Abstract
Interaction of the integrin receptors with ligands determines the molecular basis of integrin –dependent cell adhesion. Integrin ligands are typically large proteins with relatively low binding affinities. This makes direct ligand-binding kinetic measurements somewhat difficult. Here we examine several real-time methods, aimed to overcome these experimental limitations and to distinguish the regulation of integrin conformation and affinity. This chapter includes: the use of a small ligand-mimetic probe for studies of inside-out regulation of integrin affinity and unbending, real-time cell aggregation and disaggregation kinetics to probe integrin conformational states and the number of integrin-ligand bonds, as well as the real-time monitoring of ligand -induced epitopes under signaling through G-protein-coupled receptors, and others. Experimental data obtained using these novel methods are summarized in terms of the current model of integrin activation.
Keywords: Ligand-receptor interaction, Ligand mimetic, Real-time kinetics, Cells adhesion, Inside-out signal, Monoclonal antibodies, Quantitative approaches
1. Introduction
Understanding how cell adhesion and migration is regulated is essential for describing embryonic development, tissue repair, hemostasis, inflammation, cell mobilization, and metastasis. The ability to rapidly and reversibly modulate cellular adhesive properties serves as the basis for multiple biological functions of multicellular organisms. Several adhesion molecules regulate cell adhesion through de novo expression, rapid up regulation by the means of exocytosis, downregulation through proteolysis, shedding, and other mechanisms that can alter the number of molecules on the cell surface. Methods for studying these molecules are beyond the scope of this chapter. We focus here on integrins, a unique class of adhesion molecules that can rapidly change cell adhesion through a conformational change and/or clustering, without altering molecule expression.
Our current understanding of integrin conformational regulation implies the potential existence of multiple conformational states, with different binding affinities for their ligands, different degrees of unbending (extension), and different positioning of integrin domains (hybrid domain in particular). These states are expected to contribute to the lifetime of the ligand-receptor bond, and the efficiency of the bond formation. Such a model allows us to describe how an integrin such as VLA-4 can be responsible for very diverse cellular behaviors, such as a nonadhesive state, as well as rolling, cell arrest, and firm adhesion (1). The recent discovery that G-protein-coupled receptors can provide a negative (deactivating) signal, which results in cell deadhesion, adds to the number of possible conformational states and highlights the complexity of integrin conformational regulation (2).
In this chapter, we review basic methods that led to the current model of integrin activation and focus on basic techniques that are currently used in our and other laboratories to study integrin-dependent cell adhesion. Because of the limited space we will primarily focus on unique assays specifically developed for integrin studies in our laboratory. We apologize to the others whose studies contributed to the current understanding of integrin regulation and were not cited because of the lack of space.
2. Small Molecules as Tools for Integrin Studies
Interaction of the integrin receptors with ligands determines the molecular basis of integrin-dependent cell adhesion. Methods that allow monitoring of these ligand-receptor interactions in real-time on living cells under physiologically relevant signaling conditions would represent a desirable "gold standard" for these types of studies. In the best case scenario a scientist should be able to purity cells of interest, add labeled ligand, and monitor binding of the probe in real time after activation/deactivation through other types of receptors ("inside-out" or "outside-in" signal). Unfortunately, soluble integrin ligands are large proteins that have relatively low binding affinities. Therefore, direct kinetic measurements of natural integrin ligand binding are technically difficult. One of the solutions to this problem is the development of small molecule probes that exhibit higher binding affinities and, at the same time, reflect the binding of the natural ligand. These probes can be used as reporters of the affinity state of the integrin-binding pocket, as well as in other applications (see below). Fluorescently labeled molecules of this type can be used in a conventional flow cytometer to make homogeneous real-time measurements of ligand-receptor interactions (3, 4). Drug-like small molecules also appear to be good candidates for these assays. Integrins represent an attractive target for treatment of several diseases. Therefore, a number of drug-like small molecules (direct and allosteric integrin antagonists) have been developed by several pharmaceutical companies (5). Fluorescent antagonists for GPIIb/IIIa (RGD peptidomimetics) were described and used in a flow cytometer by Dr. Bednar et al. from Merck Research Labs (4). The binding of fluorescent LFA-1 antagonists has been described by Dr. Keating et al. from Genentech, Inc. (6). We took advantage of the published structure of LDV-based competitive antagonists developed by Biogen Idec Inc. (BI01211) (7, 8), and created a fluorescent probe that mimics binding of a natural VLA-4 (α4β1-integrin) ligand (9). This probe has been used for determination of rapid affinity changes of the integrin ligand-binding pocket in real time in our laboratory and others (9, 10). The assay is performed directly in a tube attached to a flow cytometer and cells are continuously sampled for periods up to several tens of minutes. For a short period of time the tube is removed from the cytometer and a signaling molecule of interest is added. Because the fluorescent probe is added at a concentration sufficient to occupy only high-affinity VLA-4 sites, additional binding of the probe is observed in response to an affinity change. The presence of the affinity change can be verified using dissociation rate analysis, where a large excess of the unlabelled competitor is added to prevent rebinding of the fluorescent probe. A strong correlation between dissociation rates for the probe and natural ligand, as well as cellular dissociation rates has been observed for the case of multiple affinity states (11, 12). The same fluorescent probe can be used to assess integrin unbending (Fig. 1). The ability to independently measure the affinity state of the ligand-binding pocket and molecular unbending permitted us to study the regulation of these two processes through "inside-out" signaling. Surprisingly, this resulted in the observation that affinity and unbending are regulated by two independent signaling pathways (1). According to these types of measurements "inside-out" signaling through different G protein-coupled receptors results in a plethora of conformational states, at a minimum the four combinations of high and low affinity with independently regulated bent and unbent states (2). Thus, the idea that a single integrin molecule can adopt states suitable for rolling (extended and low affinity of the binding pocket), arrest (high affinity), and nonadhesive (low affinity bent with hidden binding pocket) may be realistic for non I-domain-containing integrins (such as VLA-4) (13). For integrins with an inserted domain (such as LFA-l), the situation is more complicated.
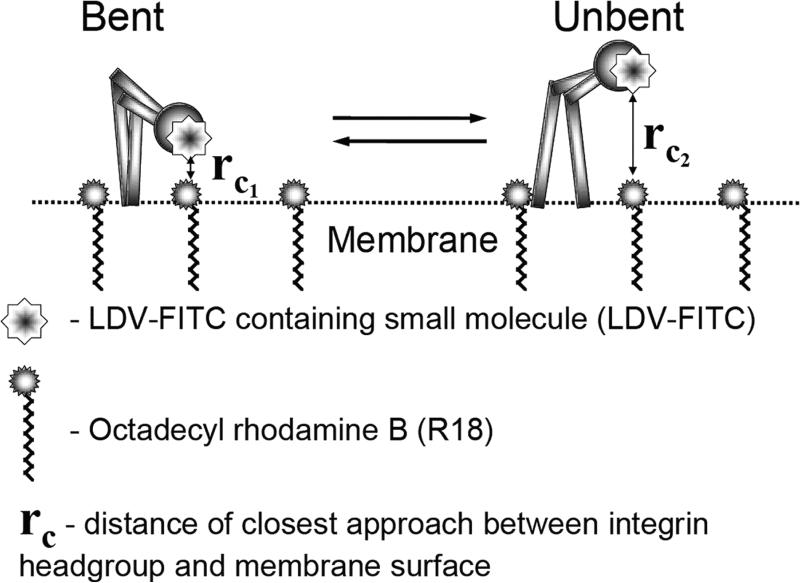
Fig. 1.
Schematic depicting the FRET assay for assessing VLA-4 conformational unbending (modified from (1)). Energy transfer between VLA-4 head groups and lipid probes incorporated into the plasma membrane provides a way of studying integrin conformational unbending. The LDV-FITC probe that specifically binds to the head group ofVLA-4 is used as a fluorescent donor at a high enough concentration to saturate all low-affinity resting binding sites. A change in VLA-4 affinity would not affect probe binding. Octadecyl rhodamine B (R18), a lipophilic probe, inserts into the membrane as an acceptor. Upon activation, VLA-4 assumes an unbent (upright) conformation. rC1 and rC2 are the distances of closest approach before and after molecular unbending. Changes in the fluorescence of the donor were measured on live cells in real time at 37°C by flow cytometry.
The development of similar fluorescent ligand-mimicking probes for other integrins appears to be very beneficial. Small molecule probes with appropriate affinity (in the nM range) can be used for detecting affinity changes and unbending in real-time on live cells after activation and/or deactivation through signaling receptors. However, only competitive antagonists, which mimic the binding of a natural ligand, can be used for the detection of the affinity change of the ligand-binding pocket. We have also used a fluorescent allosteric antagonist of LFA-l (fluorescent derivative of BIRT-377) to probe vertical extension upon activation in a FRET-based assay analogous to Fig. 1 (14). Only the reducing agent DTT caused a large FRET signal change, in a manner analogous to DTT-induced extension of VLA-4 (15). The absence of a large conformational change was explained by the fact that BIRT was shown to stabilize the inactive (bent) conformation of LFA-l (14). Nevertheless, the question remains open why β1-, and β3-integrin-specific small molecules are predominantly competitive antagonists, while the majority of β2-integrin antagonists are allosteric (at least for LFA-l) (5).
3. Single Bond Life-Times
Rapid kinetic measurements of natural integrin ligands binding and other protein-protein interaction are possible with the use of a rapid-mix flow cytometer (16-18). In a conventional flow cytometer several seconds are required for the delivery of a sample from a test tube to the flow chamber. Modern automated rapid-mix devices allow mixing and delivery under a second using microliter volume of samples (55-600 ms, 35-45 μl aliquots) (16, 18). We used a rapid-mix flow cytometer to determine the dissociation rate of soluble fluorescently labeled recombinant human VCAM from a rapidly dissociating intermediate affinity state of VLA-4 integrin. However, a direct measurement of the VCAM dissociation rate for resting VLA-4 (without activation and with physiological concentrations of divalent cations) using this technique is still elusive (12). Nonetheless, the single molecule dissociation rates appear to provide insight into the duration of cell adhesion as described below.
Single bond life-times have also been evaluated with the bioforce probe (19). When these measurements are extrapolated to 0 force, the bioforce probe and flow cytometry measurements give comparable results (Evan Evans, unpublished data).
4. Real-Time Aggregation and Disaggregation Kinetics
Another powerful method for studying real-time integrin activation and cell adhesion is the cell-suspension adhesion assay. Two types of cells, one population expressing the integrin of interest along with activating or inhibiting pathway receptors (G protein-coupled receptor) and the other cell population expressing an integrin ligand, can be stained with two fluorescent dyes (e.g., green and red). For the case of homotypic aggregation, such as neutrophil aggregation, a single color stain is sufficient (20). After cells are mixed in a tube maintained at 37°C with constant stirring, they are continuously sampled over several tens of minutes. Aggregates, which are formed over a period of time, are detected as double-positive (green and red co-fluorescent) events. Because flow cytometers also detect single cells (only green or red events), it is possible to follow cell aggregation in real time by evaluating the aggregates or depletion of "singlets." This allows eliminating the effect of multicellular aggregates that present in the double-positive gate (11).
Using this methodology it is possible to observe GPCR-dependent activation of integrin-dependent cell adhesion ("inside-out" activation), as well as rapid deactivation and cell disaggregation (1, 2, 20) (Fig. 2). Moreover, it was possible to establish a relationship between cellular disaggregation rates and ligand dissociation rates for different affinity states. Quantitative analysis of molecular and cellular dissociation rates revealed that only a small number of VLA-4-VCAM-1 bonds (~1.5 on average) was sufficient to hold together cellular aggregates (11).
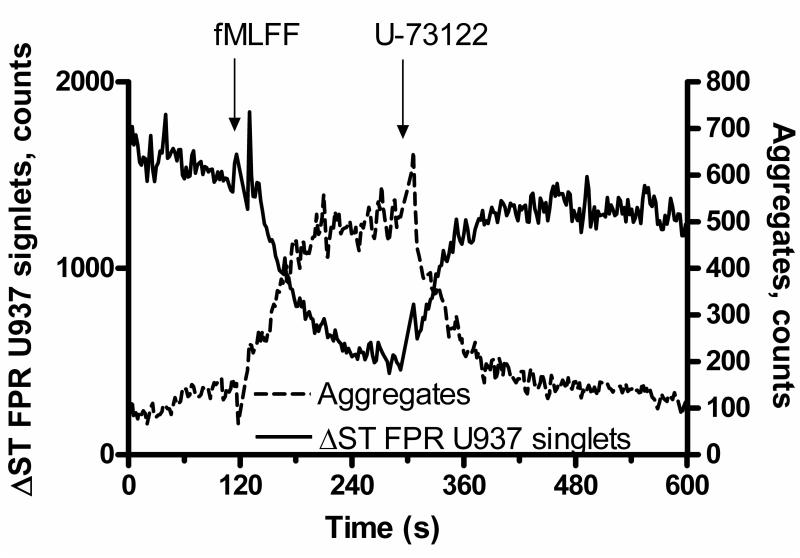
Fig. 2.
Changes in cell adhesion between formyl peptide transfected U937 cell and VCAM-1-transfected B78H1 cells at resting state and in response to receptor stimulation (modified from (1)). Addition of fMLFF (formyl peptide) induces cell aggregation. This results in U937 singlets depletion. PLC inhibitor U-73122 has the opposite effect.
This method can be also adopted to study cell aggregation and disaggregation under force. We and others have used devices which create defined shear in cone and plate as well as parallel-plate conditions (i.e., Ravenfield model EM Shear Generator (Ravenfield Designs Ltd., Heywood, UK) (12, 21). As expected, shear stress had a significant effect on cellular disaggregation rates (12). The work of Simon et al. showed how the contributions of L-selectin with PSGL and β2-integrin with ICAM -1 worked together under shear in neutrophil aggregation (20, 22).
The method can be also used to determine cellular association rates (analogous to the "forward kinetics" for the ligand binding). Based on our measurements of integrin molecule extension (using a FRET-based assay, see below), we postulated that molecular extension could facilitate integrin ligand recruitment because of the better exposure of the integrin ligand-binding pocket. We established experimental conditions to enhance integrin extension (determined using a FRET-based assay) while maintaining the affinity state of the ligand-binding pocket (determined in a ligand dissociation assay). We found that the initial rate of cell aggregation was dramatically elevated for the case of "extended" integrins (see Fig. 9 in (1)).
5. Parallel-Plate Flow Chamber
The parallel-plate flow chamber with immobilized integrin ligands or cells bearing integrin ligands has been actively used by many groups to study adhesive interactions under shear (23,24). Several important findings include the role of shear in the induction of transmigration, and the role of force exerted on the bond in the formation of shear resistant adhesion (22, 25-27). In the case of high ligand density, where multiple bonds between the cell and the ligand can be formed, it is difficult to determine the molecular mechanisms that are responsible for the changes in cell adhesion avidity. Rapid clustering of integrin molecules and formation of multivalent contacts could be indistinguishable from the changes in the properties of individual integrin-ligand contacts. Thus, the results of Alon et al. (28) are also consistent with our model in which extension regulates captures frequency while affinity regulates tether duration (1).
Using ligand at low density creates conditions, where predominantly only one integrin-ligand contact is formed. This type of experiment allows determination of individual bond kinetics based upon tether frequencies and bond life-times. The increase in the life-time of the bond can be interpreted as a decrease in bond dissociation rate and elevated affinity state of the ligand-binding pocket. It is not surprising that values of bond life-times determined in these experiments are comparable to the lifetime determined in a soluble system (compare dissociation rates for different activation states from (28) and (12)). This will be true only if a relatively small force applied is applied to the integrin bond. According to theory, bond life-time would exponentially decrease with the force. However, a recent report from Cheng Zhu group showed that force (in the 10-30 pN range) applied to the integrin actually prolonged bond life-times (29). This so-called catch bond behavior could potentially be tested in a parallel-plate chamber as well. In unpublished experiments we observed cell-cell adhesion with very long life-times, where we did not distinguish catch bonds from altered avidity due to multiple bonds formation.
Another interesting phenomenon observed in parallel-plate experiments is the rapid change in tether frequency following "inside-out" activation (e.g., see (28)). Currently, two different interpretations are considered: (1) rapid clustering of integrins and (2) rapid conformational change of individual integrin molecules. Clustering is usually determined using staining with mAbs on fixed cells. To our knowledge no widely used method for rapid real-time determination of integrin clustering exists. Fluorescence resonance energy transfer (FRET)-based methods are the most likely option right now (30). FRET has been used to study integrin subunit separation upon activation, molecule unbending (extension), and rnicroclustering (31). Rapid unbending (extension) of the integrin molecules upon activation, which leads to the rapid exposure of previously hidden ligand-binding site, can also be considered as possible mechanism for a rapid change in the tether frequency. Combining a parallel-plate flow chamber with FRET-based measurements of integrin activation would be a logical next step for studying intimate mechanisms of integrin activation under shear.
6. mAbs and Integrin Conformation
Historically, monoclonal antibodies were the first critical tool for identifying cell adhesion molecules (32). Blocking antibodies that inhibit cell-cell, or cell-soluble or immobilized ligand interactions remain invaluable for determining the specificity of molecular contributions to cell adhesion. For multiple anti-integrin antibodies, the binding epitopes are finely mapped and overlaid on 3D models of integrins (33). From a practical point of view it is important to note that certain blocking mAbs with epitopes mapped close to the ligand-binding site (but not exactly covering it) can be successfully used to block the binding of a large protein ligand, and yet can fail to block binding of a small ligand-mimicking probe.
A conformational change that enhances integrin-receptor interaction upon binding of "activating" antibodies can directly lead to the affinity change of the ligand-binding pocket. However, in our experience, the affinity state generated by this treatment can be several orders of magnitude higher than the "physiological" high -affinity state generated upon G-protein -coupled receptor signaling ("inside-out" activation) (9). Thus, the physiological role of this "artificially" generated state is not certain. Other "activating" antibodies can mimic "inside-out" activation by inducing a separation of integrin subunits, or by inducing an extended integrin conformation (34, 35). This type of mAb is particularly useful in the absence of a proper "physiological" activating pathway, such as G-protein-coupled or other receptors.
Antibodies that recognize the "activated" integrin conformation, also termed "activation reporter" mAbs, often report the ligand bound conformation of the integrin (13, 32). Therefore, they are also termed "ligand-induced binding sites" or LIBS mAbs. Fine mapping of LIBS epitopes, together with mutagenesis studies has provided valuable information about the nature of conformational changes resulting from integrin activation and ligand binding (13, 33, 36). Generation and screening of mAbs, (LIBS-type and others) is described in (32).
A major drawback for using mAbs to detect rapid conformational changes is the long incubation time, which is required to reach binding equilibrium. This is caused by a slow mAb dissociation rate, as well as the size of the protein that largely determines its slow diffusion. One possible solution to this problem is to perform binding experiments under conditions that are far from equilibrium (Fig. 3). For these types of studies, real-time detection of mAb binding can be done in a homogeneous assay in a conventional flow cytometer. Since the exposure of the LIBS antibody epitope is proportional to the occupancy of the integrin binding pocket, the rate of LIBS mAb binding at a given concentration of the ligand will be proportional to the concentration of the ligand-receptor complex. By plotting mAbs binding rate vs. ligand concentrations it is possible to determine ligand-binding affinities at rest and after "inside-out" activation through a GPCR (13). This approach has been verified for multiple VLA-4 ligands. The EC50s for βl-integrin LIBS mAbs (HUTS-21) binding determined in the mAb-binding experiments were identical to Kis, determined in the competition assay with fluorescent VLA-4 ligand (37). This novel approach can be used for determination of ligand-binding affinities for unlabeled ligands, as well as for previously unknown integrin ligands. It was also adapted to a high-throughput screening format for identification of integrin antagonists (PubChem, AID: 2617, Summary of HTS for Identification of VLA-4 Allosteric Modulators). Moreover, it is worthwhile considering, that under conditions that resolves the four states of affinity and extension regulation, the presence of ligand under these conditions creates a unique LIBS state, allowing the potential of eight distinct physiological VLA-4 states (13).
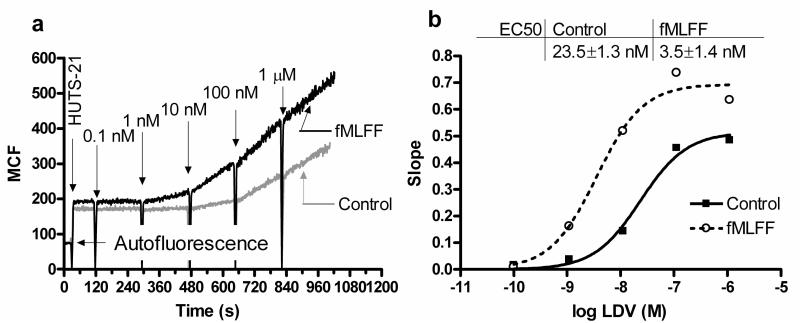
Fig. 3.
Kinetics of real-time binding of LIBS mAbs (HUTS-21) demonstrates a difference in VLA-4 ligand (LDV) binding affinity before (control) and after "inside-out" activation through formyl peptide receptor (modified from (13)). (a) U937 cells expressing the formyl peptide receptor were treated with 100 nM fMLFF (activated) or vehicle before the start of the experiment. The addition of HUTS-21 antibodies (first arrow) resulted in the rapid low-level nonspecific binding of the antibody. Next, increasing amounts of LDV ligand were added. This induced binding of mAbs resulted in different rates of antibody binding (compare slopes after LDV additions). (b) absolute rates of HUTS-21 binding (slopes of lines between sequential LDV additions calculated from panel a plotted vs. concentration of LDV in solution). The data were fitted using the sigmoidal dose-response equation with variable slope. Differences in EC50 values for resting and activated cells indicated the affinity change for LDV binding.
7. Current Models of Integrin Activation
The combination of several novel real-time approaches that include the use of a small fluorescent ligand-mimicking probe for the detection of integrin affinity change and unbending (extension), real-time analysis of LIBS mAb binding, together with the analysis of real-time aggregation and disaggregation kinetics resulted in a model of integrin activation with several novel features (see Fig. 6 in (13)). Here without going into details we highlight specific methods that provided evidence for each specific conclusion (Table 1).
Table 1.
General approaches for studying integrin-dependent cell adhesion
| Experimental methods | Conclusions | References |
|---|---|---|
| Ligand mimetic binding and dissociation, FRET-based extension method, real-time cellular aggregation, binding of a soluble integrin ligand (VCAM−1), rapid mix flow cytometry |
Independent regulation of integrin affinity and unbending (extension). Affinity regulates the life-time of the interaction, unbending regulates initial rate of aggregate formation |
(1;2;17) and unpublished data |
| Real-time binding of LIBS antibodies, ligand mimetic under activation through Galphai-coupled GPCRs |
Affinity state of the ligand binding pocket is independent of hybrid domain movement, which is solely determined by ligand occupancy |
(13;37) |
| Ligand mimetic binding and dissociation, FRET-based extension method, under activation through Galphai-coupled and Galphas-coupled GPCRs, real-time cellular aggregation /disaggregation, parallel plate flow chamber |
Galphai-coupled GPCRs provide “pro-adhesive signal”, Galphas-coupled GPCRs provide “anti-adhesive signal”. |
(2) and unpublished data |
Taken together, a combination of real-time assays performed on live cells at 37°C under physiologically relevant "inside-out" signaling through multiple GPCRs have provided a unique opportunity to study integrin conformational regulation and its role in the modulation of integrin-dependent cell adhesion and deadhesion. This approach can be extended to other integrins, and will provide valuable information about mechanisms of integrin conformational regulation.
Acknowledgments
This work was supported by R01HL081062 and U54MH084690.
References
- 1.Chigaev A, Waller A, Zwartz GJ, Buranda T, Sklar LA. Regulation of cell adhesion by affinity and conformational unbending of alpha4betal integrin. J Immunol. 2007;178:6828–6839. doi: 10.4049/jimmunol.178.11.6828. [DOI] [PubMed] [Google Scholar]
- 2.Chigaev A, Waller A, Amit 0, Sklar LA. Galphas-coupled receptor signaling actively down -regulates alpha4betalintegrin affinity: a possible mechanism for cell de-adhesion. BMC. Immunol. 2008;9:26. doi: 10.1186/1471-2172-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sklar LA, Edwards BS, Graves SW, Nolan JP, Prossnitz ER. Flow cytometric analysis of ligand-receptor interactions and molecular assemblies. Annu. Rev. Biophys. Biomol. Struct. 2002;31:97–119. doi: 10.1146/annurev.biophys.31.082901.134406. [DOI] [PubMed] [Google Scholar]
- 4.Bednar B, Cunningham ME, McQueney PA, Egbertson MS, Askew BC, Bednar RA, Hartman GD, Gould RJ. Flow cytometric measurement of kinetic and equilibrium binding parameters of arginine-glycine- aspartic acid ligands in binding to glycoprotein IIb /IIIa on platelets. Cytometry. 1997;28:58–65. doi: 10.1002/(sici)1097-0320(19970501)28:1<58::aid-cyto7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Shimaoka M, Springer TA. Therapeutic antagonists and conformational regulation of integrin function. Nat. Rev. Drug Discov. 2003;2:703–716. doi: 10.1038/nrd1174. [DOI] [PubMed] [Google Scholar]
- 6.Keating SM, Clark KR, Stefanich LD, Arellano F, Edwards CP, Bodary SC, Spencer SA, Gadek TR, Marsters JC, Jr., Beresini MH. Competition between intercellular adhesion molecule -1 and a small-molecule antagonist for a common binding site on the alphal subunit of lymphocyte function-associated antigen-I. Protein Sci. 2006;15:290–303. doi: 10.1110/ps.051583406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin K, Ateeq HS, Hsiung SH, Chong LT, Zimmerman CN, Castro A, Lee WC, Hammond CE, Kalkunte S, Chen LL, Pepinsky RB, Leone DR, Sprague AG, Abraham WM, Gill A, Lobb RR, Adams SP. Selective, tight-binding inhibitors of integrin alpha4betal that inhibit allergic airway responses. J Med. Chem. 1999;42:920–934. doi: 10.1021/jm980673g. [DOI] [PubMed] [Google Scholar]
- 8.Chen LL, Whitty A, Lobb RR, Adams SP, Pepinsky RB. Multiple activation states of integrin alpha4betal detected through their different affinities for a small molecule ligand. J BioI. Chem. 1999;274:13167–13175. doi: 10.1074/jbc.274.19.13167. [DOI] [PubMed] [Google Scholar]
- 9.Chigaev A, Blenc AM, Braaten JV, Kumaraswamy N, Kepley CL, Andrews RP, Oliver JM, Edwards BS, Prossnitz ER, Larson RS, Sklar LA. J BioI. Chem. 2001;276:48670–48678. doi: 10.1074/jbc.M103194200. [DOI] [PubMed] [Google Scholar]
- 10.Chan JR, Hyduk SJ, Cybulsky MI. Detecting rapid and transient upregulation of leukocyte integrin affinity induced by chemokines and chemoattractants. J Immunol. Methods. 2003;273:43–52. doi: 10.1016/s0022-1759(02)00417-9. [DOI] [PubMed] [Google Scholar]
- 11.Zwartz G, Chigaev A, Foutz T, Larson RS, Posner R, Sklar LA. Relationship between molecular and cellular dissociation rates for VLA-4/VCAM-l interaction in the absence of shear stress. Biophys. J. 2004;86:1243–1252. doi: 10.1016/S0006-3495(04)74198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chigaev A, Zwartz G, Graves SW, Dwyer DC, Tsuji H, Foutz TD, Edwards BS, Prossnitz ER, Larson RS, Sklar LA. Alpha4betal integrin affinity changes govern cell adhesion. J BioI. Chem. 2003;278:38174–38182. doi: 10.1074/jbc.M210472200. [DOI] [PubMed] [Google Scholar]
- 13.Chigaev A, Waller A, Amit O, Halip L, Bologa CG, Sklar LA. Realtime analysis of conformation-sensitive antibody binding provides new insights into integrin conformational regulation. J BioI. Chem. 2009;284:14337–14346. doi: 10.1074/jbc.M901178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larson RS, Davis T, Bologa C, Semenuk G, Vijayan S, Li Y, Oprea T, Chigaev A, Buranda T, Wagner CR, Sklar LA. Dissociation of I domain and global. conformational changes in LFA -1: refinement of small molecule-I domain structure-activity relationships. Biochemistry. 2005;44:4322–4331. doi: 10.1021/bi048187k. [DOI] [PubMed] [Google Scholar]
- 15.Chigaev A, Zwartz GJ, Buranda T, Edwards BS, Prossnitz ER, Sklar LA. Conformational regulation of alpha 4 beta 1-integrin affinity by reducing agents. "Inside-out" signaling is independent of and additive to reduction-regulated integrin activation. J BioI. Chem. 2004;279:32435–32443. doi: 10.1074/jbc.M404387200. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Zwartz G, Lopez GP, Sklar LA, Buranda T. Small-volume rapid-mix device for subsecond kinetic analysis in flow cytometry. Cytometry A. 2005;67:37–44. doi: 10.1002/cyto.a.20171. [DOI] [PubMed] [Google Scholar]
- 17.Nolan JP, Posner RG, Martin JC, Habbersett R, Sklar LA. A rapid mix flow cytometer with subsecond kinetic resolution. Cytometry. 1995;21:223–229. doi: 10.1002/cyto.990210302. [DOI] [PubMed] [Google Scholar]
- 18.Graves SW, Nolan JP, Jett JH, Martin JC, Sklar LA. Nozzle design parameters and their effects on rapid sample delivery in flow cytometry. Cytometry. 2002;47:127–137. doi: 10.1002/cyto.10056. [DOI] [PubMed] [Google Scholar]
- 19.Evans E. Probing the relation between force--lifetime--and chemistry in single molecular bonds. Annu. Rev. Biophys. Biomol. Struct. 2001;30:105–128. doi: 10.1146/annurev.biophys.30.1.105. [DOI] [PubMed] [Google Scholar]
- 20.Simon SI, Chambers JD, Butcher E, Sklar LA. Neutrophil aggregation is beta 2-integrin- and L-selectin-dependent in blood and isolated cells. I Immunol. 1992;149:2765–2771. [PubMed] [Google Scholar]
- 21.Zwartz GJ, Chigaev A, Dwyer DC, Foutz TD, Edwards BS, Sklar LA. Real-time analysis of very late antigen- 4 affinity modulation by shear. I BioI. Chem. 2004;279:38277–38286. doi: 10.1074/jbc.M402944200. [DOI] [PubMed] [Google Scholar]
- 22.Simon SI, Goldsmith HL. Leukocyte adhesion dynamics in shear flow. Ann. Biomed. Eng. 2002;30:315–332. doi: 10.1114/1.1467677. [DOI] [PubMed] [Google Scholar]
- 23.Brown DC, Larson RS. Improvements to parallel plate flow chambers to reduce reagent and cellular requirements. BMC Immunol. 2001;2:9. doi: 10.1186/1471-2172-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiVietro JA, Brown DC, Sklar LA, Larson RS, Lawrence MB. Immobilized stromal cell-derived factor-l alpha triggers rapid VLA-4 affinity increases to stabilize lymphocyte tethers on VCAM-l and subsequently initiate firm adhesion. I Immunol. 2007;178:3903–3911. doi: 10.4049/jimmunol.178.6.3903. [DOI] [PubMed] [Google Scholar]
- 25.Alon R, Feigelson SW. Chemokine signaling to lymphocyte integrins under shear flow. Microcirculation. 2009;16:3–16. doi: 10.1080/10739680802026076. [DOI] [PubMed] [Google Scholar]
- 26.Alon R, Ley K. Cells on the run: shear-regulated integrin activation in leukocyte rolling and arrest on endothelial cells. Curro Opin. Cell BioI. 2008;20:525–532. doi: 10.1016/j.ceb.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ley K. Cell adhesion under flow. Microcirculation. 2009;16:1–2. doi: 10.1080/10739680802644415. [DOI] [PubMed] [Google Scholar]
- 28.Grabovsky V, Feigelson S, Chen C, Bleijs DA, Peled A, Cinamon G, Baleux F, Arenzana-Seisdedos F, Lapidot T, van Kooyk Y, Lobb RR, Alon R. Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. I Exp. Med. 2000;192:495–506. doi: 10.1084/jem.192.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong F, Garcia AJ, Mould AP, Humphries MJ, Zhu C. Demonstration of catch bonds between an integrin and its ligand. I Cell BioI. 2009;185:1275–1284. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim M, Carman CV, Yang W, Salas A, Springer TA. The primacy of affinity over clustering in regulation of adhesiveness of the integrin {alpha} L{ beta} 2. I Cell BioI. 2004;167:1241–1253. doi: 10.1083/jcb.200404160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lefort CT, Kim M. Fluorescence resonance energy transfer in the studies of integrin activation. Curro Top. Membranes. 2009;64:359–388. [Google Scholar]
- 32.Cabanas C, Sanchez-Madrid F. Monoclonal antibodies specific for leukocyte adhesion molecules. Selective protocols of immunization and screening assays for generation of blocking, activating and activation reporter antibodies. Methods Mol. BioI. 1999;96:1–9. doi: 10.1385/1-59259-258-9:1. [DOI] [PubMed] [Google Scholar]
- 33.Humphries MJ, Symonds EJ, Mould AP. Mapping functional residues onto integrin crystal structures. Curro Opin. Struct. BioI. 2003;13:236–243. doi: 10.1016/s0959-440x(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 34.Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301:1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- 35.Mould AP, Travis MA, Barton SJ, Hamilton JA, Askari JA, Craig SE, Macdonald PR, Kammerer RA, Bucldey PA, Humphries MJ. Evidence that monoclonal antibodies directed against the integrin beta subunit plexin/semaphorin/ integrin domain stimulate function by inducing receptor extension. I BioI. Chem. 2005;280:4238–4246. doi: 10.1074/jbc.M412240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mould AP, Barton SJ, Askari JA, McEwan PA, Bucldey PA, Craig SE, Humphries MJ. Structure of an integrin-ligand complex deduced from solution X-ray scattering and site-directed mutagenesis. J. BioI. Chem. 2003;278:17028–17035. doi: 10.1074/jbc.M304627200. [DOI] [PubMed] [Google Scholar]
- 37.Njus BH, Chigaev A, Waller A, Wlodek D, Ostopovici-Halip L, Ursu 0, Wang W, Oprea TI, Bologa CG, Sklar LA. Conformational mAb as a tool for integrin ligand discovery. Assay. Drug Dev. Technol. 2009;7:507–515. doi: 10.1089/adt.2009.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]