Abstract
Memory can be modified when reactivated, but little is known about how the properties and extent of reactivation can selectively affect subsequent memory. We developed a novel museum paradigm to directly investigate reactivation-induced plasticity for personal memories. Participants reactivated memories triggered by photos taken from a camera they wore during a museum tour and made relatedness judgments on novel photos taken from a different tour of the same museum. Subsequent recognition memory for events at the museum was better for memories that were highly reactivated (i.e., the retrieval cues during reactivation matched the encoding experience) than for memories that were reactivated at a lower level (i.e., the retrieval cues during reactivation mismatched the encoding experience), but reactivation also increased false recognition of photographs depicting stops that were not experienced during the museum tour. Reactivation thus enables memories to be selectively enhanced and distorted via updating, thereby supporting the dynamic and flexible nature of memory.
Keywords: memory, false memory, autobiographical memory, episodic memory, long-term memory
People are frequently reminded of past experiences (Berntsen, 1998; Hintzman, 2011), but how the properties and extent of memory reactivation modify subsequent retrieval is unresolved (e.g., Diekelmann, Buchel, Born, & Rasch, 2011; Schiller & Phelps, 2011). Reactivating a memory following consolidation can bring a stable memory into a labile state, which requires a period of reconsolidation that is sensitive to pharmacological modification (Misanin, Miller, & Lewis, 1968; Nader, Schafe, & Le Doux, 2000). Moreover, reactivation preceding new learning can increase memory interference (Hupbach, Gomez, Hardt, & Nadel, 2007) and prevent a learned fear response from returning (Schiller et al., 2010). Reactivation-induced modification of memory thus provides an opportunity for memories to be enhanced or updated (Johnson & Chalfonte, 1994). However, such adaptive updating processes—critical for the operation of a dynamic memory system that flexibly incorporates relevant new information—could also contribute to certain kinds of memory distortion (Hardt, Einarsson, & Nader, 2010; Schacter, 2012; Schacter, Guerin, & St. Jacques, 2011).
It is also well known that memory can be strengthened by additional rehearsal. However, the properties of rehearsal can differentially affect later memory. For example, spaced studying has greater benefits for long-term memory than massed studying does (Ebbinghaus, 1885/1913). Further, repeated testing enhances later memory more than repeated studying does (for a review, see Roediger & Karpicke, 2006). Reminder cues may also differ in their effectiveness in reactivating a memory (e.g., Forcato, Argibay, Pedreira, & Maldonado, 2009; Hupbach, Hardt, Gomez, & Nadel, 2008). However, it remains unknown whether the properties of reactivation can selectively enhance and also distort personal memories.
We developed a museum paradigm to investigate whether the properties of reactivation affect subsequent retrieval of personal memories. People conducted a self-guided museum tour while wearing a camera that automatically took photographs using a timer (e.g., St. Jacques, Conway, Lowder, & Cabeza, 2011; St. Jacques, Rubin, LaBar, & Cabeza, 2008). Following a 48-hr delay, they were shown photos of stops from events in their tour (i.e., retrieval cues) and then rated the degree to which novel photos (taken from a different tour of the same exhibits) were related to those events (i.e., reactivation of the museum event preceded encoding of the novel photo). We manipulated whether the retrieval cues matched or mismatched the memory encoding experience at the museum (Tulving & Thompson, 1973; see Fig. 1), thus influencing the level and quality of retrieval. We predicted that reactivation would both enhance and distort memory via updating, and therefore lead to an increase in subsequent true and false memory.
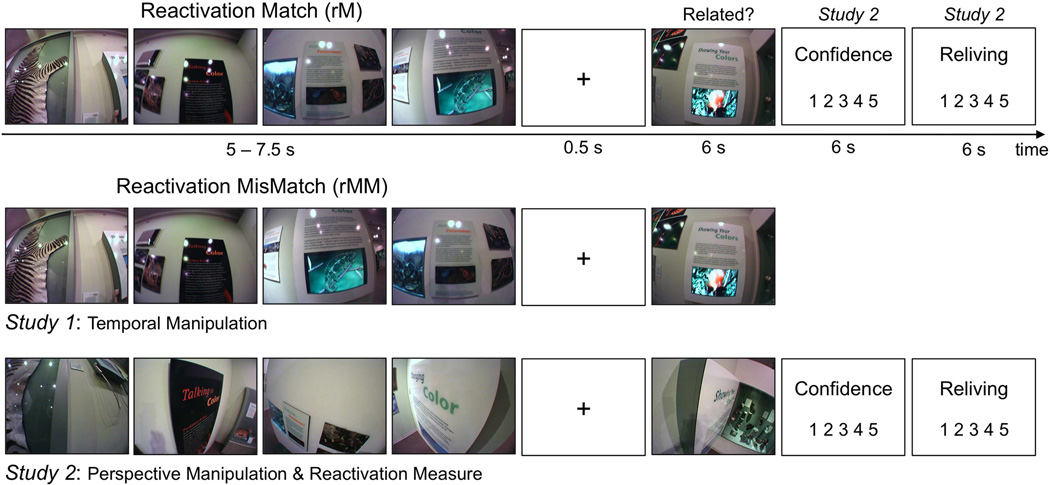
Figure 1.
Illustration of the trial sequence during the reactivation session. Forty-eight hours following the museum tour, participants were shown “movies” of events they had experienced during the tour. Each movie consisted of six (Study 1) or four (Study 2) photos of stops from that event. (Only the first four stops are included here in the examples from Study 1.) In the reactivation-match condition, the stops matched ones the participant had experienced during the tour. In the reactivation-mismatch condition, a mismatch was created by altering the temporal order of the last four stops in the event (Study 1) or by presenting photos from a control set that altered the perspective by changing the angle, height, or both. Following a 0.5-s delay, a novel photo of the same exhibit, but from an alternative tour version, was presented, and participants rated the relatedness of that photo to the museum event just shown. In Study 2 only, they then rated their confidence in the relatedness judgment and their sense of reliving the event while viewing the event movie.
Study 1
Method
Participants
Potential participants were excluded if they had previously visited the museums where the tours took place. Two additional participants were excluded because instructions were not followed or technical issues arose. The final sample consisted of 42 participants (27 women, 15 men; mean age = 21.11 years, SD = 2.87 years). All gave informed consent, and this study was approved by the ethics committee at Harvard University.
Procedure
The study involved three sessions. In Session 1, participants were given a tour booklet outlining a self-guided tour of the adjoining Harvard Natural History and Peabody museums. They were asked to wear a ViconRevue (Vicon, Oxford, United Kingdom) camera, which automatically takes photos every 15 s using a timer. The tour was composed of 32 events, each containing six discrete stops that would normally be conducted at a museum (e.g., examining a display case). There were two versions of the tour; they included the same museum events, but differed in the last two stops in each event. These alternate stops involved similar content (e.g., one video vs. another), but the alternate stops from one tour version were unseen in the context of the other tour version. Photos of the alternate stops in each event were used as lures for recognition memory, and the two tour versions were counterbalanced across participants. Participants were instructed to complete only the stops described in their tour. Photographs from each participant’s camera were inspected to ensure that the participant adhered to the instructions, and if the camera captured a photograph of an alternate stop for an event, that event was excluded from further analysis. A photograph depicting each stop within each museum event was selected to use in the later sessions.
Session 2, reactivation, took place following a 48-hr delay. On each trial, participants were shown an event “movie” consisting of six photographs of the stops from that event, presented at a rate of 1.25 s per photo. Following a 0.5-s fixation interval, participants were shown a novel photo of one of the alternate stops for that event (i.e., the stop was at the same exhibit, but the participant had not been at that particular stop during the tour). Participants made a yes/no judgment as to whether the novel photograph was related to the event in the movie. One novel photo was shown for each event included in the reactivation session. Reactivation was manipulated within participants on the basis of the principle of encoding specificity (Tulving & Thompson, 1973). Specifically, on some trials, we changed the temporal order of the photos depicting the last four stops within the event such that the order differed from the order of the stops experienced during the museum tour. Recalling memories in the correct temporal order (as opposed to the incorrect order) has been previously shown to produce more detailed and faster retrieval of personal memories (Anderson & Conway, 1993; Radvansky, Copeland, & Zwaan, 2005). Thus, we reasoned that photo cues presented in the correct temporal order would result in a greater level of reactivation than cues shown in the incorrect temporal order. Three quarters of the museum events were shown during reactivation, and the remaining were used for the baseline condition.
Session 3 took place 48 hr following reactivation. Participants were asked to make yes/no recognition decisions on pairs of photos; each pair consisted of two targets or a target and a lure. Target-target pairs depicted two of the six possible stops from the same museum event. Target-lure pairs consisted of one of the six possible stops from an event (i.e., target) along with one of the alternate stops for that event (i.e., lure). A given photo was presented only once during this session. Participants were instructed to respond “yes” to a pair only if the photos showed stops that had been experienced together during an event at the museum. Photo pairs belonged to three conditions: reactivation match (i.e., the event that the photos were taken from had been shown during reactivation with stops in their correct temporal order), reactivation mismatch (i.e., the event that the photos were taken from had been shown during reactivation with stops in incorrect temporal order), and baseline (i.e., the event that the photos were taken from had not been shown during reactivation). Participants rated their confidence in each judgment on a 4-point scale.
Results
Session 2: reactivation
Reaction times to the novel photos did not differ significantly between the reactivation-match condition (M = 3.77 s, SD = 0.37 s) and the reactivation-mismatch condition (M = 3.87 s, SD = 0.38 s). Similarly, the proportion of correct relatedness judgments did not differ between these two conditions (match: M = .85, SEM = .03; mismatch: M = .84, SEM = .03).
Session 3: recognition memory
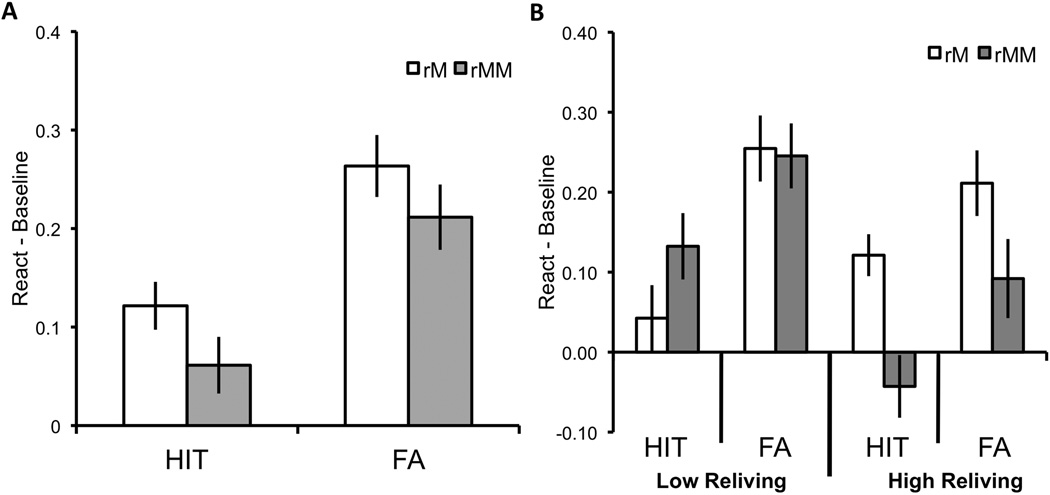
Hit and false alarm rates in all three conditions are presented in Table 1. Analyses of data from the recognition session were conducted on difference scores (reactivation condition – baseline condition). Confidence in recognition judgments and reaction times for both recognition and confidence judgments did not differ between the two reactivation conditions. A 2 (memory type: hit, false alarm) × 2 (reactivation condition: match, mismatch) repeated measures analysis of variance (ANOVA) on the difference scores for recognition responses revealed a main effect of memory type, F(1, 39) = 25.16, p < .0001, ηp2 = .39, suggesting that prior exposure had a greater influence on the proportion of false alarms than on the proportion of hits (relative to baseline). As predicted, we also found a main effect of reactivation condition, F(1, 41) = 8.59, p < .01, ηp2 = .17; both hit and false alarm rates increased more (relative to baseline) in the reactivation-match condition than in the reactivation-mismatch condition (Fig. 2a). There was no interaction.
Table 1.
Mean Proportion of Hits and False Alarms in the Two Studies
| Study and condition | Hits | False alarms | |
|---|---|---|---|
| Study 1 | |||
| Reactivation match | .77 (.02) | .64 (.03) | |
| Reactivation mismatch | .71 (.04) | .59 (.03) | |
| Baseline | .65 (.03) | .38 (.03) | |
| Study 2 | |||
| Reactivation match | .80 (.02) | .52 (.03) | |
| Reactivation mismatch | .58 (.03) | .44 (.03) | |
| Baseline match | .72 (.02) | .29 (.02) | |
| Baseline mismatch | .53 (.03) | .28 (.03) | |
Note: Standard errors of the mean are given in parentheses.
Figure 2.
Recognition memory performance in (a) Study 1 and (b) Study 2. Performance was measured by subtracting the proportion of hits and false alarms in the baseline condition from, respectively, the proportion of hits and false alarms in each reactivation condition. For Study 2, hit and false alarm rates in the two reactivation conditions are shown separately for participants who reported a greater difference in reliving between the conditions and those who reported a smaller difference (as determined by a median split on the difference in reliving ratings between the reactivation match and mismatch conditions). Error bars represent ±1 SEM.
Study 2
In Study 2, we attempted to replicate the findings in Study 1 using a different manipulation of reactivation: perspective. Personal memories recalled from a first-person perspective are associated with more vivid recall than memories recalled from a third-person perspective (e.g., Rice & Rubin, 2009). Thus, we reasoned that retrieval cues from a perspective that matched the original encoding perspective (Tulving & Thompson, 1973) would result in a greater level of reactivation than cues from a perspective that mismatched the original encoding perspective. Additionally, we directly measured reactivation by asking participants to judge the amount of reliving, or the subjective sense of recollection or reexperience, associated with viewing the museum photographs during the reactivation session.
Method
Participants
There were 41 participants (23 women, 18 men; mean age = 21.44 years, SD = 2.22 years) in Study 2. One additional participant was excluded because of technical issues.
Procedure
The procedure was similar to that of Study 1, except as noted here. The museum tour was increased to 53 events, each consisting of four stops, to accommodate an additional condition.
During Session 2, we manipulated reactivation within participants by changing the perspective of the photographs in some trials. In the reactivation-match condition, the photographs in the event movies were participants’ own photographs, whereas in the reactivation-mismatch condition, the photographs were from a control set of images in which perspective was altered by changing the angle or height (or both) from which the photographs were taken. Each event movie consisted of four photos, one for each stop in the event. The novel photo following a given event movie was from the alternate version of the tour and depicted a stop from a typical perspective (i.e., from the participant’s own camera), in the match condition, or from an atypical perspective (i.e., from a control set of photos that altered the angle, height, or both), in the mismatch condition. Participants were asked to make a yes/no relatedness decision and then to rate their confidence in their relatedness judgment and to indicate the sense of reliving associated with viewing the event movie; both response scales ranged from 1, low, to 5, high (Fig. 1). Half of the museum events were shown during reactivation, and the remaining were in the baseline conditions.
During Session 3, a single photo was shown in each trial of the yes/no recognition test. Target photos (i.e., photos of museum stops during the tour) and lure photos (i.e., photos of alternate stops from the other tour) were selected for both the reactivation conditions and the baseline conditions. Each reactivation condition had a corresponding baseline condition to control for potential differences in memory performance due to the difference in the perspectives (typical vs. atypical) of the photographs. Baseline-match photographs were the participant’s own photographs (i.e., targets) or photographs taken from the alternative museum tour (i.e., lures). Baseline-mismatch photographs were taken from the control set of photographs that altered the perspective of stops within museum events; they depicted either stops in the participant’s tour (i.e., targets) or alternate stops from the other tour (i.e., lures). Participants were instructed to respond “yes” only to photographs depicting stops they had experienced during their museum tour. They also rated their confidence in each judgment on a 5-point scale ranging from 1, low, to 5, high.
Results
Session 2: reactivation
Reaction times for relatedness judgments did not differ between the match condition (M = 2.61 s, SD = 0.66 s) and the mismatch condition (M = 2.70 s, SD = .73 s). The proportion of correct responses also did not differ between the two conditions (match: M = .85, SEM = .03; mismatch: M = .84, SEM = .03. Participants were more confident in the match condition (M = 4.01, SEM = 0.08) than in the mismatch condition (M = 3.66, SEM = 0.08), t(39) = 4.32, p < .0001; however, reaction time for the confidence judgments did not differ between the two conditions. Additionally, participants were faster to rate reliving on match trials (M = 1.38 s, SD = 0.52 s) than on mismatch trials (M = 1.54 s, SD = 0.46 s), t(40) = 4.08, p < .0001, and these ratings differed significantly between conditions (match: M = 3.79, SEM = 0.07; mismatch: M = 3.27, SEM = 0.08), t(40) = 5.82, p < .0001; these results suggest that the perspective manipulation was effective in altering the properties of reactivation.
Session 3: recognition memory
Table 1 presents the mean hit and false alarm rates in the four conditions. Data from the recognition session were analyzed using difference scores (reactivation condition – corresponding baseline condition). Confidence ratings were higher for false alarms (M = 0.52, SEM = 0.17) than for hits (M = −0.01, SEM = 0.06), F(1, 40) = 8.42, p < .01, ηp2 = .17. Reaction times for the confidence and recognition responses did not differ between the two reactivation conditions.
A median split on the difference in reliving between the two reactivation conditions was used to categorize participants as reporting high versus low levels of reliving. A 2 (memory type: hit, false alarm) × 2 (reactivation condition: match, mismatch) × 2 (reliving: high, low) mixed, repeated measures ANOVA on the difference scores for recognition responses revealed that individual differences in reliving between the reactivation-match and reactivation-mismatch conditions modulated the magnitude of the influence of reactivation on memory. There was a significant Reactivation Condition × Reliving interaction, F(1, 39) = 9.33, p < .005, ηp2 = .19 (see Fig. 2b). This interaction was explained by the fact that there was a significant difference in recognition between the reactivation-match and reactivation-mismatch conditions only in the high-reliving group, F(1, 20) = 9.12, p < . 01, ηp2 = .31; in that group, reactivation increased both hits and alarms significantly more in the reactivation-match condition than in the reactivation-mismatch condition. Thus, participants who showed the greatest difference in reliving ratings between the reactivation conditions also showed the greatest difference in the influence of the level of reactivation on subsequent recognition memory. There was an additional main effect of memory type, F(1, 39) = 25.16, p < .0001, ηp2 = .39; the influence of reactivation was greater for false alarms than for hits. The main effect of reactivation condition approached significance (p = .09); this marginal effect suggested that reactivation had a greater effect in the match condition than in the mismatch condition, but this effect was qualified by the significant Reactivation Condition × Reliving interaction. In an analysis including the data from both studies, however, we found a significant main effect of reactivation condition, F(1, 81) = 8.40, p < .005, ηp2 = .09, and no interaction between reactivation condition and study.
Discussion
In two separate studies, we found that selectively reactivating personal memories influences subsequent retrieval. Our data are consistent with the long-standing idea that retrieval is an active process that can modify memory (Semon, 1923; see also Schacter, 2001). Previous studies have shown that a contextual reminder cue preceding new learning can degrade or interfere with later memory of the originally encoded memory (e.g., Hupbach et al., 2007; Schiller et al., 2010; Schwabe & Wolf, 2009; Walker, Brakefield, Hobson, & Stickgold, 2003). Here we have shown, for the first time, that manipulating properties of reactivation selectively influences personal memories by both enhancing and distorting memory via updating.
The memory distortion we observed resembles in some respects other previously documented memory distortions involving source-memory confusion for information presented after study. For example, in the postevent misinformation paradigm (for a review, see Loftus, 2005), the misinformation effect mainly reflects a failure to recollect the source of misinformation—that is, whether it was presented during the original event or later (e.g., Zaragoza & Lane, 1994). Similarly, after viewing a videotaped event sequence, and later viewing a novel event in a photograph, participants sometimes mistakenly claim that an event shown only in the photo occurred in the original videotape, another example of source memory confusion (Schacter, Koutstaal, Johnson, Gross, & Angell, 1997).
In a general sense, our results by definition reflect a failure of source monitoring (Johnson, Hashtroudi, & Lindsay, 1993): Participants claimed that an event they saw after their museum tour occurred during the tour. However, we controlled for simple source confusion by presenting postevent information in both the reactivation-match and the reactivation-mismatch conditions. Thus, we did not examine the effect of the presence versus absence of postevent information, but rather looked at how postevent information differentially affected the false alarm rate in these two conditions.
Although simple source confusion does not account for our results, one could argue that postevent information was more similar to target information in the reactivation-match condition than in the reactivation-mismatch condition, and that source monitoring was therefore more difficult in the former condition (Johnson et al., 1993). We do not dispute this characterization, nor do we believe it is inconsistent with an account focusing on the properties of reactivation, as matching reactivation cues by definition will resemble the original episode more than mismatching ones do. Rather, our data underscore the need to explore further the relationship between the properties of reactivation and source monitoring.
Reactivation may not affect all memories equally in all contexts (Diekelmann et al., 2011; Wang, de Oliveira Alvares, & Nader, 2009); the recruitment of reactivation-induced updating mechanisms may depend on the memory strength and the nature of new information presented during reactivation (Lee, 2009; Nader & Einarsson, 2010). Nevertheless, we propose that reactivation can selectively affect subsequent retrieval of individual memories, and that such reactivation may consequently enhance and distort later memory.
Reminders of past experiences occur frequently in daily life—potentially allowing individual memories to be strengthened and updated—so that memories continue to remain relevant in the future. Here we have demonstrated that reactivation can modify memory for naturalistic events encoded during a museum tour, and we have provided evidence that the quality of reactivation is critical in supporting this dynamic and flexible aspect of memory.
Acknowledgments
We thank Annie Mitran, Morgan Ford, Christopher Olm, and the Natural History Museum and Peabody Museum of Archaeology and Ethnology at Harvard University.
Funding
This work was supported by National Institute of Health Grants MH060941 and AG08441 (awarded to D. L. S) and by National Institute on Aging Grant NRSA AG038079 and a L’Oreal USA for Women in Science Fellowship (both awarded to P. L. S.).
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Anderson SJ, Conway MA. Investigating the structure of autobiographical memories. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1993;19:1178–1196. [Google Scholar]
- Berntsen D. Voluntary and involuntary access to autobiographical memory. Memory. 1998;6:113–141. doi: 10.1080/741942071. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Buchel C, Born J, Rasch B. Labile or stable: Opposing consequences for memory when reactivated during waking and sleep. Nature Neuroscience. 2011;14:381–386. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus H. In: Memory: A contribution to experimental psychology. Ruger HA, Bussenius CE, translators. New York, NY: Columbia University, Teachers College; 1913. (Original work published 1885) [Google Scholar]
- Forcato C, Argibay PF, Pedreira ME, Maldonado H. Human reconsolidation does not always occur when a memory is retrieved: The relevance of the reminder structure. Neurobiology of Learning and Memory. 2009;91:50–57. doi: 10.1016/j.nlm.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Hardt O, Einarsson EO, Nader K. A bridge over troubled water: Reconsolidation as a link between cognitive and neuroscientific memory research traditions. Annual Review of Psychology. 2010;61:141–167. doi: 10.1146/annurev.psych.093008.100455. [DOI] [PubMed] [Google Scholar]
- Hintzman DL. Research strategy in the study of memory: Fads, fallacies, and the search for the “coordinates of truth.”. Perspectives on Psychological Science. 2011;6:253–271. doi: 10.1177/1745691611406924. [DOI] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: A subtle reminder triggers integration of new information. Learning & Memory. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Hardt O, Gomez R, Nadel L. The dynamics of memory: Context-dependent updating. Learning & Memory. 2008;15:574–579. doi: 10.1101/lm.1022308. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Chalfonte BL. Binding complex memories: The role of reactivation and the hippocampus. In: Schacter DL, Tulving E, editors. Memory systems. Vol. 1994. Cambridge, MA: MIT Press; 1994. pp. 311–350. [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychological Bulletin. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Lee JL. Reconsolidation: Maintaining memory relevance. Trends in Neurosciences. 2009;32:413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus EF. Planting misinformation in the human mind: A 30-year investigation of the malleability of memory. Learning & Memory. 2005;12:361–366. doi: 10.1101/lm.94705. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Nader K, Einarsson EO. Memory reconsolidation: An update. Annals of the New York Academy of Sciences. 2010;1191:27–41. doi: 10.1111/j.1749-6632.2010.05443.x. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Radvansky GA, Copeland DE, Zwaan RA. A novel study: Investigating the structure of narrative and autobiographical memories. Memory. 2005;13:796–814. doi: 10.1080/09658210444000412. [DOI] [PubMed] [Google Scholar]
- Rice HJ, Rubin DC. I can see it both ways: First- and third-person visual perspectives at retrieval. Consciousness and Cognition. 2009;18:877–890. doi: 10.1016/j.concog.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger HL, III, Karpicke JD. The power of testing memory: Basic research and implications for educational practice. Perspectives on Psychological Science. 2006;1:181–210. doi: 10.1111/j.1745-6916.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Forgotten ideas, neglected pioneers: Richard Semon and the story of memory. Philadelphia, PA: Psychology Press; 2001. [Google Scholar]
- Schacter DL. Adaptive constructive processes and the future of memory. American Psychologist. 2012;67:603–613. doi: 10.1037/a0029869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Guerin SA, St. Jacques PL. Memory distortion: An adaptive perspective. Trends in Cognitive Sciences. 2011;15:467–474. doi: 10.1016/j.tics.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Koutstaal W, Johnson MK, Gross MS, Angell KE. False recollection induced by photographs: A comparison of older and younger adults. Psychology and Aging. 1997;12:203–215. doi: 10.1037//0882-7974.12.2.203. [DOI] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Phelps EA. Does reconsolidation occur in humans? Frontiers in Behavioral Neuroscience. 2011;5:24. doi: 10.3389/fnbeh.2011.00024. Retrieved from http://www.frontiersin.org/Behavioral_Neuroscience/10.3389/fnbeh.2011.00024/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. New episodic learning interferes with the reconsolidation of autobiographical memories. PLoS ONE. 2009;4(10):e7519. doi: 10.1371/journal.pone.0007519. Retrieved from http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0007519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semon R. The mneme. London, England: George Allen & Unwin; 1923. [Google Scholar]
- St. Jacques PL, Conway MA, Lowder MW, Cabeza R. Watching my mind unfold versus yours: An fMRI study using a novel camera technology to examine neural differences in self-projection of self versus other perspectives. Journal of Cognitive Neuroscience. 2011;23:1275–1284. doi: 10.1162/jocn.2010.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Rubin DC, LaBar KS, Cabeza R. The short and long of it: Neural correlates of temporal-order memory for autobiographical events. Journal of Cognitive Neuroscience. 2008;20:1327–1341. doi: 10.1162/jocn.2008.20091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Thompson DM. Encoding specificity and retrieval processes in episodic memory. Psychological Review. 1973;80:352–373. [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- Wang SH, de Oliveira Alvares L, Nader K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nature Neuroscience. 2009;12:905–912. doi: 10.1038/nn.2350. [DOI] [PubMed] [Google Scholar]
- Zaragoza MS, Lane SM. Source misattributions and the suggestibility of eyewitness memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:934–945. doi: 10.1037//0278-7393.20.4.934. [DOI] [PubMed] [Google Scholar]