at the indicated times during DEN treatment. Results are means ± s.d., n = 5. p < 0.0001.
(D) Representative photographs of livers from DEN-treated rats with or without EP treatment.
(E) Number (p = 0.0264) and Largest tumor size of HCCs (≥ 2 mm, p = 0.01361) were quantified in DEN+saline and DEN+EP groups on week 16, six weeks after the end of DEN treatment. Results are means ± s.d., n = 5.
Discussion
A key feature of p53 is that its function is significantly dependent on the cellular microenvironment. The regulation of cell fate by p53 in the liver also seems to be context-dependent. p53 activation in murine liver carcinomas has been shown to trigger cell cycle arrest and cellular senescence but not apoptosis [24]. Using hepatocyte-specific Mdm2-knockout mice, however, Hayashi et al reported that persistent p53 activation in hepatocytes led to increased hepatocyte apoptosis and spontaneous liver fibrosis [25]. In addition, p53-mediated liver cell apoptosis also plays a crucial role in injury associated with primary biliary cirrhosis and cholestasis, where the accumulation of toxic bile salts within the liver is a key event [26, 27]. In our recent [7, 14] and current studies, we demonstrate that sustained p53 activation mediated by carcinogen-induced DNA damage responses leads to pro-tumorigenic liver inflammation, at least in part by triggering HMGB1 release. Cell viability and apoptosis assays revealed that sustained p53 activation resulted in late apoptosis, necrotic cells death and loss of membrane integrity, which eventually caused the release of HMGB1. This phenomenon might not represent p53-specific regulation of HMGB1 release. Constant p53 activation may promote this process by inducing cell death. Extracellular HMGB1 can act both as a chemoattractant for leukocytes and as a proinflammatory mediator to induce both recruited leukocytes and resident immune cells to release TNF, IL-1, IL-6 and other cytokines [28]. Consistent with these observations, we recently showed that inflammatory infiltration of p53+/− rat livers was much less than that of wild-type controls, as evidenced by lower numbers of neutrophils, lymphocytes and macrophages within portal tracts [14]. Accordingly, the circulating levels of HMGB1 as well as proinflammatory cytokines such as IL-6 and TNFα were detected to be lower in p53+/− rats. Importantly, immunodepletion of HMGB1 from the conditioned supernatants of ip53-hepatocytes with p53 induction eliminated TNFα release from macrophages treated with these supernatants. These results suggest that HMGB1 is a critical mediator of p53-dependent hepatic inflammation that exerts an important pathogenic role in hepatocarcinogenesis.
HMGB1 is known to activate immune cells through the differential engagement of multiple surface receptors including RAGE (receptor for advanced glycation end products) and TLR4 (Toll like receptor 4), which was recently shown to link inflammation and carcinogenesis in the chronically injured liver [12, 29, 30]. Our results support the notion that endogenous damage-associated molecular patterns (DAMPs), such as HMGB1, not only trigger protective immunity against damaged cells but also contribute to inflammation-associated tumorigenesis. Importantly, we observed that EP efficiently inhibited HMGB1 release from hepatocytes after p53 induction in vitro and in rat livers treated with DEN, which caused persistent DNA damage and p53 activation in vivo. The inhibition of HMGB1 release mitigated DEN-induced hepatic injury and hepatocarcinogenesis, giving direct evidence that HMGB1 is an important mediator of pro-tumorigenic inflammation following genetic damage and p53 activation. In line with our results, glycyrrhizin, which has been used clinically [31, 32] and experimentally [33, 34] for decades to prevent liver cirrhosis and HCC, was recently proved to be a direct inhibitor of HMGB1 [35].
Emerging data have revealed paradoxical functions of genes previously identified as prooncogenic or tumor suppressive in hepatocarcinogenesis [36]. The tumor suppressor p53 pathway has evolved to restrict malignant transformation by mounting cooperative cellular damage and immune responses. Inactivation of p53 is thought to be required for tumor maintenance, prompting efforts to restore p53 activity as an anticancer therapeutic approach. Our prior and current results, however, provide evidence that sustained p53 activation in response to a persistent DNA damage signal, as occurs in livers infected with hepatitis virus or exposed to carcinogen [37, 38], might lead to unresolved inflammation that drives progression to malignancy. These findings also have important implications for p53 restoration in cancer therapy. As restoring p53 culls only the most aggressive cells within tumors [39, 40], its reactivation in unresponsive tumor cells or adjacent normal cells could instead accelerate tumorigenesis. Strategies that specifically mitigate p53-mediated inflammatory effects, such as the use of HMGB1 inhibitors, as studied here, might represent an adjunct therapeutic approach for p53 reactivation. Based on our observation, HMGB1 inhibitors can be used before any p53 restoration therapy, and can also be used after liver injury is established but p53 is not yet mutated or otherwise inactivated.
Supplementary Material
Acknowledgements
We thank Jiaohong Wang and Shoudong Ye for technical assistance.
Financial support: This work was supported by a NIH grant to Q.-L. Y (R01OD010926) and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning to H.-X. Y.
List of abbreviations in the order of appearance
- HCC
hepatocellular carcinoma
- HMGB1
high-mobility group protein 1
- DEN
diethylnitrosamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: the authors have no potential conflicts of interest.
Reference List
- 1.Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002 Aug;3(8):461–469. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003 Dec 6;362(9399):1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001 Sep 13;345(11):784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 4.Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury- and death-induced inflammation. Immunity. 2011 Oct 28;35(4):467–477. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efeyan A, Serrano M. p53: guardian of the genome and policeman of the oncogenes. Cell Cycle. 2007 May 2;6(9):1006–1010. doi: 10.4161/cc.6.9.4211. [DOI] [PubMed] [Google Scholar]
- 6.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009 May 1;137(3):413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 7.Yan HX, Wu HP, Ashton C, Tong C, Ying QL. Rats deficient for p53 are susceptible to spontaneous and carcinogen-induced tumorigenesis. Carcinogenesis. 2012 Jul;12 doi: 10.1093/carcin/bgs238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002 Jul 11;418(6894):191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 9.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005 Apr 4;201(7):1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong C, Li P, Wu NL, Yan Y, Ying QL. Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature. 2010 Sep 9;467(7312):211–213. doi: 10.1038/nature09368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajewsky MF, Dauber W, Frankenberg H. Liver carcinogenesis by diethylnitrosamine in the rat. Science. 1966 Apr 1;152(3718):83–85. doi: 10.1126/science.152.3718.83. [DOI] [PubMed] [Google Scholar]
- 12.Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010 Oct;52(4):1322–1333. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 13.Mello T, Nakatsuka A, Fears S, Davis W, Tsukamoto H, Bosron WF, et al. Expression of carboxylesterase and lipase genes in rat liver cell-types. Biochem Biophys Res Commun. 2008 Sep 26;374(3):460–464. doi: 10.1016/j.bbrc.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan HX, Wu HP, Zhang HL, Ashton C, Tong C, Wu J, et al. DNA damage-induced sustained p53 activation contributes to inflammation-associated hepatocarcinogenesis in rats. Oncogene. 2012 Oct;15 doi: 10.1038/onc.2012.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008 Feb 15;180(4):2011–2017. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- 16.Roberts RA, Ganey PE, Ju C, Kamendulis LM, Rusyn I, Klaunig JE. Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol Sci. 2007 Mar;96(1):2–15. doi: 10.1093/toxsci/kfl173. [DOI] [PubMed] [Google Scholar]
- 17.Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AG, Wandless TJ. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell. 2006 Sep 8;126(5):995–1004. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010 May;16(5):238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009 Aug;11(8):973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007 Nov 26;204(12):2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002 Sep 17;99(19):12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou RR, Zhao SS, Zou MX, Zhang P, Zhang BX, Dai XH, et al. HMGB1 cytoplasmic translocation in patients with acute liver failure. BMC Gastroenterol. 2011;11:21. doi: 10.1186/1471-230X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007 Feb 8;445(7128):656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kodama T, Takehara T, Hikita H, Shimizu S, Shigekawa M, Tsunematsu H, et al. Increases in p53 expression induce CTGF synthesis by mouse and human hepatocytes and result in liver fibrosis in mice. J Clin Invest. 2011 Aug;121(8):3343–3356. doi: 10.1172/JCI44957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang H, Li TW, Ko KS, Xia M, Lu SC. Switch from Mnt-Max to Myc-Max induces p53 and cyclin D1 expression and apoptosis during cholestasis in mouse and human hepatocytes. Hepatology. 2009 Mar;49(3):860–870. doi: 10.1002/hep.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro RE, Amaral JD, Sola S, Kren BT, Steer CJ, Rodrigues CM. Differential regulation of cyclin D1 and cell death by bile acids in primary rat hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2007 Jul;293(1):G327–G334. doi: 10.1152/ajpgi.00093.2007. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Tracey KJ. Targeting HMGB1 in inflammation. Biochim Biophys Acta. 2010 Jan;1799(1–2):149–156. doi: 10.1016/j.bbagrm.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, et al. Promotion of Hepatocellular Carcinoma by the Intestinal Microbiota and TLR4. Cancer Cell. 2012 Apr 17;21(4):504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang HL, Yu LX, Yang W, Tang L, Lin Y, Wu H, et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol. 2012 Oct;57(4):803–812. doi: 10.1016/j.jhep.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, et al. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997 Apr 15;79(8):1494–1500. doi: 10.1002/(sici)1097-0142(19970415)79:8<1494::aid-cncr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda K, Arase Y, Kobayashi M, Saitoh S, Someya T, Hosaka T, et al. A long-term glycyrrhizin injection therapy reduces hepatocellular carcinogenesis rate in patients with interferon-resistant active chronic hepatitis C: a cohort study of 1249 patients. Dig Dis Sci. 2006 Mar;51(3):603–609. doi: 10.1007/s10620-006-3177-0. [DOI] [PubMed] [Google Scholar]
- 33.Shiota G, Harada K, Ishida M, Tomie Y, Okubo M, Katayama S, et al. Inhibition of hepatocellular carcinoma by glycyrrhizin in diethylnitrosamine-treated mice. Carcinogenesis. 1999 Jan;20(1):59–63. doi: 10.1093/carcin/20.1.59. [DOI] [PubMed] [Google Scholar]
- 34.Wan XY, Luo M, Li XD, He P. Hepatoprotective and anti-hepatocarcinogenic effects of glycyrrhizin and matrine. Chem Biol Interact. 2009 Sep 14;181(1):15–19. doi: 10.1016/j.cbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Mollica L, De MF, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, et al. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007 Apr;14(4):431–441. doi: 10.1016/j.chembiol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Feng GS. Conflicting roles of molecules in hepatocarcinogenesis: paradigm or paradox. Cancer Cell. 2012 Feb 14;21(2):150–154. doi: 10.1016/j.ccr.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikitin PA, Luftig MA. The DNA damage response in viral-induced cellular transformation. Br J Cancer. 2012 Jan 31;106(3):429–435. doi: 10.1038/bjc.2011.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Gijssel HE, Maassen CB, Mulder GJ, Meerman JH. p53 protein expression by hepatocarcinogens in the rat liver and its potential role in mitoinhibition of normal hepatocytes as a mechanism of hepatic tumour promotion. Carcinogenesis. 1997 May;18(5):1027–1033. doi: 10.1093/carcin/18.5.1027. [DOI] [PubMed] [Google Scholar]
- 39.Feldser DM, Kostova KK, Winslow MM, Taylor SE, Cashman C, Whittaker CA, et al. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature. 2010 Nov 25;468(7323):572–575. doi: 10.1038/nature09535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Junttila MR, Karnezis AN, Garcia D, Madriles F, Kortlever RM, Rostker F, et al. Selective activation of p53-mediated tumour suppression in high-grade tumours. Nature. 2010 Nov 25;468(7323):567–571. doi: 10.1038/nature09526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.