Whereas the frequency of alopecia to cytotoxic chemotherapies has been well described, the incidence of alopecia during endocrine therapies (i.e., anti-estrogens, aromatase inhibitors) has not been investigated. We performed a systematic analysis of the literature to ascertain the incidence and risk for alopecia while receiving endocrine therapies and found that alopecia is a common yet underreported adverse event of endocrine-based cancer therapies.
Keywords: Alopecia, Breast neoplasms, Antineoplastic agents, Adverse events, Aromatase inhibitors, Tamoxifen
Learning Objectives
Define the incidence and grades of alopecia to endocrine-based therapies in cancer patients.
Differentiate risk of alopecia to various endocrine agents used against cancer.
Design therapeutic, counseling, and supportive care strategies for patients requiring endocrine agents causing alopecia.
Abstract
Background.
Whereas the frequency of alopecia to cytotoxic chemotherapies has been well described, the incidence of alopecia during endocrine therapies (i.e., anti-estrogens, aromatase inhibitors) has not been investigated. Endocrine agents are widely used in the treatment and prevention of many solid tumors, principally those of the breast and prostate. Adherence to these therapies is suboptimal, in part because of toxicities. We performed a systematic analysis of the literature to ascertain the incidence and risk for alopecia in patients receiving endocrine therapies.
Methods.
An independent search of citations was conducted using the PubMed database for all literature as of February 2013. Phase II–III studies using the terms “tamoxifen,” “toremifene,” “raloxifene,” “anastrozole,” “letrozole,” “exemestane,” “fulvestrant,” “leuprolide,” “flutamide,” “bicalutamide,” “nilutamide,” “fluoxymesterone,” “estradiol,” “octreotide,” “megestrol,” “medroxyprogesterone acetate,” “enzalutamide,” and “abiraterone” were searched.
Results.
Data from 19,430 patients in 35 clinical trials were available for analysis. Of these, 13,415 patients had received endocrine treatments and 6,015 patients served as controls. The incidence of all-grade alopecia ranged from 0% to 25%, with an overall incidence of 4.4% (95% confidence interval: 3.3%–5.9%). The highest incidence of all-grade alopecia was observed in patients treated with tamoxifen in a phase II trial (25.4%); similarly, the overall incidence of grade 2 alopecia by meta-analysis was highest with tamoxifen (6.4%). The overall relative risk of alopecia in comparison with placebo was 12.88 (p < .001), with selective estrogen receptor modulators having the highest risk.
Conclusion.
Alopecia is a common yet underreported adverse event of endocrine-based cancer therapies. Their long-term use heightens the importance of this condition on patients' quality of life. These findings are critical for pretherapy counseling, the identification of risk factors, and the development of interventions that could enhance adherence and mitigate this psychosocially difficult event.
Implications for Practice:
Whereas the frequency of alopecia in the context of cytotoxic chemotherapies has been well described, its incidence with endocrine therapies (i.e., anti-estrogens, aromatase inhibitors) has not been systematically described. This lack of knowledge precludes comprehensive therapeutic decision-making, appropriate pretherapy counseling, and the establishment of interventions for patients who experience alopecia. Moreover, this lack of knowledge has negated the importance of alopecia and its associated psychosocial impact, hindering research endeavors toward its prevention, management, and the identification of individuals at risk. The data presented here reveal that alopecia is a common and likely underreported adverse event of treatment with endocrine therapies for cancer. Data also showed a higher relative risk of alopecia for those treated with selective estrogen receptor modulators than for those treated with aromatase inhibitors. This knowledge represents a step toward a heightened awareness of this condition, which may have an impact on patient adherence and persistence.
Introduction
Although the frequency of alopecia to cytotoxic chemotherapy is a well-described event, hair loss in patients treated with endocrine-based cancer therapies has not been systematically investigated. These agents are widely used at various stages of treatment and prevention for many types of solid tumors, including breast, prostate, and endocrine tumors, which have a combined incidence of more than 2.2 million cases worldwide [1]. The use of endocrine-based therapies is not limited to various stages of metastatic or advanced disease; they are also used in the preventive, adjuvant, and neoadjuvant settings. Consequently, these agents are given to a large patient population for several years, and these numbers are expected to grow as endocrine agents are used at earlier stages of disease [2, 3].
Although there are many clinical benefits of endocrine therapies, there are also adverse events (AEs). Anti-androgens such as flutamide used to treat prostate cancer may lead to hepatic damage, hot flashes, and diarrhea [4]; aromatase inhibitors (AIs) used in breast cancer can result in hot flashes, arthralgias, cardiovascular events, and bone fractures [5, 6]; and tamoxifen carries an increased risk for thromboembolic complications and endometrial cancer [7]. Most of the systemic, neoplastic, and musculoskeletal AEs that occur are reported consistently and are well known by health care providers and patients; however, alopecia is not always reported, even though patients with cancer say it is one of the topmost events that negatively affect their quality of life [8]. Anecdotal reports and observation from dermatologic clinical programs in cancer centers suggest otherwise—namely, that alopecia is indeed a frequent, albeit largely underreported, effect of treatment with endocrine-based cancer therapies (Fig. 1).
Figure 1.
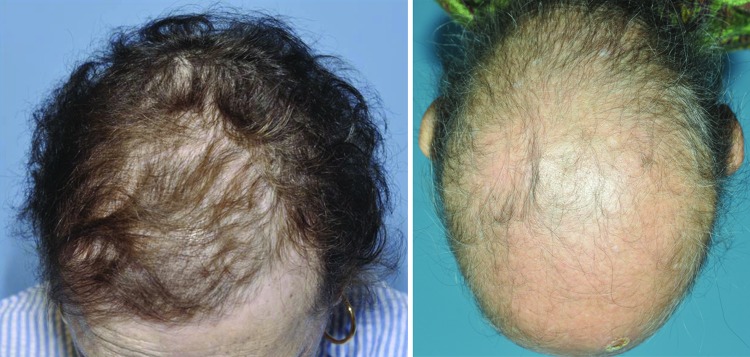
Clinical presentation of alopecia grade 1 (left panel) and grade 2 (right panel).
The psychosocial importance of alopecia resulting from cancer therapies cannot be understated. Approximately 58% of women receiving treatment for breast cancer state that alopecia is one of the most traumatic AEs during their treatment, with 8% indicating they would reject treatment because of this reaction alone. Indeed, some women refuse chemotherapy because of alopecia [9]. Decreased quality of life, social activity, self-esteem, and body image are all associated with hair loss [10, 11]. These findings have been attributed to the severe alopecia that develops during treatment with cytotoxic agents (grade 2); alopecia that occurs during treatment with endocrine agents is of lower severity, however, it tends to last for the duration of treatment (several years), which heightens the impact on patients' quality of life.
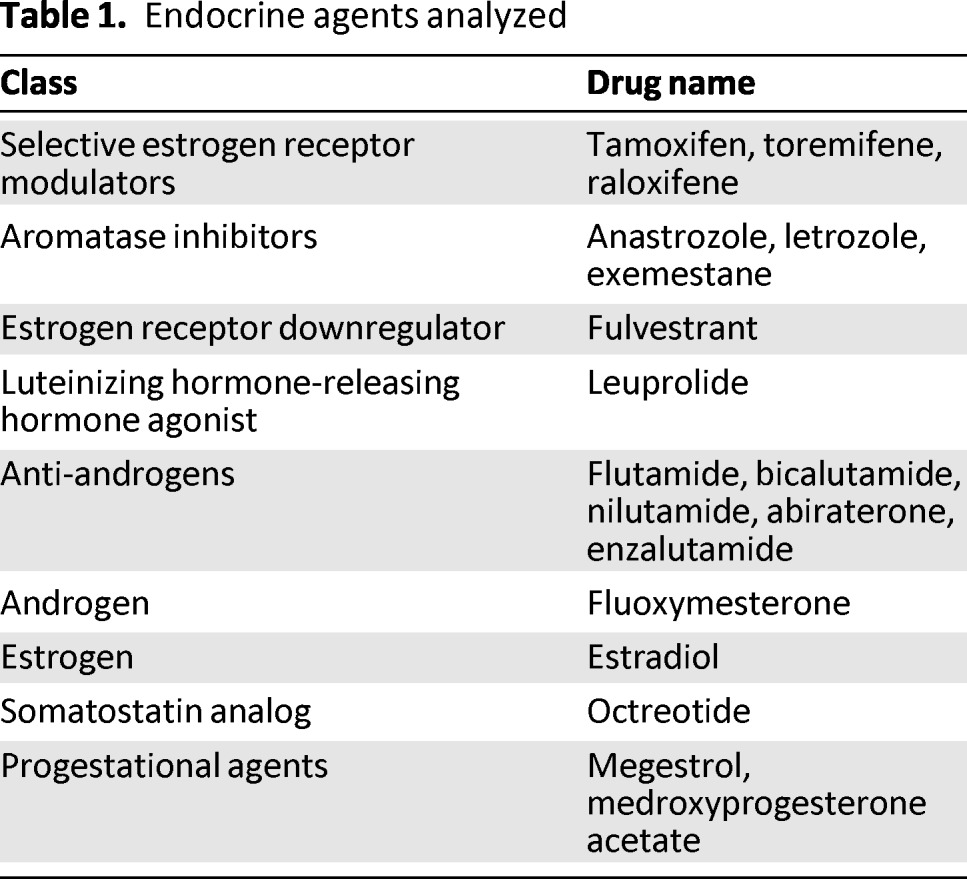
A systematic analysis of the literature was performed to determine the incidence and risk for alopecia in the context of therapy with the following: selective estrogen receptor modulators (SERMs) (tamoxifen, toremifene, raloxifene), aromatase inhibitors (Als) (anastrozole, letrozole, exemestane), an estrogen receptor downregulator (fulvestrant), a luteinizing hormone-releasing hormone agonist (leuprolide), anti-androgens (flutamide, bicalutamide, nilutamide, abiraterone, enzalutamide), an androgen (fluoxymesterone), an estrogen (estradiol), a somatostatin analog (octreotide), and progestational agents (megestrol, medroxyprogesterone acetate) (Table 1). Knowledge of the incidence and risk for alopecia with these agents represents the first step toward counseling patients, identifying individuals with greater susceptibility, and developing management and anticipatory coping strategies for patients.
Table 1.
Endocrine agents analyzed
Methods
Data Source
An independent search of citations was conducted using the PubMed database for all available literature as of February 2013 (earliest relevant citation from 1986). The terms “tamoxifen,” “toremifene,” “raloxifene,” “anastrozole,” “letrozole,” “exemestane,” “fulvestrant,” “leuprolide,” “flutamide,” “bicalutamide,” “nilutamide,” “fluoxymesterone,” “estradiol,” “octreotide,” “megestrol,” “medroxyprogesterone acetate,” “enzalutamide,” and “abiraterone” were used as keywords in the search. Results were restricted to include only phase II and phase III clinical trials. For every citation, the corresponding full article was searched for the terms “alopecia” and “hair” to determine whether hair loss or thinning was documented in the trial. In addition to the PubMed database, abstracts presented at the American Society of Clinical Oncology (ASCO) conferences up to February 2013 were searched to identify relevant clinical trials. Searches of each abstract body were performed using the listed terms from the PubMed searches, along with the keywords, “alopecia,” or “hair.” Each result was reviewed. When duplicate publications of a clinical trial were found, only the most recent report was included. If only qualitative results of hair loss were mentioned, efforts were made to contact the appropriate investigators for quantitative results. Details on study characteristics, treatment information, dosages, enrollment numbers, and rates of alopecia from selected trials were extracted.
Study Selection
The primary difficulty in study selection involved finding trials that included rates of alopecia in the context of endocrine therapy for cancer, without including confounding variables such as concurrent treatment with additional biologic therapy or chemotherapy, which could also cause alopecia. Studies were selected for the final analysis based on the following criteria: (a) prospective phase II or III clinical trial in patients with cancer; (b) assignment of participants to a particular endocrine therapy being studied and no additional biologic therapy or chemotherapy known to cause alopecia; (c) data available regarding the incidence of alopecia in the context of the endocrine therapy.
Clinical Endpoints
Clinical endpoints were determined by examining the safety profile of each trial. The National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 divides alopecia into two grades. Grade 1, which is mild, is marked by partial hair thinning, with hair loss less than 50% of what would be considered normal for that person, and not obvious from a distance but only on close inspection. For grade 1 alopecia, a different hairstyle may be required to cover the hair loss, but it does not require a wig or hairpiece for camouflage. Grade 2 alopecia is moderate to severe noticeable hair loss, with the absence of more than 50% of what would be normal for that person, and readily apparent to others. A wig or hairpiece is necessary to mask the alopecia completely.
Statistical Analysis
All statistical analyses for patients with all-grade and high-grade alopecia were performed using version 2 of the Comprehensive MetaAnalysis program (Biostat, Englewood, NJ, http://www.meta-analysis.com/); 95% confidence intervals (CI) and rates of alopecia were determined for each study. Relative risk (RR) for alopecia was calculated by comparing data from patients receiving endocrine treatments with data from controls.
Both the fixed-effects (weighted with inverse variance) and the random-effects models were given consideration for meta-analysis. To determine the heterogeneity of the relevant results, Cochran Q statistic was calculated for each meta-analysis. If the p value was found to be < .1, the random-effects model was employed because the assumption of homogeneity was considered invalid. Barring this phenomenon, findings from both the fixed-effects and random-effects models were assessed. When these findings were comparable, results from only the fixed-effects model were given. A statistically significant two-tailed p value was established when p < .05.
Results
Search Results
A literature search of PubMed retrieved a total of 1,429 results for phase II and phase III clinical trials that included the selected keywords. Of these, 1,346 did not include data on alopecia (94.2% of search results). Of the 83 results that did include descriptions of alopecia (5.8% of overall search results), 35 clinical trials (2.4% of search results) met the necessary parameters to be included in the final analysis [12–46]. A search of ASCO abstracts was also performed, but of six potentially relevant abstracts, no trials met the minimum inclusion criteria. In summary, 35 phase II and III clinical trials were considered relevant to the meta-analysis (Table 2).
Table 2.
Dosage and duration of therapy by study
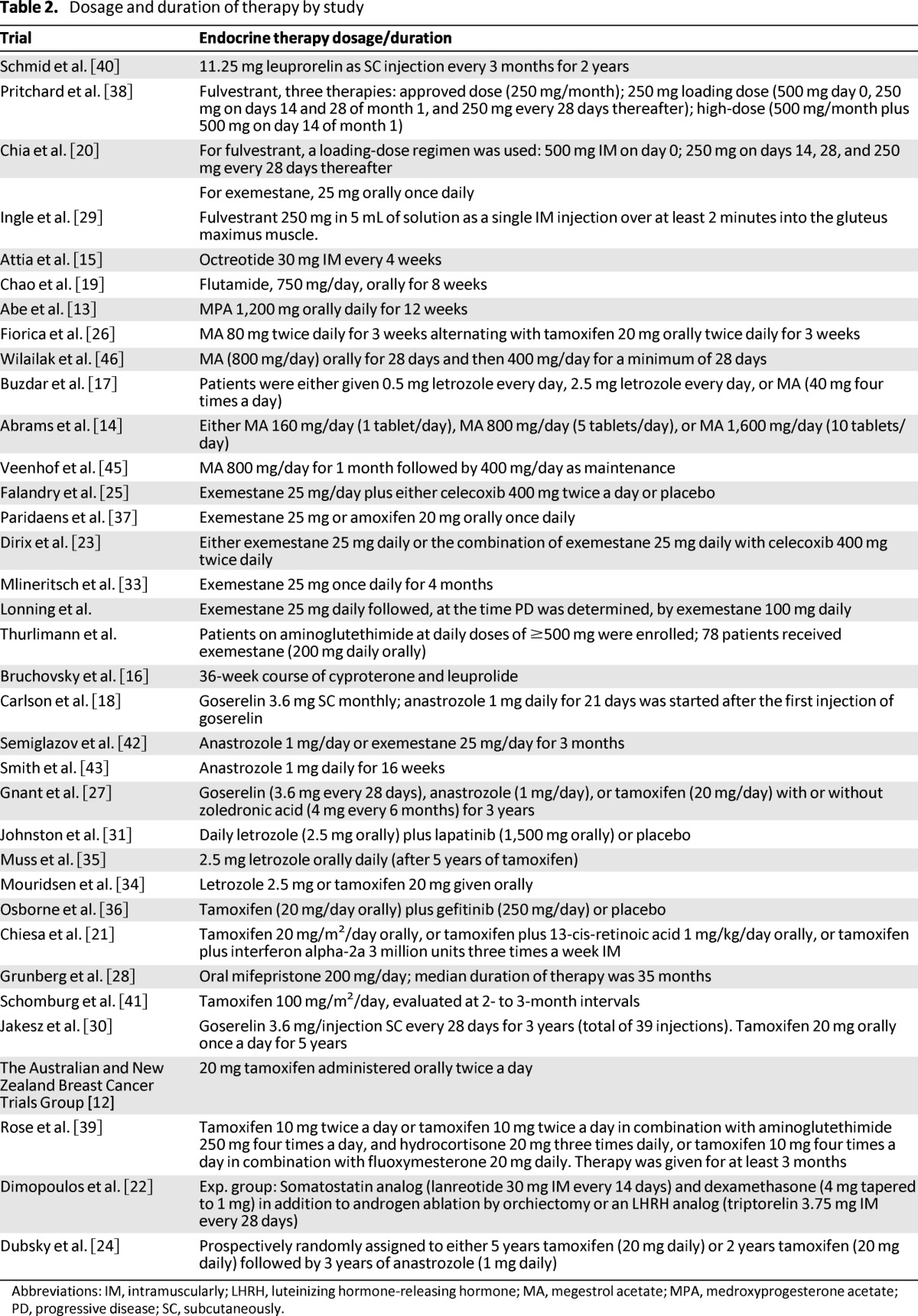
Abbreviations: IM, intramuscularly; LHRH, luteinizing hormone-releasing hormone; MA, megestrol acetate; MPA, medroxyprogesterone acetate; PD, progressive disease; SC, subcutaneously.
Patients
A total of 19,430 patients from the 35 clinical trials was available for analysis. Of these 19,430 patients, 13,415 patients received endocrine therapies and the remaining 6,015 patients were controls. Of the 35 clinical trials, 15 trials included control therapies. Alopecia was not listed as a preexisting condition in any of the selected trials. Underlying malignancies included those of the breast (26 trials) [12–14, 17, 18, 20, 21, 23–25, 27, 29–40, 42–44], hepatocellular (2 trials) [15, 19], endometrial (1 trial) [26], ovarian (2 trials) [45, 46], prostate (2 trials) [16, 22], renal cell (1 trial) [41], and meningioma (1 trial) [28].
Incidence of All-Grade Alopecia
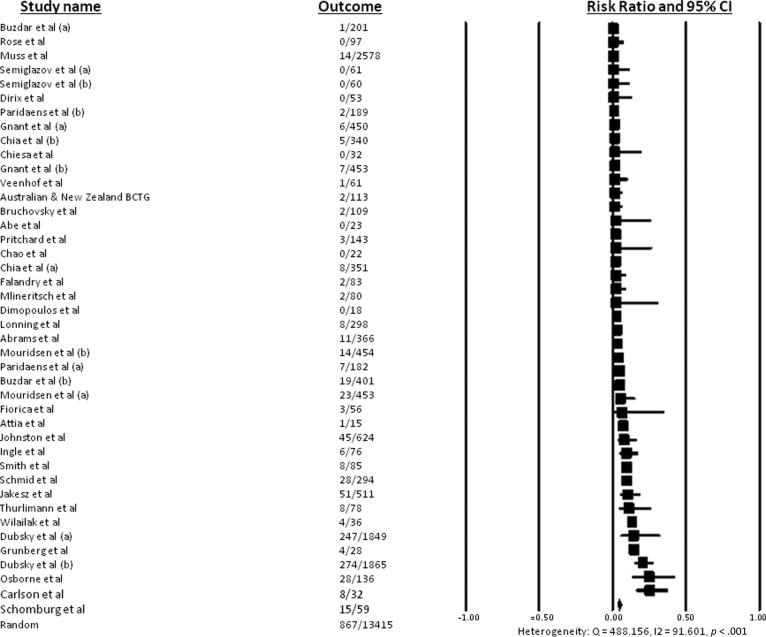
Data for all-grade alopecia in the context of specific endocrine therapies was available for 13,415 patients. The incidence of all-grade alopecia ranged from 0% to 25.4%, with no reported cases of alopecia with flutamide therapy for prostate cancer or with medroxyprogesterone acetate for breast cancer [13, 19]. In a phase II trial of anastrozole and goserelin used to treat breast cancer, 25% of patients experienced alopecia [18], and in a phase II trial using tamoxifen to treat renal cell carcinoma, 25.4% of patients experienced alopecia [41]. In the 35 relevant trials with a total of 13,415 patients, the overall incidence of all-grade alopecia was 4.4% (95% CI: 3.3%–5.9%), according to the random-effects model (heterogeneity test: Q = 488.156, I2 = 91.601, p < .001) (Fig. 2). When a single endocrine therapy agent was used, the overall incidence of all-grade alopecia was 3.6% (95% CI: 2.5%–5.3%) (heterogeneity test: Q = 399.148, I2 = 91.482, p < .001).
Figure 2.
Incidence of all-grade alopecia.
Incidence of High-Grade Alopecia
High-grade alopecia was reported in 6 of the 35 relevant clinical trials [12, 14, 31, 33, 40, 41]. The incidence of high-grade alopecia ranged between 0.2% and 16.9%; the lowest incidence (0.2%) was reported in a phase III clinical trial of letrozole therapy for breast cancer [31], and the highest incidence (16.9%) was observed in a phase II trial studying the use of tamoxifen in the treatment of renal cell carcinoma [41]. Overall, the six studies examined a total of 1,536 patients, resulting in an overall incidence of high-grade alopecia of 1.2% (95% CI: 0.2%–6.4%), according to the random-effects model (heterogeneity test: Q = 49.209, I2 = 89.839, p < .001).
Variation of Alopecia in Patients With Different Endocrine Therapies
We investigated whether different endocrine therapies resulted in varying incidences of alopecia (Table 3). Endocrine therapies were grouped as follows: selective estrogen receptor modulators (SERMs) (tamoxifen, toremifene, raloxifene); AIs (anastrozole, letrozole, exemestane); estrogen receptor downregulator (fulvestrant); luteinizing hormone-releasing hormone agonist (leuprolide); anti-androgens (flutamide, bicalutamide, nilutamide, abiraterone, enzalutamide); androgen (fluoxymesterone); estrogen (estradiol); somatostatin analog (octreotide); and progestational agents (megestrol, medroxyprogesterone acetate) (Table 1). Statistical analyses were performed for each endocrine group. There was significant difference among different classes of endocrine therapies (p = .002). The highest endocrine therapy incidence occurred with a SERM therapy (tamoxifen), followed sequentially by treatment with an AI (anastrozole) (14.7%) [24]. In contrast, anti-androgen therapy exhibited the lowest event rate, with no alopecia reported in this setting [19].
Table 3.
Incidence of all-grade alopecia by therapy
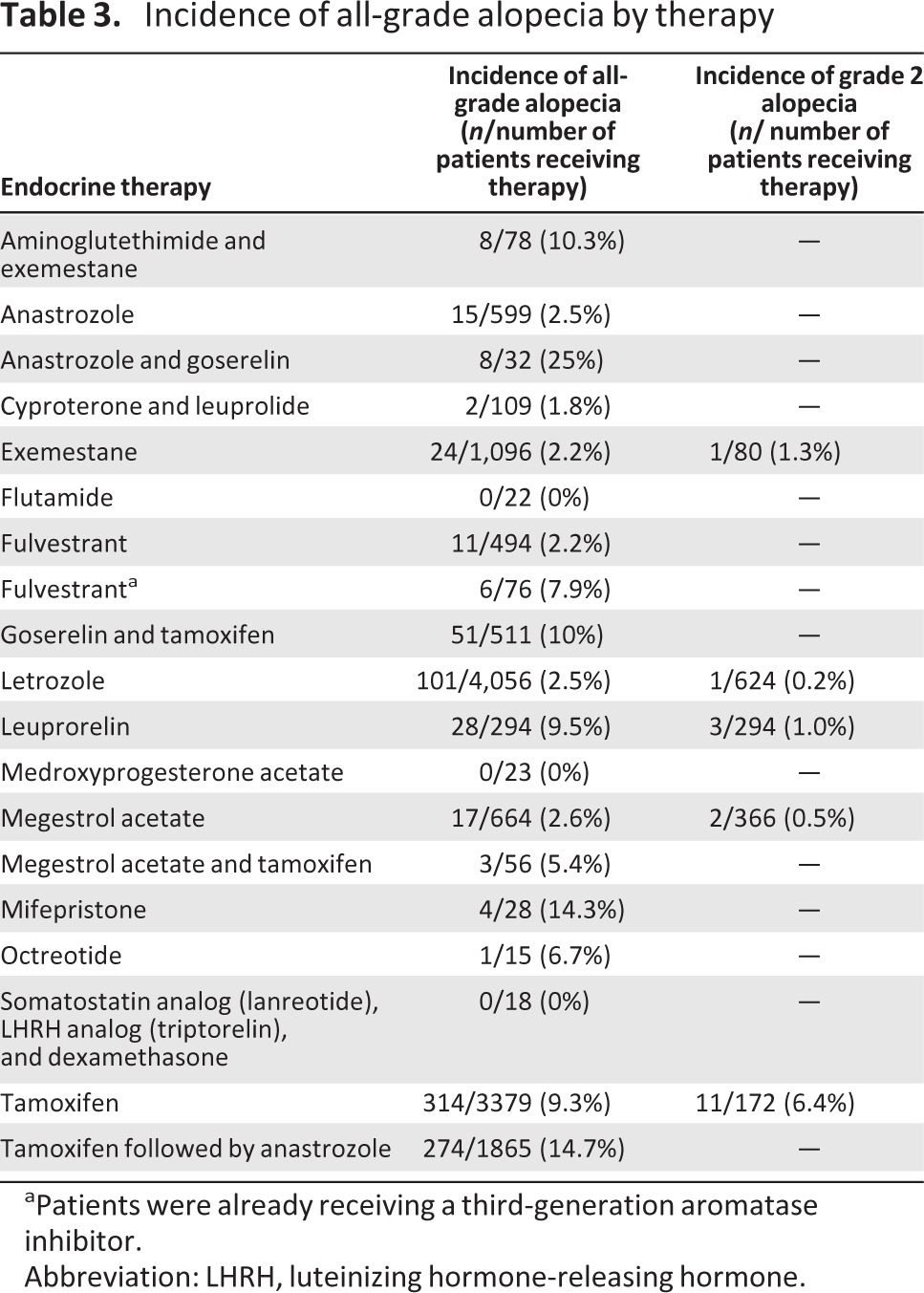
aPatients were already receiving a third-generation aromatase inhibitor.
Abbreviation: LHRH, luteinizing hormone-releasing hormone.
Increased Risk for Alopecia With Combination of Endocrine Therapies
When endocrine therapies were used in combination, the overall incidence of all-grade alopecia was 10.5% (95% CI: 7.1%–15.4%) (heterogeneity test: Q = 25.036, I2 = 76.035, p < .001) [17, 18, 22, 24, 26, 30, 44]. There was a significant difference between combination and single-agent therapies in alopecia (RR: 2.92; 95% CI: 2.56–3.38; p = .002), supporting an additive or synergistic effect in the development of alopecia when agents are combined. The highest incidence of alopecia (25%) occurred in a phase II study of anastrozole and goserelin for metastatic breast cancer [18].
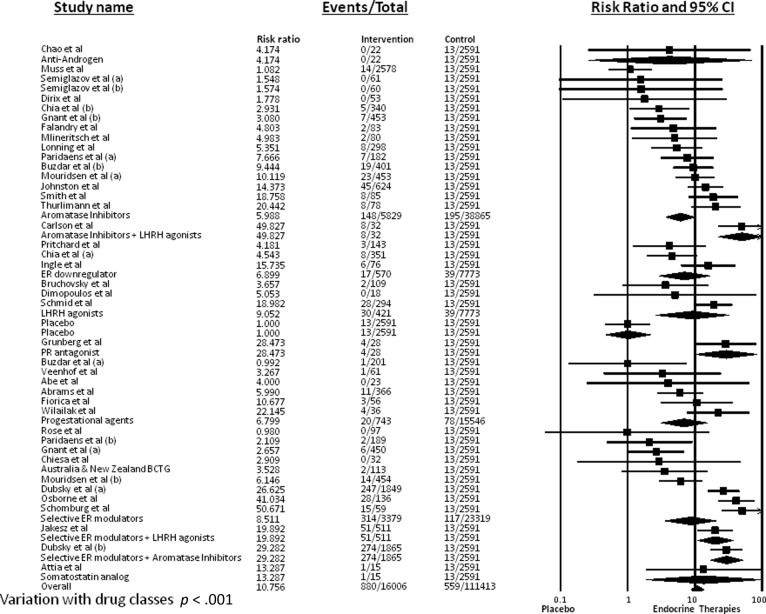
RR for Alopecia
RR for alopecia with endocrine therapy compared with placebo was calculated to account for any potential confounding variables. The incidence of alopecia in 2,591 patients receiving placebo was 0.5% (95% CI: 0.3–0.9%). RR was found to be 12.88 (95% CI: 7.46–22.24; p < .001) when compared with controls. Because of the statistically significant variation of alopecia in patients undergoing different endocrine therapies, RR was also calculated for each endocrine class (Fig. 3). Of note, RR for SERM therapies compared with controls was 8.51 (95% CI: 3.54–20.49; p < .001), RR for AIs compared with controls was 5.99 (95% CI: 3.63–9.88; p < .001), and RR for anti-androgens was 4.174 (95% CI: 0.26–68.14; p = .316) compared with controls.
Figure 3.
Relative risk for all-grade alopecia.
Discussion
Our analysis demonstrates that endocrine treatments against cancer place individuals at a significantly increased risk for alopecia. The overall incidence of all-grade alopecia was 4.4% (95% CI: 3.3%–5.9%), and the incidence of high-grade alopecia was 1.2% (95% CI: 0.2%–6.4%). The RR of 12.88 (95% CI: 7.46–22.24; p < .001) demonstrates the higher risk for alopecia when using endocrine therapies versus placebo.
The increased risk, however, is not uniform across all endocrine drug classes; no patients experienced alopecia with anti-androgens. This is not unexpected, as anti-androgens such as flutamide have been used to treat female-pattern hair loss [11], a hereditary disorder involving thinning of the hair that is believed to be caused by androgen activity [47].
Hair growth involves three defined phases: anagen, catagen, and telogen. Androgenetic alopecia is marked by a gradual shortening of the anagen or growth phase (normally lasting 2–6 years), leading to miniaturization of the hair follicles [48]. Catagen is a short (2–3 weeks) apoptosis-driven regression phase that follows anagen and signals the end of active hair growth. The telogen phase of hair growth is a resting stage, normally lasting three months [11]. The presence of a higher proportion of hair follicles in the telogen stage of hair growth may lead to excessive shedding [48]. The putative mechanisms by which endocrine therapies result in alopecia include hair loss during telogen and a decrease in the diameter of the shafts, leading to fragility, breakage, and subsequent loss [49].
The properties of hair growth and the underlying mechanisms of androgenetic alopecia may help explain why SERMs and AIs exhibited event rates of 4.79% (95% CI: 2.28%–9.78%) and 3.50% (95% CI: 2.07%–5.87%), respectively, whereas tamoxifen and anastrozole in sequence resulted in an event rate of 14.7% (95% CI: 13.2%–16.4%) [24]. Animal models treated with a tamoxifen-loaded gel experienced arrested hair growth, with no growth persisting even after discontinuation of this treatment [50]. Further, the affected hair follicles were arrested in the telogen phase [50]. Estrogens have been found to alter hair growth by means of binding with locally expressed high-affinity estrogen receptors [50]; alopecia related to tamoxifen has been shown to exhibit a distribution similar to that of female-pattern alopecia, primarily affecting the crown and frontal scalp [49]. Both men and women with androgenetic alopecia have lower levels of cytochrome P-450 aromatase in hair follicles located within the frontal region of the scalp, which would make these follicles more susceptible to AI effects [47]. Therefore, it is possible that AIs mimic the hereditary deficiency typically seen in androgenetic alopecia.
There are important limitations to consider in this meta-analysis. First, alopecia is reported infrequently as an adverse event in published manuscripts on endocrine therapies in cancer: 94.1% of the 1,429 PubMed search results did not indicate the incidence of alopecia. Notably, although 94.1% of search results for tamoxifen did not report alopecia, studies reporting it showed between 1% and 25.4% alopecia incidence [12, 21, 24, 27, 34, 36, 37, 39, 41]. In addition, only 6 of 35 relevant clinical trials differentiated between all-grade and high-grade alopecia [12, 14, 31, 33, 40, 41]. Furthermore, PubMed and abstracts from the American Society of Clinical Oncology served as the databases for biomedical literature in this meta-analysis, and it is possible that alternate sources of scientific literature may have yielded additional results. Finally, there is always a possibility that other confounding variables were not accounted for, such as prior or concurrent diseases or medications causing alopecia.
Patients presenting with alopecia during therapy with endocrine agents should be evaluated for other contributing factors, such as thyroid gland function (thyroid-stimulating hormone and free T4), iron stores (ferritin), vitamin D, and zinc levels in blood. In addition, a scalp examination must be conducted to rule out other common causes of alopecia, such as female-pattern hair loss, alopecia areata, or inflammatory (scarring) alopecia. Patients whose alopecia cannot be attributed to any of the above conditions should be treated with supportive care, including the use of minoxidil 2%–5% twice daily applied on the scalp, biotin (vitamin B7) 2.5 mg a day orally, and camouflaging sprays/powders and wigs or extensions [51, 52]. In severe cases of alopecia, spironolactone orally or hair transplants may be considered after consultation with a trichologist.
Endocrine therapies have had an integral role in the treatment and prevention of many types of solid tumors [2, 3, 5]. At the same time, alopecia caused by endocrine therapy can have a profound psychosocial effect, warranting pretherapy counseling to engage the patient in anticipatory coping to maintain persistence and adherence to therapy. This is important because there have been reports of patients refusing or discontinuing therapy because of alopecia [53, 54].
Conclusion
The results of this meta-analysis indicate that alopecia is a common but underreported adverse event associated with endocrine agents used to treat cancer, particularly of the breast. This study aims to provide information that is critical for counseling and interventions against alopecia. These findings are critical for pretherapy counseling, the identification of risk factors, and the development of interventions that could mitigate this psychosocially untoward event.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
This research was supported by the RJR Oncodermatology Fund.
Author Contributions
Conception/Design: Mario E. Lacouture
Provision of study material or patients: Mario E. Lacouture
Collection and/or assembly of data: Vishal Saggar
Data analysis and interpretation: Mario E. Lacouture, Vishal Saggar, Shenhong Wu, Maura N. Dickler
Manuscript writing: Mario E. Lacouture, Vishal Saggar, Shenhong Wu, Maura N. Dickler
Final approval of manuscript: Mario E. Lacouture, Vishal Saggar, Shenhong Wu, Maura N. Dickler
Disclosures
Shenhong Wu: Novartis (C/A); Bayer-Onyx, Novartis, Pfizer (H); Mario E. Lacouture: Genentech, Roche, BMS, Berg, Novartis, Pfizer, Merck, Lilly, AstraZeneca, GSK (C/A;H). The other authors indicated no financial relationships.
Section editors: Eduardo Bruera: None; Russell K. Portenoy: Grupo Ferrer, Purdue Pharma (C/A); Allergan, Boston Scientific, Covidien Mallinckrodt, Endo Pharmaceuticals, Medtronic, Otsuka Pharma, ProStrakan, Purdue Pharma, Salix, St. Jude Medical (RF)
Reviewer “A”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ferlay J, Shin HR, Bray F, et al. Globocan 2008: Cancer Incidence and Mortality Worldwide. [Accessed December 2, 2012]. IARC Cancer Base No. 10 Available at: http://globocan.iarc.fr/
- 2.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 3.Goss PE, Ingle JN, Alés-Martínez JE, et al. NCIC CTG MAP. 3 Study Investigators. Exemestane for breast-cancer prevention in postmenopausal women N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 4.Catalona WJ. Management of cancer of the prostate. N Engl J Med. 1994;331:996–1004. doi: 10.1056/NEJM199410133311507. [DOI] [PubMed] [Google Scholar]
- 5.Swain SM. Aromatase inhibitors—a triumph of translational oncology. N Engl J Med. 2005;353:2807–2809. doi: 10.1056/NEJMe058273. [DOI] [PubMed] [Google Scholar]
- 6.Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431–2442. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- 7.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators — mechanisms of action and application to clinical practice. N Engl J Med. 2003;348:618–629. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi M, Oishi K, Zubal B, et al. Unanticipated toxicities from anticancer therapies: Survivors' perspectives. Support Care Cancer. 2010;18:1461–1468. doi: 10.1007/s00520-009-0769-1. [DOI] [PubMed] [Google Scholar]
- 9.McGarvey EL, Baum LD, Pinkerton RC, et al. Psychological sequelae and alopecia among women with cancer. Cancer Pract. 2001;9:283–289. doi: 10.1046/j.1523-5394.2001.96007.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Lu Z, Au JL. Protection against chemotherapy-induced alopecia. Pharm Res. 2006;23:2505–2514. doi: 10.1007/s11095-006-9105-3. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro J. Clinical practice. Hair loss in women. N Engl J Med. 2007;357:1620–1630. doi: 10.1056/NEJMcp072110. [DOI] [PubMed] [Google Scholar]
- 12.The Australian and New Zealand Breast Cancer Trials Group. A randomized trial in postmenopausal patients with advanced breast cancer comparing endocrine and cytotoxic therapy given sequentially or in combination. Clinical Oncological Society of Australia. J Clin Oncol. 1986;4:186–193. doi: 10.1200/JCO.1986.4.2.186. [DOI] [PubMed] [Google Scholar]
- 13.Abe O, Asaishi K, Izuo M, et al. Effects of medroxyprogesterone acetate therapy on advanced or recurrent breast cancer and its influences on blood coagulation and the fibrinolytic system. Surg Today. 1995;25:701–710. doi: 10.1007/BF00311486. [DOI] [PubMed] [Google Scholar]
- 14.Abrams J, Aisner J, Cirrincione C, et al. Dose-response trial of megestrol acetate in advanced breast cancer: Cancer and leukemia group B phase III study 8741. J Clin Oncol. 1999;17:64–73. doi: 10.1200/JCO.1999.17.1.64. [DOI] [PubMed] [Google Scholar]
- 15.Attia S, Holen KD, Thomas JP, et al. Biologic study of the effects of octreotide-LAR on growth hormone in unresectable and metastatic hepatocellular carcinoma. Clin Adv Hematol Oncol. 2008;6:44–54. [PubMed] [Google Scholar]
- 16.Bruchovsky N, Klotz L, Crook J, et al. Quality of life, morbidity, and mortality results of a prospective phase II study of intermittent androgen suppression for men with evidence of prostate-specific antigen relapse after radiation therapy for locally advanced prostate cancer. Clin Genitourin Cancer. 2008;6:46–52. doi: 10.3816/CGC.2008.n.008. [DOI] [PubMed] [Google Scholar]
- 17.Buzdar A, Douma J, Davidson N, et al. Phase III, multicenter, double-blind, randomized study of letrozole, an aromatase inhibitor, for advanced breast cancer versus megestrol acetate. J Clin Oncol. 2001;19:3357–3366. doi: 10.1200/JCO.2001.19.14.3357. [DOI] [PubMed] [Google Scholar]
- 18.Carlson RW, Theriault R, Schurman CM, et al. Phase II trial of anastrozole plus goserelin in the treatment of hormone receptor-positive, metastatic carcinoma of the breast in premenopausal women. J Clin Oncol. 2010;28:3917–3921. doi: 10.1200/JCO.2009.24.9565. [DOI] [PubMed] [Google Scholar]
- 19.Chao Y, Chan WK, Huang YS, et al. Phase II study of flutamide in the treatment of hepatocellular carcinoma. Cancer. 1996;77:635–639. [PubMed] [Google Scholar]
- 20.Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: Results from EFECT. J Clin Oncol. 2008;26:1664–1670. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 21.Chiesa MD, Passalacqua R, Michiara M, et al. Italian Oncology Group for Clinical Research. Tamoxifen vs tamoxifen plus 13-cis-retinoic acid vs tamoxifen plus interferon alpha-2a as first-line endocrine treatments in advanced breast cancer: Updated results of a phase II, prospective, randomised multicentre trial. Acta Biomed. 2007;78:204–209. [PubMed] [Google Scholar]
- 22.Dimopoulos MA, Kiamouris C, Gika D, et al. Combination of LHRH analog with somatostatin analog and dexamethasone versus chemotherapy in hormone-refractory prostate cancer: A randomized phase II study. Urology. 2004;63:120–125. doi: 10.1016/j.urology.2003.08.041. [DOI] [PubMed] [Google Scholar]
- 23.Dirix LY, Ignacio J, Nag S, et al. Treatment of advanced hormone-sensitive breast cancer in postmenopausal women with exemestane alone or in combination with celecoxib. J Clin Oncol. 2008;26:1253–1259. doi: 10.1200/JCO.2007.13.3744. [DOI] [PubMed] [Google Scholar]
- 24.Dubsky PC, Jakesz R, Mlineritsch B, et al. Tamoxifen and anastrozole as a sequencing strategy: A randomized controlled trial in postmenopausal patients with endocrine-responsive early breast cancer from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2012;30:722–728. doi: 10.1200/JCO.2011.36.8993. [DOI] [PubMed] [Google Scholar]
- 25.Falandry C, Debled M, Bachelot T, et al. Celecoxib and exemestane versus placebo and exemestane in postmenopausal metastatic breast cancer patients: A double-blind phase III GINECO study. Breast Cancer Res Treat. 2009;116:501–508. doi: 10.1007/s10549-008-0229-5. [DOI] [PubMed] [Google Scholar]
- 26.Fiorica JV, Brunetto VL, Hanjani P, et al. Gynecologic Oncology Group study. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: A Gynecologic Oncology Group study Gynecol Oncol. 2004;92:10–14. doi: 10.1016/j.ygyno.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Gnant M, Mlineritsch B, Stoeger H, et al. Austrian Breast and Colorectal Cancer Study Group, Vienna, Austria Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12:631–641. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 28.Grunberg SM, Weiss MH, Russell CA, et al. Long-term administration of mifepristone (RU486): Clinical tolerance during extended treatment of meningioma. Cancer Invest. 2006;24:727–733. doi: 10.1080/07357900601062339. [DOI] [PubMed] [Google Scholar]
- 29.Ingle JN, Suman VJ, Rowland KM, et al. North Central Cancer Treatment Group Trial N0032. Fulvestrant in women with advanced breast cancer after progression on prior aromatase inhibitor therapy: North Central Cancer Treatment Group Trial N0032 J Clin Oncol. 2006;24:1052–1056. doi: 10.1200/JCO.2005.04.1053. [DOI] [PubMed] [Google Scholar]
- 30.Jakesz R, Hausmaninger H, Kubista E, et al. Austrian Breast and Colorectal Cancer Study Group Trial 5. Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: Evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer—Austrian Breast and Colorectal Cancer Study Group Trial 5 J Clin Oncol. 2002;20:4621–4627. doi: 10.1200/JCO.2002.09.112. [DOI] [PubMed] [Google Scholar]
- 31.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 32.Lønning PE, Bajetta E, Murray R, et al. Activity of exemestane in metastatic breast cancer after failure of nonsteroidal aromatase inhibitors: A phase II trial. J Clin Oncol. 2000;18:2234–2244. doi: 10.1200/JCO.2000.18.11.2234. [DOI] [PubMed] [Google Scholar]
- 33.Mlineritsch B, Tausch C, Singer C, et al. Austrian Breast, Colorectal Cancer Study Group (ABCSG) Exemestane as primary systemic treatment for hormone receptor positive post-menopausal breast cancer patients: A phase II trial of the Austrian Breast and Colorectal Cancer Study Group (ABCSG-17) Breast Cancer Res Treat. 2008;112:203–213. doi: 10.1007/s10549-007-9843-x. [DOI] [PubMed] [Google Scholar]
- 34.Mouridsen HT. Letrozole in advanced breast cancer: The PO25 trial. Breast Cancer Res Treat. 2007;105(Suppl 1):19–29. doi: 10.1007/s10549-007-9527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muss HB, Tu D, Ingle JN, et al. Efficacy, toxicity, and quality of life in older women with early-stage breast cancer treated with letrozole or placebo after 5 years of tamoxifen: NCIC CTG intergroup trial MA. 17. J Clin Oncol. 2008;26:1956–1964. doi: 10.1200/JCO.2007.12.6334. [DOI] [PubMed] [Google Scholar]
- 36.Osborne CK, Neven P, Dirix LY, et al. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: A randomized phase II study. Clin Cancer Res. 2011;17:1147–1159. doi: 10.1158/1078-0432.CCR-10-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paridaens RJ, Dirix LY, Beex LV, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: The European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:4883–4890. doi: 10.1200/JCO.2007.14.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pritchard KI, Rolski J, Papai Z, et al. Results of a phase II study comparing three dosing regimens of fulvestrant in postmenopausal women with advanced breast cancer (FINDER2) Breast Cancer Res Treat. 2010;123:453–461. doi: 10.1007/s10549-010-1022-9. [DOI] [PubMed] [Google Scholar]
- 39.Rose C, Kamby C, Mouridsen HT, et al. Combined endocrine treatment of elderly postmenopausal patients with metastatic breast cancer. A randomized trial of tamoxifen vs. tamoxifen + aminoglutethimide and hydrocortisone and tamoxifen + fluoxymesterone in women above 65 years of age. Breast Cancer Res Treat. 2000;61:103–110. doi: 10.1023/a:1006460925986. [DOI] [PubMed] [Google Scholar]
- 40.Schmid P, Untch M, Kossé V, et al. Leuprorelin acetate every-3-months depot versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant treatment in premenopausal patients with node-positive breast cancer: The TABLE study. J Clin Oncol. 2007;25:2509–2515. doi: 10.1200/JCO.2006.08.8534. [DOI] [PubMed] [Google Scholar]
- 41.Schomburg A, Kirchner H, Fenner M, et al. Lack of therapeutic efficacy of tamoxifen in advanced renal cell carcinoma. Eur J Cancer. 1993;29A:737–740. doi: 10.1016/s0959-8049(05)80357-0. [DOI] [PubMed] [Google Scholar]
- 42.Semiglazov VF, Semiglazov VV, Dashyan GA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110:244–254. doi: 10.1002/cncr.22789. [DOI] [PubMed] [Google Scholar]
- 43.Smith IE, Walsh G, Skene A, et al. A phase II placebo-controlled trial of neoadjuvant anastrozole alone or with gefitinib in early breast cancer. J Clin Oncol. 2007;25:3816–3822. doi: 10.1200/JCO.2006.09.6578. [DOI] [PubMed] [Google Scholar]
- 44.Thürlimann B, Paridaens R, Serin D, et al. Third-line hormonal treatment with exemestane in postmenopausal patients with advanced breast cancer progressing on aminoglutethimide: A phase II multicentre multinational study. Exemestane Study Group Eur J Cancer. 1997;33:1767–1773. doi: 10.1016/s0959-8049(97)00283-9. [DOI] [PubMed] [Google Scholar]
- 45.Veenhof CH, van der Burg ME, Nooy M, et al. Phase II study of high-dose megestrol acetate in patients with advanced ovarian carcinoma. Eur J Cancer. 1994;30A:697–698. doi: 10.1016/0959-8049(94)90548-7. [DOI] [PubMed] [Google Scholar]
- 46.Wilailak S, Linasmita V, Srisupundit S. Phase II study of high-dose megestrol acetate in platinum-refractory epithelial ovarian cancer. Anticancer Drugs. 2001;12:719–724. doi: 10.1097/00001813-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Price VH. Treatment of hair loss. N Engl J Med. 1999;341:964–973. doi: 10.1056/NEJM199909233411307. [DOI] [PubMed] [Google Scholar]
- 48.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 49.Lindner J, Hillmann K, Blume-Peytavi U, et al. Hair shaft abnormalities after chemotherapy and tamoxifen therapy in patients with breast cancer evaluated by optical coherence tomography. Br J Dermatol. 2012;167:1272–1278. doi: 10.1111/j.1365-2133.2012.11180.x. [DOI] [PubMed] [Google Scholar]
- 50.Bhatia A, Singh B, Amarji B, et al. Tamoxifen-loaded liposomal topical formulation arrests hair growth in mice. Br J Dermatol. 2010;163:412–415. doi: 10.1111/j.1365-2133.2010.09772.x. [DOI] [PubMed] [Google Scholar]
- 51.Sinclair R, Patel M, Dawson TL, Jr, et al. Hair loss in women: Medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12–18. doi: 10.1111/j.1365-2133.2011.10630.x. [DOI] [PubMed] [Google Scholar]
- 52.Van Zuuren EJ, Fedorowicz Z, Carter B. Evidence-based treatments for female pattern hair loss: A summary of a Cochrane systematic review. Br J Dermatol. 2012;167:995–1010. doi: 10.1111/j.1365-2133.2012.11166.x. [DOI] [PubMed] [Google Scholar]
- 53.Dean JC, Salmon SE, Griffith KS. Prevention of doxorubicin-induced hair loss with scalp hypothermia. N Engl J Med. 1979;301:1427–1429. doi: 10.1056/NEJM197912273012605. [DOI] [PubMed] [Google Scholar]
- 54.Nesbit RA, Woods RL, Tattersall MH, et al. Tamoxifen in malignant melanoma. N Engl J Med. 1979;301:1241–1242. doi: 10.1056/nejm197911293012218. [DOI] [PubMed] [Google Scholar]