Abstract
The transfer function HV between stapes velocity VS and sound pressure near the tympanic membrane PTM is a descriptor of sound transmission through the middle ear (ME). The ME power transmission efficiency (MEE), the ratio of sound power entering the cochlea to power entering the middle ear, was computed from HV measured in seven chinchilla ears and previously reported measurements of ME input admittance YTM and ME pressure gain GMEP [Ravicz and Rosowski, J. Acoust. Soc. Am. 132, 2437–2454 (2012); J. Acoust. Soc. Am. 133, 2208–2223 (2013)] in the same ears. The ME was open, and a pressure sensor was inserted into the cochlear vestibule for most measurements. The cochlear input admittance YC computed from HV and GMEP is controlled by a combination of mass and resistance and is consistent with a minimum-phase system up to 27 kHz. The real part Re{YC}, which relates cochlear sound power to inner-ear sound pressure, decreased gradually with frequency up to 25 kHz and more rapidly above that. MEE was about 0.5 between 0.1 and 8 kHz, higher than previous estimates in this species, and decreased sharply at higher frequencies.
INTRODUCTION
This paper continues our studies of the transmission of sound power through the external and middle ear to the sensory cells of the cochlea (Ravicz and Rosowski, 2012b, 2013). Specifically, we examine the transformation of ear canal sound pressure to stapes velocity through the middle ear of chinchilla and use these data and concurrent measurements of other middle- and inner-ear acoustical quantities in the same ears to compute (1) the input admittance of the cochlea and (2) the middle-ear efficiency, the ratio of sound power absorbed by the inner ear at the oval window (OW) to the power absorbed at the tympanic membrane (TM), the entrance of the middle ear (Rosowski et al., 1986; Rosowski, 1991, 1994). (A table of nomenclature is provided at the end of this paper.)
We focus on inner-ear sound power absorption because hearing sensitivity has been hypothesized to be directly related to the power absorbed by the inner ear (e.g., von Waetzmann and Keibs, 1936; Khanna and Tonndorf, 1969); therefore, according to this hypothesis, the effect of the middle ear on sound power transmission to the cochlea could influence the frequency range of hearing (Rosowski et al. 1986; Rosowski, 1991). Furthermore, the frequency dependence of noise-induced hearing loss produced by broadband impulse noise has been related to the frequency dependence of sound power absorption by the inner ear (Tonndorf, 1976; Rosowski, 1991; Patterson et al., 1993). If so, the absorption of harmful levels of sound power might be reduced by protecting the ear from sound in the frequency range where middle-ear power transfer is most efficient. In this study we ignore those processes that relate the sound pressure of a free-field stimulus and the amount of sound power absorbed at the entrance to the middle ear and focus on power transmission and absorption within the middle ear.
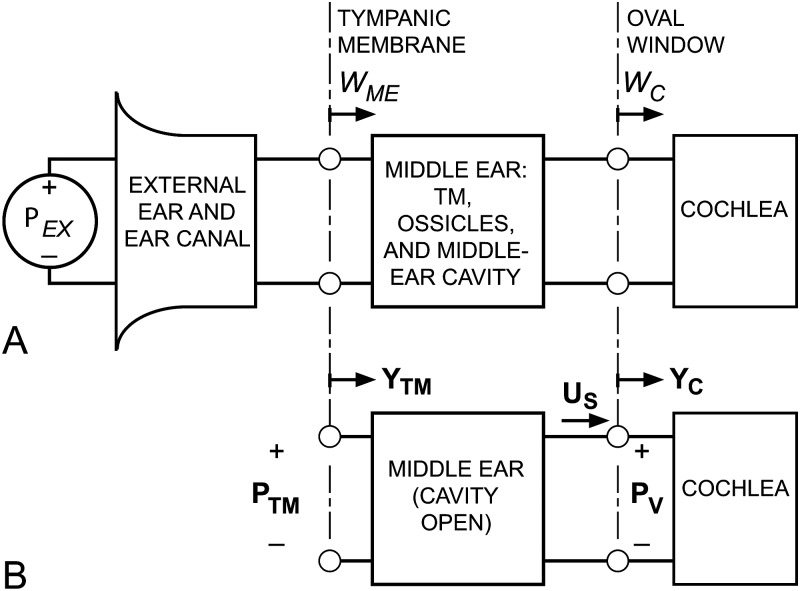
The theoretical framework we use to quantify sound power flow through the middle ear is outlined in Fig. 1, a block diagram of the mammalian auditory periphery that defines the significant acoustic input and output variables of the external, middle, and inner ears (adapted from Rosowski et al., 1986 and Rosowski, 1991). The transfer of sound power from the external environment to the cochlea (left to right in Fig. 1) is governed by sound transmission through each block and sound power delivery from one block to the next. In panel (A), sound pressure just outside the entrance to the external ear Pex produces a sound power that is incident on the tympanic membrane (TM), which we consider to be the entrance of the middle ear. As mentioned above, we ignore the processes that lead to the generation of this incident sound power (e.g., Kuhn, 1979; Shaw, 1988) and the fraction of the power incident at the TM that is reflected back down the ear canal (Rosowski et al., 1986, 1988; Rosowski, 1991; Keefe et al., 1994); instead, we concentrate on the sound power that enters the ME at the TM (WME). Some fraction of this power (WC) passes through the middle ear and is absorbed at the oval window (OW), the entrance to the cochlea and inner ear (IE). The remaining fraction (WME – WC) is lost within the middle ear through losses in the motion of the TM and/or ossicular chain or through losses associated with the compression and rarefaction of the air within the middle-ear cavity.
Figure 1.
A block diagram of the auditory periphery to quantify acoustic power flow through the external and middle ear into the inner ear. (A) The interaction of the sound pressure in the outside world Pex and the head, body, and the external and middle ear results in sound power incident on the TM. Some of this sound power enters the middle ear (WME), and some fraction of that is transmitted to the cochlea (WC). The MEE = WC/WME. (B) The acoustics of our preparation and sound power calculations. The cochlear input admittance YC, the ratio of stapes volume velocity US to sound pressure in the cochlear vestibule PV, was computed from measurements of stapes velocity in each of seven ears resulting from a sound pressure at the TM PTM in the truncated ear canal and the previously reported sound pressure in the cochlear vestibule PV (Ravicz and Rosowski, 2013) measured in the same ears. MEE was computed from PV, PTM, YC, and previously reported middle-ear input admittance YTM (Ravicz and Rosowski, 2012b) also measured in the same ears.
The middle-ear efficiency (MEE) is defined as the ratio of the sound power that enters the cochlea WC to the sound power that enters the middle ear WME (Rosowski et al., 1986, 1988):
| (1) |
Computation of MEE generally requires three measurements: The input immittances (impedance or admittance) of the middle ear and the inner ear (defined below), and a transfer function between a middle-ear input variable (typically, sound pressure or volume velocity) and an inner-ear input variable—see Fig. 1B. The sound pressure at the TM (PTM) and the mean volume velocity of the TM (UTM) are related by the middle-ear input admittance YTM = UTM/PTM.1 The sound pressure within the inner ear vestibule PV and the stapes footplate volume velocity US are related by the cochlear input admittance YC = US/PV. A complication in these computations is that some of these quantities must be estimated from measurements at nearby locations or with procedures that may modify their normal value; for instance, we were constrained to measure the sound pressure Pnear-TM 1–1.5 mm from the TM and estimate PTM from Pnear-TM using an ear-canal model.
A previous investigation computed MEE in chinchilla from the data available at the time: means of measurements of YTM and the transfer functions between stapes velocity or scala vestibuli sound pressure and PTM, each performed in different subject animals in different laboratories (Rosowski, 1991, 1994). The MEE computed from those data differed substantially from estimates in other species, but it was impossible to evaluate the statistical significance of these differences: The various sound pressures and velocities were measured in different populations, hence, any interdependence between the variances of these variables in each species could not be quantified. In this study, we compute MEE in individual ears from measurements of the necessary acoustical and mechanical quantities made in each ear; thus, we can assess directly the variance in MEE within the test population.
In this paper we present measurements of VS as the stapes velocity transfer function HV, the ratio of VS to ear canal sound pressure near the TM. We also use a previously developed model of the ear canal (Ravicz and Rosowski, 2012b, 2013) to provide better estimates of PTM and therefore HV at frequencies above 10 kHz. We use HV, simultaneous measurements of the middle-ear pressure gain GMEP = PV/PTM (Ravicz and Rosowski, 2013), and other measurements of stapes footplate area (Vrettakos et al., 1988) to compute cochlear input admittance for power computations, as described in Sec. 3B below. We compute MEE from these data and concurrent measurements of YTM in these same ears (Ravicz and Rosowski, 2012b) over the frequency range 0.1–17 kHz. We use a simple ear-canal model (Ravicz and Rosowski, 2012b, 2013) to extend our estimates of MEE to 27 kHz.
METHODS
Preparation
These experiments were performed in accordance with guidelines published by the U.S. Public Health Service and were approved by the Massachusetts Eye & Ear Infirmary Institutional Animal Care and Use Committee. Seven chinchilla ears were used in this study and in the companion papers (Ravicz and Rosowski, 2012b, 2013). Animals remained alive throughout the experiment and were euthanized after completion of the experiments. The preparation and anesthesia2 have been described in detail previously (Slama et al., 2010; Ravicz and Rosowski, 2012b). The bony ear canal was greatly shortened in order to expose the TM to view, and a short brass tube (5 mm inner diameter, 9 mm long) was glued to the skull around the bony ear canal to allow the sound source (see next section) to be coupled repeatably to the ear. A stainless steel sleeve (1.3 mm outer diameter, 19 mm long) was glued under the brass coupler to position the tip of a probe tube microphone to measure sound pressure Pnear-TM within 1–1.5 mm of the umbo in the center of the TM. A hole was made in the superior middle-ear cavity (bulla) wall to cut the tensor tympani tendon and immobilize the stapedius muscle by sectioning the tympanic segment of the facial nerve (Rosowski et al., 2006). A second hole was made in the bulla wall posterior to the ear canal and TM to view the cochlear windows and stapes. A thin bony ridge that obscured the view of the stapes and the OW and round-window (RW) niches was carefully removed. Additional bony ridges posterior and medial to the RW were removed to provide access to the bony wall of the vestibule just posterior to the OW. Several small glass beads were dropped onto the stapes and oval window to be used as reflective targets for stapes velocity measurements (Songer and Rosowski, 2006). See Fig. 1 of Slama et al. (2010) for a diagram of the preparation.
After initial measurements of VS, a small hole (170–340 μm diameter) was drilled through the vestibular wall, and a pressure sensor was inserted into the inner ear. Later, a similar small hole was made in the base of the cochlear capsule approximately 1 mm inferior to the RW (the “hook” region), and a second pressure sensor was introduced into scala tympani (ST).
Stimuli, responses, and equipment
Stimulus generation and presentation were the same as described previously (Ravicz and Rosowski, 2012b, 2013). Three sound stimuli were used: A broadband chirp with uniform component magnitude from 49 Hz to 49 kHz, a tone sequence that ranged from 98 Hz to 49 kHz at 6 points/octave (as permitted by a 49-Hz frequency spacing) or a high-frequency sequence from 14–49 kHz at 12 points/octave.3 The chirp stimulus was repeated over a period of 30–60 s. Each tone in the sequences was played for 1–2 s. Tone stimulus levels were determined by the response of the sound source to a constant-level input and were generally 95–110 dB sound pressure level over the frequency range.
Three types of responses were measured: (1) sound pressure in the ear canal near the TM (Pnear-TM), (2) velocity of the target on the stapes and of other points on nearby bone, and (3) sound pressures within the inner ear. The inner-ear sound pressure measurements were described in a previous publication (Ravicz and Rosowski, 2013) and will not be discussed here. Ear canal sound pressure was measured with a small microphone (FG23652, Knowles, Itasca, IL) attached to a thin probe tube. Sound-induced velocities were measured on the medial or posterior stapes footplate (if visible) or the posterior crus and on the petrous bone near the OW and also 2–3 mm away with a laser vibrometer (CLV-1000 with an M050 Decoder set to 2 mms−1 V−1 and an MOO2 filter module with the low-pass filter cutoff set at 100 kHz; Polytec, Waldbronn, Germany). Velocity response phases were corrected for the 44-μs delay of the filter module. Ear-canal microphone responses were amplified with a gain of 10 (P5, detuned; Grass, Warwick, RI). The amplified microphone and vibrometer signals were digitized at 400 kHz by a data acquisition board (PCI6122 or PXI6122, National Instruments, Austin, TX) and saved on a computer. Up to four response channels could be saved at a time.
The Pnear-TM microphone, including the probe tube, was calibrated against 1/4 and 1/8 in. reference microphones as described previously (Ravicz and Rosowski, 2012b). Repeated calibrations showed variations of less than 2 dB and 0.01 cycles in phase.
Noise floor, artifact, and measurement limitations
Measurements were limited at high frequencies by noise and artifact. We define sensor noise floor (microphones or vibrometer) as the smallest discriminable stimulus-related change in sensor response, usually determined practically by a comparison of the sensor outputs with and without a stimulus present. Our noise floors were generally defined from spectra of the responses to tonal stimuli, where the noise floor was estimated from the RMS average of responses at nearby frequencies. The high-frequency limit for Pnear-TM was generally around 44 kHz, and for VS, 30–40 kHz (lower for ears ch18 and ch19; see below).
An “artifact” is defined as a sensor response to stimulus-linked mechanical or acoustical signals secondary to those of interest. For instance, a potential artifact in our velocity measurements was stimulus-induced motion of the entire head. This velocity artifact was assessed by measuring motion of the petrous bone within a few mm of the footplate as described in Sec. 2B. Generally, our data were limited by the noise floor, with significant artifact only at isolated frequencies. Data in which the responses were within a factor of 3 of noise or artifact levels have been omitted.
Access to the stapes head and footplate was via the open middle ear and was limited by the tympanic ring [see Fig. 1 of Slama et al. (2010)]. VS was assessed from the velocity of a single point, along a line approximately 40° to the direction of piston-like motion. We made no attempt to correct for the angle between the measurement direction and the piston direction.
Course of experiment
Stapes velocity was measured with the inner ear intact; after making a hole into the cochlear vestibule; after inserting a pressure sensor in the hole; after making a hole into scala tympani; and after inserting a second sensor into the scala tympani hole. In all or most of these conditions, ME input admittance (Ravicz and Rosowski, 2012b) and sound pressures in the vestibule and scala tympani near the RW (Ravicz and Rosowski, 2013) were also measured.
RESULTS
Stapes velocity VS
All quantities described in this section were measured with the ME opened and are normalized by Pnear-TM.
Inner ear intact
Stapes velocity VS was measured in 6 of the 7 ears (not ch11) with the inner ear intact. Middle-ear velocity transfer functions HV (VS normalized by Pnear-TM) measured with tone sequences in each ear are illustrated in Fig. 2. The thin lines represent a single measurement in one ear or the logarithmic mean of two measurements made in the same ear under similar stimulus conditions; several measurements with similar normalized results were taken at lower stimulus levels. Variation between these measurements was generally less than a factor of 1.5. Hints of a stimulus-level-dependent nonlinearity at low frequencies (below 400 Hz; Rosowski et al., 2006) were seen in two ears but not investigated further4 as it was not the focus of this study.
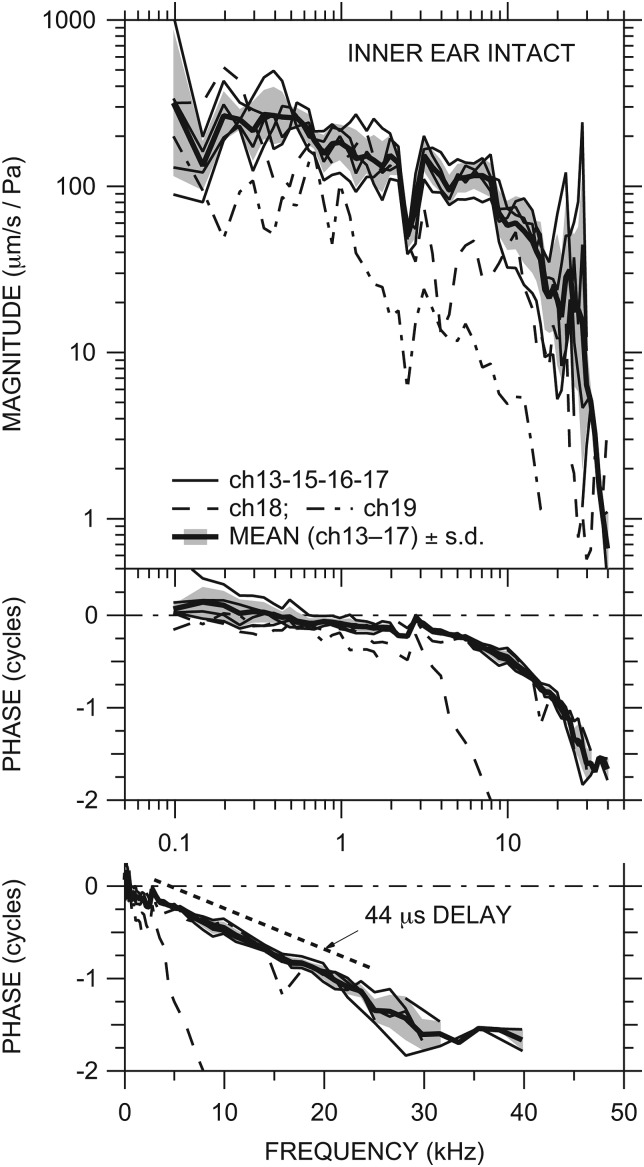
Figure 2.
Middle-ear velocity transfer function HV (stapes velocity VS normalized by Pnear-TM) in six ears with the IE intact: ch18 and ch19 (dashed and dot-dashed lines, respectively) and others (thin solid lines). Also shown is the mean HV (thick solid line) of all ears except ch18 and ch19 ± 1 standard deviation (s.d.—shaded area; see text). Top: magnitude; middle: phase; bottom: phase on a linear frequency scale. ∠HV in ear ch18 reaches −4.5 cycles by 30 kHz. The phase slope corresponding to a constant delay of 44 μs is shown between 3 and 25 kHz by the dotted line.
Between 100 Hz and about 10 kHz the magnitude of HV (|HV|) was between 30 and 500 μm s−1 Pa−1 in nearly all ears. |HV| in all ears decreased slowly with frequency over this range and the phrase angle of HV (∠HV) was slightly negative (except in ear ch18 above 3 kHz), which implies that HV is controlled primarily by resistance with some influence of mass. In all ears HV showed a sharp decrease in magnitude of ∼30% coupled with a 0.25 cycle phase step around 2.5 kHz, presumably due to a resonance between the middle-ear cavity and the bulla hole (Ravicz et al., 1992; Rosowski et al., 2006; see Sec. 4A2 below). Above 10 kHz |HV| decreased more rapidly with frequency and ∠HV continued to accumulate. Above about 18 kHz |HV| showed a peak in most ears. The frequency dependence of ∠HV between 3 and 25 kHz is well fit by a delay of 44 μs (bottom panel of Fig. 2).
Above about 25 kHz |HV| decreased rapidly, falling by a factor of 10–30 by 35 kHz in all ears. ∠HV also decreased sharply around 25–30 kHz before flattening out at the highest measurable frequencies. Data obtained with chirp stimuli with a finer frequency spacing (see Sec. 2B above) helped define this rapid phase change at high frequencies but were contaminated by noise at the highest frequencies; hence, the phase accumulation above 25 kHz could be 1 cycle greater than what is shown.
HV was similar in four of the six ears; the exceptions were ears ch18 and ch19 (dashed and dot-dashed lines, respectively). In these ears, |HV| decreased more rapidly with frequency above 2.5 kHz than in the other ears, and ∠HV was generally more negative. We have reason to believe that we damaged the ossicular chain in ear ch18;5 and since HV in ch19 shows similar behavior, we suspect ossicular chain damage in that ear as well.
Figure 2 also shows the logarithmic mean of stapes velocity in ears ch13, ch15, ch16, and ch17 (thick line) ±1 standard deviation (s.d.; shading). The mean HV is representative of HV in most ears.
The contribution of noise and stimulus artifacts to the measured HV was checked in all ears by measuring the stimulus-linked velocity of points near the OW that were not expected to respond to sound (Sec. 2C). Motion of the petrous bone was generally a factor of 100 or more lower than |VS|, which indicates that motion of the whole head was negligible. Interestingly, in some ears, motion of the bone near the OW was sometimes only a factor of 5 lower than |VS|, suggesting a compliant vestibular wall that provides a shunt path for volume velocity around the cochlear input immittance which should lead to an increase in stapes velocity (though we saw no correlation between |HV| or |YC| and vestibular wall velocity; see Sec. 3C below).
Sensor in vestibule
Middle-ear velocity transfer functions were also computed from stapes velocity measured in all 7 ears after a pressure sensor was inserted into the vestibule (). As for HV, one or two VS measurements were made in each ear under similar stimulus conditions, these measurements generally varied by less than a factor of 1.5, and only hints of low-frequency stimulus level dependence nonlinearity were seen.4 The ratio rHV = /HV, which describes the effect of opening the IE hole and placing the sensor on HV, is shown for six ears in Fig. 3A.
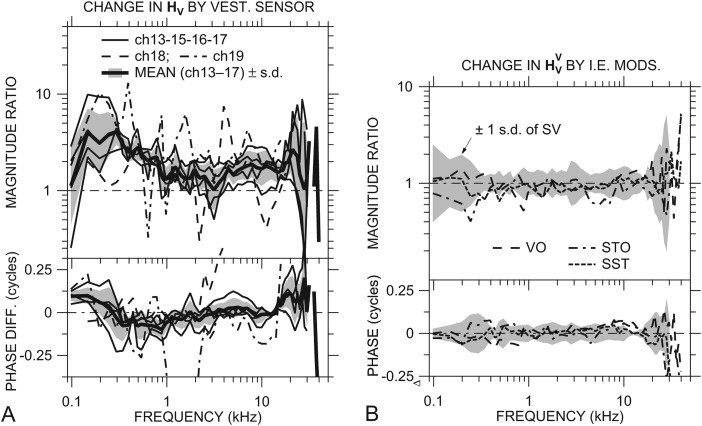
Figure 3.
(A) Effect of a sensor in the vestibule on HV in individual ears: rHV = /HV. Data for ch18 and ch19 are shown by dashed and dot-dashed lines, respectively; others by thin solid lines. Mean effects ±1 s.d. shown by thick solid lines and shading. (B) Ratio of normalized stapes velocity with various other inner-ear manipulations to with a sensor in the vestibule: the vestibule hole open but no sensor in place (VO), a sensor in the vestibule hole and a hole in scala tympani (STO), and sensors in both the vestibule and ST holes (SST). The gray regions are ±1 s.d. of || about 1 and of about 0. The result that the plotted ratios are generally within ±1 s.d. of suggests that the other manipulations had little additional effect on . Both panels—top: magnitude; bottom: phase.
Opening the small hole in the vestibule and inserting the sensor caused an increase in || relative to |HV| in all six ears at nearly all frequencies measured. The |rHV| was 2 or higher between 150 and 600 Hz (400–600 Hz in ear ch18) and near 20 kHz. There was a weak correlation between the open hole area (the area between the sensor and the surrounding bone) and |rHV| between 150 and 300 Hz. The sensor caused a substantial phase increase in ∠HV (∠rHV ≈ +0.1 cycle) in most ears at the lowest frequencies (below 250 Hz) and near 20 kHz and a slight phase decrease between 0.3 and 1 kHz. The changes in HV at the lowest frequencies resulting from opening a hole in the vestibule and inserting the sensor are consistent with a decrease in cochlear resistance allowing middle-ear compliance to become more important in determining stapes response (Slama et al., 2010).
The mean rHV (in all ears except ch18 and ch19; thick solid line) is descriptive of the effect of the vestibule hole with the sensor in place in ears ch13–ch17. In both ears ch18 and ch19, rHV shows spectral peaks and notches not seen in the other ears. The increase in | | relative to |HV| in ears ch13–ch17 was statistically significant6 at nearly all frequencies except 100 Hz, near 3 kHz, and above 20 kHz. The differences between and ∠HV were statistically significant below 200 Hz and from about 0.5 to 1 kHz.
In other conditions vs sensor in vestibule
Once a hole was made in the vestibule and a sensor was inserted, other IE modifications had much smaller effects. Figure 3B shows the mean ratios of normalized stapes velocity to under several other conditions: with the vestibule hole open but no sensor in place (VO), with a sensor in the vestibule hole and a hole in scala tympani (STO), and with a sensor in both the vestibule and ST holes (SST). The shaded area is ±1 s.d. of . The normalized stapes velocity measured in the other conditions was statistically indistinguishable from .
High-frequency correction to HV
In a previous paper we developed a correction factor to estimate ear canal sound pressure very close to the TM at high frequencies from Pnear-TM in each of the individual animals [see Ravicz and Rosowski, 2013, Fig. 5(A)] based on a simple uniform-tube acoustical model of the brass coupler and the chinchilla ear canal remnant (Ravicz and Rosowski, 2012b). Due to limitations in our analysis (Ravicz and Rosowski, 2012b), in individual ears is well-defined only at frequencies below 30 kHz. The estimated ME velocity transfer functions (with intact IE) and (with a sensor in the vestibule), defined as stapes velocity normalized by sound pressure at the TM, are computed from HV and measured in each ear:
| (2) |
The predicted mean and are shown in Figs. 4A, 4B (solid line and shaded area in each panel). Also shown are the mean uncorrected HV (from Fig. 2) and (dashed lines). The effects of the correction are to (a) reduce |HV| and || in a frequency band around the peak near 21–28 kHz in transfer functions in individual ears (see Fig. 2), which reduces the mean |HV| and || by as much as a factor of 2–4 between 10 kHz and 30 kHz and removes the peak near 23 kHz; (b) increase ∠HV and ∠ in individual ears in the 21–28 kHz range; and (c) omit data above about 30 kHz. The predicted and represent our best estimates of HV and at frequencies up to 30 kHz.
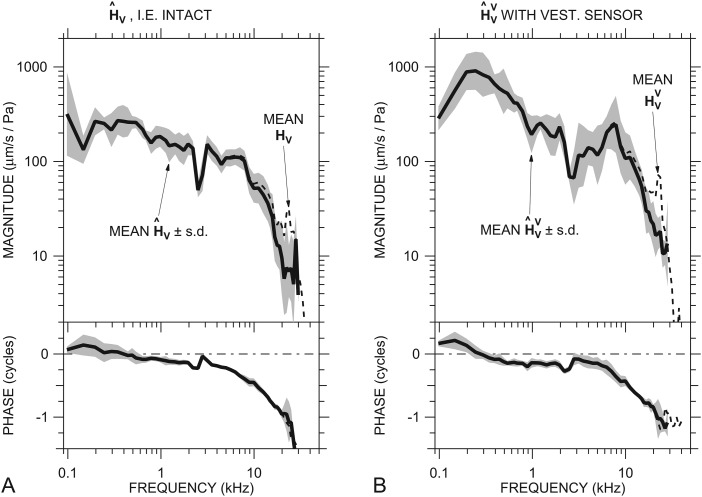
Figure 4.
Mean ME velocity transfer functions estimated using a correction for PTM derived from an ear-canal model. (A) Mean in six ears (thick solid line) ±1 s.d. (shading) estimated from HV (Fig. 2; dashed line) with the correction. (B) Mean in seven ears (thick solid line) ±1 s.d. (shading) estimated from (dashed line) with the correction. Top: magnitude; bottom: phase.
Cochlear input admittance YC
Cochlear input admittance (sensor in vestibule)
The cochlear input admittance YC is the acoustical load that the IE presents to the ME and is an important component of inner-ear power computations (see Sec. 1). We computed YC with a sensor in the vestibule from PV and VS measured simultaneously by
| (3) |
using the previously presented GMEP (Ravicz and Rosowski, 2013) and a stapes footplate area AFP of 2.0 mm2 (Vrettakos et al., 1988). We assume that stapes motion is basically piston-like so that the stapes volume velocity in the piston direction is the simple product of VS and AFP. (We test that assumption in Sec. 3C2 below.) Note that, because VS and PV were measured simultaneously in each ear, the normalization of both by either Pnear-TM or PTM is superfluous, and the YC computation is independent of any uncertainties in ear canal sound pressure. We used both and GMEP before applying the PTM correction factor discussed in Sec. 3B.
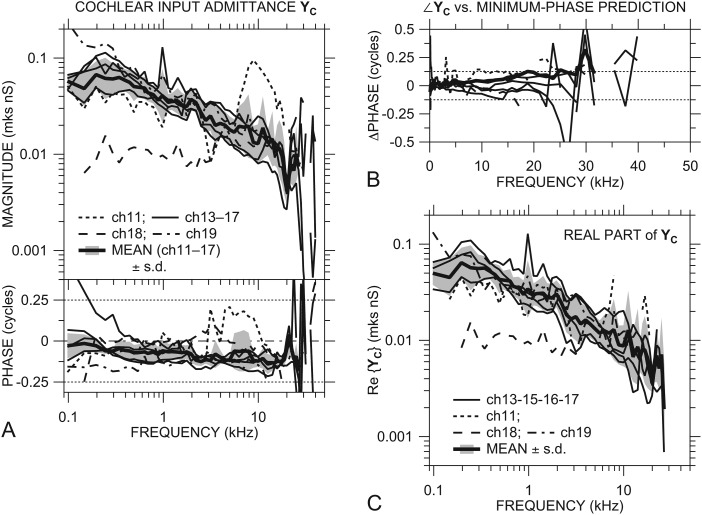
YC in the seven ears is shown in Fig. 5A. Both |YC| and ∠YC were similar among most ears over the entire frequency range of measurement: |YC| decreased slowly with frequency from 0.03–0.08 mks nS (nanosiemens = 10−9 m3 s−1 Pa−1) at 500 Hz to 0.003–0.015 nS at 20 kHz. Below 500 Hz |YC| was approximately flat with frequency and decreased at the lowest frequencies. YC in most ears showed a magnitude notch and phase peak near 20 kHz. ∠YC was generally between 0 and −0.125 cycles below 20 kHz. The |YC| slope and ∠YC in this frequency range are consistent with the admittance of a system dominated by a mixture of mass and resistance.
Figure 5.
(A) Cochlear input admittance YC with an IE sensor in all ears, computed from and GMEP [Rosowski and Ravicz, 2013, Fig. 2(A)] in the same ears. YC in ch11, ch18, and ch19 shown by dotted, dashed, and dot-dashed lines, respectively, others by thin solid lines. Also shown is the mean YC ± 1 s.d. (thick solid line and shaded area) of all ears except ch18 and ch19 (see text). Points where ∠YC was inconsistent with a passive system or differed from a minimum-phase estimate by more than 0.125 cycle are omitted from the computation of the mean. Top: magnitude; bottom: phase. (B) Deviation of ∠YC in each ear from a minimum-phase estimate based on |YC| in that ear. Line types as for panel A. (C) Real part of cochlear input admittance Re{YC}, omitting points based on the passive-system and minimum-phase criteria described above. Line types as for panel (A). Mean Re{YC} ± 1 s.d. is shown by the thick solid line and shading.
In several ears Eq. 3 produced outlier values in YC over part of the frequency range. |YC| in ear ch18 was a factor of 3–10 lower than in other ears at frequencies below 3 kHz and flat with frequency, and ∠YC in ch18 was generally closer to 0 than ∠YC in the other ears, consistent with YC being almost purely resistive. |YC| in ear ch11 showed shallow notches near 0.25, 0.5, 1, and 3 kHz and a broad peak at 9 kHz that were not present in the other ears, and ∠YC in ch11 was much higher (∼+0.2 cycles) between 3 and 10 kHz, consistent with an additional stiffness-mass resonance. |YC| in ch19 was higher below 300 Hz than in the other ears, and ∠YC in ch17 was higher below 350 Hz than in the other ears.
YC in ch19 was similar to YC in other ears above 300 Hz, even though |VS| and |GMEP| in this ear were lower than in other ears. This similarity is consistent with any ME damage in this ear being lateral to the stapes footplate and annular ligament.
Although we saw measurable motion of the vestibular wall near the OW in most ears (see Sec. 3A2 above), there was no correlation between this motion and |YC|: For instance, we would expect that higher vestibular wall motion would lead to a higher |YC| and higher |VS|, yet the vestibular wall velocity was highest in ears ch18, which had the lowest |VS| and lowest |YC| at frequencies less than 3 kHz.
Application of physical constraints
YC in all ears below 22 kHz (where not contaminated by artifact or noise) is generally consistent with physical constraints. Because we used high stimulus levels, measured PV far from the cochlear partition, and saw no signs of level-dependent nonlinearity in PV (Ravicz and Rosowski, 2013) or VS, the cochlea input immittance should appear passive. ∠YC in all ears (except ear ch17 below 200 Hz) is consistent with a driving-point admittance of a passive system: ∠YC is between −0.25 and +0.25 cycles.
Furthermore, the relationship between |YC| slope with frequency and ∠YC in all ears is consistent with the input admittance of a minimum-phase system7 in this frequency range, as seen in Fig. 5B: The discrepancy between the measured ∠YC and its minimum-phase value predicted from |YC| is generally less than 0.125 cycles below 22 kHz except at a few isolated frequencies. These observations support the validity of our HV and GMEP data and suggest that possible complications such as non-piston-like stapes motion do not affect our measurements substantially below 22 kHz.
At higher frequencies (>22 kHz), ∠YC in several ears was outside ±0.25 cycles, and |YC| was quite variable and increased sharply in some ears [Fig. 5A]. At these frequencies ∠YC differed substantially from the minimum-phase prediction [Fig. 5B]. This result suggests that our assumption that the measured VS is representative of piston-like stapes motion breaks down above 22 kHz, which can lead to errors in VS and therefore YC. Studies in other species have shown that the nature of stapes motion can deviate substantially from that of a simple piston [e.g., in gerbil (Decraemer et al., 2007; Ravicz et al., 2008) and human (Hato et al., 2003)], so it is not surprising to see evidence of such non-piston-like stapes motion at high frequencies in chinchilla as well.
Figure 5A also includes the (logarithmic) mean of YC ± 1 s.d., where those points in individual ears where ∠YC was inconsistent with a passive system or deviated from the minimum-phase estimate by more than 0.125 cycles were omitted from the mean. Data from ears ch18 and ch19 were also omitted, even though YC in ch19 was similar to YC in the other ears. The mean YC includes ear ch11, which has a YC unlike other ears above 3 kHz but which is included in the means of HV and GMEP. The mean YC captures the important features of YC in most individual ears below 24 kHz. The phase of the mean YC deviates from a minimum-phase estimate by less than 0.05 cycles below 12 kHz and by less than 0.125 cycles below 27 kHz [Fig. 5B], the high-frequency limit by the passive and minimum-phase criteria above.
Real part of cochlear input admittance Re{YC}
The real part of YC (also called the cochlear conductance) is an essential component of our sound power and efficiency calculations [see Eq. 5 below]. Figure 5C shows Re{YC}=|YC| cos ∠YC in all ears (trimmed as described above). Re{YC} generally increased slightly between 50 Hz and 250 Hz and decreased from about 0.05 nS at 500 Hz to 0.01 nS at 10 kHz and more sharply to about 0.005 nS at 20 kHz. Above about 8 kHz Re{YC} generally varies with the inverse of frequency f, consistent with a slope of −1 on a log-log scale. Above 20 kHz Re{YC} generally increased sharply, then decreased more at higher frequencies. Consistent with YC described above, Re{YC} in ear ch18 was lower below 4 kHz than Re{YC} in other ears. The logarithmic mean and s.d. of Re{YC} (omitting ch18 and ch19) are representative of Re{YC} in most individual ears.
Middle-ear efficiency MEE
The MEE, the ratio of sound power entering the cochlea to sound power entering the middle ear [Eq. 1], is an important descriptor of the role of the ME in sound transmission from the external environment to the sensory cells of the cochlea. Sound power (time-averaged) entering the cochlea WC is given by
| (4a) |
where US* is the complex conjugate of US, and the cochlear input impedance ZC = YC–1. WC can also be computed from the square of the magnitude of either PV or US and the real part of the immittance by
| (4b) |
Similarly, the time-averaged power entering the ME is given by
| (4c) |
where UTM is the volume velocity at the TM, ZTM = YTM−1, and Re{YTM} is the real part of YTM as above.
We computed MEE for individual chinchillas using our measurements of Re{YC} [Fig. 5C], GMEP with the PTM correction [Ravicz and Rosowski, 2013, Fig. 5(B)], and Re{YTM} [Ravicz and Rosowski, 2012b, Fig. 5(D)] in the same ears
| (5) |
Because MEE is a power transfer function and the middle ear is considered passive, MEE can never exceed 1. If no power enters the cochlea, MEE = 0.
An advantage of our choice of |GMEP| and YC for MEE computations is that these parameters are relatively unaffected by experimental limitations: (a) PV is a scalar quantity, and the measured GMEP is independent of measurement direction (unlike VS). (b) YC is computed from simultaneous measurements of PV and VS and is unaffected by PTM measurement errors. (c) YC is governed by physical constraints, so frequencies at which YC is inconsistent with these physical constraints (such as might result from non-piston components of stapes motion) can be identified and these suspect data can be omitted from further analysis (see Sec. 3C2 above). We showed previously that Re{YTM} varies little with small variations in measurement location at frequencies up to 17 kHz (Ravicz and Rosowski, 2012b).
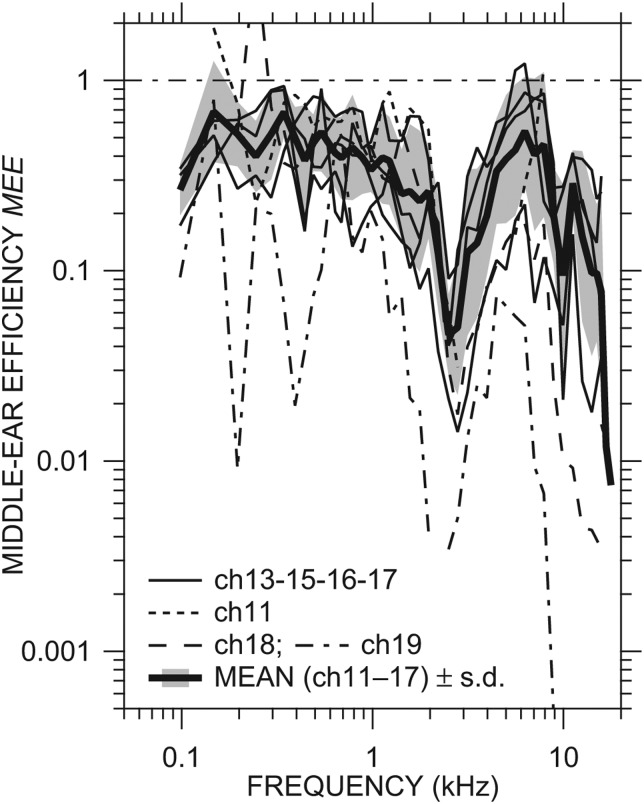
The MEE computed in individual ears with the ME open and a sensor in the vestibule is shown in Fig. 6. Our estimates of MEE are limited to frequencies below 17 kHz, the upper frequency of valid Re{YTM} measurement (Ravicz and Rosowski 2012b). In ears ch11–ch17, MEE was generally between 0.2 and 1 below 2 kHz and from 3.5 to 8 kHz and decreased at higher frequencies. Ears ch11, ch17, and ch18 had MEE > 1 at 2–3 isolated frequencies; in all other ears, MEE < 1 at all frequencies measured. All ears showed a sharp drop in MEE between 2 and 3.5 kHz, presumably due to the bulla hole resonance near 2.5 kHz (see Sec. 4A5 below for more discussion). In the two ears in which ossicular chain damage was suspected (ch18 and ch19), MEE was more variable at low frequencies than in the other ears. MEE in ear ch18 was comparable to ears ch11–ch17 below 2 kHz and lower than in most of those ears but still within their range of variation up to 10 kHz. MEE in ear ch19 was much lower than in other ears across the frequency range, consistent with other indications of poor ME transmission [HV (Fig. 3A] and GMEP (Ravicz and Rosowski, 2013)].
Figure 6.
MEE in all ears, computed from Re{YC} [Fig. 5C] and previously presented Re{YTM} and |GMEP| in the same ears. MEE in ch11, ch18, and ch19 are shown by dotted, dashed, and dot-dashed lines, respectively, others by thin solid lines. MEE = 1 indicates perfect efficiency. Also shown is the mean MEE ± 1 s.d. (thick solid line and shaded area) of all ears except ch18 and ch19.
Figure 6 also includes the (logarithmic) mean MEE from ears ch11, ch13, ch15, ch16, and ch17 ± 1 s.d. (thick line and shading). The mean MEE decreases from 0.7 at 150 and 350 Hz to 0.3 at 2 kHz, has a sharp deep notch between 2 and 3.5 kHz, is between 0.1 and 0.6 between 3 and 14 kHz, and decreases sharply between 15 and 17 kHz. The mean + 1 s.d. is slightly greater than 1 below 150 Hz and between 6 and 8 kHz, and less than 1 at all other frequencies. With the exception of the frequency region around the bulla hole resonance (2.5 kHz), MEE is approximately 0.5 over a wide frequency range, 0.1–8 kHz.
DISCUSSION
We have presented measurements of stapes velocity VS with a bulla hole open and the inner ear intact or with a sensor in the cochlear vestibule, and we have computed the ME velocity transfer function HV from VS and estimates of sound pressure at the TM. We have used simultaneous measurements of the ME pressure gain measured in the same ears (Ravicz and Rosowski, 2013) and published values of the stapes footplate area (Vrettakos et al., 1988) to compute cochlear input admittance YC and its real part Re{YC} in individual ears, and we have used concurrent measurements of ME input admittance YTM in the same ears (Ravicz and Rosowski, 2012b) to compute ME efficiency in individual ears. In this section we examine the effects of experimental conditions on our measurements and computed values. We also compare our HV and YC data and estimates of MEE to those from previous studies.
Effects of experimental conditions on HV, YC, and MEE
In these experiments we (1) made two large (∼5 mm diameter) holes in the auditory bulla for access to the stapes, OW, RW, tensor tympani tendon, and facial nerve and (2) made a 0.2-mm diameter hole into the cochlear vestibule and inserted a sensor to measure sound pressure. Our manipulations of the middle-ear cavities and the introduction of holes and sensors into the inner ear are expected to cause significant but fairly-well-defined changes in ME transfer functions, YC, and MEE.
Effect of EC sound pressure measurement location on HV and YC
In computing the stapes velocity transfer function, we initially normalized the measured VS by Pnear-TM (the sound pressure measured within 1–1.5 mm of the center of the TM). We used a correction computed from an ear canal model (Ravicz and Rosowski, 2012b, 2013) to compute a better estimate of PTM and therefore HV (Fig. 4), as we did previously for GMEP [Ravicz and Rosowski, 2013, Fig. 5(B)]. The correction from Pnear-TM to PTM has a small but significant effect on our estimates of HV at frequencies above 10 kHz as it did for GMEP.
Because we compute YC directly from the ratio of simultaneous measurements of VS and PV, PTM does not enter into the computation of YC, and the ear canal sound pressure measurements have no effect on our YC estimates.
Effect of opening the middle ear on HV and YC
Consistent with previous measurements of cochlear microphonic (Dallos, 1970), umbo velocity (Ruggero et al., 1990), and middle-ear input admittance (Rosowski et al., 2006), we expect that the effect of opening the ME cavity on middle-ear sound transfer is significant only at low and moderate frequencies. Based on these previous data we estimate that the open ME caused an increase in |HV| of about a factor of 5 at 100 Hz and a phase shift of as much as +0.25 cycle below 1 kHz relative to the intact state (Ravicz and Rosowski, 2013). Furthermore, the data of Rosowski et al. (2006) suggest that the magnitude notch and phase step in HV (and GMEP; Ravicz and Rosowski, 2013) near 2.5 kHz result from a resonance between the acoustic stiffness of the air in the ME cavity and the acoustic mass of the air associated with transmission through and radiation from the bulla hole.
The “series model” of the middle ear (Zwislocki, 1962; Møller, 1965; Huang et al., 1997; Voss et al., 2000) suggests that the ME cavity acts to restrict the motion of the TM by coupling the sound pressures produced in the middle-ear cavity to the inward and outward motions of the TM. While some have suggested that the resultant middle-ear pressures can also directly stimulate the inner ear, the magnitudes of these effects are estimated to be quite small (Peake et al., 1992; Shera and Zweig, 1992); therefore, opening the ME should have a negligible effect on YC. The cancellation of the cavity-hole-induced notches and phase steps near 2.5 kHz in GMEP and HV in the computation of YC is consistent with this view.
Effect of the angle between the VS measurement direction and piston-like stapes motion
Measurements of VS were made at an angle roughly 40° to the “piston-like” direction of stapes motion (where the piston-like direction is roughly perpendicular to the plane of the stapes footplate). This angle was necessary because the footplate is behind the TM and is at best only partly observable through the bulla holes. Measurements of stapes motion in other species (Decraemer et al., 2007 and Ravicz et al., 2008 in gerbil; Hato et al., 2003 and Chien et al., 2006 in human temporal bones) have shown that stapes motion is mostly piston-like at low and moderately high frequencies and deviates considerably from piston-like at high frequencies. Such a deviation can cause YC computed from VS measured at a single location on the stapes to appear non-minimum-phase or even non-passive (e.g., Decraemer et al., 2007).
At low frequencies, where the primary mode of motion of the stapes is simple and piston-like, motion in the piston direction can be estimated fairly accurately from measurements along another direction using the cosine of the angle between the two directions (40°), which would increase VS by a factor of (cos 40°)–1 = 1.3 at all frequencies. At higher frequencies, where other modes of motion could become significant, the relationship between stapes motion and measurements in a non-piston-like direction could be more complicated (Chien et al., 2006). Because of these uncertainties, the VS measurements we present are not corrected for the angle of measurement. Instead, we use physical constraints on the input immittance to establish limits on valid VS measurements. The observation that YC is well fit by a minimum-phase estimate at frequencies below 22 kHz (see Sec. 3C2) suggests that VS is a reasonably good description of the piston component of stapes motion to at least 22 kHz.
Effect of the vestibule hole and sensor on HV and YC
The observed changes (small increases in magnitude and phase angle) in HV caused by opening the inner ear and inserting a sensor [Fig. 3A] are qualitatively similar to the changes observed in a previous study [Slama et al., 2010, Fig. 4(B)]. However, the increases we see in |HV| below 4 kHz and in ∠HV below 250 Hz are larger than in the previous study8 and statistically significant. These increases may be the result of a larger sensor hole. We did not see the distinct peak in effect near 6 kHz seen by Slama et al. The effects we saw were virtually identical to the effect of a superior semicircular canal dehiscence (SCD) seen by Songer and Rosowski (2006, Fig. 10), including the double-humped increase in |HV| at about 150 and 300 Hz. We did not observe any diminution of effect below 150 Hz.
Because it was necessary to open the inner ear to measure PV, and we were unable to seal the hole around the sensor, we were unable to determine the effect of the IE hole and vestibule sensor directly on PV or YC. Since the impedance of the sensor is sufficiently high to have negligible effect on YC (Ravicz and Rosowski, 2013), we assume that the admittance of the hole around the sensor YH acts in parallel with , the cochlear input admittance in the intact ear [see model in Slama et al., 2010, Fig. 7(A)]. We also expect that, with an IE hole, the magnitude of the measured admittance YC = |YH| + || is greater than || alone. The computed9|YH| for hole diameters between 200 and 400 μm is comparable to our measured |YC| below 1–2 kHz and substantially smaller at higher frequencies, which suggests that the effect of the hole is significant only below 1–2 kHz. Therefore, we expect that || is lower than the measured |YC| shown in Fig. 5A below 1–2 kHz, as predicted by a mathematical analysis (Ravicz and Rosowski, 2012a); and estimates of YC from PV measured with a sensor sealed in a hole through the braincase into the vestibule show a roughly constant magnitude with frequency below 1–2 kHz (D.C. Chhan and J.J. Rosowski, private communication, 2011).
Effect of opening the middle ear on MEE
At frequencies less than 1 kHz: The decrease in middle-ear stiffness.
The air in the intact middle-ear cavity acts as a nearly ideal acoustical stiffness KMEC in series with the stiffness of the TM and ossicles KTOC, where the total stiffness KTM = KMEC + KTOC dominates the middle-ear input impedance (that governs TM and ossicular motion) at low frequencies (Zwislocki, 1962; Rosowski, 1994; Rosowski et al., 2006). If the only effect of opening the ME cavity is to remove the contribution of KMEC (Zwislocki, 1962; Møller, 1965; Ravicz et al., 1992; Rosowski, 1994), we would expect this manipulation to have a significant effect on HV, GMEP, and YTM only at frequencies below 1 kHz and very little effect on YC and MEE (see below).
The removal of the contribution of KMEC by the open bulla hole is responsible for the increase in middle-ear sound transfer and |YTM| at frequencies below 1 kHz that has been observed after opening the ME cavities in chinchilla (Dallos, 1970; Ruggero et al., 1990; Rosowski et al., 2006). A comparison of the effects of the open ME on stapes motion (Ruggero et al., 1990, Fig. 10) and Re{YTM} [Ravicz and Rosowski, 2012b, Fig. 6(B)] shows that, in the computation of MEE, the increase in the |GMEP|2 term in Eq. 5 by the open ME (Ravicz and Rosowski, 2013) is balanced by a proportionate increase in Re{YTM}. This conclusion is consistent with the removal of an ideal stiffness, which by definition is purely reactive and does not absorb power. (Deviations from a pure stiffness effect due to viscous and thermal losses to the bulla walls are expected to be small and significant only at very low frequencies.) Therefore, the reduction of middle-ear stiffness due to the open bulla hole should have very little effect on MEE.
At frequencies near 2.5 kHz: The introduction of a bulla-hole resonance.
While removing the contribution of the middle-ear air stiffness KMEC has little effect on Re{YTM}, the introduction of a hole in the bulla has a substantial effect. This occurs because the bulla holes also introduce (a) a “cavity-hole” antiresonance10 between KTM and the acoustic mass of the holes and (b) losses via the radiation of sound from the hole. This antiresonance produces a narrow deep notch in |YTM| (Rosowski et al., 2006, Fig. 3; Ravicz and Rosowski, 2012b, Fig. 6) that is associated with the narrow notches and phase steps observed in GMEP [Ravicz and Rosowski, 2013, Fig. 2(A)] and our HV (Fig. 2) near 2.5 kHz. Because the notch appears in both |GMEP| and |HV|, it does not appear in |YC| or Re{YC} (Fig. 5), consistent with the assertion in Sec. 4A2 above that |YC| is not affected by the state of the middle ear. The shape and size of the notch are comparable in |GMEP| and Re{YTM}, so the net effect on MEE (due to the |GMEP|2 term) is a notch about a factor of 3 in depth. The |YTM| notch is present only in the open-hole condition [Rosowski et al., 2006, Fig. 3(B) vs 3(A)], and therefore we expect that closing the cavity hole would remove the MEE notch near 2.5 kHz and have little effect at other frequencies.
Summary of effects of experimental methods
We have chosen the variables used in MEE computations to minimize the effects of experimental conditions and measurement errors. The openings in the vestibule should affect MEE only at low frequencies (below 1 kHz), while the opening in the ME wall should affect MEE only near the bulla hole antiresonance near 2.5 kHz. If the low-frequency effect of the vestibule hole is not large, MEE computed from measurements with an open ME and a vestibule sensor should be fairly representative of MEE in the intact ear except near the bulla antiresonance frequency.
Comparison of HV and YC to previous studies
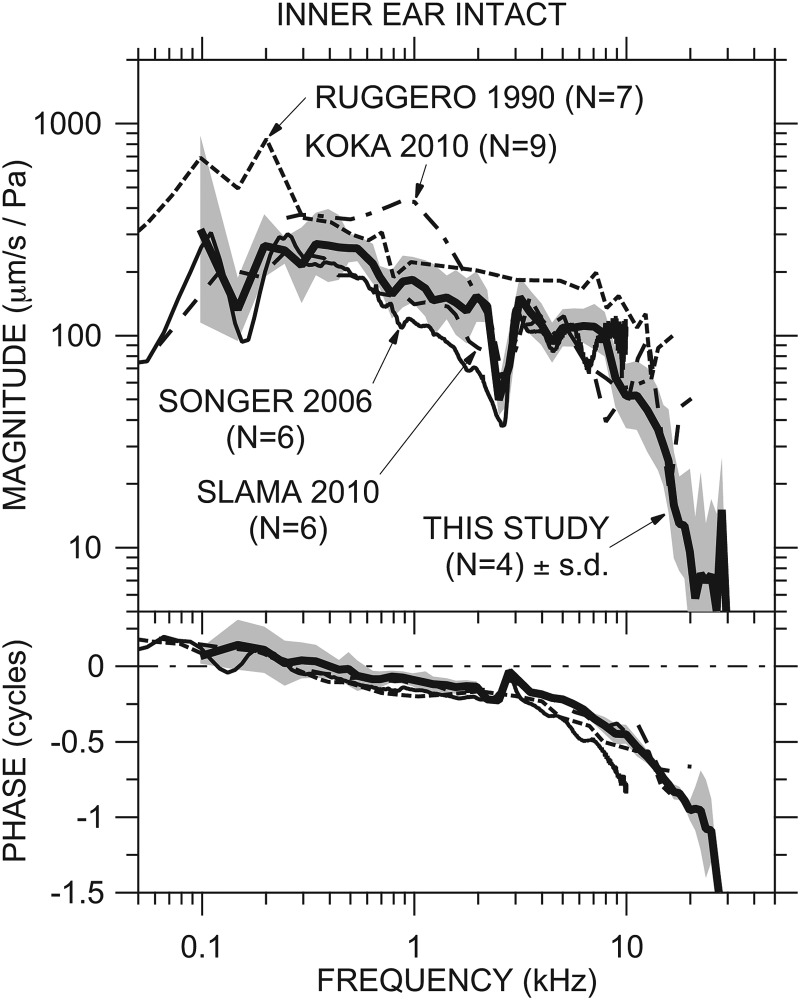
HV
Our mean HV with the ME open and IE intact [Fig. 4A] is quite similar to that measured in other studies from our laboratory (Songer and Rosowski, 2006; Slama et al., 2010) and elsewhere (Ruggero et al., 1990, Figs. 8 and 9, adjusted for stimulus level; Koka et al., 2010) in which the IE was intact and extends to higher frequencies; see Fig. 7. Our HV phase accumulates more slowly than Songer's normalized phase. As mentioned in Sec. 4A4 above, the effect of the IE sensor on HV is virtually identical to the effect of a SCD on normalized stapes velocity seen by Songer and Rosowski (2006).
Figure 7.
Comparison of our mean HV with the IE intact ± 1 s.d. [Fig. 5A; thick solid line and shading] to the mean normalized stapes velocity from Ruggero et al. (1990, Figs. 5 and 6; dotted line), Songer and Rosowski (2006, Fig. 7; thin solid line), Slama et al. (2010, Fig. 5, dashed line), and Koka et al. (2010, Fig. 6, dot-dashed line; magnitude only). Top: magnitude; bottom: phase.
YC and Re{YC}
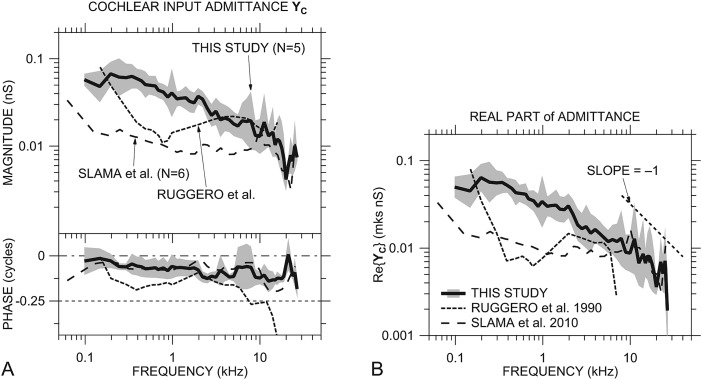
Figure 8A shows similarities and differences between our mean YC [from Fig. 5A] and previous cochlear immittance estimates (originally presented as impedance; converted to YC here). Our mean |YC| estimate is considerably higher than those computed from Slama et al. (2010, Fig. 10) and Ruggero et al. (1990, Figs. 15 and 17): by a factor of 3–4 below 2 kHz, less at higher frequencies. Our |YC| has a similar frequency dependence to that from Slama below 5 kHz, though our |YC| continues to decrease with frequency above 1.5 kHz while that from Slama flattens. Our ∠YC is similar to that from Slama across the entire frequency range. The frequency dependence of our YC is much different from that from Ruggero below 3 kHz.
Figure 8.
(A) Comparison of our mean YC ± 1 s.d. with a sensor in the vestibule [Fig. 5A; thick solid line and shading] to the cochlear admittance computed from previous measurements by Slama et al. (2010, Fig. 10; dot-dashed line) and an estimate from Ruggero et al. (1990, Figs. 15 and 17; dashed line). Top: Magnitude; bottom: phase. (B) Comparison of our mean Re{YC} ± 1 s.d. with an IE sensor [Fig. 5C; thick solid line and shading] to Re{YC} computed from the previous studies shown in panel (A). Line code as for panel (A). The thin dotted line shows an inverse relationship with frequency.
YC computed from Slama et al. (2010) supports our finding of a cochlear admittance characterizable as a combination of mass and resistance (see Sec. 3C1). This combination admittance is consistent with the Dallos (1970, p. 495) conjecture that ZC in cat and chinchilla has a significant low-frequency reactive component due to an inertance introduced by the helicotrema. Unlike Dallos' prediction of a substantial |ZC| rolloff and phase shift below 1 kHz, our results suggest a gentle |ZC| increase with frequency above 200 Hz and very little phase difference from a purely resistive cochlea.
Between 3 and 15 kHz our |YC| data are similar to those from Ruggero, and our ∠YC data are similar to those from Slama. Generally, our YC and that from Slama are approximately minimum-phase (see Sec. 3C2) over the entire frequency range. The relationship between |YC| and ∠YC computed from Ruggero's estimate (which used Ruggero's stapes velocity measurements and scala vestibuli sound pressure from Décory et al., 1990) deviates from a minimum-phase system by more than 0.1 cycle above 800 Hz and by more than 0.15 cycle above 3 kHz.
In Fig. 8B, our mean Re{YC} [from Fig. 5C] is compared to Re{YC} computed from the previous cochlear impedance estimates. Consistent with YC, our Re{YC} is higher than others below 5 kHz but shows some similarities at higher frequencies to that computed from Slama. Our Re{YC} decreases approximately as 1/f above 8 kHz (as mentioned in Sec. 3C3 above; compare to thin dotted line), whereas that from Slama is more variable. The frequency response of our Re{YC} is quite different from that from Ruggero et al. but similar to that computed from the Slama data. The Re{YC} computed from Ruggero is much lower than ours below 2 kHz and negative above 7 kHz. The consistency of our high-frequency data with those from Slama and the consistency of both these data sets with physical constraints suggest that our data and Slama's are more accurate representations of cochlear immittance at high frequencies.
MEE and power flow to the cochlea
Comparison to previous studies
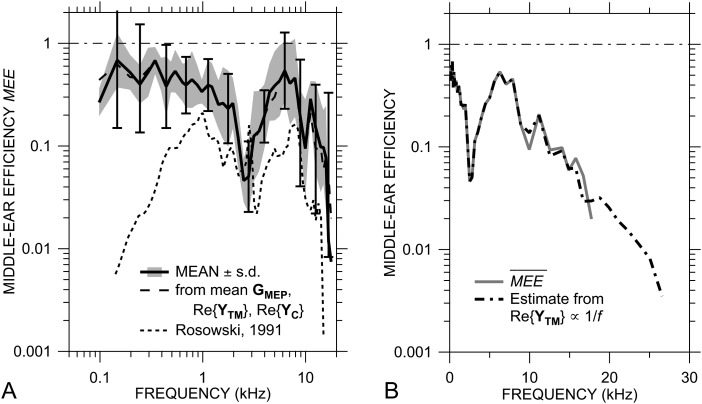
Our computed mean MEE (±s.d.; shaded area) is higher than a previous estimate of MEE in chinchilla, as seen in Fig. 9A, especially at frequencies below 1 kHz. The previous estimate (Rosowski, 1991, Fig. 7; thin dotted line) used previously published means of stapes velocity, SV sound pressure, and AFP and unpublished measurements of middle-ear input impedance, all from different laboratories.11 The previous estimate of MEE was also lower than MEE estimates in cat and guinea pig (Rosowski, 1991, 1994). Our new estimate of MEE from individuals is significantly higher than the earlier estimate, by a factor of 2 to 3 between 1 and 8 kHz, by as much as a factor of 30 below 1 kHz, and by a factor of 5 to 10 above 8 kHz. Our new estimate is more similar to MEE estimates in cat: about half of the sound power entering the middle ear is transmitted to the cochlea.
Figure 9.
(A) Comparison of the mean MEE ± 1 s.d. with the middle ear and inner ear opened (from Fig. 6; solid line and shaded area) and computed from the mean |GMEP|, Re{YC}, and Re{YTM} (dashed line; error bars show ± 1 s.d.) to a previous MEE estimate (Rosowski, 1991, Fig. 7; dotted line). (B) (solid gray line) and an estimate of high-frequency MEE using Re{YTM} = 13 nS at 9 kHz and extrapolated to 27 kHz assuming an inverse variation with frequency (as for Re{YC}; dot-dashed line).
Variance and validity of MEE estimates
One of the motivations for this study was to obtain an estimate of the variance in MEE to check the validity of our computed MEE. The standard deviation of MEE computed in the logarithmic domain from the five individuals is a factor of 1.5–3 over the entire frequency range of 0.1 to 17 kHz. As mentioned in Sec. 3D above, the mean MEE + s.d. exceeds 1 at only a few isolated frequencies, consistent with a passive middle ear, which supports the validity of our computed MEE.
Figure 9A also compares the mean of the individual MEE measurements to an estimate (dashed line) computed from the mean GMEP [Ravicz and Rosowski, 2013, Fig. 5(B)], mean Re{YTM} [Ravicz and Rosowski, 2012b, Fig. 5(D)], and mean Re{YC} [Fig. 5C]. is quite similar to the mean MEE, being slightly lower above 12 kHz but well within 1 s.d. of the mean of the individuals. The standard deviation we compute for from the variances of the individual components12 (assuming independent variances; error bars) is comparable to the s.d. of MEE between 250 Hz and 7 kHz and is about twice the s.d. of MEE (a factor of 4–5) below 250 Hz and above 8 kHz. In contrast, estimates of the variance of the previous MEE estimate (Rosowski, 1991, assuming independent variation of the measurements made in the different laboratories) were so large that they suggested that the efficiency varied at most frequencies beyond the 0 to +1 range. By measuring all quantities in individual animals, we reduce the variance of our computed MEE considerably and increase confidence in our computations.
Our new estimate appears to be robust to experimental conditions: for example, the counterbalancing effects of the open ME on |GMEP|2 and Re{YTM} demonstrate that MEE is independent of whether the middle ear is open or closed. The previous underestimate of chinchilla MEE (Rosowski, 1991, 1994) is primarily due to differences in the data used in the calculations: primarily in the estimate of YTM and YC at low frequencies (see Fig. 5 of Rosowski, 1991).
MEE estimates at high frequencies
The similarity between and the mean MEE from individuals indicates that can be used to estimate the effects of measurement corrections or ME or IE modifications on MEE. For example, our mean data suggest a rapid rolloff in above about 17 kHz; however, this conclusion is limited by the lack of Re{YTM} data at higher frequencies. Re{YC} decreases as 1/f above 10 kHz [Fig. 8B], and the decrease in Re{YTM} above 9 kHz [Ravicz and Rosowski, 2012b, Fig. 5(D)] can also be described fairly well as varying inversely with frequency. Examination of Eq. 5 shows that, if Re{YC} and Re{YTM} covary, the high-frequency rolloff in MEE is due to the rolloff in |GMEP|. Using the mean data, we can easily extrapolate MEE to higher frequencies by assuming that Re{YTM} continues to vary as 1/f above 9 kHz. The estimated using this extrapolation above 9 kHz [Fig. 9B] shows a rapid high-frequency rolloff that continues to the high-frequency limit of Re{YC} at 27 kHz.
SUMMARY AND CONCLUSIONS
-
(1)
Stapes velocity VS and sound pressure in the ear canal close to the TM Pnear-TM were measured in seven live chinchillas in which the ME was opened and the IE was intact (Fig. 2). The ME velocity transfer function HV (Fig. 4) was computed from those measurements in these individual animals using an estimate of TM sound pressure derived from Pnear-TM and an ear-canal model. HV was generally similar to previous measurements from other studies (Fig. 7).
-
(2)
Small but significant changes in HV were produced by the introduction of a hole in the vestibule and the insertion of a pressure sensor (); but once the sensor was in place in the vestibule hole, further IE modifications had little effect on (Fig. 3).
-
(3)
The cochlear input admittance YC (Fig. 5) computed from and GMEP had a higher magnitude than that computed from some previously presented cochlear input impedance data (Fig. 8). Our mean YC was consistent with the input admittance of a passive minimum-phase system at frequencies up to 27 kHz (in all individual ears, up to 22 kHz), which suggests that potential errors due to non-piston-like stapes motions are small below 22 kHz.
-
(4)
The MEE (Fig. 6) computed from Re{YC}, |GMEP|, and Re{YTM} in the same ears is substantially higher than a previous estimate in chinchilla (Rosowski, 1991), especially at low and high frequencies [Fig. 9A]. The relatively small variance of MEE due to the use of complete data sets in individual animals establishes the validity of our estimate.
-
(5)
While our estimates of MEE are limited to 17 kHz, a sharp rolloff in MEE is observed just at that limit. Examination of the factors of MEE suggests that, because Re{YC} and Re{YTM} decrease proportionally with frequency between 10 and 17 kHz, this MEE rolloff is due to the rolloff in |GMEP| at 17 kHz rather than to a change in Re{YTM}. An extrapolation of the 1/f frequency dependence of Re{YTM} predicts a sharp decrease in MEE up to 27 kHz [Fig. 9B].
ACKNOWLEDGMENTS
We thank Michael Slama for helpful discussions, Melissa McKinnon for animal surgery and assistance with experiments, and the staff of the Eaton-Peabody Laboratory for technical support. Supported by NIDCD.
Nomenclature
- AFP
Stapes footplate area
Correction factor to estimate PTM from Pnear-TM
- f
Frequency (Hz)
- GMEP
Middle-ear pressure gain
- HV
Middle-ear velocity transfer function = VS/PTM
with a pressure sensor in the vestibule
- IE
Inner ear
- KMEC
Stiffness of air in ME cavity
- KTM
Stiffness associated with YTM
- KTOC
Stiffness of TM, ME ossicles, and cochlea
- ME
Middle ear
- MEE
Middle-ear efficiency
computed from mean factors
- OW
Oval window
- P
Sound pressure
- Pex
external to the EC
- Pnear-TM
measured near the TM
- PTM
measured at the TM
- PV
measured in the cochlear vestibule
- rHV
Ratio of to HV
- Re{Y}
Real part of admittance
- RW
Round window
- s.d.
Standard deviation of the mean
- ST
Scala tympani
- TM
Tympanic membrane
- UTM
Volume velocity entering the ME
- VS
Stapes velocity (evaluated from single-point measurement)
- WC
Sound power entering the cochlea
- WME
Sound power entering the ME
- YBH
Admittance of bulla holes
- YC
Cochlear input admittance with a vestibule sensor = VSAFP/GMEP
Cochlear input admittance with the IE intact
- YH
Admittance of vestibule hole
- YTM
Middle-ear input admittance
- ZC
Cochlear input impedance = YC−1
- ZTM
Middle-ear input impedance = YTM−1
- σ
Standard deviation = variance1/2
Superscript V refers to sound pressure measured with a sensor in the vestibule. The circumflex (^) denotes quantities estimated from an EC model (Ravicz and Rosowski, 2012b, 2013).
Footnotes
In this paper, real variables (e.g., Pex) are shown in italics, and complex variables (e.g., PTM) with real and imaginary parts are shown in bold type. All complex variables are functions of frequency and are expressed as magnitude (e.g., |PTM|) and phase angle (e.g., ∠PTM).
Surgical dose: Ketamine, 40 mg/kg, administered intramuscularly and pentabarbitol (Nembutal), 50 mg/kg initial dose, administered intraperitoneally. Maintenance dose as necessary: Nembutal alternating with Ketamine at half the surgical dose. Depth of anesthesia was assessed by monitoring heart rate and toe pinch response.
The frequencies of stimulus tones were restricted to multiples of the 49-Hz minimum frequency corresponding to the 20.4-ms analysis window. Chirps allowed quick overview measurements and were useful for guiding phase unwrapping of the measured sound pressures at high frequencies and checking the stability of the measurements. Tone sequences had better signal-to-noise ratio and allowed higher stimulus levels to be used at high frequencies as necessary.
The frequency resolution of our chirp and tone-sequence measurements was not high enough to adequately observe the low-frequency nonlinearity documented in YTM by Rosowski et al. (2006).
The stapes was touched during experimental preparation, and substantially lower IE sound pressures were observed (Ravicz and Rosowski, 2013, Figs. 2 and 3). We also observed a greater range in the middle-ear input admittance in ears ch18 and ch19 (Ravicz and Rosowski, 2012b, Fig. 3) similar to that seen in other studies when the ossicular chain was interrupted (Rosowski et al., 2006).
Recognizing that our small sample size makes statistical inferences difficult, we consider a mean of one population that does not overlap ±1 s.d. of another population to indicate a statistically significant difference. For our small sample, this is roughly equivalent to the condition that the probability p that data are from the same population (the null hypothesis) is less than 5% [p(0) < 0.05].
In a minimum-phase transfer function, the phase and the natural logarithm of the magnitude (or the real and imaginary parts) are Hilbert transforms of each other. A minimum-phase system is causal and stable in time. Minimum-phase estimates were computed from |YC| by a matlab function (Ru, 1997).
Note that the velocity transfer function ratio magnitude is expressed in decibels in Slama et al. (2010) rather than in linear units as shown here.
We modeled the annular space around the sensor as an unrolled narrow slit (Backus, 1975), where the slit thickness t is the difference between the radius of the hole and the radius of the sensor and the width w is approximately the circumference of the hole, and computed the impedance ZH using Eq. (5.51) of Beranek (1986): , where ρ0 and μ are the density and dynamic viscosity of water, respectively, ω is frequency in radians, and the hole length l = 0.2 mm plus an end correction.
An antiresonance results from the parallel connection of an acoustic mass and compliance. The antiresonance produces a narrow impedance maximum in the frequency domain, where the magnitude of the peak is related to the resistance within the resonant circuit.
Rosowski's (1991) estimate of MEE used stapes velocity from Ruggero et al., (1990), scala vestibuli sound pressure from Décory (1989) (also in Décory et al., 1990), AFP from Fleischer (1973), and middle-ear input impedance from Dear (1987).
Because we used logarithmic means of |GMEP|, Re{YC}, and Re{YTM}, their standard deviations σGMEP, σRe{YC}, and σRe{YTM}, respectively, add to the means in the logarithmic domain. Then, in the logarithmic domain, Eq. 5 becomes log() = log = 2 log(|GMEP|) + log(Re{YC}) – log(Re{YTM}), and = 2, = 1, and = −1. The standard deviation was then computed by = .
References
- Backus, J. (1975). “Acoustic impedance of an annular cavity,” J. Acoust. Soc. Am. 58, 1078–1081. 10.1121/1.380767 [DOI] [Google Scholar]
- Beranek, L. L. (1986). Acoustics (Acoustical Society of America, Melville, NY: ). [Google Scholar]
- Chien, W., Ravicz, M. E., Merchant, S. N., and Rosowski, J. J. (2006). “The effect of methodological differences in the measurement of stapes motion in live and cadaver ears,” Audiol. Neurotol. 11, 183–197. 10.1159/000091815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos, P. (1970). “Low-frequency auditory characteristics: Species dependence,” J. Acoust. Soc. Am. 48, 489–499. 10.1121/1.1912163 [DOI] [PubMed] [Google Scholar]
- Dear, S. (1987). “Impedance and sound transmission in the auditory periphery of the chinchilla,” Ph.D. thesis, University of Pennsylvania. [Google Scholar]
- Décory, L. (1989). Origine des différences interspecifiques de susceptibilité au bruit [Origins of interspecies differences in susceptibility to noise], Ph.D. thesis, Université de Bordeaux, France. [Google Scholar]
- Décory, L., Franke, R. B., and Dancer, A. L. (1990). “Measurement of the middle ear transfer function in cat, chinchilla and guinea pig,” in The Mechanics and Biophysics of Hearing, edited by Dallos P., Geisler C. D., Matthews J. W., Ruggero M. A., and Steele C. R. (Springer, Berlin: ), pp. 270–277. [Google Scholar]
- Decraemer, W. F., de La Rochefoucauld, O., Dong, W., Khanna, S. M., Dirckx, J. J. J., and Olson, E. S. (2007). “Scala vestibuli pressure and three-dimensional stapes velocity measured in direct succession in gerbil,” J. Acoust. Soc. Am. 121, 2774–2791. 10.1121/1.2709843 [DOI] [PubMed] [Google Scholar]
- Fleischer, G. (1973). “Studien am Skelett des Gehörorgans der Säugetiere, einschliesslich des Menschen [Studies of the structure of the hearing organs of mammals, including human],” Säugetierkundl. Mitt. (München) 21, 131–239. [Google Scholar]
- Hato, N., Stenfelt, S., and Goode, R. L. (2003). “Three-dimensional stapes footplate motion in human temporal bones,” Audiol. Neuro-Otol. 8, 140–152. 10.1159/000069475 [DOI] [PubMed] [Google Scholar]
- Huang, G. T., Rosowski, J. J., Flandermeyer, D. T., Lynch, T. J.III, and Peake, W. T. (1997). “The middle ear of a lion: Comparison of structure and function to domestic cat,” J. Acoust. Soc. Am. 101, 1532–1549. 10.1121/1.418107 [DOI] [PubMed] [Google Scholar]
- Keefe, D. H., Bulen, J. C., Campbell, S. L., and Burns, E. M. (1994). “Pressure transfer function and absorption cross section from the diffuse field to the human infant ear canal,” J. Acoust. Soc. Am. 95, 355–371. 10.1121/1.408380 [DOI] [PubMed] [Google Scholar]
- Khanna, S., and Tonndorf, J. (1969). “Middle ear power transfer,” Arch. Klin. Exp. Ohren Nasen Kehlkopfheilkd. 193, 78–88. 10.1007/BF00417239 [DOI] [PubMed] [Google Scholar]
- Koka, K., Holland, N. J., Lupo, J. E., Jenkins, H. A., and Tollin, D. J. (2010). “Electrocochleographic and mechanical assessment of round window stimulation with an active middle ear prosthesis,” Hear. Res. 263, 128–137. 10.1016/j.heares.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Kuhn, G. F. (1979). “The pressure transformation from a diffuse sound field to the external ear and to the body and head surface,” J. Acoust. Soc. Am. 65, 991–1000. 10.1121/1.382606 [DOI] [Google Scholar]
- Møller, A. (1965). “An experimental study of the acoustic impedance of the middle ear and its transmission properties,” Acta Otolaryng. 60, 129–149. 10.3109/00016486509126996 [DOI] [PubMed] [Google Scholar]
- Patterson, J. H., Hamernik, R. P., Hargett, C. E., and Ahroon, W. A. (1993). “An isohazard function for impulse noise,” J. Acoust. Soc. Am. 93, 2860–2869. 10.1121/1.405805 [DOI] [Google Scholar]
- Peake, W. T., Rosowski, J. J., and Lynch, T. J.III. (1992). “Middle-ear transmission: Acoustic vs. ossicular coupling in cat and human,” Hear. Res. 57, 245–268. 10.1016/0378-5955(92)90155-G [DOI] [PubMed] [Google Scholar]
- Ravicz, M. E., Cooper, N. P., and Rosowski, J. J. (2008). “Gerbil middle-ear sound transmission from 100 Hz to 60 kHz,” J. Acoust. Soc. Am. 124, 363–380. 10.1121/1.2932061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravicz, M. E., and Rosowski, J. J. (2012a). “A new estimate of the middle-ear transmission matrix in chinchilla,” Abstracts of the 35th Midwinter Meeting of the Association for Research in Otolaryngology (Association for Research in Otolaryngology, Mt. Royal, NJ: ), No. 126.
- Ravicz, M. E., and Rosowski, J. J. (2012b). “Chinchilla middle-ear admittance and sound power: High-frequency estimates and effects of inner-ear modifications,” J. Acoust. Soc. Am. 132, 2437–2454. 10.1121/1.4750487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravicz, M. E., and Rosowski, J. J. (2013). “Inner-ear sound pressures near the base of the cochlea in chinchilla: Further investigation,” J. Acoust. Soc. Am. 133, 2208–2223. 10.1121/1.4792139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravicz, M. E., Rosowski, J. J., and Voigt, H. F. (1992). “Sound-power collection by the auditory periphery of the Mongolian gerbil Meriones unguiculatus: I. Middle-ear input impedance,” J. Acoust. Soc. Am. 92, 157–177. 10.1121/1.404280 [DOI] [PubMed] [Google Scholar]
- Rosowski, J. J. (1991). “The effects of external and middle ear filtering on auditory threshold and noise-induced hearing loss,” J. Acoust. Soc. Am. 90, 124–135 (erratum: J. Acoust. Soc. Am. 90, 3373–135). 10.1121/1.401306 [DOI] [PubMed] [Google Scholar]
- Rosowski, J. J. (1994). “Outer and middle ears,” in Comparative Hearing in Mammals, edited by Popper A. N. and Fay R. R. (Springer-Verlag, New York: ), pp. 173–247. [Google Scholar]
- Rosowski, J. J., Carney, L. H., Lynch, T. J., III, and Peake, W. T. (1986). “The effectiveness of external and middle ears in coupling acoustic power into the cochlea,” in Peripheral Auditory Mechanisms, edited by Allen J. B., Hall J. L., Hubbard A., Neely S. T., and Tubis A. (Springer-Verlag, New York: ), pp. 3–12. [Google Scholar]
- Rosowski, J. J., Carney, L. H., and Peake, W. T. (1988). “The radiation impedance of the external ear of cat: Measurements and applications,” J. Acoust. Soc. Am. 84, 1695–1708. 10.1121/1.397185 [DOI] [PubMed] [Google Scholar]
- Rosowski, J. J., Ravicz, M. E., and Songer, J. E. (2006). “Structures that contribute to middle-ear admittance in chinchilla,” J. Comp. Physiol., 192A, 1287–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru, P. (1997). “‘minphase.m’ Matlab function,” NSL Matlab toolbox, Neural Systems Laboratory, University of Maryland, www.isr.umd.edu/Labs/NSL/Software.htm (date last viewed 12/16/11).
- Ruggero, M. A., Rich, N. C., Robles, L., and Shivapuja, B. G. (1990). “Middle-ear response in the chinchilla and its relationship to mechanics at the base of the cochlea,” J. Acoust. Soc. Am. 87, 1612–1629. 10.1121/1.399409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, E. A. G. (1988). “Diffuse field response, receiver impedance and the acoustical reciprocity principle,” J. Acoust. Soc. Am. 84, 2284–2287. 10.1121/1.397026 [DOI] [Google Scholar]
- Shera, C. A., and Zweig, G. (1992). “Middle-ear phenomenology: The view from the three windows,” J. Acoust. Soc. Am. 92, 1356–1370. 10.1121/1.403929 [DOI] [PubMed] [Google Scholar]
- Slama, M. C. C., Ravicz, M. E., and Rosowski, J. J. (2010). “Middle ear function and cochlear input impedance in chinchilla,” J. Acoust. Soc. Am. 127, 1397–1410. 10.1121/1.3279830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songer, J. E., and Rosowski, J. J. (2006). “The effect of superior-canal opening on middle ear input admittance and air-conducted stapes velocity in chinchilla,” J. Acoust. Soc. Am. 120, 258–269. 10.1121/1.2204356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonndorf, J. (1976). “Relationship between the transmission characteristics of the conductive system and noise-induced hearing loss,” in Effects of Noise on Hearing, edited by Henderson D., Hamernik R. P., Dosanjh D., and Mills J. (Raven, New York: ), pp. 159–178. [Google Scholar]
- Voss, S. E., Rosowski, J. J., Merchant, S. N., and Peake, W. T. (2000). “Acoustic responses of the human middle ear,” Hear. Res. 150, 43–69. 10.1016/S0378-5955(00)00177-5 [DOI] [PubMed] [Google Scholar]
- Vrettakos, P. A., Dear, S. P., and Saunders, J. C. (1988). “Middle ear structure in the chinchilla: A quantitative study,” Am. J. Otolaryngol. 9, 58–67. 10.1016/S0196-0709(88)80009-7 [DOI] [PubMed] [Google Scholar]
- von Waetzmann, E., and Keibs, L. (1936). “Theoretischer und experimenteller Vergleich von Hörschwellenmessungen [Theoretical and experimental comparisons of hearing threshold determination],” Akustische Zeitschrift 1, 1–12. [Google Scholar]
- Zwislocki, J. J. (1962). “Analysis of the middle-ear function. Part I: Input impedance,” J. Acoust. Soc. Am. 34, 1514–1523. 10.1121/1.1918382 [DOI] [Google Scholar]