Incidence of invasive methicillin-resistant Staphylococcus aureus (MRSA) infections decreased substantially among dialysis patients during 2005–2011 based on surveillance data from 9 metropolitan areas. Despite decreases, an estimated 15 169 invasive MRSA infections occurred in US dialysis patients in 2011.
Keywords: invasive MRSA, dialysis, methicillin-resistant S. aureus
Abstract
Background. Approximately 15 700 invasive methicillin-resistant Staphylococcus aureus (MRSA) infections occurred in US dialysis patients in 2010. Frequent hospital visits and prolonged bloodstream access, especially via central venous catheters (CVCs), are risk factors among hemodialysis patients. We describe the epidemiology of and recent trends in invasive MRSA infections among dialysis patients.
Methods. We analyzed population-based data from 9 US metropolitan areas from 2005 to 2011. Cases were defined as MRSA isolated from a normally sterile body site in a surveillance area resident who received dialysis, and were classified as hospital-onset (HO; culture collected >3 days after hospital admission) or healthcare-associated community-onset (HACO; all others). Incidence was calculated using denominators from the US Renal Data System. Temporal trends in incidence and national estimates were calculated controlling for age, sex, and race.
Results. From 2005 to 2011, 7489 cases were identified; 85.7% were HACO infections, and 93.2% were bloodstream infections. Incidence of invasive MRSA infections decreased from 6.5 to 4.2 per 100 dialysis patients (annual decrease, 7.3%) with annual decreases of 6.7% for HACO and 10.5% for HO cases. Among cases identified during 2009–2011, 70% of patients were hospitalized in the year prior to infection. Among hemodialysis cases, 60.4% of patients were dialyzed through a CVC. The 2011 national estimated number of MRSA infections was 15 169.
Conclusions. There has been a substantial decrease in invasive MRSA infection incidence among dialysis patients. Most cases had previous hospitalizations, suggesting that efforts to control MRSA in hospitals might have contributed to the declines. Infection prevention measures should include improved vascular access and CVC care.
Healthcare-associated invasive methicillin-resistant Staphylococcus aureus (MRSA) infections have decreased in the past several years [1], yet they remain a significant public health problem with a national incidence rate of 21.8 per 100 000 population and mortality rate of 3.7 per 100 000 population [2]. Of 67 000 healthcare-associated invasive MRSA infections reported in 2010, 15 700 (23.4%) were among dialysis patients [2]. The incidence of invasive MRSA infection among patients undergoing chronic dialysis is >100 times higher than in the general population [1, 3]. Increased risk of MRSA infections in dialysis patients is related to multiple factors, including repeated vascular access for hemodialysis patients through central venous catheters (CVCs) or arteriovenous (AV) grafts or fistulae, frequent hospital visits, and high prevalence of MRSA colonization [4]. Invasive S. aureus infections among dialysis patients are associated with high mortality and cost [5–7].
Surveillance sponsored by the US Centers for Disease Control and Prevention (CDC) showed that the incidence of invasive MRSA infections among dialysis patients decreased by 6.4% annually during 2005–2008 [1]. Understanding the current epidemiology of MRSA infections in dialysis patients and whether decreases in incidence have persisted in recent years is important to guide prevention efforts. We used population-based surveillance data to evaluate recent changes in invasive MRSA infection incidence, estimate the national burden of disease among dialysis patients, and characterize invasive MRSA infections among this patient group.
METHODS
Surveillance System
We analyzed data reported to the CDC's Active Bacterial Core surveillance (ABCs) system for invasive MRSA infections during 2005–2011. ABCs MRSA methodology has been previously described [8]. In brief, ABCs MRSA is an active, population-based surveillance covering a population of 19 million persons from selected counties across 9 US states (California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New York, Oregon, and Tennessee). Surveillance personnel at participating sites investigate all laboratory reports of MRSA isolated from normally sterile sites. For each invasive MRSA case identified, a standard case report is completed including demographic and clinical data.
Case Definition
ABCs defines a case as MRSA isolated from a normally sterile body site in a surveillance area resident. In this analysis, only ABCs cases that occurred in persons who received dialysis within the year before the positive MRSA culture were included. Cases were classified as hospital-onset (HO) if the culture was obtained >3 calendar days after a hospital admission (admission day is considered day 1), or healthcare-associated community-onset (HACO) if the culture was obtained as an outpatient or ≤3 calendar days after a hospital admission [2, 8]. Because all cases had healthcare exposure (ie, dialysis) prior to culture, no community-associated cases were included. Cases were categorized as bloodstream infection (BSI) if MRSA was isolated from blood. One patient may represent >1 case in the system if he or she had ≥2 positive cultures >30 days apart.
Invasive MRSA Incidence Calculations
Invasive MRSA infection incidence was calculated from the 27 counties that reported data continuously for every month during 2005–2011 among all 9 ABCs sites. The numerator was the number of cases reported in a calendar year. The denominator was the point prevalence of dialysis patients on December 31 of the previous year in the corresponding surveillance counties and was obtained from the US Renal Data System (USRDS), which included patients enrolled in the Medicare End-Stage Renal Disease (ESRD) program. ESRD covers 83% of the US chronic dialysis population [9]. Annual incidences of HO and HACO MRSA were calculated using the same denominators.
Trend Analysis
Changes in incidence over time (2005–2011) were assessed by fitting a Poisson regression model across surveillance sites, treating MRSA case counts as the outcome variable and time (ie, year) as the independent variable. As both the 2005 (ie, baseline) invasive MRSA incidence and the annual change in incidence varied by area, a mixed model was fitted to include the intercept (ie, 2005 incidence) and the slope for year (ie, temporal change) at each area as random effects. Models were fitted for all invasive MRSA infections and for MRSA BSI only. As patients of older age, male sex, and black race have been shown to have higher infection incidence [8], the models were controlled for age (≥65 years, 50–64 years, or ≤49 years), race (black or nonblack), and sex. The modeled annual change and its 95% confidence interval (CI) were calculated in each model. P values <.05 were considered statistically significant. Analyses were performed using SAS software version 9.3 (SAS Institute Inc, Cary, North Carolina).
Estimation of National Burden of Invasive MRSA Infections Among Dialysis Population
To estimate the national burden and CI of invasive MRSA infections among all dialysis patients in the United States in 2011, we used the number of infections in 2011 from ABCs data and 2010 US dialysis population from USRDS and applied an approximation method based on γ distribution [10]. The estimate and 95% CI were adjusted for sex, race, and age corresponding to the overall US dialysis population.
Descriptive Epidemiology of Invasive MRSA Infections Among Dialysis Patients
To describe characteristics of cases, demographic and clinical data from ABCs were analyzed. The data included age, sex, race, clinical syndromes associated with the MRSA culture, and cases' underlying conditions. The Charlson comorbidity index [11–15] was calculated with and without age. The χ2 test was used to compare proportions between groups; P < .05 was considered significant.
Starting in 2009, additional variables were available to indicate whether patients received chronic dialysis (as opposed to any dialysis) prior to MRSA-positive culture, the type of chronic dialysis (ie, hemodialysis or peritoneal dialysis), and vascular access type used for hemodialysis (ie, CVC or AV graft/fistula). These variables permitted better identification of risk groups and potential risk factors associated with invasive MRSA. The addition of these new variables allowed us to focus our descriptive analysis on cases in patients undergoing chronic dialysis who were cultured between January 2009 and December 2011; these cases represented 93.4% of all dialysis cases during this time frame.
MRSA Strain Typing
As part of surveillance, a convenience sample of MRSA isolates was sent to the CDC for further testing, including pulsed field gel electrophoresis (PFGE). Submitted isolates were characterized either by PFGE or by inference of PFGE patterns based on staphylococcal cassette chromosome, presence of Panton-Valentine leukocidin, and antimicrobial susceptibility results [16].
Human Subjects Considerations
The ABCs surveillance protocol underwent ethical review at the CDC and was determined to be nonresearch. It was also reviewed at each participating surveillance area and approved by local institutional review boards.
RESULTS
Incidence of Invasive MRSA Infections, 2005–2011
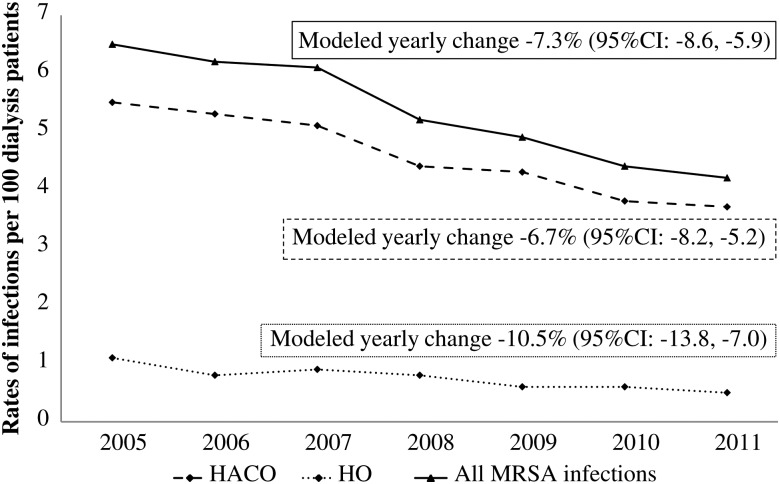
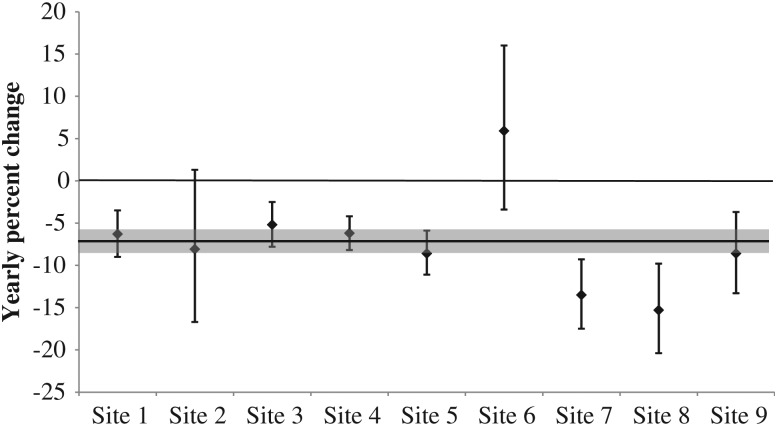
From 2005 to 2011, 7489 cases of MRSA were identified in ABCs; 85.7% of them were HACO and 93.2% were BSI. Overall crude incidence of invasive MRSA infections per 100 dialysis patients decreased from 6.5 in 2005 to 4.2 in 2011 (Figure 1). The modeled change, controlling for age, sex, and race, decreased 7.3% per year (95% CI, 5.9%–8.6%; Figure 1). This translates to an overall decrease in invasive MRSA infection of 51.1% over the 7-year period. The decrease in incidence during 2005–2011 was also observed in MRSA BSIs (modeled yearly decrease of 7.9% [95% CI, 6.5%–9.3%], and overall decrease of 55.3%). Statistically significant annual decreases in invasive MRSA incidence were noted in 7 of 9 surveillance areas (Figure 2).
Figure 1.
Rates and modeled yearly changes of invasive methicillin-resistant Staphylococcus aureus infections per 100 dialysis patients by epidemiologic class, Active Bacterial Core surveillance system, 2005–2011. Abbreviations: CI, confidence interval; HACO, healthcare-associated community-onset; HO, hospital-onset; MRSA, methicillin-resistant Staphylococcus aureus.
Figure 2.
Yearly change in invasive MRSA incidence in each surveillance area, 9 Active Bacterial Core surveillance system areas, 2005–2011. Each bar denotes yearly percent change and its 95% confidence interval for each site. The gray bar denotes the change across all sites.
Incidence among HO cases decreased 10.5% (95% CI, 7.0%–13.8%) and among HACO cases 6.7% (95% CI, 5.2%–8.2%) per year (Figure 1). The proportion of all cases that were HACO increased between 2005 and 2011 (82.8% vs 88.0%, P = .002).
National Estimate of Invasive MRSA Infections Among Dialysis Population, 2011
Projecting our 2011 data to the national level, an estimated 15 169 invasive MRSA infections and 12 823 MRSA BSIs occurred in the United States among dialysis patients, and the estimated 2011 national incidence was 3.7 invasive MRSA infections per 100 dialysis patients (95% CI, 2.8–4.7).
MRSA Strains
Between 2005 and 2011, strain data were available on 1397 MRSA isolates (18.7% of cases). USA100 accounted for 820 (58.7%) and USA300 accounted for 377 (27.0%) isolates. The remaining isolates belonged to other strain types. USA300 was identified in 28.3% of HACO and 19.6% of HO cases. The proportion of USA300 isolates by year was 15.8% (2005), 25.2% (2006), 22.5% (2007), 27.9% (2008), 33.5% (2009), 33.3% (2010), and 31.1% (2011).
Case Characteristics, 2009–2011
From January 2009 through December 2011, 2940 MRSA cases in patients undergoing chronic dialysis were reported to ABCs. These cases occurred in 2158 unique patients, of whom 496 (23.0%) had >1 episode during 2009–2011. Among the cases, 2608 (88.7%) were categorized as HACO, and 332 (11.3%) as HO (Table 1). Hemodialysis was the major dialysis type (n = 2854 [97.3%]) and most cases were BSIs (n = 2699 [91.8%]). Additional demographic characteristics, underlying conditions, and infection types of cases are described in Table 1.
Table 1.
Characteristics of Chronic Dialysis Cases With Invasive Methicillin-Resistant Staphylococcus aureus Infections, Active Bacterial Core Surveillance System, 2009–2011
| Characteristic | HACO (n = 2608) | HO (n = 332) | All Cases (n = 2940) | P Value |
|---|---|---|---|---|
| Male sex | 1435 (55.0) | 184 (55.4) | 1619 (55.1) | .82 |
| Age | ||||
| Median | 60 | 60.5 | 60 | .97 |
| Range | 7–97 | 0–95 | 0–97 | |
| ≥65 y | 37.5% | 39.8% | 37.8% | .42 |
| Race | ||||
| White | 774 (29.7) | 121 (36.5) | 895 (30.5) | .01 |
| Black | 1502 (57.6) | 180 (54.2) | 1682 (57.2) | .24 |
| Other(s) | 332 (12.7) | 31 (9.3) | 363 (12.3) | … |
| Dialysis type | ||||
| Hemodialysis | 2540 (97.6) | 314 (94.9) | 2854 (97.3) | .004 |
| Peritoneal | 63 (2.4) | 17 (5.1) | 80 (2.7) | … |
| Unknown/missing | 5 | 1 | 6 | |
| Vascular access among hemodialysis (%) | ||||
| CVC | 1424 (60.6) | 171 (58.8) | 1595 (60.4) | .55 |
| AV graft or fistula | 927 (39.4) | 120 (41.2) | 1047 (39.6) | … |
| Unknown/missing | 189 | 23 | 212 | |
| Types of infectiona | ||||
| Bloodstream infection | 2446 (93.8) | 253 (76.2) | 2699 (91.8) | <.0001 |
| Catheter site infections | 491 (18.8) | 30 (9.0) | 521 (17.7) | <.0001 |
| AV F/G infections | 256 (9.8) | 26 (7.8) | 282 (9.6) | .24 |
| Septic shock | 238 (9.1) | 37 (11.1) | 275 (9.4) | .23 |
| Pneumonia | 215 (8.2) | 38 (11.5) | 253 (8.6) | .05 |
| Endocarditis | 201 (7.7) | 28 (8.4) | 229 (7.8) | .64 |
| Osteomyelitis | 162 (6.2) | 35 (10.5) | 197 (6.7) | .003 |
| Underlying conditionsa | ||||
| Diabetes | 1610 (61.7) | 212 (63.9) | 1822 (62.0) | .45 |
| Heart failure | 837 (32.1) | 130 (39.2) | 967 (32.9) | .01 |
| ASCVD | 592 (22.7) | 82 (24.7) | 674 (22.9) | .41 |
| CVA/stroke | 437 (16.8) | 50 (15.1) | 487 (16.6) | .43 |
| COPD | 239 (9.2) | 38 (11.5) | 277 (9.4) | .18 |
| Chronic liver disease | 95 (3.6) | 25 (7.5) | 120 (4.1) | .0007 |
| Charlson score, mean ± SD | 4.0 ± 1.7 | 4.3 ± 1.7 | 4.0 ± 1.7 | .02 |
| Charlson score with age, mean ± SD | 5.6 ± 2.3 | 5.9 ± 2.3 | 5.6 ± 2.3 | .01 |
Data are presented as No. (%) unless otherwise specified. P values represent the comparison between HACO and HO cases. The χ2 test was used to compare proportions and Student t test to compare continuous variables. P < .05 was considered statistically significant
Abbreviations: AV, arteriovenous; ASCVD, arteriosclerotic cardiovascular disease (ie, coronary artery disease); AV F/G, arteriovenous graft or fistula; CVA, cerebrovascular accident; CVC, central venous catheter; COPD, chronic obstructive pulmonary disease; HACO, healthcare-associated community-acquired; HO, hospital-onset; SD, standard deviation.
a Infection types were classified based on chart review. Infection types and underlying conditions were not mutually exclusive.
Vascular Access Types
Among 2642 hemodialysis cases with available vascular access data in 2009–2011, 1595 (60.4%) infections occurred in patients who had a CVC, and 1047 (39.6%) were in patients who had an AV graft or fistula (Table 1). Among the 2489 hemodialysis cases with a BSI and available vascular access data, 1543 (62.0%) patients had a CVC and 946 (38.0%) had an AV graft or fistula.
Previous Healthcare Exposures and Outcomes of Cases
Of 2940 cases, 1311 (44.6%) had a history of previous MRSA infection or colonization. Cases' other healthcare exposures are described in Table 2. Among HACO cases, 1817 (69.7%) had hospitalization within a year prior to positive culture.
Table 2.
Previous Healthcare Exposure Among Chronic Dialysis Cases With Invasive Methicillin-Resistant Staphylococcus aureus Infection, Active Bacterial Core Surveillance System, 2009–2011
| Exposure | HACO (n = 2608) | HO (n = 332) | All Cases (n = 2940) | P Value |
|---|---|---|---|---|
| Had previous MRSA infections or colonization | 1167 (44.8) | 144 (43.4) | 1311 (44.6) | .63 |
| Had surgery within a year prior | 771 (29.6) | 140 (42.2) | 911 (31.0) | <.0001 |
| In an LTCF within a year prior | 763 (29.3) | 94 (28.3) | 857 (29.2) | .72 |
| Had hospitalization within a year prior | 1817 (69.7) | 241 (72.6) | 2058 (70.0) | .27 |
| Duration from previous hospitalization to this positive culture ≤90 d | 1062 (60.6) | 138 (60.5) | 1200 (60.6) | .98 |
| Missing/unknown date | 65 | 13 | 78 | |
| Had at least 1 healthcare exposurea | 2067 (79.3) | 281 (84.6) | 2348 (79.9) | .02 |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: HACO, healthcare-associated community-acquired; HO, hospital-onset; LTCF, long-term care facility; MRSA, methicillin-resistant Staphylococcus aureus.
a Either previous hospitalization or surgery or LTCF within a year prior to culture (in addition to dialysis exposure). P values represent the comparison between HACO and HO cases. The χ2 test was used to compare proportions and Student t test to compare continuous variables. P < .05 was considered statistically significant.
Outcomes of cases and characteristics of recurrent infections are described in Table 3. Although case fatality was higher among HO cases than HACO cases, mortality was similar among patients with CVCs and those with AV grafts or fistulae (12.0% vs 11.8%; P = .7).
Table 3.
Outcomes of Invasive Methicillin-Resistant Staphylococcus aureus Infections Among Chronic Dialysis Patients, Active Bacterial Core Surveillance System, 2009–2011
| Outcome | HACO (n = 2608) | HO (n = 332) | All Cases (n = 2940) | P Value |
|---|---|---|---|---|
| Died | 263 (10.1) | 90 (27.1) | 353 (12.0) | <.0001 |
| Died within 7 d of culture | 120 (4.6) | 38 (11.5) | 158 (5.4) | <.0001 |
| Survival time since culture, d, median (range) | 7 (3–16) | 9.5 (3–30) | 7 (3–21) | .004 |
| Hospitalization | 2324 (89.6) | 332 (100) | 2656 (90.8) | … |
| Hospital stay, d, median (range) | 9 (6–15) | 25 (13–55) | 10 (6–17) | <.0001 |
| Had recurrent infection | 983 (37.7) | 111 (33.4) | 1094 (37.2) | .09 |
| No. of patients | 1887 | 271 | 2158 | … |
| Had 1 MRSA infection | 1443 (76.5) | 219 (80.8) | 1662 (77.0) | … |
| Had 1 recurrent infectiona | 297 (15.7) | 43 (15.9) | 340 (15.8) | … |
| Had >1 recurrent infection | 147 (7.8) | 9 (3.3) | 156 (7.2) | … |
| Time between 1st and 2nd infections, d, median (IQR) | 92 (56–167) | 87 (37–142) | 91 (55–166) | … |
| Time between infections, d, median (IQR) | 89 (55–167) | 79 (40–142) | 88 (55–165) | … |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: HACO, healthcare-associated community-acquired; HO, hospital-onset; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus.
a HACO/HO classification based on the last infection. P values represent the comparison between HACO and HO cases. The χ2 test was used to compare proportions and Student t test to compare continuous variables. P < .05 was considered statistically significant.
DISCUSSION
In this study, the incidence of invasive MRSA infections among dialysis patients decreased from 2005 to 2011, consistent with the prior findings in all healthcare-associated invasive MRSA infections, including among dialysis patients from 2005 to 2008 [1]. The majority of infections (93.2%) were BSIs. The incidence also decreased among HACO and HO infections, indicating that reductions in both contributed to the decreasing rates. Because yearly decreases were more pronounced in HO infections, we observed a proportional increase in HACO infections. The majority of HACO cases (69.7%) had recent hospitalizations, suggesting that efforts in hospitals to prevent BSI and MRSA transmission could have led to decreases in not only in HO, but also HACO infections. The potential relationship between hospital multidrug-resistant organism transmission prevention and decreasing colonizations in outpatient dialysis settings has been described previously [17].
There are several possible explanations for the observed decrease in HACO and HO infection rates. Better adherence to recommended MRSA prevention measures in hospitals have led to reduced MRSA infections [18]. These efforts have included prioritizing infection control through administrative, financial, and human resource support and more consistent adherence to good hand hygiene and use of personal protective equipment; detecting cases early and initiating appropriate treatment, isolation, and contact precautions; and performing MRSA surveillance and feedback [19, 20]. Another potential contributing factor to the rate decrease is the implementation of BSI prevention efforts in hospitals, which have been shown to significantly reduce central line–associated BSI [21–24]. Those efforts have included reductions in catheter use, better catheter insertion and maintenance practices through implementation of “catheter care bundles” comprised of multiple evidence-based interventions, use of standard tools to record adherence to catheter care practice, and staff education on BSI prevention. Finally, CVC use in hemodialysis outpatients has decreased whereas AV fistula use has increased [25]. AV fistulas and grafts are associated with lower risk for infection compared to CVCs [26–29] and are the preferred vascular access types over CVCs [4, 30]. Data obtained from the Fistula First Breakthrough Initiative on CVC use from 2005 to 2011 among the 9 ABCs reporting areas showed a consistent reduction in the proportion of hemodialysis patients with a CVC from 27.8% in 2005 to 18.8% in 2011, with a dramatic reduction after 2007 (Fistula First Prevalent US Data 2005–2011, unpublished data), coincident with the observed reduction in MRSA incidence (Figure 1). Therefore, this may have also contributed to the observed decrease.
Although the incidence of MRSA infections among dialysis patients is decreasing, the burden of these infections, including MRSA BSI, continues to be high, as demonstrated by the 15 169 invasive MRSA infections estimated to have occurred in US chronic dialysis patients in 2011. Moreover, this study did not evaluate the impact of methicillin-sensitive S. aureus, which may account for two-thirds of S. aureus infections among dialysis patients and may also cause severe morbidity, mortality, and high cost [5, 6].
With the availability of new variables in the system during 2009–2011, we were able to assess the vascular access type among cases. Of hemodialysis patients with invasive MRSA infections during 2009–2011, the majority (60.4%) had a CVC instead of other access types. This is in contrast to the 18.8% of all chronic hemodialysis patients in the surveillance areas who had a CVC in 2011 (Fistula First Prevalent US Data 2005–2011, unpublished data). Evidence has shown that patients with a CVC are at high risk for BSI and other adverse outcomes [26, 27, 31–35], and conversion from a CVC to an AV fistula can reduce risk of death [31]. Although CVC use among the chronic hemodialysis population has been decreasing, CVCs are still commonly used to initiate hemodialysis in the United States [27, 36–38]. The disproportionate use of CVCs among patients with MRSA infection may point to prevention opportunities to improve CVC maintenance and reduce CVC use in favor of lower-risk vascular access types, while also pursuing strategies to prevent infections in patients with fistulae and grafts.
In addition to dialysis, the surveillance population had other prior healthcare exposures (ie, hospitalization, surgery, or long-term care facility stay). Many cases had prior MRSA infection or colonization, and although we were unable to ascertain whether patients became infected or colonized during their previous hospitalizations, interrupting MRSA transmission in both outpatient and inpatient healthcare settings may be important for preventing invasive infections.
The strengths of the ABCs data include population-based rates that represent large and diverse geographic areas throughout the United States, and standardized surveillance methods.
The analysis was subject to several limitations. Metropolitan areas covered by ABCs may not be nationally representative. However, when calculating national estimates, we were able to adjust for regional differences in sex, age, and race to produce estimates that more accurately represent the entire US dialysis population. Although the proportion of MRSA infections caused by USA300 increased during the study period, we did not have strain types for all cases; instead, only a convenient sample of the isolates was available for PFGE testing.
Although surveillance sites perform annual audits of participating hospital laboratories to ensure completeness of reporting, increased awareness of invasive MRSA infections among dialysis patients diagnosed in outpatient settings led to a more thorough audit, including nonhospital laboratories that served outpatient dialysis centers in the surveillance areas in 2011. Through this assessment, we determined that as many as 9.1% of dialysis cases were missed because they were identified by laboratories outside of the surveillance area. However, the inclusion of those missed cases in the 2011 data did not change the decreasing trend in MRSA infections (data not shown). Moreover, the denominators used represent only patients in the Medicare ESRD Program, which covers 83% of US dialysis patients. Finally, ABCs data on vascular access was available for only 3 years (2009–2011), which limited our ability to assess if observed decreases in invasive MRSA infection incidence were due in part to reductions of MRSA infection among patients with CVCs.
CONCLUSIONS
Great progress has been made in reducing invasive MRSA infection among dialysis patients. However, the infection burden remains substantial among this patient population, especially those with CVCs. Therefore, prevention activities should focus on improving CVC maintenance and reducing CVC use, while also improving care of other vascular access types. Adherence to current infection prevention guidelines should be encouraged and reinforced to help sustain the decreasing trend of invasive MRSA infections.
Notes
Acknowledgments. The authors acknowledge the following persons for their contributions with data collection and input on surveillance methods and validation: Emerging Infections Program/ABCs MRSA Surveillance Personnel: Art Reingold, MD, Lauren Pasutti, MPH, California Emerging Infections Program; Ken Gershman, MD, Deborah Aragon, MPH, Colorado Emerging Infections Program; Heather Altier, MPH, Carmen Marquez, MPH, Connecticut Emerging Infections Program; Monica Farley, MD, Janine Ladson, MPH, Georgia Emerging Infections Program; Joanne Benton, MPH, Rosemary Hollick, MPH, Kim Holmes, MPH, Elisabeth Vaeth, MPH, Maryland Emerging Infections Program; Lindsey Lesher, MPH, Minnesota Emerging Infections Program; Anita Gellert, RN, New York Emerging Infections Program; Ann Thomas, MD, John Townes, MD, Robert Vega, MPH, Oregon Emerging Infections Program; Brenda Barnes, RN, Terri McMinn, RN, Tennessee Emerging Infections Program. The authors also wish to acknowledge the following CDC contributors: Scott Fridkin, MD, for his critical review of the manuscript, Sandra Bulens, MPH, for her contribution with data collection and cleaning, Brandi Limbago, PhD, and Valerie Albrecht, MPH, for processing the MRSA isolates in this surveillance. The authors thank William Rullo and Shannon Wright from ESRD Network 2 and Edwin Huff, PhD, from the Centers for Medicare and Medicaid Services for providing the Fistula First data. Dr Duc Nguyen would like to thank the Emory AIDS International Training and Research Program (NIH/FIC D43 TW01042).
Financial support. This work was supported by the Center for Disease Control's Emerging Infections Program Cooperative Agreement.
Disclaimer. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the US CDC. Some of the data reported herein have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of the US government. Some materials were prepared by the ESRD division of IPRO, Network Information Technology Support, under contract with the Centers for Medicare and Medicaid Services (CMS), an agency of the US Department of Health and Human Services. The contents presented do not necessarily reflect CMS policy.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kallen AJ, Mu Y, Bulens S, et al. Health care-associated invasive MRSA infections, 2005–2008. JAMA. 2010;304:641–8. doi: 10.1001/jama.2010.1115. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Active Bacterial Core surveillance report, Emerging Infections Program Network, methicillin-resistant Staphylococcus aureus. 2010. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/mrsa10.html. Accessed 14 February 2012.
- 3.Centers for Disease Control and Prevention. Invasive methicillin-resistant Staphylococcus aureus infections among dialysis patients—United States, 2005. MMWR Morb Mortal Wkly Rep. 2007;56:197–9. [PubMed] [Google Scholar]
- 4.Patel PR, Kallen AJ, Arduino MJ. Epidemiology, surveillance, and prevention of bloodstream infections in hemodialysis patients. Am J Kidney Dis. 2010;56:566–77. doi: 10.1053/j.ajkd.2010.02.352. [DOI] [PubMed] [Google Scholar]
- 5.Engemann JJ, Friedman J, Reed SD, et al. Clinical outcomes and costs due to Staphylococcus aureus bacteremia among patients receiving long-term hemodialysis. Infect Control Hosp Epidemiol. 2005;26:534–9. doi: 10.1086/502580. [DOI] [PubMed] [Google Scholar]
- 6.Inrig JK, Reed SD, Szczech LA, et al. Relationship between clinical outcomes and vascular access type among hemodialysis patients with Staphylococcus aureus bacteremia. Clin J Am Soc Nephrol. 2006;1:518–24. doi: 10.2215/CJN.01301005. [DOI] [PubMed] [Google Scholar]
- 7.Nissenson AR, Dylan ML, Griffiths RI, et al. Clinical and economic outcomes of Staphylococcus aureus septicemia in ESRD patients receiving hemodialysis. Am J Kidney Dis. 2005;46:301–8. doi: 10.1053/j.ajkd.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 9.US Renal Data System. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. USRDS 2011 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. [Google Scholar]
- 10.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16:791–801. doi: 10.1002/(sici)1097-0258(19970415)16:7<791::aid-sim500>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med. 2000;108:609–13. doi: 10.1016/s0002-9343(00)00371-5. [DOI] [PubMed] [Google Scholar]
- 14.Fried L, Bernardini J, Piraino B. Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis. 2001;37:337–42. doi: 10.1053/ajkd.2001.21300. [DOI] [PubMed] [Google Scholar]
- 15.Rattanasompattikul M, Feroze U, Molnar MZ, et al. Charlson comorbidity score is a strong predictor of mortality in hemodialysis patients. Int Urol Nephrol. 2012;44:1813–23. doi: 10.1007/s11255-011-0085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Use of an inferred PFGE algorithm, Emerging Infections Program/Active Bacterial Core (ABCs) surveillance invasive MRSA project. Available at: http://www.cdc.gov/HAI/settings/lab/inferred-PFGE-algorithm.html. Accessed 15 July 2013.
- 17.D'Agata EM, Horn MA, Webb GF. The impact of persistent gastrointestinal colonization on the transmission dynamics of vancomycin-resistant enterococci. J Infect Dis. 2002;185:766–73. doi: 10.1086/339293. [DOI] [PubMed] [Google Scholar]
- 18.Siegel JD, Rhinehart E, Jackson M, Chiarello L The Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in healthcare settings. 2006. Available at: http://www.cdc.gov/hicpac/mdro/mdro_0.html. Accessed 10 May 2013. [DOI] [PubMed]
- 19.Harrington G, Watson K, Bailey M, et al. Reduction in hospitalwide incidence of infection or colonization with methicillin-resistant Staphylococcus aureus with use of antimicrobial hand-hygiene gel and statistical process control charts. Infect Control Hosp Epidemiol. 2007;28:837–44. doi: 10.1086/518844. [DOI] [PubMed] [Google Scholar]
- 20.Huang SS, Yokoe DS, Hinrichsen VL, et al. Impact of routine intensive care unit surveillance cultures and resultant barrier precautions on hospital-wide methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2006;43:971–8. doi: 10.1086/507636. [DOI] [PubMed] [Google Scholar]
- 21.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Reduction in central line-associated bloodstream infections among patients in intensive care units—Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep. 2005;54:1013–6. [PubMed] [Google Scholar]
- 23.Furuya EY, Dick A, Perencevich EN, Pogorzelska M, Goldmann D, Stone PW. Central line bundle implementation in US intensive care units and impact on bloodstream infections. PLoS One. 2011;6:e15452. doi: 10.1371/journal.pone.0015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerin K, Wagner J, Rains K, Bessesen M. Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control. 2010;38:430–3. doi: 10.1016/j.ajic.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Fistula First. Fistula First Prevalent US Data. Available at: http://www.fistulafirst.org/LinkClick.aspx?fileticket=vdZB_GadCFU%3d&tabid=204. Accessed 5 August 2013.
- 26.Hoen B, Paul-Dauphin A, Hestin D, Kessler M. EPIBACDIAL: a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol. 1998;9:869–76. doi: 10.1681/ASN.V95869. [DOI] [PubMed] [Google Scholar]
- 27.Foley RN, Chen SC, Collins AJ. Hemodialysis access at initiation in the United States, 2005 to 2007: still “catheter first. Hemodial Int. 2009;13:533–42. doi: 10.1111/j.1542-4758.2009.00396.x. [DOI] [PubMed] [Google Scholar]
- 28.Lafrance JP, Rahme E, Lelorier J, Iqbal S. Vascular access-related infections: definitions, incidence rates, and risk factors. Am J Kidney Dis. 2008;52:982–93. doi: 10.1053/j.ajkd.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Klevens RM, Edwards JR, Andrus ML, et al. Dialysis surveillance report: National Healthcare Safety Network (NHSN)—data summary for 2006. Semin Dial. 2008;21:24–8. doi: 10.1111/j.1525-139X.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- 30.National Kidney Foundation–Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines and clinical practice recommendations 2006 updates. Available at: http://www.kidney.org/professionals/kdoqi/pdf/12-50-0210_JAG_DCP_Guidelines-HD_Oct06_SectionA_ofC.pdf. Accessed 5 August 2013.
- 31.Lacson E, Jr, Wang W, Lazarus JM, Hakim RM. Change in vascular access and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2009;54:912–21. doi: 10.1053/j.ajkd.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Pisoni RL, Arrington CJ, Albert JM, et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis. 2009;53:475–91. doi: 10.1053/j.ajkd.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 33.Taylor G, Gravel D, Johnston L, et al. Prospective surveillance for primary bloodstream infections occurring in Canadian hemodialysis units. Infect Control Hosp Epidemiol. 2002;23:716–20. doi: 10.1086/501999. [DOI] [PubMed] [Google Scholar]
- 34.Taylor G, Gravel D, Johnston L, et al. Incidence of bloodstream infection in multicenter inception cohorts of hemodialysis patients. Am J Infect Control. 2004;32:155–60. doi: 10.1016/j.ajic.2003.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Ishani A, Collins AJ, Herzog CA, Foley RN. Septicemia, access and cardiovascular disease in dialysis patients: the USRDS Wave 2 study. Kidney Int. 2005;68:311–8. doi: 10.1111/j.1523-1755.2005.00414.x. [DOI] [PubMed] [Google Scholar]
- 36.Pisoni RL, Young EW, Dykstra DM, et al. Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int. 2002;61:305–16. doi: 10.1046/j.1523-1755.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 37.Ng LJ, Chen F, Pisoni RL, et al. Hospitalization risks related to vascular access type among incident US hemodialysis patients. Nephrol Dial Transplant. 2011;26:3659–66. doi: 10.1093/ndt/gfr063. [DOI] [PubMed] [Google Scholar]
- 38.Ethier J, Mendelssohn DC, Elder SJ, et al. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2008;23:3219–26. doi: 10.1093/ndt/gfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]