Abstract
OBJECTIVES
Aortic valve replacement (AVR) in geriatric patients (>75 years) with small aortic roots is a challenge. Patient–prosthesis mismatch and the long cross-clamp time necessary for stentless valves or root enlargement are matters of concern. We compared the results of AVR with sutureless valves (Sorin Perceval), against those with conventional biological valves.
METHODS
Between April 2007 and December 2012, 120 isolated AVRs were performed in patients with a small annulus (<22 mm) at our centre. In 70 patients (68 females, age 77.4 ± 5.5 years), conventional valves (C group) and in 50 patients (47 females, age 79.8 ± 4.5 years), sutureless valves (P group) were implanted. The Logistic EuroSCORE of the C group was 16.7 ± 10.4 and that of the P group 20.4 ± 10.7, (P = 0.054). Minimal-access surgery was performed in 4.3% (3/70) patients in the C group and 72% (36/50) patients in the P group.
RESULTS
The cardiopulmonary bypass (CPB) and cross-clamp times of the C group were 75.3 ± 23 and 50.3 ± 14.2 min vs 58.7 ± 20.9 and 30.1 ± 9 min in the P group, (P < 0.001). In the C group, two annulus enlargements were performed. Thirty-day mortality was 4.3% (n = 3) in the C group and 0 in the P group, (n.s.). At follow-up (up to 5 years), mortalities were 17.4% (n = 12) in the C group and 14% (n = 7) in the P group, (n.s.).
CONCLUSIONS
This study highlights the advantages of sutureless valves for geriatric patients with small aortic roots reflected by shorter cross-clamp and CPB times, even though most of these patients were operated on via a minimally invasive access. Moreover, due to the absence of a sewing ring, these valves are also almost stentless, with greater effective orifice area (EOA) for any given size. This may potentially result in better haemodynamics even without the root enlargement. This is of advantage, as several studies have shown that aortic root enlargement can significantly increase the risks of AVR. Moreover, as seen in this series, these valves may also enable a broader application of minimally invasive AVR.
Keywords: Small aortic root, Elderly patients, Aortic valve stenosis, Sutureless aortic valve
INTRODUCTION
Since 1960 when the first operation was performed, aortic valve replacement (AVR) has been the gold standard for the treatment of symptomatic aortic valve stenosis. Although various valves have been designed, the basic technique has remained similar. After the diseased native aortic valve is removed, the new valve prosthesis is anchored in the aortic annulus with the help of sutures under extracorporal circulation (ECC) and cross-clamping of the aorta. A significant part of the cross-clamp time is spent in placing these sutures in the aortic annulus and the valve prosthesis and then tying the knots. The conventional valves have a sewing ring for this purpose. Therefore, for any given valve size, at least some part of the external valve area is taken up by this sewing ring. This may lead to patient–prosthesis mismatch (PPM), particularly in patients with small aortic roots. Rahimtoola [1] first described this condition, in which the effective orifice area (EOA) of the impacted prosthesis may be inadequate for the patient's body size. This means that patients undergoing AVR for aortic valve stenosis need a valve prosthesis of an adequate size to avoid a possible patient-prosthesis mismatch (PPM) phenomenon.
In recent years, due to the ageing population, the incidence of aortic valve stenosis has increased. A majority of these geriatric patients are females of small stature with corresponding small aortic roots. Several authors have suggested that PPM may be associated with less regression of left ventricular hypertrophy and lower survival [2]. Although some other authors such as Blackstone et al. [3] have disputed this suggestion, surgeons have tried to avoid PPM with various options in patients with small aortic roots by the following means:
Implantation of stentless valves.
Full root replacement either with homograft, porcine grafts or the so-called Ross operation.
Implantation of a bigger-sized prosthesis after aortic root enlargement (ARE).
Both stentless valve implantation and aortic root replacements are technically more challenging than isolated AVR with conventional stented valves. Although these techniques may result in better haemodynamics, they are not widely used. ARE to implant a larger-sized prosthesis has been described by several surgeons [4–6]. However, many surgeons are still reluctant to use these techniques routinely. There have also been conflicting reports about the risks associated with these techniques [7, 8]. Moreover, Kulik et al. [9] reported that although ARE is a safe procedure, it does not appreciably improve long-term clinical outcome. All these above-mentioned techniques do prolong the cross-clamp and cardiopulmonary bypass (CPB) times even in experienced hands.
Recently, sutureless valves have been proposed as an ideal solution for this subset of patients [10]. As these valves do not need to be ‘sutured’, shorter cross-clamp and CPB times are potentially possible. Moreover, due to the absence of a sewing ring, these valves are also almost ‘stentless’, with a greater valve EOA for any given size. This may therefore result in better haemodynamics even without the root enlargement.
In this study, we compared the perioperative and mid-term follow-up results of AVR with sutureless valves (Sorin Perceval), against those with conventional biological valves in geriatric patients (>75 years) with small aortic roots (<22 mm).
MATERIALS AND METHODS
Between April 2007 and December 2012, 120 isolated AVR were performed in geriatric patients with small annulus (<22 mm) at our centre. Seventy patients (68 females, age 77.4 ± 5.5 years) received conventional stented biological valves (C group). An additional 50 patients (47 females, age 79.8 ± 4.5 years), fulfilling the inclusion and exclusion criteria for a European, multicentre, prospective, non-randomized, clinical feasibility trial, received the Perceval S sutureless aortic valve prosthesis (P group). The Logistic EuroSCRORE of patients in the C group was 16.7 ± 10.4 and that of the P group 20.4 ± 10.7, (P = 0.0054). Minimal-access surgery was performed in 4.3% (3/70) patients in the C group and 72% (36/50) patients in the P group, (<0.001). Patient characteristics are listed in Table 1. All patients involved in the Perceval trial also gave informed consent. Approval for this study was granted by the Institutional Review Board of our University Hospital.
Table 1:
Preoperative patient characteristics
| Parameters | Conventional group | Perceval group | P-value |
|---|---|---|---|
| Number of patients (n) | 70 | 50 | 0.014 |
| Gender (m/f) | 2/86 | 3/47 | n.s. |
| Age (years) | 77.4 ± 5.5 | 79.8 ± 4.5 | n.s. |
| Height (cm) | 159 ± 6.3 | 160 ± 6.8 | n.s. |
| Weight (kg) | 69.5 ± 12.3 | 70.7 ± 15.2 | n.s. |
| BMI | 27.5 ± 4.9 | 27.7 ± 5.5 | n.s. |
| BSA | 1.8 ± 0.17 | 1.7 ± 0.18 | n.s. |
| Mean gradient (mmHg) | 50.8 ± 16.5 | 4.84 ± 16.3 | n.s. |
| NYHA status | |||
| I (n) | 0 | 0 | |
| II (n) | 11 (15.9%) | 3 (6.1%) | |
| III (n) | 53 (76.8%) | 44 (89.8%) | |
| IV (n) | 5 (7.2%) | 2 (4.1%) | |
| EOA (cm2) | 0.65 ± 0.16 | 0.65 ± 0.19 | n.s. |
m/f: male/female; BSA: body surface area; BMI: body mass index; NYHA: New York Heart Association; EOA: effective orifice area; n.s.: not significant.
Statistical analysis
All data analyses were performed with IBM SPSS Statistic 20 for Windows (IBM Corporation, 1 New Orchard Road, Armonk, New York, NY, USA). The most continuous variables were normally distributed. They were expressed as mean with standard deviation. The not normally distributed variables were expressed as median. Statistical comparisons were made by independent-samples T-test, one-way analysis of variance, Mann–Whitney U-test, Kruskal–Wallis H-test, Pearson χ2-test, Fisher's exact test depending on the scale level, the distribution and the number of groups. Cox-regression was chosen for univariate and multivariate prognostic analysis of mid-time survival. Prognostic variables of postoperative death within 30 days and hospital stay were evaluated by logistic regression. A value of P < 0.05 was considered significant.
RESULTS
Perioperative data are presented in Table 2. The Kaplan–Meier survival curve is shown in Fig. 1. The follow-up data are given in Table 3.
Table 2:
Perioperative data
| Parameters | Conventional group | Perceval group | P-value |
|---|---|---|---|
| Number of patients (n) | 70 | 50 | |
| Ministernotomy (n) | 3 (4.3%) | 36 (72%) | <0.001 |
| Cross-clamp time (min) | 50.3 ± 14.2 | 30.1 ± 9.0 | <0.001 |
| CPB time (min) | 75.3 ± 23.0 | 58.7 ± 20.9 | <0.001 |
| Operation time (min) | 141.5 ± 33.5 | 125.4 ± 30.5 | 0.008 |
| Annulus enlargement (n) | 2 (2.8%) | 0 | |
| Conversion to full sternotomy (n) | 1 | 0 | |
| Mean gradient (mmHg) | 12.7 ± 4.3 | 15.9 ± 5.0 | n.s. |
| EOA (cm2) | 1.5 ± 0.18 | 1.5 ± 0.26 | n.s. |
| ICU stay (days) | 2.0 ± 2.6 | 1.8 ± 1.8 | n.s. |
| Hospital stay (days) | 15.9 ± 10.9 | 14.1 ± 7.5 | n.s. |
| Rethoracotomy due to postoperative (n) bleeding | 2 (2.85%) | 2 (4%) | n.s. |
| Para valvular leak (Grade 1), (n) | 1 (2.3%) | 4 (8.2%) | n.s. |
| 30-POD mortality (n) | 3 (4.3%) (cardiac: 2, pulmonary: 1) | 0 (0%) | n.s. |
CPB: cardiopulmonary bypass; EOA: effective orifice area; ICU: intensive care unit; POD: postoperative day.
Figure 1:
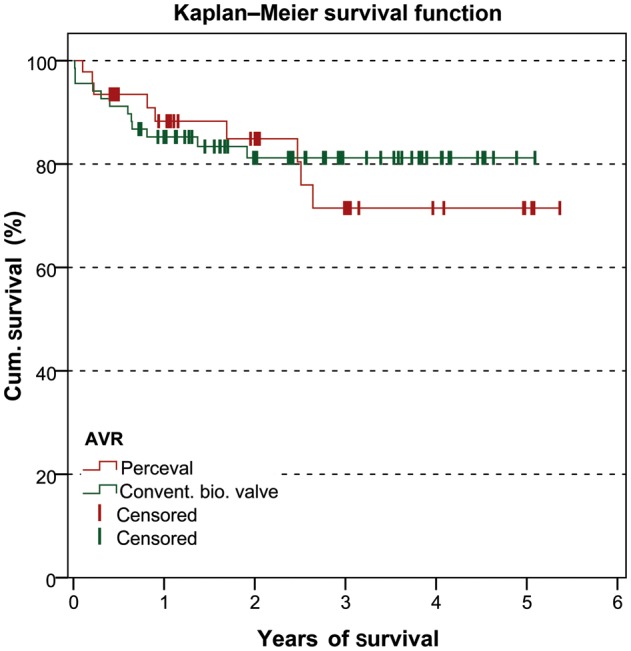
Kaplan–Meier survival curve.
Table 3:
The follow-up data
| Parameters | Conventional group | Perceval group | P-value |
|---|---|---|---|
| Lost to follow-up (n) | 3 (2.9%) | 3 (6.1%) | |
| Mean follow-up time (months) | 32.7 ± 15.5 | 22.7 ± 17.5 | 0.002 |
| Mean gradient (mmHg) | 15.4 ± 6.3 | 13.6 ± 5.4 | n.s. |
| EOA (cm2) | 1.3 ± 0.2 | 1.5 ± 0.25 | <0.001 |
| Mortality at 1 year (n) | 10 (16.4%) | 5 (13.2%) | n.s. |
| Cause (cardiac, pulmonary, renal, Ca, unknown) | (4/1/2/1/2) | (1/2/1/1/0) | |
| Mortality at 3 years (n) | 12 (34.3%) | 9 (39.1%) | n.s. |
| Cause (cardiac, pulmonary, renal, Ca, sepsis, unknown) | 4/1/2/1/1/3 | 1/2/1/1/1/1/3 | |
| Endocarditis (n) | 1 (1.4%) | 3 (6%) | n.s. |
| Re-AVR (n) | 1 (1.4%) | 2 (4%) | n.s. |
EOA: effective orifice area; Re-AVR: Re-aortic valve replacement.
Conventional group
Perioperative results
The CPB and cross-clamp times were 75.3 ± 23 and 50.3 ± 14.2 min. The mean operation time was 141.5 ± 33.5 min (full sternotomy: 139.8 ± 32.6 min, ministernotomy: 181.3 ± 34.1). Two patients received additional AREs. Only 4.3% (3/70) of these patients underwent AVR via an upper ministernotomy. The rest of the patients (67/70) underwent AVR via a full median sternotomy. The patients received either size 19 mm valve (n = 6) or size 21 valves (n = 64). The majority of them are Sorin Mitroflow (size 19: n = 6, size 21: n = 44). The rest of the size 21 valves were Carpentier Edwards Perimount (n = 12), St. Judes Epic Supra (n = 4), Medtronic Hancock II Ultra (n = 2) and Medtronic Mosaic Cinch (n = 2). The valves were implanted in a supra-annular position using pledgeted sutures.
Thirty-day mortality was 4.3% (n = 3). The causes of death were cardiac in 2 patients and respiratory in 1. The mean gradient and the EOA of the valve prosthesis at discharge were 12.7 ± 4.3 mmHg and 1.5 ± 0.18 cm2, respectively. The ICU and hospital stays were 2.0 ± 2.6 days and 15.9 ± 10.9 days. The left ventricular ejection fraction at discharge was 55.2 ± 9.7%.
Follow-up
The mean follow-up time was 32.7 ± 15.5 months. Two patients were lost to follow-up. The mean gradient and the EOA of the valve prosthesis at follow-up were 15.4 ± 6.3 mmHg and 1.3 ± 0.2 cm2, respectively. The left ventricular ejection fraction at follow-up was 60.9 ± 10.3%. The mortalities at 1 year and 3 years were 16.4% (n = 10) and 34.3% (n = 12), respectively. One patient developed prosthesis endocarditis at follow-up. Another patient had a paravalvular leak grade 1.
Perceval group
Perioperative results
The valves were successfully and firmly positioned under visual control in all patients; no death occurred during the procedure. The CPB and cross-clamp times were 58.7 ± 20.9 and 30.1 ± 9 min, respectively. Thirty-day mortality was 0%. The mean operation time was 25.4 ± 30.3 min (full sternotomy: 105.9 ± 13.1 min, ministernotomy: 133.0 ± 31.7 min). Of note, 72% (36/50) of these patients underwent AVR via an upper ministernotomy. The rest of the patients (14/50) underwent AVR via a full median sternotomy.
These patients received either a size 21 mm valve (n = 12) or a size 23 valve (n = 38). The mean gradient and the EOA of the valve prosthesis at discharge were 15.9 ± 5 mmHg and 1.5 ± 0.26 cm2, respectively. The ICU and hospital stays were 1.8 ± 1.8 days and 14.1 ± 7.5 days.
Follow-up
The mean follow-up time was 22.7 ± 17.5 months. Three patients were lost to follow-up. The mean gradient and the EOA of the valve prosthesis at follow-up were 13.6 ± 5.4 mmHg and 1.5 ± 0.25 cm2, respectively. The left ventricular ejection fraction at follow-up was 62.2 ± 7.6%. The mortalities at 1 year and 3 years were 13.2% (n = 5) and 39.1% (n = 9), respectively. At follow-up, 3 patients developed prosthesis endocarditis and 4 had Grade 1 leakage without haemolysis.
Comparison of the two groups
Tables 1–3 give an overview of the comparison of the two groups. In short, the preoperative characteristics of the two groups did not differ significantly. The Logistic EuroSCRORE of the C group was 16.7 ± 10.4 and that of the P group 20.4 ± 10.7, respectively (P = 0.054). The cross-clamp times (C group 50.3 ± 14.2 min vs P group 30.1 ± 9.0 min, P < 0.001) and CBP times (C group 75.3 ± 23.0 min vs P group 58.7 ± 20.9 min, P < 0.001) were significantly shorter in the P group. Total operational times also tended to be shorter in the P group, albeit, without reaching statistical significance (C group 141.5 ± 33.5 vs P group 125.4 ± 30.5, P = 0.008). The early postoperative course was comparable in terms of ICU stay, complications, mortality and paravalvular leakages. Mean gradient (C group 12.7 ± 4.3 mmHg vs P group 15.9 ± 5.0 mmHg, n.s) as well as EOA (C group 1.5 ± 0.18 cm2 vs P group 1.5 ± 0.26 cm2, n.s.) did not differ postoperatively.
The follow-up of the C group was significantly longer than the P group (C group 32.7 ± 15.5 months vs P group 22.7 ± 17.5 months, P < 0.002). Mean gradient was comparable during the follow-up time (C group 15.4 ± 6.3 mmHg vs P group 13.6 ± 5.4 mmHg, n.s.). The EOA was significantly higher in the P group compared with the C group (C group 1.3 ± 0.2 vs P group 1.5 ± 0.25, P < 0.001), due to a decrease in EOA in the C group. There were no differences between mortality rates at 1- and 3-year follow-up.
DISCUSSION
Surgical AVR has been the gold standard of treatment in patients with symptomatic aortic valve stenosis [11, 12]. The primary goal of this operation is to alleviate the pressure overload on the left ventricle and allow regression as well as remodelling of the left ventricular mass. Logically, a larger-sized valve would reduce the gradient by a greater margin, leading to greater regression of the hypertrophied left ventricle. A smaller-sized prosthetic valve may result in so-called PPM. Therefore, different options have been proposed for patients with small aortic root presenting for AVR. As discussed above, these options are stentless valves, ARE and even complete aortic root replacement. However, all these options are technically more complex and take longer than isolated ‘conventional’ AVR with stented valve prostheses. Therefore, these techniques have not been popular with surgeons.
Due to the changing population demographics, the age of the patients presenting for AVR is also increasing. According to the data base of the German Society of Cardiothoracic and Vascular Surgery (GSCTVS), >50% of the patients presenting for cardiac surgery in Germany are above the age of 70 years and >10% are the above the age of 80 [13]. The situation is similar in most of the industrialized countries. A large proportion of these geriatric patients are small women. In addition to the presence of significant comorbidities, these patients are also usually small in stature and have calcified aortic roots. Conventional AVR in these geriatric patients with small and calcified aortic roots present a unique challenge to the surgical community. On the one hand, is the need for implantation of the biggest possible prosthesis to maximize the alleviation of the pressure gradient and, on the other hand is the need to keep the cross-clamp and CPB times as short as possible due to the higher perioperative risks. Due to the second reason, many surgeons implant conventional stented valves and accept the possibility of PPM in such patients. Several studies have shown that better results are obtained with minimal-access AVR compared with full sternotomy patients [14–16]. However, limited exposure to the operative field increases the technical complexity for the surgeon and is reflected in longer operative times in minimally invasive cases. Moreover, minimal-access AVR is technically even more difficult in these geriatric patients. This may be one of the reasons that only ∼10% of the isolated AVR in Germany are done via a minimally invasive access as reflected in the database of the GSCTVS [13]. Therefore, there is a need for new options of AVR to treat these patients optimally. The idea of a ‘sutureless’ valve implantation itself is not new. Magovern and Cromie [17] performed the first implantation of such a valve in 1962. These early sutureless valves were bulky and technically difficult to implant, particularly in a small aortic annulus. Moreover, paravalvular leakage was frequent. Thrombosis and ball variance resulting in poppet failure was a common late occurrence. Due to these reasons, these early sutureless valves fell out of favour [18]. The modern sutureless Perceval valve has overcome these disadvantages. Additionally, these valves may have following advantages:
The absence of anchoring sutures should reduce the cross-clamp and consequently ECC times.
The absence of a ‘suturing ring’ potentially results in increased functional valvular diameter for any given valve size. This may reduce the risks of PPM.
The absence of the need for sutures may potentially make minimal-access AVR technically easier and more reproducible.
Between April 2007 and December 2011, we implanted 100 Perceval sutureless valves as a part of a prospective, European, multicentre, non-randomized, clinical feasibility pilot trial. This trial was performed in three stages with time intervals between them. Only a limited number of Perceval valves were available for the study, so not all patients who fitted the inclusion criteria could be included in the trial. This is the reason for a shorter mean follow-up time in this group of patients. This study highlights the advantages of the Perceval S self-anchoring valve. It is a technically simple and reproducible alternative to AVR. As the valve does not need to be sutured, the limited exposure is not a disadvantage even in patients with calcified or small aortic roots. The lack of necessity for the valve prosthesis to be anchored with sutures potentially reduces the cross-clamp and CPB times. This valve may enable a broader application of minimally invasive AVR. Further longer-term experience is needed to determine the potential clinical benefits and durability of the Perceval S self-anchoring valve.
CONCLUSION
In summary, the data presented here highlight the advantages of the Perceval S sutureless valve in geriatric patients with small aortic roots both in the short- as well as mid-term. Furthermore, this device appears to be ideal for patients with severe calcification of the aortic roots. Further experience is needed to determine the potential clinical benefits of these sutureless valves.
LIMITATIONS
The main limitation of this study is that it is not a prospective randomized study. Another limitation is that the patient cohorts are small. The third limitation is that the Perceval valves were implanted only by two senior surgeons while AVRs with conventional valves were done by all of the staff surgeons, including the two senior surgeons.
Funding
The institution received an unrestricted research grant from Sorin for the conduct of the study.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr U. Lockowandt (Stockholm, Sweden): When you operate on elderly ladies with small aortas, you have several problems. One could be the valve, another problem could be the ascending aorta, which could be small and very calcified. I wonder, if you do a Perceval stentless implantation, is there any possibility of making the aortic incision smaller in order to place it far down or far up if you find a place with less calcification, or perhaps even asymmetrical, left or right, if you can find places with less calcification?
Secondly, I don't know what it is like in Hannover, but in Sweden health care is on a very tight fiscal budget. As alluded to earlier, the introduction of new techniques is difficult if they are more costly. Have you done any cost comparisons between the conventional and the stentless solutions?
Dr Shrestha: In answer to the first question, the Perceval is a sutureless valve. Because of the stent above the leaflets, the aortotomy has to be done a little bit higher, and we use a transverse aortotomy and, because the valve is collapsed and mounted, you see all around. You don't need to do a total transection of the aorta, just a small incision. If the ascending aorta is very calcified, you can always find a place where you can cut, because you don't need to put the sutures all around. Technically it is a lot easier with this valve.
Concerning your second question, of course you are right. In my previous talk I also said that the limiting factor right now is the cost. We hope that it will go down in the future. That is why, at least in Hannover, we are putting this valve in only very old patients, because in the younger age patient the cost is also a factor. If the cost comes down, I think that this valve will be implanted in even more patients.
REFERENCES
- 1.Rahimtoola SH. The problem of valve prosthesis-patient mismatch. Circulation. 1978;58:20–4. doi: 10.1161/01.cir.58.1.20. [DOI] [PubMed] [Google Scholar]
- 2.Mohty D, Dumesnil JG, Echahidi N, Mathieu P. Impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: influence of age, obesity, and left ventricular dysfunction. J Am Coll Cardiol. 2009;53:39–47. doi: 10.1016/j.jacc.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Blackstone EH, Cosgrove DM, Jamieson WR, Birkmeyer NJ, Lemmer JH, Miller DC, et al. Prosthesis size and long-term survival after aortic valve replacement. J Thorac Cardiovasc Surg. 2003;126:783–93. doi: 10.1016/s0022-5223(03)00591-9. [DOI] [PubMed] [Google Scholar]
- 4.Nicks R, Cartmill T, Bernstein L. Hypoplasia of the aortic root: the problem of aortic valve replacement. Thorax. 1970;25:339–346. doi: 10.1136/thx.25.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manouguian S, Seyblod-Epting W. Patch enlargement of the aortic valve ring by extending the aortic incision to the anterior mitral leaflet. J Thorac Cardiovasc Surg. 1979;78:402–12. [PubMed] [Google Scholar]
- 6.Konno S, Imai Y, Lida Y, Nakajima M, Tatsuno K. A new method for prosthetic valve replacement in congenital aortic stenosis associated with hypoplasia of the aortic ring. J Thorac Cardiovasc Surg. 1975;70:909–17. [PubMed] [Google Scholar]
- 7.Sommers KE, David TE. Aortic valve replacement with patch enlargement of the aortic annulus. Ann Thorac Surg. 1997;63:1608–12. doi: 10.1016/s0003-4975(97)00127-6. [DOI] [PubMed] [Google Scholar]
- 8.Coutinho GF, Correia PM, Pauperio G, de Oliveira F, Antunes MJ. Aortic root enlargement does not increase the surgical risk and short-term patient outcome? Eur J Cardiothorac Surg. 2011;40:441–7. doi: 10.1016/j.ejcts.2010.11.064. [DOI] [PubMed] [Google Scholar]
- 9.Kulik A, Al-Saigh M, Chan V, Masters RG. Enlargement of the small aortic root during aortic valve replacement: is there a benefit? Ann Thorac Surg. 2008;85:94–101. doi: 10.1016/j.athoracsur.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 10.Shrestha M, Folliguet T, Meuris B, Dibie A, Bara C, Herregods MC, et al. Sutureless Perceval S aortic valve replacement: a multicenter prospective pilot trial. J Heart Valve Dis. 2009;18:11. [PubMed] [Google Scholar]
- 11.Brown JM, O'Brien SM, Wu C, Sikora JA. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137:82–90. doi: 10.1016/j.jtcvs.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Eusanio M, Fortuna D, Palma R, Dell'Amore A, Lamarra M, Gontini G, et al. Aortic valve replacement: results and predictors of mortality from a contemporary series of 2256 patients. J Thorac Cardiovasc Surg. 2011;141:940–7. doi: 10.1016/j.jtcvs.2010.05.044. [DOI] [PubMed] [Google Scholar]
- 13.Gummert JF, Funkat AK, Beckmann A, Ernst M, Hekmat K, Beyersdorf F, et al. Cardiac Surgery in Germany during 2010: a report on behalf of the German Society for Thoracic and cardiovascular Surgery. Thorac Cardiovasc Surg. 2011;59:259–67. doi: 10.1055/s-0030-1271191. [DOI] [PubMed] [Google Scholar]
- 14.Mihaljevic T, Cohn LH, Unic D, Aranki SF, Couper GS, Byrne JG. One thousand minimally invasive valve operations: early and late results. Ann Surg. 2004;240:529–34. doi: 10.1097/01.sla.0000137141.55267.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonacchi M, Prifti E, Giunti G, Frati G, Sani G. Does ministernotomy improve postoperative outcome in aortic valve operation? A prospective randomized study. Ann Thorac Surg. 2002;73:460–5. doi: 10.1016/s0003-4975(01)03402-6. [DOI] [PubMed] [Google Scholar]
- 16.Tabata M, Umakanthan R, Cohn LH, Bolman RM, III, Shekar PS, Chen FY, et al. Early and late outcomes of 1000 minimally invasive aortic valve operations. Eur J Cardiothorac Surg. 2008;33:537–41. doi: 10.1016/j.ejcts.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 17.Magovern GJ, Cromie HW. Sutureless prosthetic heart valves. J Thorac Cardiovasc Surg. 1963;46:726–736. [PubMed] [Google Scholar]
- 18.Scott SM, Sethi GK, Flye MW, Takaro T. The sutureless aortic valve prosthesis: experience with and technical considerations for replacement of the early model. Ann Surg. 1976;184:174–8. doi: 10.1097/00000658-197608000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]


