Abstract
Intravascular catheters used in clinical practice can activate platelets, leading to thrombus formation and stagnation of blood flow. Nitric oxide (NO)-releasing polymers have been shown previously to reduce clot formation on a number of blood contacting devices. In this work, trilaminar NO-releasing silicone catheters were fabricated and tested for their thrombogenicity. All catheters had specifications of L = 6 cm, inner diameter = 21 gauge (0.0723 cm), outer diameter = 12 gauge (0.2052 cm), and NO-releasing layer thickness = 200 ± 11 μm. Control and NO-releasing catheters were characterized in vitro for their NO flux and NO release duration by gas phase chemiluminescence measurements. The catheters were then implanted in the right and left internal jugular veins of (N = 6 and average weight = 3 kg) adult male rabbits for 4 hours thrombogenicity testing. Platelet counts and function, methemoglobin (metHb), hemoglobin (Hb), and white cell counts and functional time (defined as patency time of catheter) were monitored as measured outcomes. Nitric oxide-releasing catheters (N = 6) maintained an average flux above (2 ± 0.5) × 10−10 mol/min/cm2 for more than 24 hours, whereas controls showed no NO release. Methemoglobin, Hb, white cell, and platelet counts and platelet function at 4 hours were not significantly different from baseline (α = 0.05). However, clots on controls were visibly larger and prevented blood draws at a significantly (p < 0.05) earlier time (2.3 ± 0.7 hours) into the experiment, whereas all NO-releasing catheters survived the entire 4 hours test period. Results indicate that catheter NO flux levels attenuated thrombus formation in a short-term animal model.
There are 500,000 admissions to neonatal intensive care units in the United States each year. Most of these babies require management through central venous, umbilical venous, or umbilical artery catheter access for the administration of either one or a combination of the following: total parenteral nutrition, chemotherapy, fluid, blood products, and life-saving medications.1 These catheters are commonly made up of poly(vinyl chloride), polyurethane, or silicone rubber. Despite best practices, these catheters are often compromised because of infection, thrombosis, and complications leading to an increase in morbidity, extended hospital stay, and mortality, in some cases.2–5 The risk of complication associated with catheterization is even higher in population of predominantly premature neonates6 whose haemostatic system is not yet matured.7,8 The overall rate of catheter occlusion is estimated to be 2.0 per 1,000 catheter days.9
Current attempts in clinical practice to prevent clot formation involve heparin flushing through indwelling catheters. Although its use can be appreciated, heparin is a systemic-acting anticoagulation agent associated with bleeding in patients.10 Its effect can be especially problematic in infants because of the potential risk of inadvertent overdose. Occluded catheters that can not be cleared with heparin are locked with thrombolytics like urokinase to break down clots. The catheters are locked for hours11 before flushing, and thereby interrupt vascular access for as long as it takes to flush out clots from indwelling catheters if they are simply not removed. Despite the relatively high potency of thrombolytics, there are still cases where catheters remain partially occluded after 2 hours of thrombolytic locking.11
Our attempt to improving antithrombotic properties of catheters is through surface release or generation of nitric oxide (NO). Nitric oxide is a free radical gas produced by the endothelium to maintain hemostasis.12–15 To inhibit clot formation, the gas reduces platelet activation by inhibiting agonist binding to their surface receptors. Nitric oxide freely diffuses into platelets to initiate the NO/cyclic guanosine monophosphate (cGMP)16–20 pathway that, in turn, phosphorylates G protein-coupled surface receptors, changing their conformation to decrease binding affinities of agonists. Commonly known G protein-coupled receptors on platelets include thrombin, thromboxane A2, and adenosine diphosphate receptors. The gas also reduces secondary activation of circulating platelets by inhibiting the release of platelets’ intracellular granules. This is achieved by blocking the release of calcium stores needed for actin– myosin interaction required for platelets to change shape and release their granules. Unlike other platelet inhibitors, NO has a very short half-life (milliseconds), as it is quickly taken up by red blood cells, platelets, and other NO scavengers. Thus, the anticoagulant effect occurs near the NO-releasing/generating surface and elicits no effect on coagulation downstream.
In this work, silicone rubber catheters were extruded with chemistry incorporated within that enables postextrusion charging with NO to create NO-releasing dizeniumdiolate structures within the walls of the extrude catheter. The controlled NO release from the catheters was measured in vitro by chemiluminescence and finally a 4-hour biocompatibility testing of NO-secreting catheters and controls was conducted using a rabbit model of thrombogenicity without systemic anticoagulation.
Materials and Methods
Materials
N-(6-aminohexyl) aminopropyl trimethoxysiloxane (DACA-6), potassium tetrakis(4-chlorophenyl) borate, phosphate buffer saline, and tetrahydrofuran (THF) were purchased from Sigma Aldrich (St Louis, MO). The two part silicone resin was purchased from NuSil Silicone Tech (Carpinteria, CA). The fumed silica particles were purchased from Sigma Aldrich and the stainless steel mandrel was a product of Small Parts Inc. (Marimar, FL). The 21 gauge angiocaths that were bonded to the extruded catheters were purchased from Infusion Therapy Systems Inc. (Sandy, UT).
Structure and Fabrication of NO-releasing Catheters
All extruded catheters had a trilayer structure: a base layer, an active layer, and a top layer. The active layer, where NO molecules are immobilized, is sandwiched between the base and top layers as shown in Figure 1.
Figure 1.
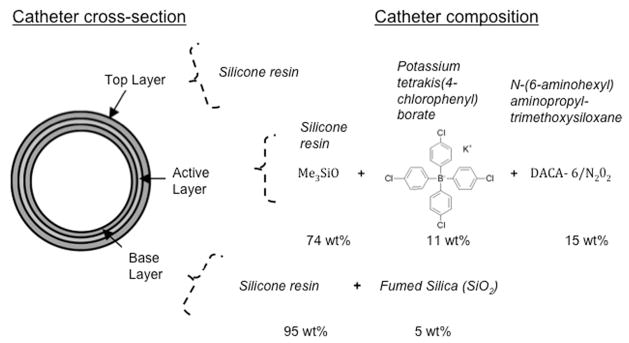
Cross-sectional composition of nitric oxide-releasing catheters.
The compositions of the base, active, and top layers are shown in Table 1. The base layer consisted of silicone resin oligomers A and B in a 1:1 ratio and 5 wt% fumed silica to add stiffness to the catheters. The active layer contained 1:1 silicone resin A to B, 15 wt% DACA-6, and 11 wt%/mole of DACA-6 of potassium tetrakis(4-chlorophenyl) borate. Only silicone (1:1 resin A to B) was used as the top-coating material.
Table 1.
Chemical Composition of Base, Active, and Top Layers of Nitric Oxide-Releasing Catheter
| Catheter Layer | Resin A | Resin B | Fumed silica | DACA-6 | Borate |
|---|---|---|---|---|---|
| Base layer | 1 part | 1 part | 5 wt% | — | — |
| Active layer | 1 part | 1 part | — | 15 wt% | 11 wt% by mole of DACA-6 |
| Top layer | 1 part | 1 part | — | — | — |
DACA-6, N-(6-aminohexyl) aminopropyl trimethoxysiloxane.
The base, active, and top layers were sequentially extruded during fabrication of the catheters. The catheter fabrication process is shown in Figure 2. Stainless steel mandrels (21 gauge) were used as cores over which catheter material was extruded using a setup with a fixed aperture size through which mandrels are passed. After extrusion and curing, the stainless steel cores were removed leaving behind the experimental catheters. For extrusion of the base layer, 10 cm long stainless steel mandrels were hand-fed through the catheter fabrication setup. After three passes, a uniform coat over the mandrels was achieved. The mandrels are then stationed vertically and cured at 500°F for 30 min. The cured base layers were then slid off the mandrels after immersion in water for 2 hours. The newly formed catheter base layers were left to dry in a drying hood for 30 min before the active layer was extruded over the base layer. The thickness of the active layer was achieved after two passes through the extruder. The two layers were dried in ambient temperature for 24 hours. Finally, to remove any surface irregularities, the top layer was added with a single pass. The extruded catheters were then dried in a fume hood for 48 hours before charging the NO-releasing group with NO.
Figure 2.
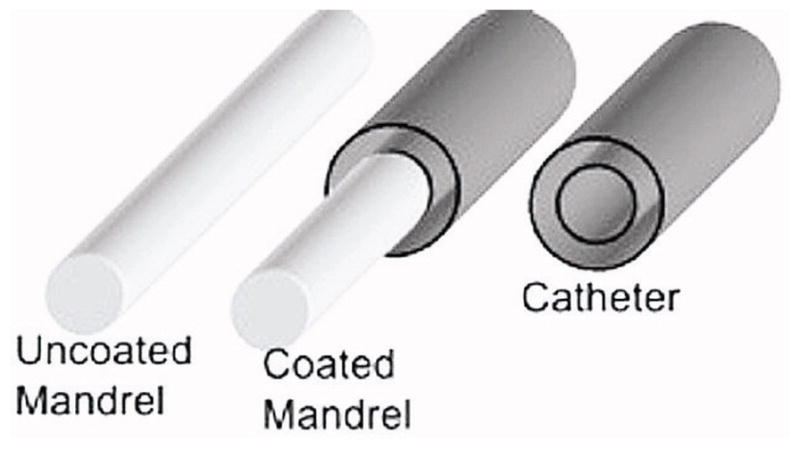
Fabrication process of nitric oxide-releasing silicone catheters.
NO Charging of Catheters and NO-release Testing
The method used to charge the active layers with NO has been previously described.21 In brief, the NO-releasing catheter group was charged with NO using a NO reactor (80 psi of NOg), whereas the control group, having the same structure and composition as the NO-releasing group, was not charged with NO. Catheters were kept in the reactor for 72 hours to allow the NO molecules to react with DACA-6. The catheters were then immediately tested for their NO release by chemiluminescence. Nitric oxide release testing by chemiluminescence has also been described.22 In brief, 1 cm long sections of the NO-reacted catheters were bathed in a chelex-treated phosphate-buffered saline (PBS), pH 7.34, at 37°C. Nitric oxide released was then measured with a Sievers Nitric Oxide Analyzer 280i (GE Instruments, Boulder, CO). The NO release signal was integrated over a given time, and knowing the surface area of the catheter, the flux of NO from the surface can be readily calculated as NO concentration per unit time divided by the total surface area of the catheters. Because all catheters were implanted 24 hours after charging, their NO release after 24 hours of dry storage in −20°C environment was also tested.
To test whether DACA-6 is essential for immobilizing NO molecules during charging, catheters without DACA-6 (N = 3) were charged and tested for their NO release. In addition, to test whether borate was needed to stabilize NO release, catheters prepared with active layers of 5% and 15% DACA-6 without borate (N = 3 each) were charged and tested for NO release.
Preparing Catheters for Nonthrombogenicity Evaluation
All catheters, controls, and NO-releasing were cut to 6 cm long sections and connected to a 21 gauge angiocatheter. Part of the commercial catheter was dipped in THF and inserted into one end of the extruded catheter to bond the interface. This assembly process was necessary for controlled and leak-free blood draws and infusions using luer-lock syringes. The catheters were then stored in a −20°C fridge to reduce spontaneous NO loses.
Rabbit Thrombogenicity Model for Testing of Catheters
The animal handling and surgical procedures were approved by the University Committee on the Use and Care of Animals in accordance with university and federal regulations. A total of six New Zealand white rabbits (Myrtle’s Rabbitry, Thompson’s Station, TN) were used in this study. All rabbits (2.5–3.5 kg) were initially anesthetized with intramuscular injections of 5 mg/kg xylazine injectable (AnaSed Lloyd Laboratories, Shenandoah, IA) and 30 mg/kg ketamine hydrochloride (Hospira, Inc., Lake Forest, IL). Maintenance anesthesia was administered via a diluted intravenous (IV) infusion of ketamine at a rate of 15 mg/kg/hour. To maintain blood pressure stability, IV lactated Ringer’s solution was given at a rate of 10 ml/kg/hour. The paralytic, pancuronium bromide (0.2 mg/kg, IV) was administered to have the animal totally dependent on mechanical ventilation which was done via a tracheotomy and using a Sechrist Infant Ventilator Model IV-100 (Sechrist, Anaheim, CA). For monitoring blood pressure and collecting blood samples, the rabbit right carotid artery was cannulated using a 16 gauge IV angiocatheter (Jelco, Johnson & Johnson, Cincinnati, OH). Blood pressure and derived heart rate were monitored with a Series 7000 Monitor (Marquette Electronics, Milwaukee, WI). Before placement of the catheters, the rabbits’ left and right internal jugular veins were isolated and baseline hemodynamics were recorded. Arterial blood pH, pCO2, pO2, total Hb, and metHb were determined using an ABL 725 blood gas analyzer and OSM3 Hemoximeter (Radiometer Copenhagen, Copenhagen, DK). In addition, baseline blood samples were collected for platelet and total white blood cell (WBC)counts using a Coulter Counter Z1 (Coulter Electronics, Hialeah, FL), activated clotting time (ACT) was determined using a Hemochron Blood Coagulation System Model 801 (International Technidyne Corp., Edison, NJ), and platelet function was determined using a Chrono-Log optical aggregometer model 490 (Havertown, PA). Animals had no systemic anticoagulation throughout the experiment.
After baseline blood measurements, catheters were placed 1 cm distal to the bifurcation of the right and left jugular vein. A control and an NO-releasing catheter were placed in each animal, and the placement was varied so that three animals had controls on the right internal jugular vein and three animals had controls in the left internal jugular vein. The catheters were locked with 0.9% saline (Hospira, Inc.). At 20 min intervals, each catheter was tested for its ability to allow blood draw and saline infusion using a 1 ml luer-lock syringe. Each animal received a 400 U/kg sodium heparin dose before euthanasia to prevent necrotic thrombosis. The animals were euthanized using a dose of Fatal Plus (130 mg/kg sodium pentobarbital) (Vortech Pharmaceuticals, Dearborn, MI). The internal jugular veins were dissected out from the site of catheter entry to approximately 1 cm below the end of the catheter. The jugular vein was cut parallel to the length of the catheter, and the catheter was removed. The catheters were photographed, and a 1 cm section of the tip of the catheter was preserved in glutaral-dehyde for obtaining a scanning electron micrograph (SEM).
Blood Sampling
Rabbit whole blood samples were collected in nonanticoagulated 1 ml syringes for ACT, 10% anticoagulant containing sodium citrate, sodium phosphate, and dextrose (ACD) (Hospira, Inc.) by volume of blood collected in 3 ml syringes for cell counts, and aggregometry, and 1 ml syringes containing 40 U/ml of sodium heparin (APP Pharmaceuticals, LLC, Schaumburg, IL) for blood–gas analysis. Every hour up to 4 hours, after placement of the catheters, blood samples were collected for in vitro measurements. Samples were used within 2 hours of collection to avoid any artifactual activation of platelets and monocytes. Also all data collected after baseline were corrected for the hemodilution effect of IV fluids.
Platelet Aggregometry
A previously published method21 for platelet aggregometry was used to assess platelet function. In brief, rabbit platelet aggregation was assayed based on Born’s turbidimetric method using a Chrono-Log optical aggregometer. Platelet-rich plasma (PRP) was obtained by centrifugation of 6 ml blood collected (1:10 blood to ACD) at 110 g for 15 min. Platelet-poor plasma was obtained by another centrifugation of the PRP-removed blood sample at 2730 g for 15 min and was used as the blank for aggregation. Platelet-rich plasma was incubated for 10 min at 37°C and then 40 mg/ml collagen (Chrono-PAR No 385, Chrono-Log, Havertown, PA) was added. The percentage of aggregation was determined 3 min after the addition of collagen using Chrono-Log Aggrolink software.
Statistical Analysis
To compare catheter group patency, a Kaplan-Meier Survival analysis was conducted between control and NO-releasing groups. Baseline and 4 hours thrombogenicity outcome measures of the controls and NO-releasing groups were compared using a single-factor analysis of variance. A p < 0.05 was regarded as significant.
Results
Extruded Catheter
The finished catheter shown in Figure 3A was partly filled with a mixture of water and blue food dye for perspective visualization. The extruded NO-releasing portion of the catheter, left of the demarcation, was connected to a 21 guage angiocath. All implanted catheters had the following dimensions: L = 6 cm, ID = 21 guage (0.723 mm), and OD = 12 gauge (2.052 mm). A cross-sectional representative is shown in Figure 3B. The lumen is on the top left corner followed by the base, active, and top layers. Because the NO flux of the catheters is dependent on the amount of DACA-6 and subsequent in situ diazeniumdiolation of the secondary amine site of this species, the thickness of the active layers was measured. The opaque active layer thickness averaged 200 ± 11 μm (N = 6 catheters).
Figure 3.
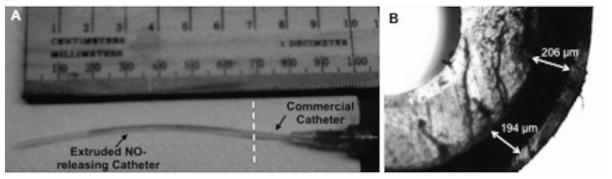
Extruded silicone-rubber nitric oxide-releasing catheter (A) and cross section of catheter (B).
NO Release from DACA-6 Doped Silicone Catheters
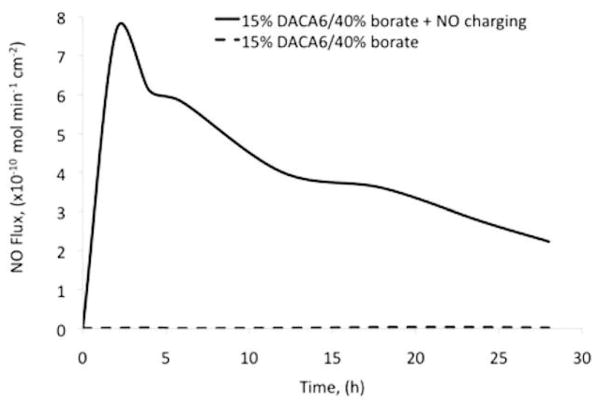
Nitric oxide release from control and NO-charged catheter groups is shown in Figure 4. As expected control catheters did not release NO, whereas NO-charged catheter secreted NO for the test duration. On average (N = 6), NO-releasing catheters released NO at a flux of approximately 6 ± 0.92 × 10−10 mol/min/cm2 steadily for first 4 hours of testing. After 24 hours of dry storage in a −20°C freezer, the flux levels dropped to approximately 2 ± 0.5 × 10−10 mol/min/cm2 on average (N = 6). In brief, the NO flux from catheters increased for the first 2 hours until the release rate peaked. Thereafter, the NO stores within the walls of the catheters started to be depleted gradually for the remainder of the in vitro test period.
Figure 4.
Nitric oxide release from control catheters (15% DACA-6, 40% borate by mole of DACA-6, and uncharged) and NO-releasing catheters (15% DACA-6, 40% borate by mole of DACA-6, and NO charged) as measured by chemiluminescence. Control catheter samples were bathed in a chelex-treated phosphate buffer saline (pH 7.34, 37°C) medium and NO release was measured with a nitric oxide analyzer.
The outcomes of the effects of DACA-6 and borate on NO release are shown in Figures 5 and 6. Catheters without borate did release larger bursts of NO within an hour but thereafter released relatively lower flux levels of NO. The addition of borate helped reduce this burst for a more sustained NO release profile (Figure 4). The catheters prepared without DACA-6 did not release significant levels of NO after charging. Low levels of NO release were measured from these catheter types, likely because of NO gas has high solubility in silicone rubber materials. However, this temporary storage of NO dissipates very quickly during NO release testing. Nitric oxide flux density (× 10−8 mol/cm2) of catheters was function of wt% DACA-6 according to the relationship NO = 0.38 (wt% DACA-6) + 0.21, R2 = 0.99 (see Figure 6). Higher NO flux levels were sustained for more than 24 hours at 40 wt% borate inclusion compared to no borate.
Figure 5.
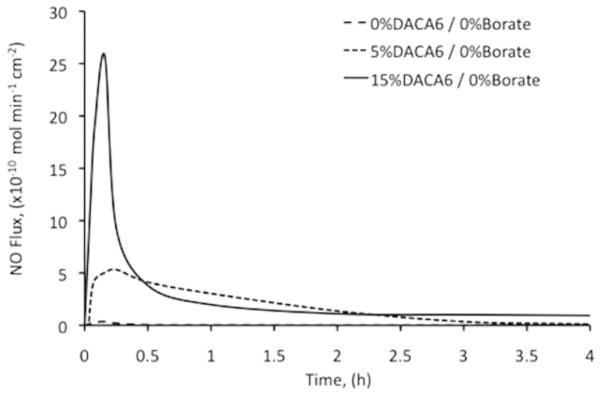
Nitric oxide release from catheters (0% DACA-6 and 0% borate, 5% DACA-6 and 0% borate, and 15% DACA-6 and 0% borate) as measured by chemiluminescence. Catheter samples were bathed in a chelex-treated phosphate-buffered saline (pH 7.34, 37°C) medium. NO released was measured with a nitric oxide analyzer.
Figure 6.
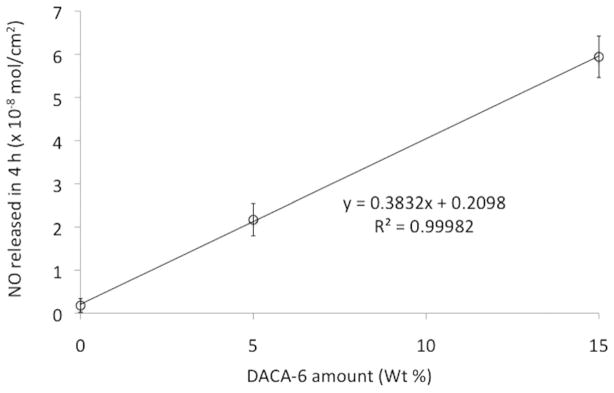
NO flux density of NO-releasing catheters as a function of their wt% of DACA-6 amounts.
Thrombogenicity Testing Outcomes
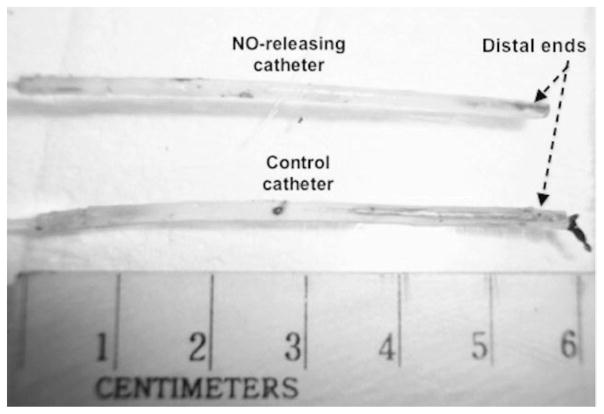
All NO-releasing silicone catheters remained patent for the entire duration of the thrombogenicity testing. There were no blood-drawing or saline-infusion incidents with any of the NO-releasing catheters. However, four out of six control catheters became occluded before the end of the in vivo testing experiments. Catheter occlusion was interpreted as the first time when blood draw was totally impeded. Further investigations for clots on the occluded catheters using SEMs and photography were also performed after carefully dissecting out the implanted catheters. Representative images of clots on implanted control catheters and explanted control, and NO-releasing catheters are shown in Figures 7 and 8. Scanning electron micrograph analysis also showing occlusive clots on control catheters (A) as well as small amounts of platelet microaggregates on control and NO catheters’ surfaces (B) are shown in Figure 9. The microaggregates were further observed to travel up the lumen of the control catheters.
Figure 7.
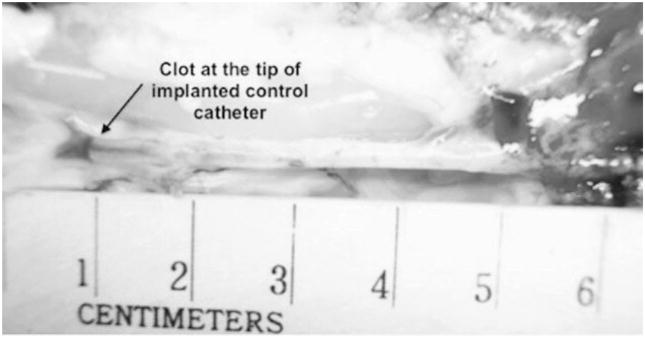
Clots on control catheters after 4 hours of implantation in the jugular vein of adult New Zealand male rabbits.
Figure 8.
Clots on control and NO-releasing catheters. All NO-releasing catheters showed no occlusive clots at their tips after explantation (top) whereas control catheters (bottom) got completely occluded by clots before the end of the 4-hour experiments.
Figure 9.

Scanning electron microscopy analysis of clots on explanted catheters: (A) control catheter with an occlusive clot and (B) sparse microaggregates that were present on both control and NO-releasing catheter surfaces.
Biological markers such as metHb, Hb, WBC count, and platelet count and platelet function, frequently used to measure NO toxicity, inflammation, and blood activation, were collectively used for a general evaluation of the biocompatibility of the catheters. Baseline metHb, Hb, WBC counts, and platelet counts did not change significantly over the course of the experiment. Hemoglobin level at the end of the experiment was however significantly lower than baseline level (p < 0.05). This was because of the fact that IV lactated Ringer’s solution was given at a rate of 10 ml/kg/hour to maintain a stable blood pressure (see Table 2). Basically, the surface area (3.9 ± 0.4 cm2) of the catheters are so small that 4 hours of blood exposure is not long enough to effect significant changes in those biological markers. Hemoglobin, however, did decrease by a significant amount (14%, p < 0.05) after 4 hours because of hemodilution.
Table 2.
Four-hour Hemocompatibility of Implanted Catheters
| Implantation (h) | metHb (%) | Hb (g/dl) | WBC (cell/μl) | Platelets (×108 cell/ml) | Platelet function (%) |
|---|---|---|---|---|---|
| 0 | 0.48 ± 0.08 | 10.14 ± 0.16 | 3502.6 ± 459 | 3.66 ± 0.56 | 82 ± 4.9 |
| 4 | 0.34 ± 0.07* (p = 0.1) | 8.68 ± 0.14 (p < 0.05) | 3338.8 ± 581* (p = 0.8) | 2.87 ± 0.28* (p = 0.2) | 78.3 ± 9.5* (p = 0.9) |
Corrected for hemodilution.
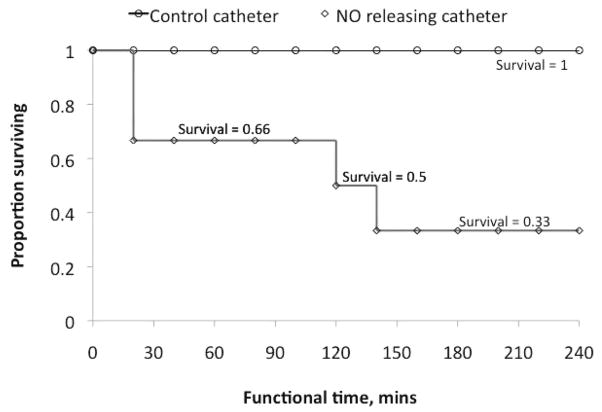
An important evaluation parameter of any catheter is its ability to remain patent. In our study, the average time until the first blood draw incident occurred was termed the patency time. As shown in the Kaplan-Meier survival analysis (Figure 10), all NO-releasing catheters remained functional after 4 ± 0 hours whereas the controls’ functional time averaged 2.3 ± 0.7 hours. There was a 100% survival in the NO-releasing group and 33.33% in the control group.
Figure 10.
In vivo functional time of control and NO-releasing catheter groups. Functional time was interpreted as average time until first blood draw incident per catheter group.
Discussion
Research is needed to develop small (21–24 gauge) intravascular catheters that function similar to vascular endothelium, producing NO locally, preventing development of biofilm, repelling platelets, and preventing thrombus formation. The risks of clotting and infection with current catheters occur in all patients (including children and adults), but the problem is most serious in newborns because the vessels are so narrow that even a small thrombus can occlude the catheter, and because the newborn’s host response to bacterial infection is not fully developed.
Preventing these risks would have a major impact on neonatal care, and even greater impact on all patients who require intravascular catheters. It would decrease morbidity and mortality and decrease costs by shortening hospital stay while increasing survival. Creating catheters from materials that are highly resistant to thrombus and biofilm formation would solve these major problems. Current approaches do not eliminate these problems. Catheters with heparin bonded to the surfaces are available,23–25 but systemic heparin injections/infusions are still required. Catheters coated with antiseptics or antibiotics are available26 and do decrease the risk of infection somewhat, but do not prevent biofilm formation on the outer surface of the catheter in contact with blood/tissue. Catheter-related sepsis (or the possibility of catheter sepsis requiring catheter removal) is still a major problem in the hospital setting.26
The trilaminar layer fabrication process is a new approach used for developing NO-releasing catheters for the following reasons: 1) initial catheter prototypes fabricated entirely out of the active layer material deformed on removal from mandrels, 2) application of a base layer of silicone blended with fumed silica to the mandrel before other layers allowed damage-free catheter removal from mandrels, and 3) addition of top layer prevents surface charging of catheter because of NO release and also dampened surface asperities.
The results of this study suggest that NO-releasing catheters attenuate thrombus formation in a short-term animal model and that such catheters can be fabricated by extrusion with the precursors for NO release chemistry incorporated into the walls of the catheters. Key properties of the extruded catheters are the level of NO flux they secrete and the duration of the NO secretion. The levels of NO release from catheters (6 × 10 −10 mol/min/cm2) after NO charging and (approximately 2 × 10−10 mol/min/cm2) after 24 hours of dry storage were comparable to endothelial level of NO flux. Although the catheters were stored dry and at a low temperature (−20°C) to reduce spontaneous release of NO, there was a 66.7% decrease in flux levels when tested after 24 hours. Therefore, such storage condition may not be ideal for long-term storage as most of the stored NO may be lost. In this study, however, the levels of NO release after 24 hours storage proved adequate for maintaining catheter patency.
If further improvements in the NO release profile are necessary, they might be achieve by adjusting the thickness of the active layer, the availability of anionic borate sites in the active layer, and the NO charging conditions.
The thickness of the active layer will affect the amount of NO that a catheter can release and may also affect its mechanical strength. Thicker active layer will allow for a substantially higher catheter NO payload and hence a relatively longer NO release duration. For thinner walls the contrary will be true. As the active layer material received the most chemical additives, its silicone base material achieves less cross-linkages resulting in a more complaint layer. The manufacturing conditions for such catheters become more challenging when fabrication NO release catheters for pediatric use. Their diameters can be as small as 21 g (0.723 mm), and therefore their base layer thicknesses must be small and rigid enough so that majority of the pediatric catheter wall thickness is taken up by the active layer. The inclusion of anionic sites in the active layer sustains NO release by keeping the microenvironment of the catheters in a more acidic condition.27 Anionic agents such as borate (used in this study), dibutyltin dilaurate, and poly(lactic-co-glycolic acid) (PLGA) used in many Food and Drug Administration approved medical devices are currently being investigated in detail to determine what levels allow sustained NO release from hydrophobic polymers doped with diazeniumdiolates for a 30 day period. Their stability may also be important in keeping the acidic conditions necessary for sustained release. For instance, slow-degradation-rate PLGAs may serve as a better regulator of NO release than a faster-degradation-rate PLGA. Lastly, the optimal charging time of these catheters are, at this point, unknown and needs to be examined in detail. The method of loading NO into catheters was based on previous work on silicone membranes.21 A combination of longer NO charging and high NO-payload amines with stable anionic sites may improve the longevity of NO release from catheters.
The short-term rabbit model used to test the biocompatibility of catheters is a well-developed model that allowed for monitoring an array of biological markers. There are however some improvements that can be made. For example, the 20 min blood withdrawal test interval may mask true differences between control and test catheters, as forming clots maybe broken up before they grow into larger occlusive masses. Blood draws could be done every hour, instead, such that clots have a greater duration to form. This methodology would be more aggressive in teasing out differences in performance between catheter test groups but would require an animal model with catheter placement lasting for a longer period of time (e.g., 8–24 hours). Protein deposition was not measured in this study although it may affect NO diffusion by increasing the diffusion distance of the gas. In this setting, the protein layer will increase diffusion distance, but however minimally. The ratio of adsorbed protein layer thickness (up to 5 μm) to catheter wall thickness (665 μm) is very small, less than 1%. As no metric for measuring NO diffusion was included in our experimental protocol, no correlation between NO diffusion and adsorbed protein thickness could have been made. In addition, data on the mechanical strength of NO-releasing silicone catheters should be studied in long-term study protocols, as the mechanical strength of silicone modified with NO-releasing material is currently unknown. None of the catheters (controls and NO releasing) however failed during explantation. Therefore, the next step in testing these extruded NO-releasing catheters should focus on longer-term outcomes using appropriate animal models. The longer-term model would also allow for examination of the antimicrobial effects of the NO-releasing catheters. This measure will be added during the longer-term thrombogenicity testing.
Conclusion
The goals of this study were to investigate the following: fabrication of silicone catheters, NO charging of catheters to release endothelial levels of NO in a controlled environment, characterization, and investigation of how to control NO flux and nonthrombogenic evaluation of the NO-releasing catheters in vivo.
The catheter fabrication process was adequate for the manufacturing of silicone catheters for nonthrombogenic evaluation. Quality controls built into the fabrication process ensured the manufacturing of 12 gauge outer diameter catheters that could be implanted into the right and left internal jugular veins in adult rabbits. Because the catheters’ walls were fabricated in layers, chemistry could be incorporated into their active layers to allow loading of NO into their walls. Nitric oxide-charged catheters also released endothelial levels (>2 × 10−10 mol/min/cm2) of NO upon stimulation in a PBS solution (37°C, pH = 7.34). Their NO flux, as measured by chemiluminescence, showed that 1) NO flux depended of DACA-6 concentration and 2) high levels of sustained NO release depended on borate inclusion. Nitric oxide flux density (× 10−8 mol/cm2) of catheters was function of wt% DACA-6 according to the relationship NO = 0.38(wt% DACA-6) + 0.21, R2 = 0.99. In addition, higher NO flux levels were sustained for more than 24 hours at 40 wt% borate inclusion compared to no borate.
Overall, 100% of the NO-releasing catheters survived the 4 hour test duration compared to a 33% survival of the non-NO-releasing controls. For NO-releasing catheters to find use in both pediatric and adult care, fabrication of NO-releasing catheters with ODs as small as 21 gauge should be investigated. The duration of NO release should also be studied to extend their patency up to 30 days and beyond. To get there, different NO immobilizing compounds and anionic sites must be investigated.
Acknowledgments
Supported by National Institutes of Health grants 2R01 Hl069420-06, #R01 HD01534, and EB-000783.
Footnotes
The authors except Dr. Robert Bartlett confirm that there is no known conflict of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome. Dr. Robert Bartlett has an equity interest in Accord Biomaterials, Inc. but no financial support from this company was used in any of this paper’s work.
References
- 1.Bass J, Halton J, Drouet Y, Ni A, Barrowman N. Central venous catheter database: An important issue in quality assurance. J Pediatr Surg. 2011;46:942–945. doi: 10.1016/j.jpedsurg.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 2.Wiener ES, McGuire P, Stolar CJ, et al. The CCSG prospective study of venous access devices: An analysis of insertions and causes for removal. J Pediatr Surg. 1992;27:155–63. doi: 10.1016/0022-3468(92)90304-p. discussion 163. [DOI] [PubMed] [Google Scholar]
- 3.Hollyoak MA, Ong TH, Leditschke JF. Critical appraisal of surgical venous access in children. Pediatr Surg Int. 1997;12:177–182. [PubMed] [Google Scholar]
- 4.Dillon PA, Foglia RP. Complications associated with an implantable vascular access device. J Pediatr Surg. 2006;41:1582–1587. doi: 10.1016/j.jpedsurg.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Male C, Chait P, Andrew M, Hanna K, Julian J, Mitchell L. PARKAA Investigators: Central venous line-related thrombosis in children: Association with central venous line location and insertion technique. Blood. 2003;101:4273–4278. doi: 10.1182/blood-2002-09-2731. [DOI] [PubMed] [Google Scholar]
- 6.Grisoni ER, Mehta SK, Connors AF. Thrombosis and infection complicating central venous catheterization in neonates. J Pediatr Surg. 1986;21:772–776. doi: 10.1016/s0022-3468(86)80364-5. [DOI] [PubMed] [Google Scholar]
- 7.Peters M, ten Cate JW, Koo LH, Breederveld C. Persistent anti-thrombin III deficiency: Risk factor for thromboembolic complications in neonates small for gestational age. J Pediatr. 1984;105:310–314. doi: 10.1016/s0022-3476(84)80138-9. [DOI] [PubMed] [Google Scholar]
- 8.Peters M, ten Cate JW, Jansen E, Breederveld C. Coagulation and fibrinolytic factors in the first week of life in healthy infants. J Pediatr. 1985;106:292–295. doi: 10.1016/s0022-3476(85)80308-5. [DOI] [PubMed] [Google Scholar]
- 9.Journeycake JM, Buchanan GR. Catheter-related deep venous thrombosis and other catheter complications in children with cancer. J Clin Oncol. 2006;24:4575–4580. doi: 10.1200/JCO.2005.05.5343. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett RH. Extracorporeal Life Support Registry Report 1995. ASAIO J. 1997;43:104–107. [PubMed] [Google Scholar]
- 11.Winthrop AL, Wesson DE. Urokinase in the treatment of occluded central venous catheters in children. J Pediatr Surg. 1984;19:536–538. doi: 10.1016/s0022-3468(84)80098-6. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Rojas AP, Griffith GW, et al. Improving blood compatibility of intravascular oxygen sensors via catalytic decomposition of S-Nitrosothiols to generate nitric oxide in situ. Sens Actuators B Chem. 2007;121:36–46. doi: 10.1016/j.snb.2006.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh BK, Meyerhoff ME. Catalytic generation of nitric oxide from nitrite at the interface of polymeric films doped with lipophilic CuII-complex: A potential route to the preparation of thrombo-resistant coatings. Biomaterials. 2004;25:283–293. doi: 10.1016/s0142-9612(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 14.Boudko DY. Bioanalytical profile of the L-arginine/nitric oxide pathway and its evaluation by capillary electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:186–210. doi: 10.1016/j.jchromb.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murad F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling. Biosci Rep. 1999;9:453–474. doi: 10.1023/a:1020265417394. [DOI] [PubMed] [Google Scholar]
- 16.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 17.Ghaffari A, Miller CC, McMullin B, Ghahary A. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide. 2006;14:21–29. doi: 10.1016/j.niox.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Yoo JW, Choe ES, Ahn SM, Lee CH. Pharmacological activity and protein phosphorylation caused by nitric oxide-releasing microparticles. Biomaterials. 2010;31:552–558. doi: 10.1016/j.biomaterials.2009.09.058. [DOI] [PubMed] [Google Scholar]
- 19.Kushwaha M, Anderson JM, Bosworth CA, et al. A nitric oxide releasing, self assembled peptide amphiphile matrix that mimics native endothelium for coating implantable cardiovascular devices. Biomaterials. 2010;31:1502–1508. doi: 10.1016/j.biomaterials.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramamurthi A, Robson SC, Lewis RS. Effects of nitric oxide (NO) and soluble nucleoside triphosphate diphosphohydrolase (NTPDase) on inhibition of platelet deposition in vitro. Thromb Res. 2001;102:331–341. doi: 10.1016/s0049-3848(01)00244-4. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Annich GM, Miskulin J, et al. Nitric oxide releasing silicone rubbers with improved blood compatibility: Preparation, characterization, and in vivo evaluation. Biomaterials. 2002;23:1485–1494. doi: 10.1016/s0142-9612(01)00274-5. [DOI] [PubMed] [Google Scholar]
- 22.Major TC, Brant DO, Reynolds MM, et al. The attenuation of platelet and monocyte activation in a rabbit model of extracorporeal circulation by a nitric oxide releasing polymer. Biomaterials. 2010;31:2736–2745. doi: 10.1016/j.biomaterials.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anton N, Cox PN, Massicotte MP, et al. Heparin-bonded central venous catheters do not reduce thrombosis in infants with congenital heart disease: A blinded randomized, controlled trial. Pediatrics. 2009;123:e453–e458. doi: 10.1542/peds.2008-1508. [DOI] [PubMed] [Google Scholar]
- 24.Krafte-Jacobs B, Sivit CJ, Mejia R, Pollack MM. Catheter related thrombosis in critically ill children: Comparison of catheters with and without heparin bonding. J Pediatr. 1995;126:5054–5060. doi: 10.1016/s0022-3476(95)70499-x. [DOI] [PubMed] [Google Scholar]
- 25.Massicotte MP, Dix D, Monagle P, Adams M, Andrew M. Central venous catheter related thrombosis in children: Analysis of the Canadian Registry of Venous Thromboembolic Complications. J Pediatr. 1998;133:770–776. doi: 10.1016/s0022-3476(98)70149-0. [DOI] [PubMed] [Google Scholar]
- 26.Riley KD, Classen CD, Stevens LE, Burke JP. A large randomized clinical trial of a silver-impregnated urinary catheter: Lack of efficacy and staphylococcal superinfection. J Amer Med. 1995;98:349–356. doi: 10.1016/S0002-9343(99)80313-1. [DOI] [PubMed] [Google Scholar]
- 27.Batchelor MM, Reoma SL, Fleser PS, et al. More lipophilic dialkyldiamine-based diazeniumdiolates: Synthesis, characterization, and application in preparing thromboresistant nitric oxide release polymeric coatings. J Med Chem. 2003;46:5153–5161. doi: 10.1021/jm030286t. [DOI] [PubMed] [Google Scholar]