In a trial substudy of 1000 IU vitamin D3 supplementation, 759 participants completed daily health diaries for up to 17 months. Supplementation provided no significant reduction in upper respiratory tract infections, colds, or influenza-like illness during winter or overall.
Keywords: upper respiratory tract infection, vitamin D, randomized controlled trial
Abstract
Background. Randomized controlled trials testing the association between vitamin D status and upper respiratory tract infection (URTI) have given mixed results. During a multicenter, randomized controlled trial of colorectal adenoma chemoprevention, we tested whether 1000 IU/day vitamin D3 supplementation reduced winter episodes and duration of URTI and its composite syndromes, influenza-like illness (ILI; fever and ≥2 of sore throat, cough, muscle ache, or headache) and colds (no fever, and ≥2 of runny nose, nasal congestion, sneezing, sore throat, cough, swollen or tender neck glands).
Methods. The 2259 trial participants were aged 45–75, in good health, had a history of colorectal adenoma, and had a serum 25-hydroxyvitamin D level ≥12 ng/mL. They were randomized to vitamin D3 (1000 IU/day), calcium (1200 mg/day), both, or placebo. Of these, 759 participants completed daily symptom diaries. Secondary data included semiannual surveys of all participants.
Results. Among those who completed symptom diaries, supplementation did not significantly reduce winter episodes of URTI (rate ratio [RR], 0.93; 95% confidence interval [CI], .79–1.09) including colds (RR, 0.93; 95% CI, .78–1.10) or ILI (RR, 0.95; 95% CI, .62–1.46), nor did it reduce winter days of illness (RR, 1.13; 95% CI, .90–1.43). There was no significant benefit according to adherence, influenza vaccination, body mass index, or baseline vitamin D status. Semiannual surveys of all participants (N = 2228) identified no benefit of supplementation on ILI (odds ratio [OR], 1.14; 95% CI, .84–1.54) or colds (OR, 1.03; 95% CI, .87–1.23).
Conclusions. Supplementation with 1000 IU/day vitamin D3 did not significantly reduce the incidence or duration of URTI in adults with a baseline serum 25-hydroxyvitamin D level ≥12 ng/mL.
Upper respiratory tract infections (URTIs) are a frequent cause of illness, with an estimated 500 million episodes annually in the United States, and an economic burden of $40 billion [1]. Most colds are mild, but influenza and influenza-like illness (ILI), characterized by high fever, headache, and myalgia, account for approximately 9% of URTIs and affect one-fifth of the US population annually, causing 226 000 hospitalizations and 36 000 deaths [2].
In 1926, Smiley hypothesized that the seasonality of URTI was due to fluctuations in the “vitamine” found in dairy foods and produced in humans on sunlight exposure [3]. Others have since reiterated variations of this hypothesis [4, 5]. Although observational studies show an inverse association between vitamin D status and URTI risk [6–8], the few randomized controlled trials (RCTs) of vitamin D supplementation and respiratory infection have yielded mixed results [9–20].
Evidence from animal models and human observational studies suggested that vitamin D may inhibit carcinogenesis [21–22]. We therefore conducted a large RCT of vitamin D3 and calcium chemoprevention of colorectal adenoma. During the trial, we investigated whether vitamin D3 supplementation (1000 IU/day) would reduce the number of episodes and duration of URTI in winter and throughout the year, and the number of episodes and duration of winter ILI and of colds.
METHODS
We conducted a randomized, double-blind, placebo-controlled multicenter trial of supplementation with vitamin D3 (1000 IU/day) and/or calcium carbonate (1200 mg elemental calcium/day) for the prevention of large-bowel adenomas.
Participants recruited from May 2004 through November 2008 at 11 clinical centers had at least 1 colorectal adenoma recently removed and none remaining after complete colonoscopy. Eligible participants were 45–75 years old, in good general health with no contraindications to study treatment, and had no familial colorectal cancer syndromes or history of serious intestinal disease. Individuals with serum vitamin D levels <12 ng/mL were excluded. All participants provided written informed consent; the research was approved by institutional review boards at each clinical center.
A detailed health questionnaire was administered at enrollment. Participants agreed to avoid taking vitamin D supplements outside the trial, although after April 2008, recognizing the increasing use of vitamin D supplementation in the community and its potential impact on study retention, the study procedures were revised to allow personal supplementation of up to 1000 IU/day vitamin D while continuing randomized treatment. Enrollment was followed by a placebo run-in period of 56–84 days to identify and exclude before randomization participants who were unlikely to be adherent, including those who had taken <80% of study pills. Study treatment was scheduled to continue until the surveillance colonoscopy either 3 or 5 years after the qualifying examination, according to recommendations by each participant's gastroenterologist.
Eligibility for randomization after the run-in period was confirmed by blinded coordinators, and a web-based, random number generator assigned treatment within blocks, stratified by study center, sex, and colonoscopy interval (3 or 5 years) in a modified 2 × 2 factorial design to identical-looking pills containing vitamin D3, calcium carbonate, both, or placebo (“4-arm study”). Women who declined to forego calcium supplementation were randomized to calcium alone or calcium plus vitamin D (“2-arm study”). Participants and investigators were blinded; the computer programming staff, 1 pharmacy technician, statistician, and statistical analyst were unblinded.
After randomization, all participants were telephoned every 6 months to assess adherence to study agents, illness, and use of medications and supplements. In September 2007, we added a question to assess a secondary endpoint in all participants: “Have you had a cold in the last 2 weeks? (By a cold, we mean at least 2 of the following: runny nose, congestion, sneezing, sore throat, dry cough, or swollen or tender neck glands, lasting for at least 2 days, and which was not caused by allergies).” In December 2009, in response to the H1N1 pandemic of April 2009–August 2010, we added a question to assess an additional secondary endpoint: “Since your last study questionnaire, have you had the flu or an influenza-like illness (fever plus 2 or more of the following: sore throat, cough, muscle ache, headache)?” Responses through September 2012 were analyzed.
Serum was tested for 25-hydroxyvitamin D [25(OH)D] at enrollment, 1 year after randomization, and 3 years after randomization for participants with a 5-year colonoscopy surveillance cycle. We measured 25(OH)D using the IDS (Fountain Hills, Arizona) liquid phase radioimmunoassay kit.
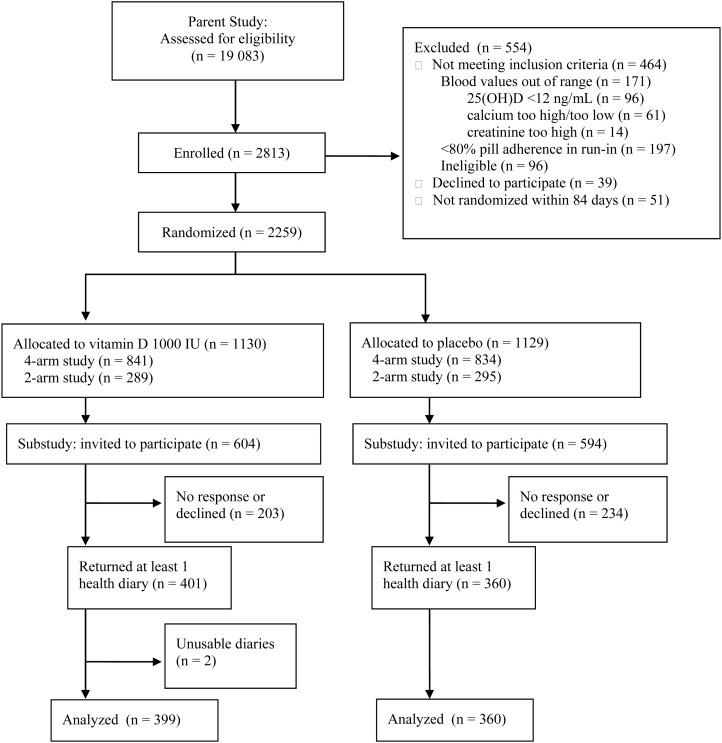
Beginning in November 2009, participants with at least 6 months remaining on study were invited to participate in a “Colds and Flu” (CAF) substudy (Figure 1) by completing daily health diaries regarding fever, headache, muscle aches, chills, cough, runny nose, and allergies. Information was also collected each month on influenza and pneumococcal vaccines, antibiotics and antiviral medications, and medical care sought for URTI symptoms. Initially, all diaries were completed on paper while a web-based application was being programmed; the web-based option was also offered in June 2010. In summer 2010, because we had not reached our target enrollment of 800 participants, we asked existing participants to continue completing health diaries for a second winter season through March 2011 or until 2 months after the end of treatment. Participants were compensated $5 for reviewing informational materials and $5 for each completed diary.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram. Abbreviation: 25(OH)D, 25-hydroxyvitamin D.
Definitions and Assumptions
URTI was defined as either a cold or ILI. ILI was any episode with at least 1 day of fever (≥100°F [37.8°C] or participants reported feeling hot) and ≥2 of sore throat, cough, muscle ache, or headache [23]. A cold required absence of ILI and ≥2 of the following on a single day: runny nose, nasal congestion, sneezing, sore throat, cough, and swollen or tender neck glands.
Three seasons were defined: winter 1 (1 November 2009–31 March 2010), nonwinter (1 April 2010–31 October 2010), and winter 2 (1 November 2010–31 March 2011). We defined the start of an episode as the first day the syndrome case definition was fulfilled, provided the preceding 6 days were syndrome-free or not recorded [24]; if a day with symptoms occurred within 6 days of a previous episode, it was considered to be part of the previous episode. Episodes were attributed to the season in which they began. Days of moderate illness were those fulfilling the definition of a cold but not ILI, even if part of an ILI episode; days of severe illness fulfilled the definition of ILI.
We defined optimal trial adherence as the reported taking of at least 80% of study pills during the CAF period, no personal vitamin D supplementation, and no gaps in pill-taking of ≥7 days.
For each year, illness episodes in participants vaccinated before November were compared with those who received no influenza vaccination from August through March. Participants receiving vaccine during the 2 winters (November through March) were excluded from that analysis. If >1 vaccination date was reported, we used the earliest.
Subjects were allocated into 8 subgroups of baseline vitamin D exposure according to the season of enrollment (winter/spring or summer/fall) and supplemental vitamin D intake at enrollment (>400 IU, 201–400 IU, 1–200 IU, or none). We calculated tertiles of 25(OH)D for each subgroup and used these to rank each CAF participant's vitamin D status within their subgroup. Participants with the same rank were pooled and the effect of supplementation evaluated separately for those with low, medium, or high baseline vitamin D status.
Statistical Analysis
Assuming 800 participants would participate for 1 year, and that placebo participants would experience 0.6–0.9 episodes and 1–3 days ill with winter URTI per year on average [1], we estimated we could detect a rate ratio of ≤0.7 for the number of episodes (power >90%), and ≤0.8 for the number of days ill (power >80%), with a 2-sided, 5% significance level.
We computed rate ratios with 95% confidence intervals (CIs) for illness episodes among participants randomized to vitamin D vs placebo, using generalized estimating equations (GEEs) with robust Poisson errors and exchangeable correlation matrices to adjust for overdispersion. Season-specific rates were estimated using person-years of observation during that season—for example, 1 episode during 4 winter months is equivalent to 3 episodes per person-year. Days of illness were frequently zero, so GEEs with negative binomial errors were used for these rate ratios. Analyses were adjusted for calcium treatment and the stratifying variables center, colonoscopy surveillance interval (3 or 5 years), a 3-category term for sex and participation in the 4- or 2-arm study, and season (winter1, winter 2, and other). We also analyzed the associations between symptoms and serum 25(OH)D on treatment, independent of randomized treatment assignment, adjusting for variables that were significant predictors of baseline serum level.
Analyses were conducted using Stata version 12 [25] according to intent-to-treat except as indicated. Sensitivity analyses to explore effects of missing data and episode definitions are described in the Supplementary Appendix.
RESULTS
We identified 19 083 patients potentially eligible for the parent trial; 2813 were enrolled and 2259 were randomized (Figure 1, Table 1). Of the 2259 randomized participants, 1198 (53.0%) were invited to complete health diaries, and 759 (63.4%) returned at least 1 correctly completed diary (Figure 1, Table 1). The mean numbers of diaries returned were similar between the groups. In the most recent telephone survey before participants' first health diaries, 660 of 759 (87.0%) reported having taken at least 85% of study pills; 77.7% were classified as optimally adherent during the CAF substudy period. The relative increase in 25(OH)D associated with supplementation was 8.7 ng/mL in substudy participants and 7.8 ng/mL in all participants (Table 1).
Table 1.
Characteristics of Participants Who Provided Cold and Influenza-like Illness Data
| Characteristic | Participants, No. (%) |
||||
|---|---|---|---|---|---|
| Polyp Prevention Studya |
Colds and Flu Substudyb |
||||
| Vitamin D | Placebo | Vitamin D | Placebo | ||
| n = 1130 | n = 1129 | n = 399 | n = 360 | P Valuec | |
| Age at enrollment, y, mean (SD) | 58.1 (6.9) | 58.0 (6.8) | 57.9 (6.7) | 57.8 (6.3) | .89 |
| Age as of 1 November 2009, mean (SD) | 61.5 (7.0) | 61.3 (6.9) | 60.7 (6.7) | 60.5 (6.4) | .78 |
| Male sex | 712 (63.0) | 711 (63.0) | 237 (59.4) | 201 (55.8) | .32 |
| Race | .22 | ||||
| White | 951 (88.1) | 949 (87.8) | 352 (90.5) | 309 (88.8) | |
| Black | 96 (8.9) | 88 (8.1) | 27 (6.9) | 26 (7.5) | |
| Asian | 25 (2.3) | 26 (2.4) | 9 (2.3) | 7 (2.0) | |
| Other | 7 (28.0) | 18 (1.7) | 1 (0.3) | 6 (1.7) | |
| Missing | 51 | 48 | 10 | 12 | |
| Hispanic ethnicity | 76 (6.7) | 70 (6.2) | 20 (5.0) | 22 (6.1) | .50 |
| Missing | 1 | 2 | 0 | 1 | |
| Body mass index at enrollment, kg/m2 | |||||
| Mean | 28.9 (5.0) | 29.1 (5.3) | 28.6 (5.2) | 28.6 (5.4) | |
| <25.0 | 272 (24.1) | 245 (21.7) | 112 (28.1) | 94 (26.2) | |
| 25.0–29.9 | 458 (40.6) | 458 (40.6) | 159 (40.0) | 147 (41.0) | |
| 30.0–34.9 | 266 (23.6) | 288 (25.6) | 82 (20.6) | 81 (22.6) | |
| ≥35.0 | 133 (11.8) | 136 (12.1) | 45 (11.3) | 37 (10.3) | |
| Missing | 1 | 2 | 1 | 1 | |
| Smoking status at enrollment | .70 | ||||
| Never | 590 (52.2) | 604 (53.5) | 228 (57.1) | 201 (55.8) | |
| Former | 421 (37.3) | 429 (38.0) | 141 (35.3) | 136 (37.8) | |
| Current | 119 (10.5) | 96 (8.5) | 30 (7.5) | 23 (6.4) | |
| Pretrial supplements | |||||
| Multivitamin | 633 (56.0) | 634 (56.3) | 222 (55.6) | 222 (61.8) | .08 |
| Vitamin D | 158 (14.0) | 137 (12.2) | 66 (16.5) | 66 (18.4) | .50 |
| Vitamin C | 219 (19.4) | 271 (24.0) | 66 (16.6) | 82 (22.8) | .03 |
| Zinc | 49 (4.3) | 38 (3.4) | 16 (4.0) | 11 (3.1) | .48 |
| Serum 25(OH)D at enrollment, ng/mL | |||||
| Mean | 24.6 (8.2) | 24.5 (8.5) | 24.8 (8.3) | 25.3 (8.8) | .39 |
| <20 | 380 (33.6) | 402 (35.6) | 127 (31.8) | 112 (31.1) | |
| 20–29.9 | 474 (42.0) | 452 (40.0) | 167 (41.9) | 151 (41.9) | |
| ≥30 | 276 (24.4) | 275 (24.4) | 105 (26.3) | 97 (26.9) | |
| Serum 25(OH)D, ng/mLd | |||||
| Mean | 31.8 (9.9) | 23.9 (8.9) | 33.3 (9.9) | 25.1 (9.1) | <.0001 |
| <20 | 108 (9.9) | 429 (39.0) | 25 (6.3) | 122 (33.9) | |
| 20–29.9 | 413 (37.7) | 431 (39.2) | 143 (35.9) | 136 (37.8) | |
| ≥30 | 575 (52.5) | 239 (21.8) | 230 (57.8) | 102 (28.3) | |
| Missing | 34 | 30 | 1 | 0 | |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; SD, standard deviation.
a These participants provided symptom data by telephone every 6 months from 2007 to 2012.
b These participants also provided symptom data via daily health diaries from 2009 to 2011.
c P values are shown for comparisons in the Colds and Flu (CAF) substudy participants.
d The blood draw closest in time to the start of participation in the CAF substudy.
During winter months, CAF substudy participants reported on average 0.25 episodes of URTI per month of observation (Table 2); 28.6% of participants reported none. There were no significant differences between treatment groups in episodes of URTI (rate ratio [RR], 0.93; 95% confidence interval [CI], .79–1.09), colds (RR, 0.93; 95% CI, .78–1.10), or ILI (RR, 0.95; 95% CI, .62–1.46) in winter, nor over the entire 17-month CAF period (Table 2). During winter, the mean number of days of URTI was 1.8 per person-month in the vitamin D group and 1.6 per person-month in the placebo group (RR, 1.13; 95% CI, .90–1.43), with no significant differences in moderate or severe days ill. Calcium supplementation was not associated with incidence or duration of any of the 3 syndromes, nor did it modify the vitamin D treatment effect (not shown). Similar results were seen in winter 1 and winter 2 (during and after the H1N1 pandemic, respectively; data not shown).
Table 2.
Episodes and Days of Illness of Upper Respiratory Tract Infection
| Episode and Days of Illness | No. Reporting Illness |
Mean Episodes per Person-month (SD) |
Adjusted Rate Ratioa (95% CI) | ||
|---|---|---|---|---|---|
| Vitamin D | Placebo | Vitamin D | Placebo | ||
| Health diary participants (n = 759) | |||||
| Winter episodes | n = 387 | n = 351 | |||
| URTI | 275 (71.1%) | 252 (71.8%) | 0.24 (0.30) | 0.27 (0.31) | 0.93 (.79–1.09) |
| Colds | 267 (69.0%) | 239 (68.1%) | 0.22 (0.28) | 0.24 (0.31) | 0.93 (.78–1.10) |
| ILI | 46 (11.9%) | 51 (14.5%) | 0.02 (0.09) | 0.02 (0.06) | 0.95 (.62–1.46) |
| All seasons episodes | n = 399 | n = 360 | |||
| URTI | 303 (75.9%) | 276 (76.7%) | 0.20 (0.26) | 0.23 (0.27) | 0.94 (.79–1.11) |
| Colds | 295 (73.9%) | 266 (73.9%) | 0.19 (0.25) | 0.21 (0.27) | 0.94 (.79–1.12) |
| ILI | 62 (15.5%) | 62 (17.2%) | 0.02 (0.07) | 0.02 (0.04) | 0.99 (.68–1.43) |
| Mean Days Ill per Person-month (SD) |
Adjusted Rate Ratioa (95% CI) | ||||
| Vitamin D | Placebo | ||||
| Winter days of illness | |||||
| All days ill | 1.78 (3.33) | 1.68 (2.84) | 1.13 (.90–1.43) | ||
| Days of moderate illness | 1.70 (3.13) | 1.62 (2.80) | 1.14 (.90–1.44) | ||
| Days of severe illness | 0.08 (0.60) | 0.06 (0.20) | 0.92 (.56–1.50) | ||
| All seasons days of illness | |||||
| All days ill | 1.52 (2.98) | 1.56 (2.87) | 1.03 (.81–1.31) | ||
| Days of moderate illness | 1.46 (2.79) | 1.51 (2.84) | 1.04 (.81–1.33) | ||
| Days of severe illness | 0.06 (0.56) | 0.05 (0.20) | 0.78 (.49–1.25) | ||
| No. Ever Reporting Illness/No. |
Odds Ratiob (95% CI) | ||||
| Vitamin D | Placebo | ||||
| All participants (N = 2228) | |||||
| Influenza or ILI since last semiannual phone call | 106/1113 (9.5%) | 96/1115 (8.6%) | 1.14 (.84–1.54) | ||
| A cold during the 2 weeks before semiannual phone call | 423/1113 (38.1%) | 417/1111 (37.5%) | 1.03 (.87–1.23) | ||
Abbreviations: CI, confidence interval; ILI, influenza-like illness; SD, standard deviation; URTI, upper respiratory tract infection (cold or ILI).
a Intent-to-treat analysis adjusted for study center, follow-up colonoscopy interval, randomization arm (female 2-arm, female 4-arm, male 4-arm), calcium treatment assignment, season (winter 1, nonwinter [when appropriate], winter 2) and clustering by participant due to longitudinal data.
b Intent-to-treat analysis. Odds ratio adjusted for study center, follow-up colonoscopy interval, and randomization arm (female 2-arm, female 4-arm, male 4-arm).
A variety of secondary and sensitivity analyses were conducted (Supplementary Data). The effect of vitamin D supplementation on episodes or days of illness was not significantly modified by 25(OH)D status at enrollment. In particular, supplementation did not significantly protect against winter URTI episodes among participants with the lowest baseline vitamin D (RR, 0.82; 95% CI, .62–1.10). There was no statistically significant modification of the effect of vitamin D by influenza vaccination, adherence, or body mass index, on either episodes or days of illness, although there were nonsignificant protective effects on severe illness when analyses were restricted to the unvaccinated group, the optimally adherent, and the obese (Supplementary Table 1). There were no significant differences between treatment groups, during winter or overall, in the proportions of participants who sought medical help for their symptoms or were prescribed antibiotics or antiviral medicines. Sensitivity analyses did not change these conclusions.
Among all participants in the parent trial, semiannual telephone surveys identified an episode of ILI since the previous survey among 9.5% of the vitamin D group and 8.6% of the placebo group (adjusted odds ratio [OR], 1.14; 95% CI, .84–1.54; Table 2). A cold in the previous 2 weeks was reported on at least 1 survey by 38.1% of participants in the vitamin D group and 37.5% in the placebo group (OR, 1.03; 95% CI, .87–1.23). Similar results were seen after restricting the analysis to the participants and timeframe of the CAF study (November 2009–March 2011; data not shown).
The mean serum levels measured closest in time to the start of the CAF study showed no significant differences between those who did and those who did not report colds or ILI during all telephone interviews (colds: 28.0 and 27.8 ng/mL; ILI: 27.3 and 27.9 ng/mL, respectively) or winter health diaries (URTI: 29.7 and 29.1 ng/mL) (all P > .10). In an observational analysis, the risk of URTI based on quartiles of serum 25(OH)D suggested a significantly higher risk of ILI (but not colds) in the lowest quartile of serum 25(OH), and a similar association with the number of days of severe (but not moderate) illness, after adjustment for multiple factors not including randomized treatment (Table 3).
Table 3.
Serum 25-Hydroxy Vitamin D and Risk of Upper Respiratory Tract Infection in Health Diary Participants (n = 758)
| Episodes | All Rate Ratioa (95% CI) | Quartiles of Serum Vitamin D Closest to 1 November 2009b |
||||
|---|---|---|---|---|---|---|
| 1: Ref | 2: RR (95% CI) | 3: RR (95% CI) | 4: RR (95% CI) | P Value for Heterogeneity | ||
| Winter | ||||||
| URTI | 0.93 (.86–1.01) | 1.0 | 0.94 (.74–1.19) | 0.97 (.76–1.23) | 0.86 (.68–1.10) | .66 |
| Colds | 0.94 (.87–1.02) | 1.0 | 1.01 (.79–1.30) | 1.01 (.79–1.29) | 0.92 (.72–1.18) | .83 |
| ILI | 0.87 (.68–1.10) | 1.0 | 0.41 (.23–0.73) | 0.70 (.39–1.25) | 0.50 (.27–.94) | .01 |
| All seasons | ||||||
| URTI | 0.91 (.83–.99) | 1.0 | 0.87 (.66–1.13) | 0.86 (.66–1.12) | 0.79 (.61–1.02) | .34 |
| Colds | 0.91 (.83–1.00) | 1.0 | 0.91 (.69–1.20) | 0.90 (.68–1.18) | 0.82 (.63–1.07) | .54 |
| ILI | 0.84 (.68–1.04) | 1.0 | 0.47 (.28–.78) | 0.59 (.36–.98) | 0.51 (.30–.85) | .01 |
| Days of illness | ||||||
| Winter | ||||||
| Any | 0.96 (.85–1.10) | 1.0 | 0.96 (.65–1.40) | 1.07 (.73–1.57) | 0.91 (.61–1.34) | .79 |
| Moderate | 0.97 (.85–1.11) | 1.0 | 0.97 (.66–1.43) | 1.10 (.74–1.63) | 0.93 (.62–1.40) | .78 |
| Severe | 0.84 (.63–1.11) | 1.0 | 0.58 (.30–1.12) | 0.60 (.32–1.12) | 0.49 (.24–.99) | .13 |
| All seasons | ||||||
| Any | 0.95 (.84–1.07) | 1.0 | 0.90 (.60–1.34) | 0.96 (.66–1.46) | 0.86 (.58–1.27) | .85 |
| Moderate | 0.95 (.84–1.08) | 1.0 | 0.91 (.61–1.37) | 0.99 (.67–1.45) | 0.87 (.59–1.30) | .86 |
| Severe | 0.82 (.63–1.07) | 1.0 | 0.52 (.28–.95) | 0.52 (.29–.92) | 0.49 (.26–.91) | .04 |
Abbreviations: CI, confidence interval; ILI, influenza-like illness; RR, rate ratio; URTI, upper respiratory tract infection (cold or ILI).
a Rate ratio per 10 ng/mL change in serum 25-hydroxy vitamin D, adjusted for season of closest blood draw (December–February, March–May, June–August, September–November), age, sex, race (white, black, Asian, other), Hispanic ethnicity, body mass index, northern latitude, smoking status (never, former, current), activity level (low, moderate, high), artificial tanning (no/yes), total annual vacation days in a warm climate.
b Quartiles based on distribution of 758 participants: 25th, 50th, and 75th percentiles (21.75, 28.42, 35.4 ng/mL).
DISCUSSION
We found no evidence that 1000 IU/day vitamin D3 supplementation reduced episodes or duration of URTI during a large randomized, placebo-controlled chemoprevention trial of older adults. Our results were consistent when measured through detailed daily health diaries in a subset of 759 participants, or via simple, periodic surveys of all participants. We found no significant associations either during the first winter, which overlapped with the 2009–2010 H1N1 influenza pandemic, or the second winter after the pandemic. Vitamin D supplementation conferred no significant protection against colds, ILI, or any URTI overall, nor among those with the lowest baseline serum 25(OH) vitamin D, although participants whose baseline concentration was <12 ng/mL were specifically excluded from our trial. Our results were statistically nonsignificant during the winter and over all seasons.
We know of 3 previous, high-quality RCTs of vitamin D supplementation that collected URTI symptom data prospectively from healthy adults. None has shown a significant benefit. Murdoch et al compared 100 000 IU/month oral vitamin D3 or placebo for 18 months in 322 adults in New Zealand [9]. This dose, equivalent to 3300 IU/day, did not significantly reduce episodes of acute respiratory infection (RR, 0.97; 95% CI, .85–1.11). In the United States, Li-Ng et al reported 1.4% (95% CI, −2.4% to 3.4%; P = .57) fewer episodes of URTI in adults given 2000 IU/day vitamin D3 for 12 weeks compared with placebo [10]. This trial may have been underpowered and too short to allow vitamin D levels to reach a steady state. Laaksi et al compared a small dose, 400 IU/day vitamin D3 or placebo, for 6 months in 164 Finnish soldiers and reported 2.2 days missed from work due to respiratory tract infection in the vitamin D group and 3.0 in the placebo group (P = .096) [11].
Trials in other populations have given mixed results. A 1-year trial of 140 adults at a Swedish immunodeficiency clinic reported better infection scores in those receiving 4000 IU/day vitamin D3 than in those receiving placebo (P = .04) [12]. Two trials in predominantly vitamin D–deficient children in Mongolia [13] and Afghanistan [14] showed lower rates of URTI and recurrence of pneumonia, respectively, although a later trial in Afghanistan showed no effect on radiographically confirmed pneumonia in children aged <1 year [15]. In 334 healthy Japanese children, 4 months' supplementation with 1200 IU/day vitamin D3 was associated with fewer infections with laboratory-confirmed influenza A (RR, 0.58; 95% CI, .34–.99), but not with influenza B or other viruses [16]. Influenza vaccination status was not assessed in that trial, so it is unclear if that would have modified the effects of vitamin D supplementation. Other trials were limited by substantial methodological issues including retrospective data collection, which can predispose to recall bias. Two reported a benefit in their primary results [17, 18] and 2 did not [19, 20]. Thus, several trials suggest a benefit of vitamin D supplementation on RTI prevention among those with vitamin D deficiency or other predisposition to infection; further studies of RTI and potentially other infections could prove important in those populations. However, it may not be wise to generalize the results from those who are vitamin D deficient to those who are replete; from children to adults; or from those with predisposition to infection to those without.
Our negative results were perhaps unexpected in view of previous observational data suggesting significantly increased risks of URTI with serum 25(OH)D levels below 16 ng/mL [6], 30 ng/mL [7], or 38 ng/mL [8]. Our data illustrate the potential for conflicting results between observational studies and randomized trials. In observational analyses, we saw significantly higher risks of ILI in the lowest quartile of serum 25(OH)D, whereas the randomized analysis showed no supplementation benefit in participants whose low serum levels should have put them at greatest risk. We speculate that observational studies cannot adequately control unmeasured confounding by exercise, outdoor lifestyle, and other factors. Similar challenges prevent researchers from understanding whether winter increases in URTI incidence are due to seasonal changes in host susceptibility, viral transmission, or other factors, and whether vitamin D plays any role [26].
Our study has the advantages of a large sample size; detailed reporting of daily symptoms using health diaries in a large subgroup during an average of 13 months' observation including 2 winter seasons; serum 25(OH)D measurements; excellent adherence to pill-taking; and use of a common route and dose of supplementation. All participants were randomized for at least 12 months before completing symptom diaries, so failure to attain a steady state did not account for our negative findings. We present extensive sensitivity analyses and other secondary data to support our primary results; these include semiannual surveys of 2228 randomized participants over multiple seasons, which, although potentially susceptible to recall bias, also show no significant association between vitamin D supplementation and either colds or ILI.
A limitation of our study was the collection of health diaries from only one-third of the participants randomized in the parent trial; nevertheless, all participants were invited to take part irrespective of adherence to pill taking, and 759 participants gave sufficient power to identify relative risks of 0.7 for URTI episodes and 0.8 for duration. It is possible, but unlikely, that self-selection of participants from the parent study was influenced by any early effect (or lack of effect) of study treatment on URTI symptoms. Limitations in some of our analyses include our use of semiannual self-reported adherence to pill-taking; lack of laboratory confirmation of URTI; and potential misclassification of colds and ILI by symptom-based case definitions.
CONCLUSIONS
Supplementation with 1000 IU/day vitamin D3 supplementation conferred no significant benefit on the incidence, duration, or severity of URTI symptoms in our study population of adults aged 45 years and older without preexisting vitamin D deficiency. Our results suggest that supplementation at this level, or by inference, at the level of the Recommended Dietary Allowance in North America [27] and the European Union [28], and contained in many commonly used supplements, is unlikely to have any noticeable benefit in URTI prevention in the nondeficient adult population. The effects on URTI of supplementation in adults with vitamin D deficiency (<12 ng/mL) should be addressed in future trials.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors are grateful to the participants, coinvestigators, and study coordinators in the Vitamin D/Calcium Polyp Prevention Study who made this research possible.
The Polyp Prevention Trial is registered at www.clinicaltrials.gov (NCT00153816).
Financial support. This work was supported by the National Cancer Institute at the National Institutes of Health (grant numbers CA098286 and CA098286-S to J. A. B.). Study pills were provided by Pfizer Consumer Healthcare.
Potential conflicts of interest. Together with Dartmouth College, J. A. B. holds a use patent for the chemopreventive use of calcium supplementation. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163:487–94. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 2.Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Smiley D. Seasonal factors in the incidence of the acute respiratory infections. Am J Hygiene. 1926;6:621–6. [Google Scholar]
- 4.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hope-Simpson RE. The role of season in the epidemiology of influenza. J Hyg (Lond) 1981;86:35–47. doi: 10.1017/s0022172400068728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laaksi I, Ruohola JP, Tuohimaa P, et al. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–7. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 7.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–90. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin D and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5:e11088. doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333–9. doi: 10.1001/jama.2012.12505. [DOI] [PubMed] [Google Scholar]
- 10.Li-Ng M, Aloia JF, Pollack S, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect. 2009;137:1396–404. doi: 10.1017/S0950268809002404. [DOI] [PubMed] [Google Scholar]
- 11.Laaksi I, Ruohola JP, Mattila V, Auvinen A, Ylikomi T, Pihlajamaki H. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. J Infect Dis. 2010;202:809–14. doi: 10.1086/654881. [DOI] [PubMed] [Google Scholar]
- 12.Bergman P, Norlin AC, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2(pii):e001663. doi: 10.1136/bmjopen-2012-001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camargo CA, Jr, Ganmaa D, Frazier AL, et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics. 2012;130:e561–7. doi: 10.1542/peds.2011-3029. [DOI] [PubMed] [Google Scholar]
- 14.Manaseki-Holland S, Qader G, Isaq Masher M, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health. 2010;15:1148–55. doi: 10.1111/j.1365-3156.2010.02578.x. [DOI] [PubMed] [Google Scholar]
- 15.Manaseki-Holland S, Maroof Z, Bruce J, et al. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet. 2012;379:1419–27. doi: 10.1016/S0140-6736(11)61650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–60. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 17.Rehman PK. Sub-clinical rickets and recurrent infection. J Tropical Pediatrics. 1994;40:38. doi: 10.1093/tropej/40.1.58. [DOI] [PubMed] [Google Scholar]
- 18.Aloia JF, Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiol Infect. 2007;135:1095–6. doi: 10.1017/S0950268807008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avenell A, Cook JA, Maclennan GS, Macpherson GC. Vitamin D supplementation to prevent infections: a sub-study of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438) Age Ageing. 2007;36:574–7. doi: 10.1093/ageing/afm091. [DOI] [PubMed] [Google Scholar]
- 20.Jorde R, Witham M, Janssens W, Rolighed L, et al. Vitamin D supplementation did not prevent influenza-like illness as diagnosed retrospectively by questionnaires in subjects participating in randomized clinical trials. Scand J Infect Dis. 2012;44:126–32. doi: 10.3109/00365548.2011.621446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira F, Larriba MJ, Muñoz A. Vitamin D and colon cancer. Endocr Relat Cancer. 2012;19:R51–71. doi: 10.1530/ERC-11-0388. [DOI] [PubMed] [Google Scholar]
- 22.Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:827–38. doi: 10.7326/0003-4819-155-12-201112200-00005. [DOI] [PubMed] [Google Scholar]
- 23.Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–7. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 24.Payment P, Richardson L, Siemiatycki J, Dewar R, Edwardes M, Franco E. A randomized trial to evaluate the risk of gastrointestinal disease due to consumption of drinking water meeting current microbiological standards. Am J Public Health. 1991;81:703–8. doi: 10.2105/ajph.81.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.StataCorp. Stata Statistical Software: release 12. College Station, TX: StataCorp LP,; 2011. [Google Scholar]
- 26.Tamerius J, Nelson MI, Zhou SZ, Viboud C, Miller MA, Alonso WJ. Global influenza seasonality: reconciling patterns across temperate and tropical regions. Environ Health Perspect. 2011;119:439–45. doi: 10.1289/ehp.1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 28.The Commission of the European Communities. Commission Directive 2008/100/EC of 28 October 2008. Official Journal of the European Union; L285/9–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.