We report the clinical manifestations and management of continuous-flow left ventricular assist device (LVAD) infections from a large multicenter cohort. On the basis of these observations, a management algorithm is derived to assist clinical decision making for LVAD infection.
Keywords: device-related infections, LVAD, endocarditis, driveline infections, heart failure
Abstract
Background. Infection is a serious complication of left ventricular assist device (LVAD) therapy. Published data regarding LVAD-associated infections (LVADIs) are limited by single-center experiences and use of nonstandardized definitions.
Methods. We retrospectively reviewed 247 patients who underwent continuous-flow LVAD implantation from January 2005 to December 2011 at Mayo Clinic campuses in Minnesota, Arizona, and Florida. LVADIs were defined using the International Society for Heart and Lung Transplantation criteria.
Results. We identified 101 episodes of LVADI in 78 patients (32%) from this cohort. Mean age (± standard deviation [SD]) was 57±15 years. The majority (94%) underwent Heartmate II implantation, with 62% LVADs placed as destination therapy. The most common type of LVADIs were driveline infections (47%), followed by bloodstream infections (24% VAD related, and 22% non-VAD related). The most common causative pathogens included gram-positive cocci (45%), predominantly staphylococci, and nosocomial gram-negative bacilli (27%). Almost half (42%) of the patients were managed by chronic suppressive antimicrobial therapy. While 14% of the patients had intraoperative debridement, only 3 underwent complete LVAD removal. The average duration (±SD) of LVAD support was 1.5±1.0 years. At year 2 of follow-up, the cumulative incidence of all-cause mortality was estimated to be 43%.
Conclusion. Clinical manifestations of LVADI vary on the basis of the type of infection and the causative pathogen. Mortality remained high despite combined medical and surgical intervention and chronic suppressive antimicrobial therapy. Based on clinical experiences, a management algorithm for LVADI is proposed to assist in the decision-making process.
With increasing prevalence of heart failure in the United States and limited availability of donor organs [1], implantable left ventricular assist devices (LVADs) are increasingly being used as a bridge to heart transplantation and as a long-term myocardial surrogate, termed as destination therapy. However, infection is a major complication associated with LVAD therapy, with reported rates ranging from 25% to 80% [2]. Clinical manifestations of LVAD-associated infections (LVADIs) range from local driveline exit site and pocket infections to endovascular infections of the valves or blood-contacting surfaces of the LVAD, so-called “endocardial” LVAD-related bloodstream infections (BSIs) [1].
Prior publications that examined different aspects of LVADI have significant limitations in methodology, have small sample sizes, or focused on outdated first-generation pulsatile–flow LVADs. Even the most recently published prospective cohort of LVAD recipients had only 33 infected cases [3]. Moreover, LVADI were often loosely defined, including infections that are not directly related to LVADs, such as pneumonia or urinary tract infections [4–7]. Similarly, some investigators defined infections by the presence of a positive culture result alone, which failed to distinguish infection from colonization or contamination [4, 8, 9]. Even in the largest LVAD database of 4000 cases—the INTERMACS registry—LVADIs were loosely defined [10, 11].
In the current investigation, we report clinical manifestations and management of LVADI from a large, multicenter cohort of patients with continuous-flow (CF) LVADs, using standardized consensus definitions proposed by the International Society of Heart Lung Transplant (ISHLT) [12].
METHODS
We retrospectively reviewed 247 patients who underwent CF LVAD implantation at Mayo Clinic campuses in Minnesota, Arizona, and Florida between January 2005 and December 2011. These devices included Heartmate II (Thoratec), Jarvik 2000 (Jarvik Heart), and VentrAssist (Ventracor). Cases were identified from the hospital surgical index. Patients in whom LVADs were not initially implanted at Mayo Clinic, and those with concurrent right ventricular assist devices were excluded to minimize confounders. All patients had consented to the use of their medical records for research purposes. Study proposal was reviewed and approved by the Mayo Foundation Institutional Review Board.
Definitions
LVADIs were classified on the basis of the recent consensus guidelines proposed by the ISHLT [12]. LVADIs were defined as including all infections that occurred in the presence of LVAD that may or may not have been directly attributable to the LVAD therapy but warranted special consideration because of the presence of LVAD. For example, a catheter-related bloodstream infection (CRBSI) that was ultimately deemed not to have seeded the LVAD (and, hence, a non–VAD-related BSI) still required special consideration in the presence of an LVAD. LVADIs, therefore, encompassed all LVAD-specific infections, LVAD-related infections, and non–LVAD-related BSIs. Infections unrelated to LVAD, such as pneumonia and urinary tract infections that did not result in BSI, were excluded. Driveline infection was defined as infection involving the soft tissues surrounding the driveline exit site, typically accompanied by erythema, warmth, and purulent discharge. Pocket infection was defined as infection involving the body cavity that holds the LVAD pump. BSI and CRBSI were defined using Centers for Disease Control and Prevention/National Healthcare Safety Network criteria [13]. Central venous catheter (CVC)–associated BSI was defined as infection involving positive blood cultures in the presence of a CVC without another attributable source of infection that did not meet the strict criteria for CVC-related BSI, such as differential time to positivity or a positive catheter tip culture (>15 colony-forming units). LVAD-associated endocarditis was defined as having clinical evidence of pump and/or cannula infection and the presence of vegetation on echocardiography or a vascular phenomenon as defined by modified Duke criteria [14]. Cases with a high suspicion for device or valvular involvement but no discernible vegetations on echocardiography were classified as pump and/or cannula infection. The classification of all cases was discussed in detail with senior investigators (ie, R. C. W., A. J. W., and M. R. S.).
Each infection episode was defined as a new infection involving a new site and/or new microorganism. The episodes excluded relapses, which were defined as infections that recurred at the same site, were caused by the same organism (with similar susceptibilities) within 1 year of treatment of the original infection, and occurred after clinical resolution of the initial episode of infection. If the susceptibilities of the cultured organism showed new resistance to an antibiotic to which the organism was previously susceptible and with which the patient was treated, this was considered a relapse due to emerging resistance while receiving antibiotic therapy. However, if a patient initially developed a driveline infection that subsequently evolved into a pocket infection, a BSI, and then endocarditis, all involving the same microorganism(s), the most severe of these episodes was used for the purposes of this analysis. Similarly, in patients with multiple relapses of infections, only data from the most-severe episodes were analyzed.
Patients whose episode had >1 organisms, all of which were gram-positive cocci (GPC), were categorized as the GPC group; similarly, patients whose episode had >1 organisms, all of which were gram-negative bacilli (GNB), were categorized as the GNB group. Patients who had multiple organisms from different organism categories were categorized as the mixed group.
Cardiovascular implantable electronic device (CIED) infections were classified as pocket infections or CIED-related endocarditis, as previously described [15–17].
The time to onset of infection was defined from the date of implantation to the date of diagnosis of infection or onset of symptoms, if known, whichever came first. The microbiologic etiology of LVADI was defined on the basis of culture results for specimens obtained from sterile sites, such as bloodstream or intraoperative specimens. Superficial swab culture of the driveline site was included only if the patient also met other criteria for driveline infection in accordance with ISHLT definitions. Local infections included driveline and pocket infections, mediastinitis without BSI, and CIED pocket infection. Endovascular infections included pump and/or cannula infections, infective endocarditis, all BSIs, and CIED lead infections. Chronic suppressive antimicrobial therapy was used to suppress LVADI after primary antimicrobial therapy, when the LVAD is deemed to be seeded, and was typically continued indefinitely until death, device explantation, or transfer to hospice care.
Follow-up
In our practice, LVAD coordinators closely follow all patients who undergo LVAD implantation at Mayo Clinic and carefully document in the electronic medical record any additional events that occurred at outside institutions. All patients had a minimum of 6 months of follow-up from the time of LVAD implantation. Driveline exit site care consisted of daily cleansing with chlorhexidine solution via sterile technique and securing of the driveline by an abdominal binder. A major change during the study period was the implementation of the abdominal binder around 2009.
Statistical Analysis
Descriptive statistics for categorical variables are presented as counts and percentages, and those for continuous variables are presented as either means with standard deviations (SD) or medians with interquartile ranges (IQRs), depending on whether the data were skewed. For follow-up events such as all-cause mortality, the event rate at 2 years following the LVAD implantation was estimated using the Kaplan-Meier method. Comparisons of subgroups were made using the Student t test or Wilcoxon rank sum test for continuous variables, as appropriate, and a χ2 test or Fisher exact test for categorical variables. Statistical analyses were performed using the software package SAS, version 9.2 (SAS Institute, Cary, NC). All statistical tests were 2-sided, and P values of < .05 were considered statistically significant.
RESULTS
Epidemiology and Baseline Characteristics
Based on follow-up of 247 patients who underwent CF LVAD implantation (307.5 total person-years), 101 episodes of LVADI were identified in 78 patients, for an overall incidence of 32.8 cases (95% confidence interval, 26.7–39.9) per 100 person-years of LVAD support. The classification and incidence rates for the different infection types for all 101 episodes of LVADI are summarized in Table 1. For simplicity and ease of analytical considerations, the rest of the results section is limited to the first episode of infection for each of the 78 patients.
Table 1.
Incidence of Left Ventricular Assist Device–Associated Infection, by Infection Type
| Infection Type | Cases per 100 Person-Years of LVAD Support (95% CI) |
|---|---|
| All | 32.8 (26.7–39.9) |
| Endocarditis | 1.6 (.5–3.8) |
| Pump and/or cannula infection | 4.9 (2.7–8.0) |
| Bloodstream infection | |
| VAD related | 7.5 (4.7–11.2) |
| Non–VAD related | 6.8 (4.2–10.4) |
| CVC related | 3.3 (1.6–6.0) |
| CVC associated | 1.6 (.5–3.8) |
| Pocket infection | 2.3 (.9–4.7) |
| Driveline infection | 15.0 (10.9–20.0) |
| CIED infection | 1.6 (.5–3.8) |
| Mediastinitis, VAD related | 2.0 (.7–4.2) |
These data represent all 101 episodes of infections in 78 patients.
Abbreviations: CI, confidence interval; CIED, cardiovascular implantable electronic device; CVC, central venous catheter; LVAD, left ventricular assist device; VAD, ventricular assist device.
Demographic characteristics, device features, and comorbidities are summarized in Table 2 for the overall group of 78 LVADI patients and for subgroups with systemic (n = 41) and local (n = 37) infections. Heartmate II (94%) was the most commonly used device, with the majority of the driveline exit site tunneled to the right (87%). Of note, 21% of the patients had an active infection and were receiving antibiotic therapy at the time of LVAD implantation.
Table 2.
Demographic Characteristics, Device-Related Features, and Comorbidities Among 78 Patients With Left Ventricular Assist Device–Associated Infection
| Variable | Total (n = 78) | Endovascular (n = 41) | Local (n = 37) |
|---|---|---|---|
| Patient characteristic | |||
| Male sex | 62 (79) | 35 (85) | 27 (73) |
| Age at implantation, y | 56.8 ± 14.9 | 59.1 ± 10.9 | 54.3 ± 18.1 |
| Ethnicity | |||
| White | 66 (87) | 33 (85) | 33 (89) |
| African American | 5 (7) | 2 (5) | 3 (8) |
| Asian or Hispanic | 5 (7) | 4 (10) | 1 (3) |
| Location of LVAD placement | |||
| Mayo Clinic Rochester, MN | 54 (69) | 34 (83) | 20 (54) |
| Mayo Clinic Scottsdale, AZ | 17 (22) | 3 (7) | 14 (38) |
| Mayo Clinic Jacksonville, FL | 7 (9) | 4 (10) | 3 (8) |
| LVAD implantation characteristic | |||
| Device type | |||
| Heartmate II (Thoratec) | 73 (94) | 37 (90) | 36 (97) |
| Jarvik 2000 (Jarvik Heart) | 4 (5) | 3 (7) | 1 (3) |
| VentrAssist (Ventracor) | 1 (1) | 1 (2) | 0 (0) |
| Destination therapy | 48 (62) | 23 (56) | 25 (68) |
| Driveline exit site (right) | 67 (87) | 37 (93) | 30 (81) |
| Presence of CIED at time of implantation | 68 (87) | 35 (85) | 33 (89) |
| Prosthetic valve or vascular graft | 21 (27) | 12 (29) | 9 (24) |
| Active infection, receiving antibiotic treatment at implantation | 16 (21) | 8 (20) | 8 (22) |
| Comorbidity | |||
| Charlson comorbidity indexa | 4.5 (3.0–6.0) | 6.0 (4.0–7.0) | 4.0 (3.0–5.0) |
| Ejection fraction at time of implantation | 18.0 ± 10.1 | 19.5 ± 12.5 | 16.3 ± 6.2 |
| Body mass indexb | 29.4 ± 6.1 | 30.3 ± 6.1 | 28.4 ± 5.9 |
| Ischemic cardiomyopathy | 37 (47) | 21 (51) | 16 (43) |
| Diabetes mellitus | |||
| Without end-organ damage or on oral agents | 16 (21) | 8 (20) | 8 (22) |
| With end-organ damage or on insulin | 14 (18) | 11 (27) | 3 (8) |
| Renal disease | |||
| Not undergoing hemodialysis | 53 (68) | 32 (78) | 21 (57) |
| Undergoing hemodialysis | 2 (3) | 2 (5) | 0 (0) |
| Baseline creatinine level, mg/dLa | 1.3 (1.0–1.6) | 1.4 (1.1–1.9) | 1.2 (1.0–1.4) |
| Liver disease | 5 (6) | 5 (12) | 0 (0) |
| Autoimmune/connective tissue disease | 5 (6) | 2 (5) | 3 (8) |
| Immunosuppressive drug use | 4 (5) | 4 (10) | 0 (0) |
| Coronary artery disease | 49 (63) | 28 (68) | 21 (57) |
| Myocardial infarction | 37 (47) | 21 (51) | 16 (43) |
| Coronary artery bypass grafting | 26 (33) | 16 (39) | 10 (27) |
| Peripheral vascular disease | 9 (12) | 3 (7) | 6 (17) |
| Cerebrovascular disease | 19 (24) | 9 (22) | 10 (27) |
| Splenectomy | 2 (3) | 1 (2) | 1 (3) |
| Chronic obstructive pulmonary disease | 11 (14) | 7 (17) | 4 (11) |
| Anticoagulation | 52 (68) | 26 (63) | 26 (72) |
| Chronic skin condition | 9 (12) | 7 (18) | 2 (5) |
Categorical data are summarized as No. (%) of patients, and continuous data are summarized as mean values ± standard deviation, unless noted otherwise. There was a single episode per patient.
Abbreviations: CIED, cardiovascular implantable electronic device; LVAD, left ventricular assist device.
a Median values (interquartile ranges) are reported because of skewed distributions.
b Calculated as the weight in kilograms divided by the square of the height in meters.
Clinical Presentation
Driveline infection (47%) was the most common LVADI, followed by BSI, similarly distributed between VAD- and non–VAD-related BSI (24% and 22%, respectively; Table 3). The median time from LVAD implantation to the first episode of infection was 4.4 months (IQR, 1.0–13.5 months). Compared with patients who had local infections, patients with endovascular infections appeared to present with infections earlier (median, 1.6 vs 7.1 months after implantation; P = .006) and more frequently with fever (73% vs 19%; P < .001), leukocytosis (71% vs 29%; P < .001), and Systemic inflammatory response syndrome (SIRS) criteria (39% vs 8%; P = .002). Interestingly, all 12 patients who developed driveline trauma subsequently developed driveline infections. The most common sources of BSI were driveline site (49%) and CVC (10%). The driveline was deemed a source when the same organism was cultured from the driveline and the bloodstream.
Table 3.
Clinical Presentation of Different Left Ventricular Assist Device–Associated Infections
| Variable | Total (n = 78) | Endovascular (n = 41) | Local (n = 37) |
|---|---|---|---|
| Infection type | |||
| Pump and/or cannula infection | 11 (14) | … | … |
| Pocket infection | 4 (5) | … | … |
| Percutaneous driveline infection | 37 (47) | … | … |
| Infective endocarditis | 4 (5) | … | … |
| Bloodstream infection | |||
| VAD related | 19 (24) | … | … |
| Non–VAD related | 17 (22) | … | … |
| Mediastinitis (VAD related) | 5 (6) | … | … |
| CIED infection | 4 (5) | … | … |
| Clinical manifestation | |||
| Time to infection onset, moa | 4.4 (1.0–13.5) | 1.6 (0.3–7.3) | 7.1 (3.0–19.8) |
| Initial presentation at outside institution | 9 (12) | 6 (15) | 3 (8) |
| Fever | 37 (48) | 30 (73) | 7 (19) |
| Met SIRS criteriab | 19 (25) | 16 (39) | 3 (8) |
| WBC count >10×109 cells/L | 39 (51) | 29 (71) | 10 (29) |
| Anemiac | 52 (68) | 36 (88) | 16 (46) |
| Serum creatinine level, mg/dLa | 1.2 (0.9–1.5) | 1.4 (1.2–1.9) | 1.0 (0.9–1.2) |
| Albumin level <3.5 mg/dL | 17/57 (30) | 14/31 (45) | 3/26 (12) |
| Prealbumin level <17 mg/dL | 10/33 (30) | 5/11 (45) | 5/22 (23) |
| Driveline trauma | 12 (16) | 4 (10) | 8 (24) |
| Erythema | … | … | 32/37 (86) |
| Tenderness | … | … | 25/35 (71) |
| Purulent Drainage | … | … | 21/29 (72) |
Categorical data are summarized as No. or proportion (%) of patients, and continuous data are summarized as mean values ± standard deviation, unless noted otherwise. There was a single episode per patient.
Abbreviations: CIED, cardiovascular implantable electronic device; SIRS, systemic inflammatory response syndrome; VAD, ventricular assist device; WBC, white blood cell.
a Median values (interquartile ranges) are reported because of skewed distributions.
b SIRS was defined as the presence of ≥2 of the following: temperature >38°C or <36°C, heart rate >90 beats/minute, respiratory rate >20 breaths/minute or a partial pressure of carbon dioxide (arterial) of <32 mm Hg, or a leukocyte count of >12 or <4 × 10∧9 cells/L/L or >10% immature bands.
c Defined as a hematocrit of <38% for males and <35% for females.
Microbiology
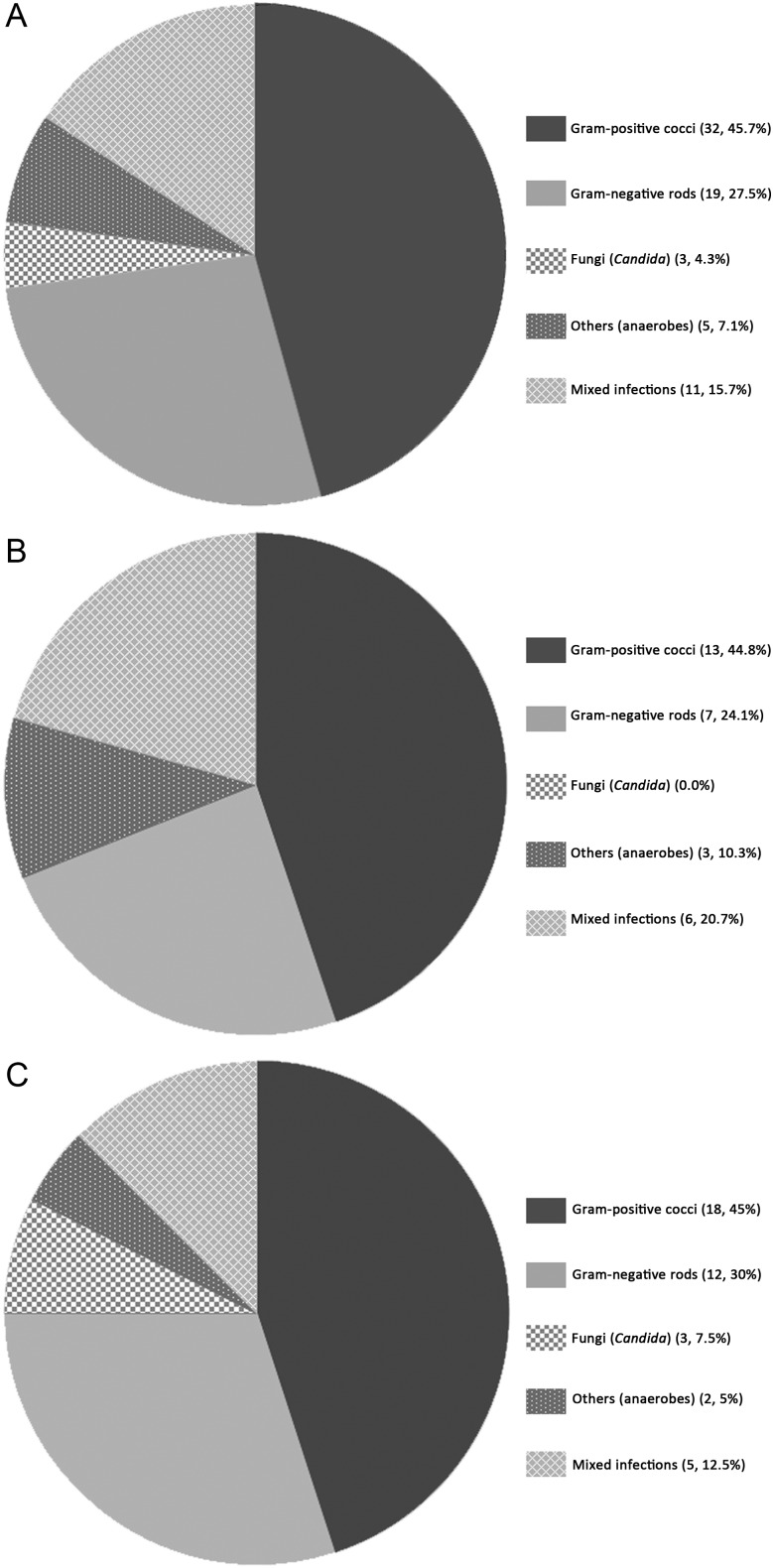
The distribution of causative pathogens for the first episodes of LVADI in the overall 78 patients and their local versus endovascular infection subgroups are shown in Figure 1. Staphylococci and nosocomial GNB were the most common causative pathogens.
Figure 1.
Distribution of causative pathogens of left ventricular assist device (LVAD)–associated infection in 78 patients. A, First or most-severe episodes (n = 78; no culture obtained for 8). B, Local infections (n = 37; no culture obtained for 8). C, Endovascular infections (n = 40; no culture obtained for 0). Data are No. of episodes of infections (%). Gram-positive cocci included methicillin-susceptible Staphylococcus aureus (15), coagulase-negative staphylococci (18), methicillin-resistant S. aureus (4), and Enterococcus species (5). The most common gram-negative bacilli were Pseudomonas aeruginosa (7), Klebsiella species (7), Escherichia coli (6), Stenotrophomonas species (4), and Serratia species (4). Fungal infections included 1 Candida albicans and 2 C. glabrata. Mixed infection included a concurrent infection with C. albicans and Mycobacterium smegmatis in the blood. “Others” included anaerobes, Propionibacterium species, diphtheroids, and Corynebacterium species. The distribution of the 78 single episodes is similar to that for all 101 episodes of infection in the 78 patients.
Management and Outcome
Management interventions for the first episodes of LVADI in 78 patients are summarized in Table 4. The majority of the patients (76%) were managed without surgical intervention. Only 13% of the patients underwent intraoperative debridement with device retention. The LVAD was eventually removed in 3 patients after failure of conservative management. The overall median duration of antimicrobial therapy was 28.0 days. Patients with local infections were more likely to receive oral antibiotics (57% vs 5%; P < .001), although the duration of antimicrobial therapy was not significantly different between local and endovascular infections (median, 23 vs 33 days; P = .182). The median duration of antimicrobial therapy for driveline infections (n = 36) was 24.5 days (IQR, 13.5–45.5 days).
Table 4.
Management and Outcome of Left Ventricular Assist Device–Associated Infections
| Variable | Total (n = 78) | Endovascular (n = 41) | Local (n = 37) | |
|---|---|---|---|---|
| Antibiotic therapy duration, da | ||||
| Overall | n = 76 | 28.0 (14.5–42.5) | 33.0 (15.5–42.5) | 23.5 (13.0–44.0) |
| Parenteral antibiotics (primarily)a | n = 53 | 36.0 (17.0–49.0) | 34.5 (16.0–43.0) | 39.0 (26.0–132.0) |
| Oral antibiotics (primarily)a | n = 23 | 14.0 (10.0–24.0) | 15.5 (14.0–17.0) | 14.0 (10.0–24.0) |
| Receipt of suppressive oral antimicrobials | 29 (37) | 16 (39) | 13 (35) | |
| Receipt of suppressive parenteral antimicrobialsb | 4 (5) | 2 (5) | 2 (5) | |
| Surgical management | n = 76 | |||
| None | 58 (76) | 33 (80) | 25 (71) | |
| Bedside debridement | 3 (4) | 1 (2) | 2 (6) | |
| Intraoperative debridement with device retention | 10 (13) | 4 (10) | 6 (17) | |
| LVAD removal | 3 (4) | 1 (2) | 2 (6) | |
| Otherc | 2 (3) | 2 (5) | 0 (0) | |
| Duration of LVAD support follow-up, y | 1.6 ± 1.1 | 1.3 ± 0.8 | 2.0 ± 1.2 | |
| Death at 2-y follow-upd | 25 (40) | 19 (53) | 6 (26) | |
| Heart transplantation at 2-y follow-up (among bridge-to-transplant patientsd | n = 30 | 10 (47) | 5 (40) | 5 (55) |
Categorical data are summarized as No. or proportion (%) of patients, and continuous data are summarized as mean values ± standard deviation, unless noted otherwise. There was a single episode per patient.
Abbreviation: LVAD, left ventricular assist device.
a Median values (interquartile ranges) are reported because of skewed distributions.
b Used because of the lack of oral options.
c Included marsupialization of skin edges, ultrasonography-guided aspiration, and pericardiocentesis.
d No. (%) of events (as estimated by the Kaplan-Meier method).
Thirteen patients had 1 or more relapses of LVADI. Of patients who were receiving suppressive antimicrobials, 29% (8/28) developed relapse, compared with 11% (5/45) of those not receiving suppressive antimicrobials. Interestingly, among the 37 patients whose LVADs were not considered seeded (ie, there was no surgical intervention, suppressive antimicrobials, or relapse), the majority had either driveline infections (38%) or non–VAD-related BSI (35%) and received antimicrobials for a median duration of 16 days (IQR, 14–28 days).
The mean follow-up duration (±SD) of LVAD support was 1.6 ±1.1 years. At the 2-year follow-up point from the time of implantation, the rate of all-cause mortality was 43%. Among the 30 patients who received LVAD as a bridge to transplantation, the cumulative incidence of heart transplantation at the 2-year follow-up point was 47%.
Pathogen-Specific LVADI
While small numbers preclude robust statistical analysis between subgroups of LVAD recipients with GPC (n = 31) versus those with GNB (n = 16; Table 5), it is interesting to note that patients with GNB infections had fairly high Charlson comorbidity indexes (median, 6). A quarter of those with GNB infections were receiving antibiotics for active infections at the time of LVAD implantation, and they also had high rates of destination therapy (75%) and non–VAD-related BSI (44%). In contrast, many of the GPC patients had VAD-related BSI (39%). A majority of GPC patients were treated with primary antibiotic therapy, primarily parenteral, for >1 month (median, 38.5 days; 84%) and received suppressive oral antimicrobials (65%).
Table 5.
Clinical Features of Left Ventricular Assist Device Infections Due to Gram-Positive and Gram-Negative Pathogens
| Variable | Gram Negative (n = 16) | Gram Positive (n = 31) | ||
|---|---|---|---|---|
| Baseline characteristic | ||||
| Male sex | 13 (81) | 25 (81) | ||
| Age at implantation, y | 59.9 ± 16.0 | 56.0 ± 13.8 | ||
| White ethnicity/race | 14 (93) | 27 (87) | ||
| Body mass indexa | 30.7 ± 5.3 | 29.4 ± 4.9 | ||
| Charlson comorbidity indexb | 6.0 (4.0–6.0) | 4.0 (3.0–6.0) | ||
| Ischemic cardiomyopathy (vs nonischemic) | 9 (56) | 16 (52) | ||
| Diabetes mellitus | 6 (38) | 12 (39) | ||
| Renal disease | ||||
| Not undergoing hemodialysis | 13 (81) | 19 (61) | ||
| Undergoing hemodialysis | 1 (6) | 0 (0) | ||
| Peripheral vascular disease | 4 (25) | 2 (7) | ||
| Active infection receiving antibiotic treatment at implantation | 4 (25) | 3 (10) | ||
| Device type, Heartmate II | 16 (100) | 30 (97) | ||
| Destination therapy (vs bridge) | 12 (75) | 15 (48) | ||
| Exit site, right (vs left) | 15 (94) | 26 (87) | ||
| Duration of LVAD support follow-up, y | 2.0 ± 1.4 | 1.5 ± 0.9 | ||
| LVADI type | ||||
| Pump and/or cannula infection | 2 (13) | 5 (16) | ||
| Pocket infection | 0 (0) | 3 (10) | ||
| Percutaneous driveline infection | 7 (44) | 12 (39) | ||
| Infective endocarditis | 0 (0) | 3 (10) | ||
| Bloodstream infection | ||||
| VAD related | 2 (13) | 12 (39) | ||
| Non–VAD related | 7 (44) | 4 (13) | ||
| Mediastinitis | ||||
| VAD related | 0 (0) | 4 (13) | ||
| Non–VAD related | 0 (0) | 0 (0) | ||
| CIED infection | 1 (6) | 1 (3) | ||
| Clinical presentation | ||||
| Time to onset of infection, mob | 5.4 (0.5–24.1) | 3.0 (1.0–12.9) | ||
| Fever | 9 (60) | 20 (65) | ||
| Driveline trauma | 2 (13) | 4 (13) | ||
| Met SIRS criteria | 3 (20) | 11 (35) | ||
| Among local infections | n = 7 | n = 15 | ||
| Warmth | 2/5 (40) | 8/12 (67) | ||
| Fluctuance | 0/6 (0) | 2/12 (17) | ||
| Purulent drainage | 5/5 (100) | 6/10 (60) | ||
| WBC count >10 ×109 cells/L | 8 (53) | 17 (57) | ||
| HCT <38% (for males), <35% (for females) | 10 (67) | 26 (87) | ||
| Serum creatinine level, mg/dLb | n = 15 | 1.3 (0.8–1.7) | n = 30 | 1.3 (1.0–1.6) |
| Albumin level <3.5/prealbumin level <17 mg/dL | n = 12 | 2 (17) | n = 24 | 9 (38) |
| Management and outcome | ||||
| Antibiotic therapy duration, db | ||||
| Overall | n = 15 | 16.0 (14.0–36.0) | n = 30 | 38.5 (24.0–49.0) |
| Parenteral antibiotics (primarily)b | n = 9 | 33.0 (16.0–72.0) | n = 26 | 40.0 (28.0–49.0) |
| Oral antibiotics (primarily)b | n = 6 | 14.0 (13.0–17.0) | n = 4 | 27.5 (17.0–71.5) |
| Lifelong receipt of suppressive parenteral antimicrobials | 2 (13) | 1 (3) | ||
| Lifelong receipt of suppressive oral antimicrobials | 4 (25) | 20 (65) | ||
| Surgical management | n = 15 | n = 30 | ||
| None | 13 (87) | 19 (63) | ||
| Bedside debridement | 0 (0) | 2 (7) | ||
| Intraoperative debridement with device retention | 1 (7) | 7 (23) | ||
| LVAD removalc | 1 (7) | 0 (0) | ||
| Other | 0 (0) | 2 (7) | ||
| Death at 2-y follow-upd | 6 (43) | 10 (40) | ||
| Heart transplantation at 2-y follow-up (among bridge to transplant patients)d | n = 4 | 2 (50) | n = 16 | 5 (40) |
Categorical data are summarized as No. or proportion (%) of patients, and continuous data are summarized as mean values ± standard deviation, unless noted otherwise. There was a single episode per patient.
Abbreviations: CIED, cardiovascular implantable electronic device; HCT, hematocrit; LVAD, left ventricular assist device; LVADI, left ventricular assist device–associated infections; SIRS, systemic inflammatory response syndrome; WBC, white blood cell.
a Calculated as the weight in kilograms divided by the square of the height in meters.
b Median values (interquartile ranges) are reported because of skewed distributions.
c The patient had their LVAD removed after treatment failure.
d No. (%) of events (as estimated by the Kaplan-Meier method) of events.
DISCUSSION
Our study is among the largest multicenter cohorts of patients with CF LVADI that uses the new ISHLT definitions and one of the first to describe pathogen-specific features in the clinical presentation of LVADI. Our findings are consistent with previous studies that found that CF LVADs have lower rates of overall (0.32 vs 0.9–3.36 cases per patient-year) and individual types of infections than PF LVADs [4–6, 18–20]. Similarly, the microbiology of LVADI appears to be comparable between the 2 types of LVADs, with a predominance of staphylococci, followed by nosocomial gram-negative pathogens such as Pseudomonas, Enterobacter, and Serratia species [4, 20, 21]. However, the rate of fungal infections was lower in our cohort (6.6%), compared with previous PF LVAD studies (16%–23%) [21, 22]. The lower rate of infections in these devices is likely attributable to the fact that CF LVADs require a smaller pump pocket, thinner drivelines, longer driveline tunneling, and simpler mechanics without the need for a mechanical valve. In addition, general increases in provider experience with the management of LVADs and their complications over time may play a role.
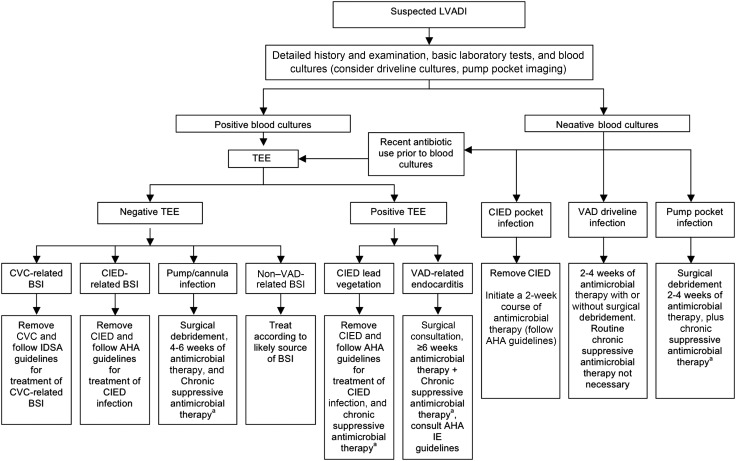
On the basis of our current analysis, experience from the Mayo Clinic, and data from published literature, we propose a treatment algorithm for the management of LVADI, based on the type and extent of the infection (Figure 2). The classification of LVADI, although challenging, is integral to the management of these complex infections. The primary decision point in our treatment algorithm is the presence or absence of BSI. Blood cultures should be obtained for all patients with suspected LVADI, even in the absence of systemic signs and symptoms of infection and normal laboratory findings, as systemic manifestations of infection such as fever, leukocytosis, or SIRS criteria were present in only half of the patients with LVADI.
Figure 2.
Mayo Clinic guidelines for the management of left ventricular assist device (LVAD)–associated infections (LVADIs). Guidelines are referenced from [23–25]. These are general guidelines only, and LVADI management should be individualized based on clinical presentation and host factors. aChronic suppressive antimicrobial therapy is highly recommended if there is (1) failure to clear bloodstream infection (BSI) despite appropriate antibiotic therapy or removal of the suspect catheter in the setting of catheter-related BSI or (2) relapse of BSI after completing an appropriate antibiotic course, but it should ultimately be guided by clinical judgment. Abbreviations: AHA, American Heart Association; BSI, bloodstream infection; CIED, cardiovascular implantable electronic device; CVC, central venous catheter; IDSA, Infectious Diseases Society of America; IE, infective endocarditis; LVADI, left ventricular assist device–associated infections; TEE, transesophageal echocardiography; VAD, ventricular assist device.
Driveline infections were the most frequent type of LVADI in our cohort and may be difficult to distinguish from local skin irritation due to either driveline trauma or adhesive tape. Interestingly, all reported cases of driveline site trauma in our cohort were followed by driveline infections. Driveline site trauma, a known risk factor for driveline infections [19, 20], can be minimized by the use of abdominal binder. A positive culture result for a swab specimen from the driveline site must be interpreted with caution because of frequent growth of skin colonizers. Additional imaging, such as ultrasonography or computed tomography, may be warranted if underlying abscess is suspected.
The second most common LVADI in our cohort was BSI. As the most common source of BSI, driveline infections should be treated aggressively to prevent secondary endovascular seeding. A decisive factor in the management of BSI in the setting of an LVAD is whether the LVAD itself is infected. This can be difficult to determine without direct intraoperative culture of the device, which cannot be easily performed. While transesophageal echocardiography (TEE) is recommended in most cases of BSI, vegetations can be missed on TEE because of the reflective internal metal surfaces of the LVAD [26]. TEE should also be considered when blood culture results may be negative because of recent antibiotic use. Despite a lack of vegetations on TEE, LVAD seeding is still a concern if there is (1) failure to clear BSI despite appropriate antibiotic therapy or removal of the suspect catheter in the setting of CRBSI or (2) relapse of BSI after completion of an appropriate antibiotic course. However, the management of each case should be individualized on the basis of clinical judgment.
The optimal duration of antimicrobial therapy for LVAD-specific infections is unclear. While localized LVADI may have trended toward shorter courses of antibiotic therapy in our cohort, treatment duration varies with the causative microorganism, the extent and severity of the infection, and the need for surgical debridement [1, 27]. LVAD-related endovascular infections are generally treated with ≥4-week courses of antimicrobials, based on the type of infection (Figure 2), modeled after the existing guidelines for catheter-related BSI [23] and endocarditis [25]. Any BSI in the setting of LVAD therapy should be treated aggressively, as the same strain has been shown to cause recurrent BSI in LVAD recipients [28].
While surgical debridement and hardware removal are integral in the management of most prosthesis-related infections, removal of an LVAD cannot be easily accomplished. Complete LVAD removal or exchange is associated with significant morbidity [29] and must be followed by immediate device exchange or heart transplantation. Therefore, device removal is usually reserved as a last resort after lifelong antimicrobial suppression fails, as observed in 3 patients in our cohort. Even after device exchange, suppressive antimicrobial therapy is continued, because the new device is placed in a presumably contaminated operative field.
In general, chronic suppressive antimicrobial therapy is indicated when the LVAD is believed to be seeded. These would include pump and/or cannula infection, endocarditis, pump pocket infection, or persistent or recurrent BSI despite appropriate antibiotic therapy. For patients with just driveline infection or non–VAD-related BSI, chronic suppression is usually not necessary. Failure of antimicrobial suppression may result from the development of drug resistance or nonadherence.
We also noted some interesting pathogen-specific findings in our LVADI study cohort; however, limited number of cases precluded a robust statistical analysis. Staphylococci were the most common pathogens responsible for LVADI, likely because of multiple surface binding proteins that enhance their ability to attach to prosthetic surfaces [30]. The high prevalence of nosocomial GNB LVADI, especially in destination therapy patients who are not transplant candidate due to poor prognosis, likely reflects the prolonged healthcare exposure that patients with end-stage heart failure experience before LVAD implantation. Because of the high level of drug resistance in nosocomial GNB, some patients with LVAD seeding with GNB required parenteral suppressive therapy.
Our study is retrospective in design and is thus subject to inherent limitations, such as information and recall bias. We rely on objective data and on standardized and reproducible case definitions proposed by the ISHLT to minimize these biases. Mayo Clinic likely has referral bias for sicker and more complex patients; however, patients with end-stage heart failure who require LVADs are typically quite ill and thus are usually managed in tertiary referral centers. Furthermore, despite incorporating patients from geographically diverse Mayo Clinic campuses, our study cohort remained predominantly white. Nevertheless, we believe that our findings should be applicable to LVAD recipients in general. The small sample size also made statistical testing and formal comparisons less feasible. Finally, our proposed treatment algorithm is for general guidance and should be tailored based on individual cases.
In summary, the clinical manifestations and management of LVADI vary based on the extent of infection and the causative pathogens. Understanding these differences is critical in making timely diagnoses and providing appropriate management interventions. We hope that the proposed Mayo Clinic LVADI guideline will be helpful for clinicians managing these complicated infections. Future analysis looking at clinical predictors for LVADI and mortality among LVAD recipients will provide further insight into the management of LVADI.
Notes
Disclaimer. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the Department of Medicine, Mayo Foundation for Medical Education and Research (Career Development Award to M. R. S.); the Division of Infectious Diseases, Mayo Clinic College of Medicine (Small Grants award); and the National Center for Research Resources, National Institutes of Health (Clinical and Translational Science Awards grant UL1 RR024150). The study database was created and maintained using REDCap (grant UL1 TR000135).
Potential conflicts of interest. M. R. S. received funding from TyRx for prior research unrelated to this study that was administered according to a sponsored research agreement that prospectively defined the scope of the research effort and corresponding budget. L. M. B. has received royalty payments from UpToDate for serving as an author and receives payment from the Massachusetts Medical Society for serving as the editor in chief of NEJM Journal Watch Infectious Diseases. S. J. P. is a consultant for Thoratec. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gordon RJ, Quagliarello B, Lowy FD. Ventricular assist device-related infections. Lancet Infect Dis. 2006;6:426–37. doi: 10.1016/S1473-3099(06)70522-9. [DOI] [PubMed] [Google Scholar]
- 2.Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108:2015–31. doi: 10.1161/01.CIR.0000093201.57771.47. [DOI] [PubMed] [Google Scholar]
- 3.Gordon RJ, Weinberg AD, Pagani FD, et al. Prospective, multicenter study of ventricular assist device infections. Circulation. 2013;127:691–702. doi: 10.1161/CIRCULATIONAHA.112.128132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossi P, Dalla Gasperina D, Pagani F, Marone P, Vigano M, Minoli L. Infectious complications in patients with the Novacor left ventricular assist system. Transplant Proc. 2001;33:1969–71. doi: 10.1016/s0041-1345(00)02757-3. [DOI] [PubMed] [Google Scholar]
- 5.Malani PN, Dyke DBS, Pagani FD, Chenoweth CE. Nosocomial Infections in Left Ventricular Assist Device Recipients. Clin Infect Dis. 2002;34:1295–300. doi: 10.1086/340052. [DOI] [PubMed] [Google Scholar]
- 6.Sinha P, Chen JM, Flannery M, Scully BE, Oz MC, Edwards NM. Infections during left ventricular assist device support do not affect posttransplant outcomes. Circulation. 2000;102:III194–9. doi: 10.1161/01.cir.102.suppl_3.iii-194. [DOI] [PubMed] [Google Scholar]
- 7.Topkara VK, Kondareddy S, Malik F, et al. Infectious complications in patients with left ventricular assist device: etiology and outcomes in the continuous-flow era. Ann Thorac Surg. 2010;90:1270–7. doi: 10.1016/j.athoracsur.2010.04.093. [DOI] [PubMed] [Google Scholar]
- 8.Deng MC, Loebe M, El-Banayosy A, et al. Mechanical circulatory support for advanced heart failure: effect of patient selection on outcome. Circulation. 2001;103:231–7. doi: 10.1161/01.cir.103.2.231. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein DJ, el-Amir NG, Ashton RC, Jr, et al. Fungal infections in left ventricular assist device recipients. Incidence, prophylaxis, and treatment. ASAIO J. 1995;41:873–5. [PubMed] [Google Scholar]
- 10.Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) Available at: http://www.intermacs.org. Accessed 3 June 2012.
- 11.Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) J Am Coll Cardiol. 2011;57:1890–8. doi: 10.1016/j.jacc.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 12.Hannan MM, Husain S, Mattner F, et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant. 2011;30:375–84. doi: 10.1016/j.healun.2011.01.717. [DOI] [PubMed] [Google Scholar]
- 13.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 15.Le KY, Sohail MR, Friedman PA, et al. Clinical predictors of cardiovascular implantable electronic device-related infective endocarditis. Pacing Clin Electrophysiol. 2011;34:450–9. doi: 10.1111/j.1540-8159.2010.02991.x. [DOI] [PubMed] [Google Scholar]
- 16.Sohail MR, Uslan DZ, Khan AH, et al. Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator infection. Mayo Clin Proc. 2008;83:46–53. doi: 10.4065/83.1.46. [DOI] [PubMed] [Google Scholar]
- 17.Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007;49:1851–9. doi: 10.1016/j.jacc.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 18.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 19.Holman WL, Park SJ, Long JW, et al. Infection in permanent circulatory support: experience from the REMATCH trial. J Heart Lung Transplant. 2004;23:1359–65. doi: 10.1016/j.healun.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Simon D, Fischer S, Grossman A, et al. Left ventricular assist device-related infection: treatment and outcome. Clin Infect Dis. 2005;40:1108–15. doi: 10.1086/428728. [DOI] [PubMed] [Google Scholar]
- 21.Gordon SM, Schmitt SK, Jacobs M, et al. Nosocomial bloodstream infections in patients with implantable left ventricular assist devices. Ann Thorac Surg. 2001;72:725–30. doi: 10.1016/s0003-4975(01)02888-0. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein JA, Kern MJ. Percutaneous mechanical support for the failing right heart. Cardiol Clin. 2012;30:303–10. doi: 10.1016/j.ccl.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458–77. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 25.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394–434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 26.Nurozler F, Argenziano M, Oz MC, Naka Y. Fungal left ventricular assist device endocarditis. Annals Thorac Surg. 2001;71:614–8. doi: 10.1016/s0003-4975(00)01444-2. [DOI] [PubMed] [Google Scholar]
- 27.Bomholt T, Moser C, Sander K, et al. Driveline infections in patients supported with a HeartMate II: Incidence, aetiology and outcome. Scand Cardiovasc J. 2011;45:273–8. doi: 10.3109/14017431.2011.577236. [DOI] [PubMed] [Google Scholar]
- 28.Vilchez RA, McEllistrem MC, Harrison LH, McCurry KR, Kormos RL, Kusne S. Relapsing bacteremia in patients with ventricular assist device: an emergent complication of extended circulatory support. Ann Thorac Surg. 2001;72:96–101. doi: 10.1016/s0003-4975(01)02690-x. [DOI] [PubMed] [Google Scholar]
- 29.Haj-Yahia S, Birks EJ, Dreyfus G, Khaghani A. Limited surgical approach for explanting the HeartMate II left ventricular assist device after myocardial recovery. J Thorac Cardiovasc Surg. 2008;135:453–4. doi: 10.1016/j.jtcvs.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Rivera J, Vannakambadi G, Hook M, Speziale P. Fibrinogen-binding proteins of Gram-positive bacteria. Thromb Haemost. 2007;98:503–11. [PubMed] [Google Scholar]