Abstract
Background.
Src, EphA2, and platelet-derived growth factor receptors α and β are dysregulated in pancreatic ductal adenocarcinoma (PDAC). Dasatinib is an oral multitarget tyrosine kinase inhibitor that targets BCR-ABL, c-Src, c-KIT, platelet-derived growth factor receptor β, and EphA2. We conducted a phase II, single-arm study of dasatinib as first-line therapy in patients with metastatic PDAC.
Methods.
Dasatinib (100 mg twice a day, later reduced to 70 mg twice a day because of toxicities) was orally administered continuously on a 28-day cycle. The primary endpoint was overall survival (OS). Response was measured using the Response Evaluation Criteria in Solid Tumors. Circulating tumor cells (CTCs) were also collected.
Results.
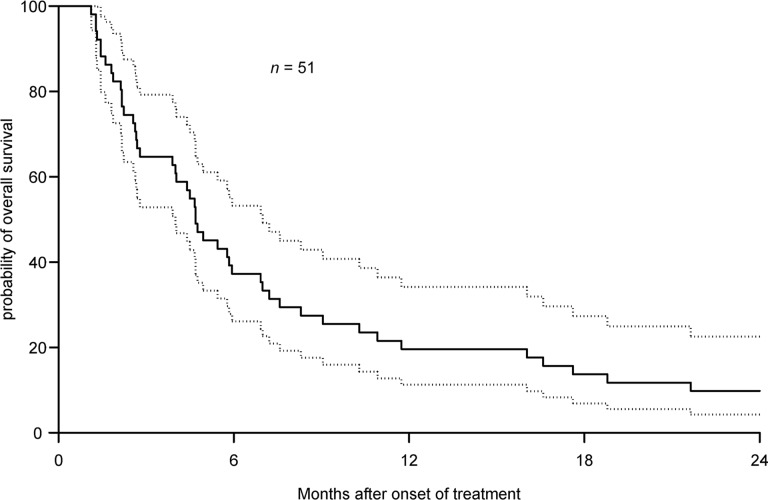
Fifty-one patients enrolled in this study. The median OS was 4.7 months (95% confidence interval [CI]: 2.8–6.9 months). Median progression-free survival was 2.1 months (95% CI: 1.6–3.2 months). In 34 evaluable patients, the best response achieved was stable disease in 10 patients (29.4%). One patient had stable disease while on treatment for 20 months. The most common nonhematologic toxicities were fatigue and nausea. Edema and pleural effusions occurred in 29% and 6% of patients, respectively. The number of CTCs did not correlate with survival.
Conclusion.
Single-agent dasatinib does not have clinical activity in metastatic PDAC.
Author Summary
Discussion
This phase II study used a targeted agent as first-line monotherapy for metastatic pancreatic ductal adenocarcinoma (PDAC). Despite being a chemotherapy-naïve study, the authors met the accrual goal of 49 patients. At the time of this study, single-agent gemcitabine was considered the standard of care in the first-line setting for metastatic PDAC. With the postulated mechanism of action of dasatinib in preclinical PDAC models, this agent was felt to be promising. Unfortunately, single-agent dasatinib did not show clinical activity in patients with metastatic PDAC (median overall survival: 4.7 months; 95% confidence interval: 2.8–6.9 months) (Fig. 1). A sustained durable response was observed in one patient who received 20 months of dasatinib. Six patients lived for more than 20 months after discontinuation of therapy. It is unknown whether this result could be attributed to sustained response from dasatinib, subsequent lines of therapy, or disease biology.
Figure 1.
Kaplan-Meier estimates of overall survival (median: 4.7 months; 95% confidence interval [CI]: 2.8–6.9 months) with 95% CI (dashed lines).
The adverse events at least possibly related to dasatinib were as expected based on prior studies with dasatinib [1, 2]. Fluid retention is a common side effect of dasatinib. The rate of pleural effusion in this study was lower (6%) compared with prior studies with dasatinib (10%–26%) [1, 2], possibly related to the short duration that patients were on dasatinib in this study (31–49.5 days) compared with patients on dasatinib for chronic myelogenous leukemia (42 weeks) [3]. The rates of grade 1–2 edema were higher in this study (29%) compared with studies of dasatinib in non-small cell lung cancer (3%) [1] and chronic myelogenous leukemia (9%) [2]. One possible explanation for worsening rates of edema observed in this study is that patients with PDAC often have low albumin levels, which can contribute to edema. For hematologic toxicities, the adverse events were comparable to other solid tumor studies with dasatinib [1]. In 19 patients with available samples, circulating tumor cells (CTC) number at baseline, measured by CellSearch technology (Veridex LLC, Raritan, NJ, https://www.cellsearchctc.com/), did not correlate with survival. This was likely because of the small number of patients and the lack of sensitivity of the detection platform in pancreas cancer (median CTC number: 1; range in 7 mL of blood: 0–5).
In conclusion, single-agent dasatinib did not show clinical activity as first-line therapy in patients with metastatic PDAC. The limited single-agent activity of dasatinib likely results from the mechanisms of resistance to Src inhibition that have been associated with a lack of inhibition of activated STAT3 signaling [4].
Supplementary Material
Access the full results at: Chee-13-255.theoncologist.com
ClinicalTrials.gov Identifier: NCT00474812 Sponsor(s): NIH Grants U01CA062502 and P30CA043703
Principal Investigator: Charles J. Nock IRB Approved: Yes
Disclosures
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.