Abstract
Endovascular occlusion of blood vessels is an important part of interventional therapy concepts. Here, we evaluate the feasibility, procedural safety and efficacy of the novel endovascular occlusion system (EOS) in the arterial system in a porcine model. Thirteen devices were deployed in the iliac and femoral arteries (diameter: 4–5 mm) of five adult swine. Post-deployment angiography was performed at 1, 5 and 10 min and 6 h. All devices (n = 13) could be successfully delivered without any complications, such as dissection, perforation or rupture. The devices could be easily advanced to the target vessel segment, deployed at the intended target location and produced immediate and complete vessel occlusion which was confirmed to be maintained after 6 h. No leaks, recanalization or device migration was observed. In this pilot study, we demonstrate the feasibility, safety and efficacy of immediate vessel occlusion with the EOS device in the peripheral arterial system in a porcine animal model. Our data indicate that this novel device allows precise delivery without the occurrence of cardiovascular complications. Owing to its long-term safety and efficacy the EOS may represent a promising and effective alternative to currently available devices for vessel occlusion during vascular interventions.
Keywords: Vessel occlusion, Endovascular, Occlusion device, Embolization
INTRODUCTION
Endovascular occlusion of blood vessels is an essential aspect of interventional vascular therapy concepts [1–3]. However, immediate and durable vessel occlusion remains a challenge when using the currently available devices and may often necessitate use of a large number of devices as well as long interventional procedures with high radiation exposure. In the present study, we assess a novel endoluminal occlusion system (EOS) for transcatheter vessel occlusion in the peripheral arterial system and demonstrate technical feasibility and procedural safety in a porcine model.
MATERIALS AND METHODS
Device description
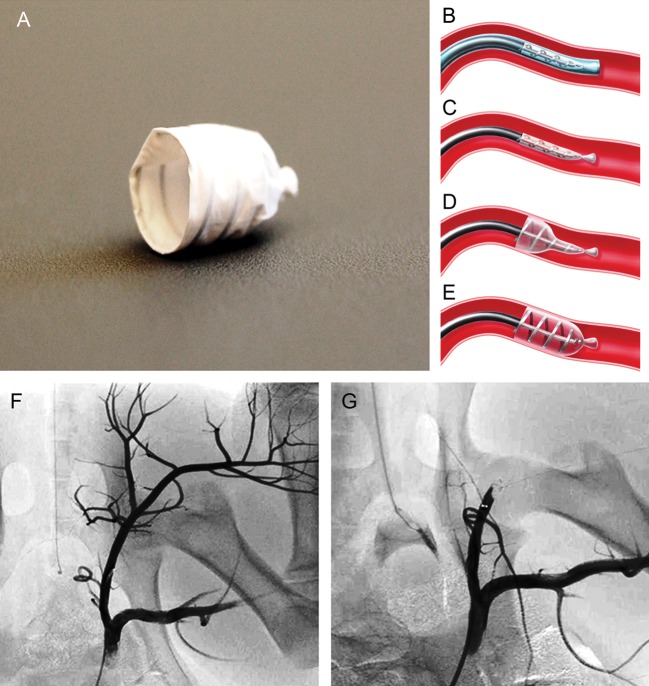
The EOS (ArtVentive Medical Group, Inc., Carlsbad, CA, USA) device allows percutaneous occlusion of the peripheral arterial and venous vasculature. The EOS system is comprised of an implant carrier catheter with a preloaded self-expandable implant and a guide sheath with a removable core (Fig. 1A–E). The EOS implant is made of a Nitinol scaffold covered with an impermeable expanded Polytetrafluorethylen membrane, which is composed to maintain complete impermeability in order to occlude blood flow reliably, precisely and immediately. The scaffold design is based on a flat helical structure. The EOS implant is available in two sizes: 6 mm for treatment of vessels between 3.0 and 4.8 mm in diameter, and 9 mm for treatment of vessels between 4.5 and 7.8 mm in diameter, with a calculated oversizing of at least 15%. It generates sufficient radial force to provide good vessel wall apposition and to minimize post-deployment migration (Fig. 1A–E). The device design generates low local pressure that lowers the risk of vessel damage and rupture. The device is self-expandable and highly flexible per design. It is also highly adoptable to the vessel geometry. Therefore, it actually adopts the vessel configuration as every segment of the device along its length takes shape of target vessel. The device maximum length as deployed is <20 mm. The delivery mechanism and the steps of delivery are summarized in Fig. 1B–E. The device is delivered via a 6-Fr guide sheath. The guide sheath is advanced using a 0.035′ guide wire.
Figure 1:
EOS device, illustration of the EOS occlusion mechanism and angiography before and after EOS device deployment. EOS device shown in deployed configuration (A). The EOS is delivered through the guide sheath into the target position of the vessel (B) before the EOS device is unsheathed for deployment (C). The deployment process is initiated by release of the proximal end (Step 1). At this stage, the operator is still in full control to refine the exact target position (D). Finally, the distal end of the implant is deployed (Step 2), leading to immediate vessel occlusion. Thereafter, the carrier catheter can be easily removed (E). Predeployment angiography was performed to define the target vessel and to assess the vessel diameter to determine the optimal EOS device size (F). After deployment of the EOS device, immediate and complete vessel occlusion was achieved (G)
Animal study
To assess the acute safety and technical feasibility of the EOS, 13 devices (11 × 6 mm and 2 × 9 mm) were tested in the preclinical setting and deployed in the iliac and femoral arteries of five adult female Landrace swine (67 ± 3 kg).
Animal care and anaesthesia
All procedures were approved by the Institutional Ethics Committee (Cantonal Veterinary Office, license no. 138/2010), and all animals received humane care in compliance with the ‘Principles of Laboratory Animal Care’ and according to the ‘Guide to the Care and Use of Experimental Animals’ published by the US National Institutes of Health. The adult swine (n = 5) were fasted overnight prior to the procedure. Anaesthesia induction was performed with an intramuscular injection of ketamine (20 mg/kg), azaperone (2 mg/kg) and atropine (0.04 mg/kg). Anaesthesia was deepened with bolus injection of propofol (1–3 mg/kg) intravenously, and the animals were then intubated. Anaesthesia was maintained with inhaled isoflurane (1–3%) in 50% oxygen. For intraoperative analgesia, buprenorphine (Temgesic; 0.005 mg/kg) was used.
Device delivery and vessel occlusion
Prior to the procedures, the animals were placed in the supine position, and an 8-Fr sheath surgically introduced in one of the carotid arteries. All procedures were carried out in an animal hybrid operation suite using a Philips Allura FD 20/20 Imaging System. Predeployment angiography with three-dimensional (3D) vessel reconstruction analysis was performed to define the target vessel diameter and to determine the optimal size of the EOS device. A corresponding post-deployment angiogram was performed directly after the procedure (at 1, 5 and 10 min) as well as 6 h later to assess the acute safety and efficacy of the EOS device.
Under fluoroscopic guidance, the ArtVentive guide catheter was introduced through the sheath and advanced into the target vessel segment of the left or right iliac or femoral arteries. Thereafter, predeployment angiography along with 3D reconstruction and subtraction analysis was performed to assess the vasculature and to determine the vessel diameter. In total, 13 devices were used, with nominal diameters of 6 mm (n = 11) or 9 mm (n = 2). In addition, a roadmap image was acquired and used throughout device delivery and deployment. To deploy the EOS, the device was introduced into the delivery catheter, and advanced to its tip. The catheter was then withdrawn, leaving the device unsheathed but still un-deployed, at the target vessel segment. Finally, the EOS device was deployed and detached utilizing a controlled, two-step release mechanism (with the proximal end opening prior to the distal end).
RESULTS
All EOS devices (n = 13) were successfully delivered into the intended segments of the left and right iliac arteries of the animals and produced immediate and complete vessel occlusion (Fig. 1F and G; Supplementary Video 1). There were no major or minor complications, such as vascular dissection, perforation or rupture along the arterial access path or at the site of EOS delivery. In particular, all EOS devices could be easily introduced into the delivery catheter, advanced to the target vessel segment and deployed at the intended target location. Post-deployment angiography at 1 min showed immediate and complete vessel occlusion, which was confirmed at the 5- and 10-min angiography. At the 6-h post-implantation angiography, complete occlusion could be shown to be maintained and importantly, there were no signs of acute migration of the EOS device, leaks and recanalization. Table 1 summarizes the procedural and outcome data.
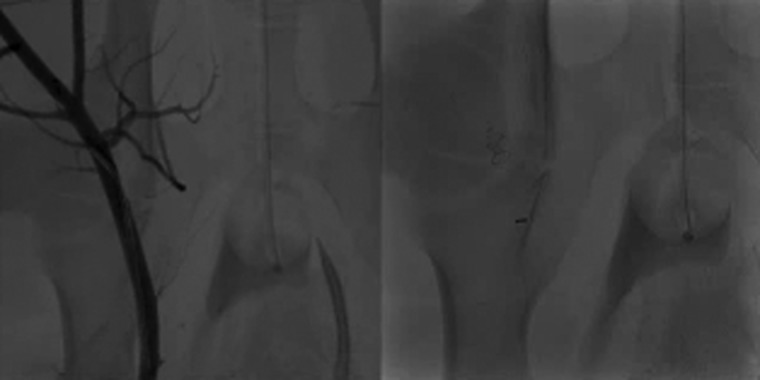
Supplementary Video 1:
Pre-deployment angiography was performed to define the target vessel and to assess the vessel diameter to determine the optimal EOS device size (left). After deployment of the EOS device, immediate and complete vessel occlusion was achieved (right).
Table 1:
Animal, device and procedural data
| Study table | |
|---|---|
| Animals | n = 5 |
| Animal weight (kg) | 67 ± 3 |
| No. and size of devices used (6/9 mm) | 11/2 |
| Target vessels | Iliac and femoral artery |
| Vessel diameter (mm) | 4–5 |
| Duration of entire procedure (min) | 12 (range 9–15) |
| Duration of EOS delivery (min) | 8 (range 6–12) |
| Cumulative fluoroscopic time for entire procedure (min) | 8 ± 2 |
| Cumulative dose area product (DAP) for the entire procedure (Gy cm2) | ∼38 |
| Mortality (%) | 0 |
| Procedural complications | 0 |
| Vessel occlusion (at 1 min) | |
| Immediate (%) | 100 |
| Complete (%) | 100 |
| Complete vessel occlusion at 6 h (%) | 100 |
| Cumulative fluoroscopic time for entire procedure (min) | 8 ± 2 |
DISCUSSION
Endovascular vessel occlusion represents an important aspect of interventional therapy strategies for a wide range of vascular and non-vascular conditions [1–3]. However, the currently available strategies have been reported to come with a limited efficacy with regard to a complete and durable vessel occlusion. While coils have been commonly used for vessel occlusion, a large number of these devices may be necessary to achieve complete occlusion and to minimize the risk of recanalization. To overcome these limitations, new devices have been developed [4–6]; however, some of these devices require large delivery sheaths and catheters [7], have complicated delivery systems and do not always achieve a fast and durable vessel occlusion [8–10].
Here, we introduce a novel endoluminal occlusion system for transcatheter vessel occlusion. Our results demonstrate the feasibility and safety of immediate vessel occlusion in the arterial system with the EOS device in an acute porcine model. Our data indicate that this novel device allows precise delivery and deployment at the intended location site without the occurrence of cardiovascular complications, such as dissection, perforation or rupture. Due to its simple and efficient design, the EOS device technology is multifaceted and thus may have other indications in the venous and arterial system of different body regions. While this was a pilot study focusing on technical feasibility, procedural safety and acute efficacy, long-term data and comparative studies of other devices are necessary. Its long-term safety and efficacy proven, the EOS may represent a promising and effective alternative to currently available devices for vessel occlusion during vascular interventions.
SUPPLEMENTARY MATERIAL
Funding
This study was funded by the ArtVentive Medical Group, Inc.
Conflict of interest: Anthony Venbrux, Martin G. Radvany and Andre Plass are members of ArtVentive SAB. Leon Rudakov and Philippe Gailloud are founders of ArtVentive.
Supplementary Material
REFERENCES
- 1.Kim HS, Malhotra AD, Rowe PC, Lee JM, Venbrux AC. Embolotherapy for pelvic congestion syndrome: long-term results. J Vasc Interv Radiol. 2006;17:289–97. doi: 10.1097/01.RVI.0000194870.11980.F8. [DOI] [PubMed] [Google Scholar]
- 2.Radvany MG, Gailloud P. Endovascular management of neurovascular arterial injuries in the face and neck. Semin Intervent Radiol. 2010;27:44–54. doi: 10.1055/s-0030-1247888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velmahos GC, Chahwan S, Hanks SE, Murray JA, Berne TV, Asensio J, et al. Demetriades D. angiographic embolization of bilateral internal iliac arteries to control life-threatening hemorrhage after blunt trauma to the pelvis. Am Surg. 2000;66:858–62. [PubMed] [Google Scholar]
- 4.Kipshidze N, Sadzaglishvili K, Panarella M, Rivera EA, Virmani R, Leon MB. Evaluation of a novel endoluminal vascular occlusion device in a porcine model: early and late follow-up. J Endovasc Ther. 2005;12:486–94. doi: 10.1583/05-1543.1. [DOI] [PubMed] [Google Scholar]
- 5.Rossi M, Rebonato A, Greco L, Stefanini G, Citone M, Speranza A, et al. A new device for vascular embolization: report on case of two pulmonary arteriovenous fistulas embolization using the Amplatzer vascular plug. Cardiovasc Intervent Radiol. 2006;29:902–6. doi: 10.1007/s00270-005-0160-7. [DOI] [PubMed] [Google Scholar]
- 6.Salamat M, Brown PR, Magee CA, Reyes DK, Peters DN, Venbrux AC. Experimental evaluation of a new transcatheter vascular embolization device in the swine model. J Vasc Interv Radiol. 2002;13:301–12. doi: 10.1016/s1051-0443(07)61724-2. [DOI] [PubMed] [Google Scholar]
- 7.Rao PS, Kim SH, Choi JY, Rey C, Haddad J, Marcon F, et al. Follow-up results of transvenous occlusion of patent ductus arteriosus with the buttoned device. J Am Coll Cardiol. 1999;33:820–6. doi: 10.1016/s0735-1097(98)00610-x. [DOI] [PubMed] [Google Scholar]
- 8.Guillon R, Garcier JM, Abergel A, Mofid R, Garcia V, Chahid T, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Intervent Radiol. 2003;26:256–60. doi: 10.1007/s00270-003-1948-y. [DOI] [PubMed] [Google Scholar]
- 9.Fidelman N, Gordon RL, Bloom AI, LaBerge JM, Kerlan RK., Jr Reperfusion of pulmonary arteriovenous malformations after successful embolotherapy with vascular plugs. J Vasc Interv Radiol. 2008;19:1246–50. doi: 10.1016/j.jvir.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Dorenberg EJ, Hafsahl G, Andersen R, Krohg-Sorensen K. Recurrent rupture of a hypogastric aneurysm caused by spontaneous recanalization of an amplatzer vascular plug. J Vasc Interv Radiol. 2006;17:1037–41. doi: 10.1097/01.RVI.0000222821.56922.03. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.