Abstract
OBJECTIVES
The aim of this study is to compare thoracoscopic mobilization of the oesophagus in the lateral decubitus position and the semiprone position and to identify potential differences between the two techniques.
METHODS
A retrospective review of a prospectively maintained oesophagectomy database identified 150 patients undergoing combined thoracoscopic and laparoscopic oesophagectomy (TLO). Of these, 90 cases underwent thoracoscopic oesophageal mobilization in the left lateral decubitus position. The remaining 60 cases underwent thoracoscopic oesophageal mobilization in the semiprone position.
RESULTS
There were no differences in the clinicopathological factors and tumour characteristics between the two groups. There was no significant difference in the blood loss, operation time, the incidence of conversion, length of hospital stay or in the number of retrieved mediastinal and abdominal nodes between the two groups. There was no significant difference with regard to the incidence of respiratory complications, anastomotic leaks, vocal cord palsy, chylothorax, delayed gastric emptying, arrhythmia and intestinal obstruction between the two groups.
CONCLUSIONS
The semiprone and lateral decubitus positions each have their inherent advantages and disadvantages. Our initial experience confirmed that while the semiprone position is associated with superior surgical ergonomics and better exposure of the posterior mediastinum, there is no convincing evidence that semiprone thoracoscopic oesophagectomy is superior to the left lateral decubitus positioning with respect to the major surgical outcomes and oncological clearance.
Keywords: Thoracoscopy, Oesophageal cancer, Oesophageal surgery
INTRODUCTION
Cuschieri et al. [1] first performed thoracoscopic oesophagectomy with the patient in the left lateral decubitus position (LLD) in 1992. Since lateral positioning is familiar to surgeons and allows for conversion to an open procedure if necessary, our centre has adopted this technique when performing minimally invasive oesophagectomy. In 1994, Cuschieri et al. [2] once again described thoracoscopic mobilization of the oesophagus, but in the prone position in 6 patients. The author commented that the prone position is associated with excellent exposure of the posterior mediastinum due to the effects of gravity pooling blood outside the operative view and the reduced need for lung retraction. However, conversion to a classic thoracotomy is more difficult in the prone position. To overcome this problem while retaining the benefits of the fully prone position, a modified semiprone position (SP) has been used for thoracoscopic oesophagectomy in our centre since August 2011. The patient is fixed in the LLD and leaned forward 45°. However, there is currently no evidence with which to judge whether the thoracoscopic phase of a three-stage thoracolaparoscopic oesophagectomy is best performed in the LLD or SP. In this retrospective analysis, we sought to compare thoracoscopic mobilization of the oesophagus in the lateral decubitus position and the SP to identify potential differences between the two techniques.
PATIENTS AND METHODS
Patients
Following Institutional Review Board approval at the Affiliated Union Hospital of Fujian Medical University, a retrospective review of a prospectively maintained three-stage thoracoscopic and laparoscopic oesophagectomy (TLO) database was performed. All patients were evaluated and underwent disease staging. Clinical staging was based on oesophagography, oesophagoscopy, colour ultrasound of the neck and enhanced computed tomography (CT) of the chest and abdomen. Positron emission tomography or bronchofibrescopy was also performed if indicated for the determination of individual staging. The tumour stages were classified according to the tumour node metastasis classification of the International Union Against Cancer (UICC 2009 7th edition). All medically fit patients with resectable thoracic oesophageal cancers (T1–T3 tumours), with or without N1 nodal status, were included in the study, whereas patients with T4 tumours were not included.
A cohort of 150 patients with thoracic oesophageal cancer undergoing TLO was enrolled in this study. Of these, 90 cases from October 2009 to June 2011 had thoracoscopic oesophageal mobilization performed in the left lateral decubitus position, Group LLD. From August 2011 to June 2012, 60 cases underwent thoracoscopic oesophageal mobilization in the semiprone position, Group SP. To avoid our early experience with TLO-affected outcomes (a plausible learning curve), the initial 20 consecutive TLO patients in each group were excluded from evaluation. Furthermore, all members of the surgical team, including the assistants, remained the same to ensure consistency and to obtain early and maximal efficiency in performing TLO during the initial period of this study. Data were recorded prospectively in an electronic database. Clinicopathological factors and surgical outcomes, including time of operation, thoracoscopic and abdominal estimated blood loss, number of retrieved mediastinal and abdominal lymph nodes, length of hospital stay and incidence of major complications, were compared between the two groups. Major complications were considered if they required any medical or surgical intervention or prolonged recovery.
Surgical technique
The TLO was performed in three stages that began with thoracoscopic oesophageal mobilization and mediastinal lymph node dissection. This was performed in either the LLD or the SP and is described below. The patient was then placed in the supine position and laparoscopy was performed to mobilize and create the gastric conduit. Through a left cervical neck incision along the anterior border of the sternocleidomastoid muscle, a cervical oesophagogastric anastomosis was created. Abdominal and cervical procedures were similar in the two groups.
Thoracoscopic oesophagectomy in the LLD
After intubation with a double-lumen tube, the patient is positioned in the LLD. We make four incisions: a 1-cm incision at the seventh intercostal space (ICS) on the anterior axillary line for a camera (Port I). The surgeon, facing the patient's back, uses a 2-cm incision at the eighth ICS on the middle axillary line (Port III) for the ultrasonic scalpel or endostapler and a 1.5-cm incision at the sixth ICS on the posterior axillary line (Port IV) for other instruments. The assistant faces the patient's chest, using a 4-cm utility incision at the fourth ICS on the anterior axillary line (Port II) for retraction and counter traction during the oesophageal dissection (Fig. 1).
Figure 1:
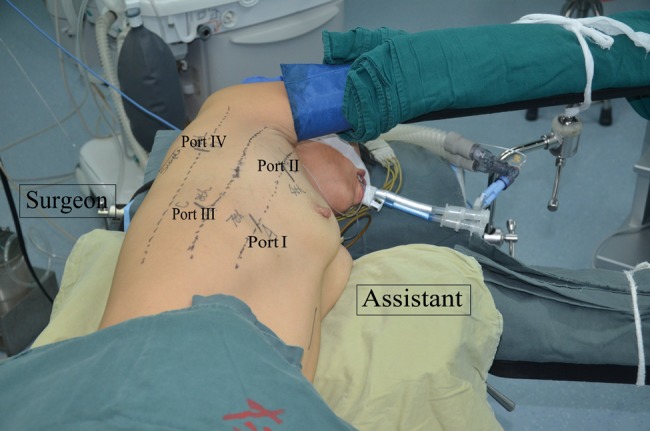
The position of the patient and the four ports for thoracoscopic oesophagectomy in the LLD.
Our surgical technique for the lateral decubitus approach has previously been described in detail [3]. Basically, the same methods as used for oesophagectomy under thoracotomy were applied. Circumferential mobilization of the oesophagus was undertaken caudorostrally with surrounding lymph nodes and perioesophageal tissue to expose the aortic wall, left mediastinal pleura, pericardium, membranous portion of the tracheobronchus and the diaphragm (Supplementary Video 1). After the entire oesophagus was mobilized, the trachea was rotated by a self-made smoothed tip retractor [3]. The retractor consists of a 3-cm wide tip, narrow intermediate pole and a hand grip. The tip was inserted through the 4-cm utility incision at the fourth ICS. It was rotated to retract the trachea or the main bronchus to allow meticulous dissection of lymph nodes deep in the upper mediastinal space. The table was rotated 15° towards the assistant if necessary. A 30° telescope presented a good view. The lymph nodes around the bilateral recurrent laryngeal nerves were completely removed, with identification and preservation of those nerves. Subsequently, the tracheobronchial nodes and subcarinal nodes were dissected separately.
Supplementary video 1:
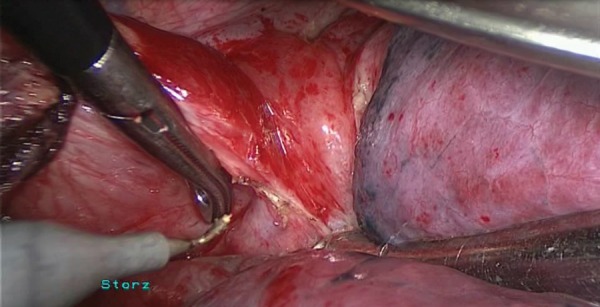
Thoracoscopic oesophageal mobilization in the LLD.
Thoracoscopic oesophagectomy in SP
Following a double-lumen endotracheal intubation, the patient is initially placed in the LLD and leaned forward 45°, with the arm raised on an arm rest. The surgeon and camera operator face the patient's chest, and the assistant faces the patient's back. Four thoracoscopic ports were used: a 10.5-mm camera port (Port I) is created at the seventh ICS on the posterior axillary line, and the remaining three incisions are placed at the fourth ICS (Port II) on the mid-axillary line, and the seventh (Port III) and ninth ICS (Port IV) inferior to the scapular tip (Fig. 2). The chest cavity is inflated via the trocar (Port III) by means of a carbon dioxide (CO2) insufflation pressure of 8 mmHg, if needed.
Figure 2:
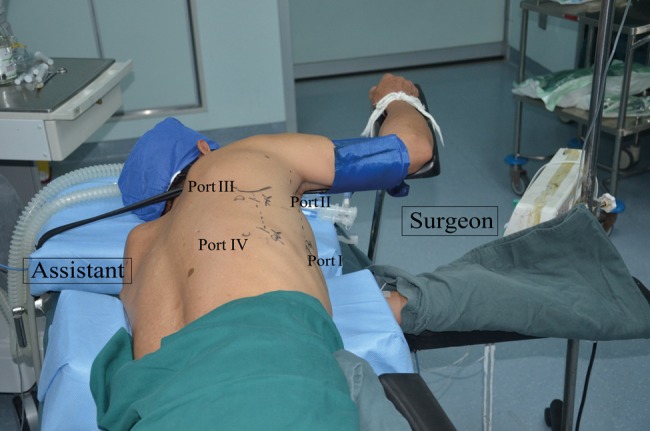
The position of the patient and the four ports for thoracoscopic oesophagectomy in the SP.
In the semiprone approach, the surgeon operates in a plane parallel to the view of the camera. That is, the oesophageal mobilization is dissected with a ‘rolling type’ of motion (Supplementary Video 2). The procedure is begun by dissection of the middle to lower oesophagus. The mediastinal pleura overlying the oesophagus is opened from the level of the azygous vein to the crus. The oesophagus and peripheral lymph nodes are circumferentially mobilized from the descending aorta, pericardium and the left mediastinal pleura. During this procedure, the surgeon uses a grasper in the left hand within Port IV and an electrocautery or harmonic scalpel in the right hand within Port II. The arch of the azygos vein is routinely ligated and transected for better visualization. The mediastinal pleura is incised cranially along the right vagus nerve. The original portion of the right recurrent laryngeal nerve is identified just caudal to the right subclavian artery and surrounding lymph nodes are dissected. Retracting the oesophagus dorsally and pressing the trachea ventrally, dissection of the lymph nodes around the left recurrent laryngeal nerve is simplified. After complete mobilization of the oesophagus, the subcarinal and bilateral bronchial lymph nodes are completely dissected. During this procedure of the upper thorax, the surgeon uses a grasper in the left hand within Port III and an electrocautery or harmonic scalpel in the right hand within Port II.
Supplementary video 2:
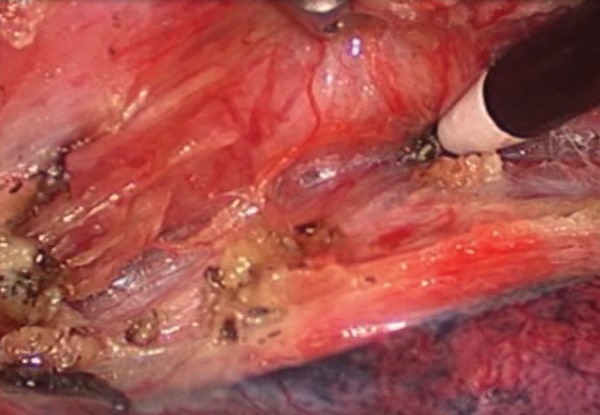
Thoracoscopic oesophageal mobilization in the SP.
Statistics
All statistical analyses were performed with the Statistical Package for Social Sciences version 11.5 (SPSS, Chicago, IL, USA). Continuous variables, including age, operation time, the amount of blood loss, length of hospital stay and number of dissected nodes, were tested for normal distribution first. If the assumptions of normality were met, continuous variables were expressed as means ± standard deviation. Student's t-test was used for comparison between groups. The amount of blood loss, length of hospital stay and the operation time did not show a normal distribution and are expressed as medians (inter quartile range). Wilcoxon rank-sum tests were used for comparison between groups. Discrete variables, including gender, tumour location, pathology type, accidental conversion and the incidence of complications, are described as numbers. Comparisons of discrete variables between groups were done using Fisher's exact test. Wilcoxon rank-sum tests were adopted for comparison of difference of pathology stage between groups. P-values of <0.05 were considered to be statistically significant.
RESULTS
Patient and tumour characteristics are listed in Table 1. There were no differences in the clinicopathological factors and tumour characteristics between the two groups (Table 1).
Table 1:
Demographics and clinicopathological factors of patients
| LLD | SP | P-value | |
|---|---|---|---|
| Cases | 90 | 60 | |
| Age (years) | 59.8 ± 9.1 | 57.7 ± 8.6 | 0.11 |
| Sex | |||
| Male:Female | 65:25 | 45:15 | 0.71 |
| Tumour location | |||
| Upper | 4 (4%) | 8 (13%) | 0.11 |
| Middle | 68 (76%) | 38 (63%) | |
| Lower | 18 (20%) | 14 (24%) | |
| Pathology type | |||
| Squamous cell | 87 (97%) | 57 (95%) | 0.68 |
| Others | 3 (3%) | 3 (5%) | |
| Pathology stage | |||
| I (Ia + Ib) | 20 (22%) | 14 (23%) | 0.72 |
| II (IIa + IIb) | 31 (34%) | 22 (37%) | |
| III (IIIa + IIIb + IIIc) | 39 (44%) | 24 (40%) | |
There was no intraoperative mortality in either group. Conversion to open thoracotomy was required for 4 patients in Group LLD. The specific indications for conversion were as follows: bulk tumour (n = 2), dense pleural adhesions (n = 1) and intraoperative left main bronchial membranous injury (n = 1). In Group SP, there were two conversions to open thoracotomy due to dense pleural adhesions (n = 1) and intraoperative bleeding from the azygos vein (n = 1). Once conversion was needed in the SP, the patient was turned to a standard lateral decubitus position to complete the procedure. Port II was extended to convert to a lateral thoracotomy. One patient in each group was converted to a conventional open laparotomy; 1 because of severe obesity (Group LLD) and the other because of dense adhesions derived from previous chronic cholecystitis (Group SP). The surgical outcomes in Group LLD were compared with those in Group SP and there was no significant difference between the two groups in blood loss, operation time, incidence of accidental conversion, length of hospital stay or the number of retrieved mediastinal and abdominal nodes (Table 2).
Table 2:
Surgical results of a thoracolaparoscopic oesophagectomy
| Factor | LLD (n = 90) | SP (n = 60) | P-value |
|---|---|---|---|
| Amount of blood loss (ml) | |||
| Thorax | 150 (100–200) | 150 (150–200) | 0.22 |
| Abdominal and neck | 20 (20–30) | 25 (20–50) | 0.40 |
| Total operation time (min) | |||
| Chest | 120 (110–150) | 120 (110–180) | 0.26 |
| Abdomen and neck | 105 (95–120) | 110 (100–120) | 0.66 |
| Conversion (cases) | |||
| Thoracotomy | 4 (4%) | 2 (3%) | 1.00 |
| Laparotomy | |||
| Number of retrieved nodes | 1 (1%) | 1 (2%) | 1.00 |
| Mediastinal nodes | 15 ± 6 | 14 ± 6 | 0.24 |
| Recurrent laryngeal nerve nodes | 3 ± 2 | 3 ± 2 | 0.90 |
| Abdominal nodes | 8 ± 5 | 8 ± 4 | 0.51 |
| Length of hospital stay | 13 (11–16) | 14 (12–16) | 0.19 |
Postoperative major complications developed in 30 of the 90 patients in Group LLD (33%) and in 22 of the 60 patients in Group SP (37%). There was no significant difference with regard to the incidence of respiratory complications, anastomotic leaks, vocal cord palsy, chylothorax, delayed gastric emptying, arrhythmia and intestinal obstruction between the two groups (Table 3). Pneumonia was the most common complication in both groups, seen in 10 patients in Group LLD (11%) and in 7 patients in Group SP (12%). Of these cases, there was 1 patient in each group who developed respiratory failure and required mechanical ventilation. Anastomotic leak was seen in 5 patients in Group SP (8%), but in only 3 patients in Group LLD (3%). All of these patients were treated conservatively with drainage and nutritional support. Postoperative hoarseness was observed in 9 patients in Group LLD (10%) vs 5 patients in Group SP (8%). All patients had recovered from hoarseness at six months’ follow-up. In Group LLD, 2 patients had postoperative intestinal obstructions due to enteroparalysis (n = 1) and intestinal adhesions (n = 1), which required second operations for adhesion release. In Group SP, 1 patient developed a chylothorax postoperatively, which was explored and repaired thoracoscopically. Each group had 1 patient dying from respiratory failure caused by pneumonia.
Table 3:
Major complications after a thoracolaparoscopic oesophagectomy
| LLD (n = 90) | SP (n = 60) | P-value | |
|---|---|---|---|
| Pneumonia | 10 (11%) | 7 (12%) | 1.00 |
| Anastomotic leak | 3 (3%) | 5 (8%) | 0.27 |
| Vocal cord palsy | 9 (10%) | 5 (8%) | 0.78 |
| Chylothorax | 2 (2%) | 2 (3%) | 1.00 |
| Delayed gastric emptying | 2 (2%) | 2 (3%) | 1.00 |
| Arrhythmia | 6 (7%) | 4 (7%) | 1.00 |
| Intestinal obstruction | 2 (2%) | 0 (0%) | 0.52 |
| Overall | 30 (33%)a | 22 (37%)b | 0.73 |
aTwo patients had two complications; 1 patient had three complications.
bFour patients had two complications.
COMMENT
There are several patient positions that can be used for thoracoscopic oesophageal surgery, including the lateral decubitus, full-prone position and SP. To date three retrospective studies [4–6] have compared the prone and lateral techniques, and the clinical outcomes were not significantly different. Most authors conclude that there is no convincing evidence that prone thoracoscopic oesophagectomy is superior to the left lateral decubitus positioning. In our centre, the modified SP is a preferred alternative to the full-prone position for thoracoscopic oesophagectomy. However, whether the theoretical advantages of the semiprone technique might translate into actual clinical practice has remained unclear. This study represents the first comparison between the lateral decubitus position and SP used for thoracoscopic mobilization. To evaluate the benefits and limitations of the two positions, technical feasibility and security, surgical ergonomics as well as oncological clearance should all be considered.
Minimally invasive oesophageal cancer surgery, including TLO, was confirmed to be safe and comparable with an open approach with respect to postoperative recovery and cancer survival [7, 8]. There was no intraoperative mortality in our 150 patients. No significant difference between the semiprone and lateral decubitus groups was found with regard to blood loss, operative time, length of hospital stay and the number of lymph nodes retrieved. There was also no difference in terms of perioperative complications including pneumonia, anastomotic leak or recurrent laryngeal nerve palsy between the two groups. The surgical results of our study have confirmed that both approaches are feasible, safe and have reasonable outcomes compared with the results reported in the literature.
In terms of the surgical ergonomics, the lateral decubitus position and SP both have benefits and limitations. We were trained, and had experience, in performing right lateral thoracotomy. With the lateral decubitus approach, the anatomic orientation was the same not only for the operating surgeon but also for the entire team. We believe that the familiar position and anatomic orientation may help a team to adapt to thoracoscopic oesophagectomy and may also reduce the learning curve. In our institution, we routinely create a 4-cm utility thoracic incision at the anterior axillary line at the fourth ICS. This port allows the fellow to assist more effectively, using retraction for improved exposure or for simultaneous insertion of added instruments. The surgeon operates in a plane perpendicular to the view of the camera. The oesophageal mobilization is carried cranially from the diaphragmatic reflection to the thoracic inlet. Besides, conversion to open surgery, if required, is better achieved with the patient in this position. However, in the lateral position, the oesophagus lays at the most dependent portion of the chest, where it is often obscured by the overlying lung. The technical skill of the assistant is critical to exposing the operative views quickly and gently by the use of the retractor and suction.
Compared with the lateral decubitus position, the SP gives better exposure of the posterior mediastinum, subcarinal and paratracheal spaces due to the effects of gravity reducing the need for lung retraction. That is to say, this position partly eliminates the need for a skilled assistant who is critical to exposure of these areas with the patient in the lateral position. In terms of the surgical ergonomics, this position also shortens the length and decreases the angle between the working ports and the cephalad and caudal extremes of the oesophagus. The surgeon's wrists are in a neutral position in relation to the forearms, which minimizes fatigue and maximizes ergonomic function. Compared with the full-prone position, the port incisions are sited in more anterior positions in the posterior axillary lines and in the triangle of safety, where the ICSs are wider and less muscular. Therefore, the semiprone port positions are better for surgical manipulation and subsequent chest tube placement [9]. In addition, the patient under general anaesthesia is less prone to injuries to the critical nerves and vital organs, such as the eyes. Crucially, it is also easier to convert to a posterolateral thoracotomy, if needed. However, barriers to adopt the SP are multiplied by the fact that oesophageal surgeons spend many years learning traditional resection techniques with an already significant learning curve and semiprone thoracoscopic views during surgery are likely to be unfamiliar, especially during the initial learning phase.
It was reported that a transitory pneumothorax using CO2 at a pressure of 8 mmHg allowed rapid collapse of the lung. The enhanced operative view is such that some surgeons have been able to operate without the use of one-lung ventilation [6]. However, in our institution, we prefer to place a double-lumen endotracheal tube. A potential disadvantage of placing a single-lumen tube is that if an emergency conversion to thoracotomy is required, one-lung ventilation would be facilitated by the presence of the double-lumen tube. Furthermore, a shortcoming of an artificial pneumothorax is that the use of suction and traditional thoracic instruments is limited. Good care should be taken to avoid injury of the contralateral mediastinal pleura. Therefore, an artificial pneumothorax is not an absolute need. Surgeons could apply this technique based on their own experiences and habits.
Lymph node involvement is an important prognostic indicator in oesophageal carcinoma. Extensive lymphadenectomy allows accurate staging, reduces local/regional recurrence and increases long-term survival [10, 11]. We highlight that, regardless of the position in which the patient is fixed, surgical resection of T1–T3 lesions could be achieved successfully via thoracoscopy. Nevertheless, the removing of mediastinal lymph nodes, especially the lymph nodes along the left recurrent laryngeal nerve, is technically difficult. With the patient in the SP, gravity is used for non-traumatic lung retraction. We were able to obtain a good working space for lymph node dissection along the left recurrent laryngeal nerve. Better surgical exposure was secured in the upper mediastinum by retracting the oesophagus dorsally and pressing the trachea ventrally. We routinely used a right-sided double-lumen tube to make the distal trachea and left mainstem bronchus more flexible and allow deep dissection in the left tracheoesophageal groove. A transitory pneumothorax at a pressure of 8 mmHg helps to open the loose connective tissue surrounding the nerve and makes dissection easier. However, our study shows that the number of dissected mediastinal lymph nodes in the semiprone group appears to be equivalent to the lateral decubitus group, with a mean number of 15 ± 6 vs 14 ± 6 (P = 0.24). In addition, the number of retrieved lymph nodes along the recurrent laryngeal nerve was similar between the lateral decubitus group and the semiprone group: 3 ± 2 in both groups (P = 0.90). The reason for our success was our technical innovations applied to the lateral decubitus group. A self-made smoothed tip retractor, which is inserted through the 4-cm utility incision at the fourth ICS, is rotated to retract the trachea or the main bronchus to allow dissection of lymph nodes deep in the upper mediastinal space. The lymph nodes around the left recurrent laryngeal nerves could be dissected meticulously by utilizing the magnifying effect of the video while keeping a 30° telescope in close proximity and at an adequate angle to the area of the dissection. In our experience, with a technically skilled assistant exposing the operative views, the lateral decubitus position allows comparable dissection of the mediastinal lymph nodes in the SP, and is oncologically equivalent.
In conclusion, semiprone and lateral decubitus positions each have their inherent advantages and disadvantages. Our initial experience confirmed that while the SP was associated with superior surgical ergonomics and better exposure of the posterior mediastinum, there is no convincing evidence that semiprone thoracoscopic oesophagectomy is superior to the left lateral decubitus positioning with respect to the major surgical outcomes and oncological clearance. Surgeons could apply either technique based on their own experience and habits.
This study has some limitations, such as its retrospective design, possibly an insufficient number of patient encounters to identify small differences in operation outcomes. Furthermore, to evaluate whether the application of SP is better for the surgeon's comfort and ergonomics compared with lateral decubitus position, objective physiological and physical parameters should be recorded in further studies [12, 13]. In addition, the influence of the SP and lateral decubitus positions on haemodynamics and oxygenation during oesophagectomy was not investigated in this study.
SUPPLEMENTARY DATA
Funding
This work was supported by Scientific Research Fund of Fujian Provincial Education Department [JK 2010024].
Conflict of interest: none declared.
Supplementary Material
REFERENCES
- 1.Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb. 1992;37:7–11. [PubMed] [Google Scholar]
- 2.Cuschieri A. Thoracoscopic subtotal oesophagectomy. Endosc Surg Allied Technol. 1994;2:21–5. [PubMed] [Google Scholar]
- 3.Lin J, Kang M, Chen C, Lin R, Zheng W, Zhug Y, et al. Thoracolaparoscopy oesophagectomy and extensive two-field lymphadenectomy for oesophageal cancer: introduction and teaching of a new technique in a high-volume centre. Eur J Cardiothorac Surg. 2013;43:115–21. doi: 10.1093/ejcts/ezs151. doi:10.1093/ejcts/ezs151. [DOI] [PubMed] [Google Scholar]
- 4.Noshiro H, Iwasaki H, Kobayashi K, Uchiyama A, Miyasaka Y, Masatsugu T, et al. Lymphadenectomy along the left recurrent laryngeal nerve by a minimally invasive esophagectomy in the prone position for thoracic esophageal cancer. Surg Endosc. 2010;24:2965–73. doi: 10.1007/s00464-010-1072-4. doi:10.1007/s00464-010-1072-4. [DOI] [PubMed] [Google Scholar]
- 5.Kuwabara S, Katayanagi N. Comparison of three different operative methods of video-assisted thoracoscopic esophagectomy. Esophagus. 2010;7:23–9. doi:10.1007/s10388-009-0218-8. [Google Scholar]
- 6.Fabian T, Martin J, Katigbak M, McKelvey AA, Federico JA. Thoracoscopic esophageal mobilization during minimally invasive esophagectomy: a head-to-head comparison of prone versus decubitus positions. Surg Endosc. 2008;22:2485–91. doi: 10.1007/s00464-008-9799-x. doi:10.1007/s00464-008-9799-x. [DOI] [PubMed] [Google Scholar]
- 7.Luketich JD, Pennathur A, Awais O, Levy RM, Keeley S, Shende M, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg. 2012;256:95–103. doi: 10.1097/SLA.0b013e3182590603. doi:10.1097/SLA.0b013e3182590603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palanivelu C, Prakash A, Senthilkumar R, Senthilnathan P, Parthasarathi R, Rajan PS, et al. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position—experience of 130 patients. J Am Coll Surg. 2006;203:7–16. doi: 10.1016/j.jamcollsurg.2006.03.016. doi:10.1016/j.jamcollsurg.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Agasthian T. Revisiting the prone position in video-assisted thoracoscopic surgery. Asian Cardiovasc Thorac Ann. 2010;18:364–7. doi: 10.1177/0218492310375857. doi:10.1177/0218492310375857. [DOI] [PubMed] [Google Scholar]
- 10.Kang CH, Kim YT, Jeon SH, Sung SW, Kim JH. Lymphadenectomy extent is closely related to long-term survival in esophageal cancer. Eur J Cardiothorac Surg. 2007;31:154–60. doi: 10.1016/j.ejcts.2006.10.033. doi:10.1016/j.ejcts.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Hulscher JBF, Van Sandick JW, De Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–9. doi: 10.1056/NEJMoa022343. doi:10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 12.Tchartchian G, Dietzel J, Bojahr B, Hackethal A, De Wilde R. Decreasing strain on the surgeon in gynecologic minimally invasive surgery by using semi-active robotics. Int J Gynecol Obstet. 2011;112:72–5. doi: 10.1016/j.ijgo.2010.08.002. doi:10.1016/j.ijgo.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 13.van der Schatte Olivier RH, van't Hullenaar CDP, Ruurda JP, Broeders IAMJ. Ergonomics, user comfort, and performance in standard and robot-assisted laparoscopic surgery. Surg Endosc. 2009;23:1365–71. doi: 10.1007/s00464-008-0184-6. doi:10.1007/s00464-008-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


