Abstract
Background. Tuberculosis is transmitted by patients with pulmonary disease. Matrix metalloproteinases (MMPs) drive lung destruction in tuberculosis but the resulting matrix degradation products (MDPs) have not been studied. We investigate the hypothesis that MMP activity generates matrix turnover products as correlates of lung pathology.
Methods. Induced sputum and plasma were collected prospectively from human immunodeficiency virus (HIV) positive and negative patients with pulmonary tuberculosis and controls. Concentrations of MDPs and MMPs were analyzed by ELISA and Luminex array in 2 patient cohorts.
Results. Procollagen III N-terminal propeptide (PIIINP) was 3.8-fold higher in induced sputum of HIV-uninfected tuberculosis patients compared to controls and desmosine, released during elastin degradation, was 2.4-fold higher. PIIINP was elevated in plasma of tuberculosis patients. Plasma PIIINP correlated with induced sputum MMP-1 concentrations and radiological scores, demonstrating that circulating MDPs reflect lung destruction. In a second patient cohort of mixed HIV seroprevalence, plasma PIIINP concentration was increased 3.0-fold above controls (P < .001). Plasma matrix metalloproteinase-8 concentrations were also higher in tuberculosis patients (P = .001). Receiver operating characteristic analysis utilizing these 2 variables demonstrated an area under the curve of 0.832 (P < .001).
Conclusions. In pulmonary tuberculosis, MMP-driven immunopathology generates matrix degradation products.
Keywords: lung, mycobacteria, immunopathology, extracellular matrix, matrix metalloproteinase
The global tuberculosis pandemic continues despite an intense biomedical research effort to improve control [1]. Tuberculosis is spread by aerosol and patients with cavitary lung disease are the most highly infectious [1, 2]. Pulmonary tuberculosis is characterized by extensive remodeling of the lung extracellular matrix [3], with both destruction and compensatory synthesis of matrix, resulting in pulmonary cavitation with an extensive fibrous wall [4]. Current investigations into pathological correlates of pulmonary tuberculosis tend to focus on either host immunological mediators [5–8] or mycobacterial components [9–12].
We have recently identified matrix metalloproteinase-1 (MMP-1) as a dominant collagenase causing lung tissue destruction in tuberculosis [13]. MMPs are proteases uniquely capable of degrading all components of the lung extracellular matrix at neutral pH [14]. MMP expression can be up-regulated by pro-inflammatory cytokines and extracellular matrix metalloproteinase inducer (EMMPRIN) [15]. MMPs are suppressed in advanced human immunodeficiency virus (HIV) infection, where lung cavitation is uncommon [16], further implicating these proteases in tuberculosis-driven pathology [17]. Proteolytic extracellular matrix destruction by collagenases will release immunoreactive peptides from intact collagen fibrils [18]. Elastases such as MMP-7 and MMP-9 may generate desmosine and isodesmosine from elastin fibers [19], which are highly stable and usually are not degraded during adult life [20]. These matrix degradation products (MDPs) are elevated in other destructive pulmonary pathologies. For example, desmosine and procollagen III N-terminal propeptide (PIIINP) production are increased in chronic obstructive pulmonary disease [21, 22], and type I and III collagen degradation products are elevated in the acute respiratory distress syndrome, pulmonary fibrosis, and sarcoidosis [23–25]. However, despite the extensive matrix remodeling that occurs in pulmonary tuberculosis, MDPs and their relation to MMP activity have not been studied in this disease.
We hypothesized that extracellular matrix turnover products will be generated by MMP activity in tuberculosis. We investigated collagen and elastin turnover products in HIV-uninfected patients with tuberculosis compared to controls. PIIINP and desmosine were elevated in induced sputum of patients with tuberculosis. PIIINP was also elevated in plasma of tuberculosis patients, and plasma PIIINP was elevated in a second independent clinical cohort of mixed HIV seroprevalence. These matrix degradation products (MDPs) correlated with immunopathological MMPs that degrade the intact fibrils. Receiver operating characteristic analysis demonstrates significant differentiation between controls and tuberculosis patients using a matrix-based model. MDPs are pathological markers of lung destruction in pulmonary tuberculosis.
MATERIALS AND METHODS
Patient Recruitment
The initial cohort recruited in Cape Town has been reported elsewhere [17]. The study was approved by the University of Cape Town Research Ethics Committee (REC REF 509/2009). Participants were recruited at Ubuntu HIV/Tuberculosis clinic and GF Jooste Hospital, and written informed consent was obtained. The second patient cohort was recruited at McCord Hospital, Durban. The study was approved by the University of Kwazulu-Natal Research Ethics Committee (REF BFC 115/09). All tuberculosis patients were sputum smear positive and were of mixed HIV seroprevalence. For controls, all sputum samples obtained (minimum 1 sputum) were smear and culture negative for AFB, and there was a low clinical suspicion for active tuberculosis after symptom review and physical examination.
Sample Collection and Processing
Induced sputum was collected after informed consent and processed as described elsewhere [17]. Plasma was collected after consent and centrifuged within 2 hours. Plasma samples were then frozen at −80°C in aliquots to minimize freeze-thaw cycles prior to analysis.
Desmosine and Isodesmosine ELISA
All analyses were performed blinded to the patient data. Desmosine and isodesmosine ELISAs were purchased from Cusabio Biotech (Wuhan, China) and performed according to manufacturer's instructions. Samples were diluted 1 in 5. The lower levels of sensitivity of the assays were 0.04 ng/mL and 0.012 ng/mL, respectively.
Collagen Peptide Analysis
Type I collagen crosslaps ELISAs were purchased from Immunodiagnostic Systems Limited (Boldon, UK) and were performed according to manufacturer's instructions. The level of sensitivity for C-terminal telopeptides of type I collagen (CTX-I) was 0.020 ng/mL and for non-isomerized fragments of C-terminal telopeptides of type I collagen (α-CTX-I) was 0.80 ng/mL. Other collagen ELISAs were purchased from USCN Life Sciences (Wuhan, China). The lower level of sensitivity of the assays was: procollagen I N-terminal propeptide (PINP) 12.6 pg/mL, procollagen III N-terminal propeptide (PIIINP) 9.8 pg/mL, procollagen III C-terminal propeptide (PIIICP) 4.4 pg/mL, and cross-linked C-telopeptide of type III collagen (CTX-III) 46.4 pg/mL, respectively. Positive controls for the assays were reconstituted lyophilized standard. Negative controls were standard diluent alone. Both were provided with the assay kit.
Luminex Assay
Samples were analyzed on the Luminex 200 platform. MMP beads were purchased from R&D Systems, Abingdon, UK (MMP-1,2,3,7,8,9, EMMPRIN), and Millipore, Watford, UK (MMP-10). The inflammatory 30-plex panel was from Invitrogen (interleukin [IL]-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12, IL-13, IL-15, IL-17, tumor necrosis factor [TNF]-α, interferon [IFN]-α, IFN-γ, IL-1RA, IL-2R, IL-8, MIP-1α, MIP-1β, Eotaxin, MIG, MCP-1, RANTES, IP-10, EGF, HGF, VEGF, FGF, G-CSF, and GM-CSF; Invitrogen, Paisley, UK). For circulating MMP analysis, plasma samples were studied as these are optimal for MMP measurement [26]. For this study, all beads were from R&D.
Statistics
Baseline statistical analysis was performed with Graphpad Prism 5. Clinical data were analyzed by the 2-tailed Mann-Whitney U test and by Spearman correlation. Scatter dot plot charts demonstrate the mean ± SEM, Box-and whisker plots show the median, 25th and 75th percentiles with whiskers at minimum to maximum values. Scatter plots include the Spearman correlation coefficient (r) and P value to show correlation between 2 continuous variables. The univariate, multivariate, and ROC analysis was performed in SPSS v19. A P value of < .05 was considered significant.
RESULTS
Procollagen III N-terminal Propeptide (PIIINP) Is Elevated in Induced Sputum of Patients With Tuberculosis
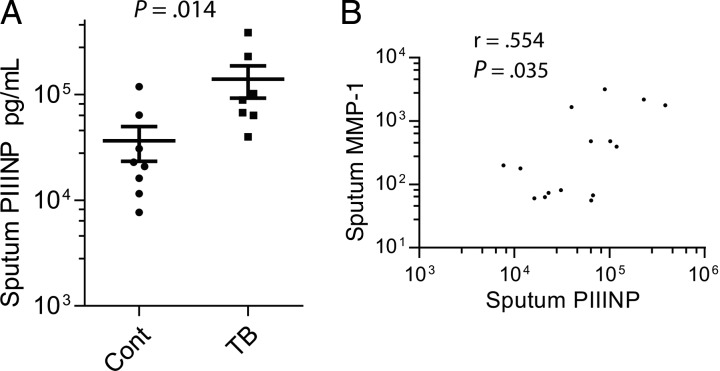
Because collagenases appear to be key in the immunopathology of tuberculosis, we first studied products released during collagen turnover in induced sputum samples from a patient cohort that we have reported [17], analyzing HIV-negative patients only. Total C-terminal telopeptides of type I collagen (CTX-1, Crosslaps), released during type I collagen degradation, and isomerised α-CTX-I were not detected in induced sputum by ELISA (data not shown). In contrast, sputum concentration of procollagen III N-terminal propeptide (PIIINP), which is released during both the synthesis and breakdown of type III collagen, was significantly elevated in patients with tuberculosis. Mean sputum PIIINP concentrations were 3.8-fold higher than controls (Figure 1A). To determine which MMPs were likely degrading the PIIINP, we analyzed correlations between sputum PIIINP and MMP concentrations in induced sputum. Sputum PIIINP concentrations correlated with sputum MMP-1 (interstitial collagenase) concentrations (Figure 1B). Conversely, PIIINP concentrations did not correlate with any other MMPs or induced sputum cytokine concentrations analyzed by Luminex array (data not shown), suggesting that MMP-1 is the key collagenase degrading type III collagen in the lung.
Figure 1.
Procollagen III N-terminal propeptide (PIIINP) concentrations are elevated in patients with tuberculosis (TB). (A) PIIINP was measured by ELISA in induced sputum samples from human immunodeficiency virus (HIV) negative patients with pulmonary TB and uninfected controls. (B) Sputum PIIINP concentrations correlated with sputum MMP-1 concentrations measured by luminex array, on Spearman analysis.
Desmosine Concentrations Are Increased in Tuberculosis Patients
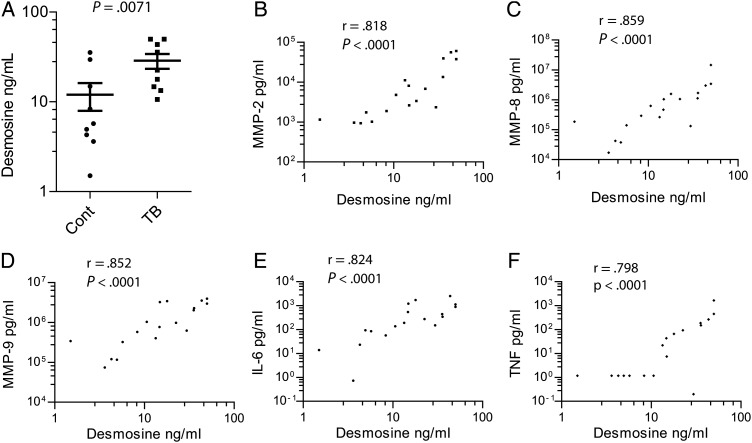
Next, we studied desmosine, an amino acid unique to mature elastin. Desmosine concentrations were increased 2.4-fold in induced sputum of HIV-negative patients with tuberculosis (P = .0071, Figure 2A). We also analyzed isodesmosine concentrations by ELISA, but levels were mainly below the level of sensitivity of the assay (data not shown). Sputum desmosine concentrations correlated with multiple MMPs in the sputum, though most closely MMP-8 (neutrophil collagenase, r = 0.859), MMP-9 (gelatinase B, r = 0.852), and MMP-2 (gelatinase A, r = 0.818; Figure 2B–D). Desmosine correlated with MMP-1 and MMP-3 (stromelysin 1) concentrations in sputum, though less closely (Supplementary Figure 1) and was weakly correlated with MMP-7 (matrilysin). MMP-9 has elastase activity and so may be the key protease degrading elastin. Desmosine also correlated with induced sputum cytokine concentrations, in particular IL-6 (r = 0.824) and TNF-α (r = 0.798; Figure 2E and 2F).
Figure 2.
Desmosine is elevated in the induced sputum of patients with pulmonary tuberculosis (TB) and correlates with MMP and cytokine concentrations. (A) Desmosine concentration was analyzed by ELISA in induced sputum from human immunodeficiency virus (HIV) negative patients with pulmonary tuberculosis. Mean desmosine concentrations were 2.4-fold higher in tuberculosis. (B–D) MMPs in induced sputum were measured by luminex multiplex array. MMP-2, -8 and -9 correlated most closely with desmosine concentrations (E and F). Cytokine concentrations were measured by luminex array and IL-6 and TNF-α associated most closely with sputum desmosine concentration.
PIIINP Is Elevated in the Plasma of Patients With Tuberculosis
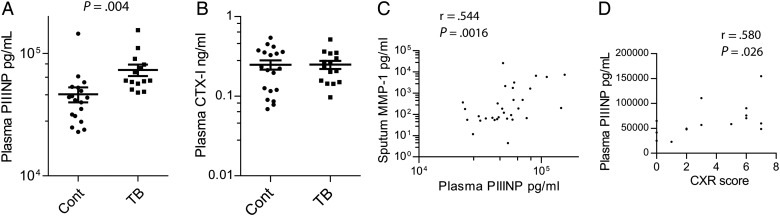
Next, we investigated whether MDPs are found in the circulation of patients with active tuberculosis by analyzing plasma samples. We studied HIV-negative patients and HIV-positive patients with relatively preserved CD4 counts of >200, as below this level lung matrix destruction is reduced [16]. Plasma PIIINP was significantly elevated in the plasma of patients with tuberculosis compared to controls (Figure 3A, P = .004). Total C-terminal telopeptides of type I collagen (CTX-I) were no different between control and tuberculosis patients (Figure 3B), and non-isomerized fragments of C-terminal telopeptides of type I collagen (α-CTX-I) were generally below the level of sensitivity of the assay (data not shown). Plasma PIIINP correlated most closely with induced sputum MMP-1 (interstitial collagenase), demonstrating that it was a peripheral marker of collagenase activity at the site of disease (Figure 3C). MMP-2 (gelatinase A) and MMP-3 (stromelysin 1) correlated weakly with plasma PIIINP, but no collagenase other than MMP-1 in induced sputum correlated with plasma PIIINP concentrations (data not shown). There was also a relatively weak correlation between plasma PIIINP and sputum TNF-α (r = 0.442). Plasma desmosine and isodesmosine were below the level of sensitivity of the ELISA assay (data not shown). To determine whether PIIINP correlated with radiological markers of tissue destruction, plasma concentrations were analyzed against chest radiographic involvement scored on a scale of 1–10 as described elsewhere [17]. Greater radiographic inflammation correlated with higher plasma PIIINP concentrations (Figure 3D).
Figure 3.
Procollagen III N-terminal propeptide (PIIINP) concentrations are elevated in the plasma of patients with tuberculosis (TB). (A) PIIINP concentration was measured by ELISA in plasma from patients of mixed human immunodeficiency virus (HIV) seroprevalence with a CD4 cell count of >200. PIIINP was significantly elevated in patients with pulmonary tuberculosis compared to controls. (B) Plasma Total C-terminal telopeptides of type I collagen (CTX-I) was no different between patients with tuberculosis and controls. (C) Plasma PIIINP correlated with induced sputum MMP-1 on Spearman's analysis. (D) Plasma PIIINP correlated with chest X-ray (CXR) infiltration scored on a scale of 1–10.
Plasma PIIINP Is Elevated in a Second, Independently Recruited Cohort of Tuberculosis Patients
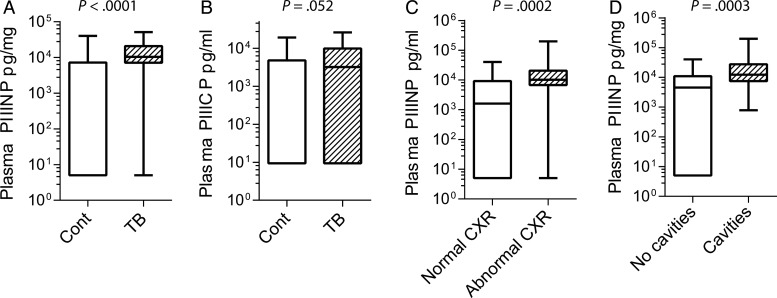
To determine whether plasma PIIINP was consistently elevated in pulmonary tuberculosis, we analyzed a larger, entirely separate cohort that was prospectively recruited in Durban, South Africa (Clinical characteristics, Supplementary Table 1). The cohort was of mixed HIV seroprevalence, with 58.9% patients HIV infected, although the CD4 cell count was relatively well preserved, with a mean CD4 count 328.1 cells/μL in HIV-positive patients. Plasma PIIINP concentration was increased in patients with tuberculosis, with mean values 2.98-fold higher (Figure 4A, P < .0001). We also analyzed a number of other matrix degradation products in this cohort to determine whether they were also markers of active tuberculosis. Procollagen I N-terminal propeptide (PINP) and cross-linked C-telopeptide of type III collagen (CTX-III) were primarily below the level of detection of the assay (data not shown). Procollagen III C-terminal propeptide (PIIICP) tended to be increased in patients with tuberculosis, but a high proportion of samples had values below the level of detection of the assay (Figure 4B). Next, we investigated whether plasma PIIINP correlated with radiographic indicators of tissue destruction. PIIINP concentrations were elevated in patients with abnormal chest radiographs compared to those with normal radiographic appearances (Figure 4C). Similarly, patients with lung cavities visible on their radiographs had higher concentrations of plasma PIIINP that those without cavitation (Figure 4D), further confirming that plasma matrix breakdown products are peripheral markers of tuberculosis-related pathology in the lung.
Figure 4.
Plasma PIIINP is elevated in a second cohort of patients with tuberculosis (TB) of mixed human immunodeficiency virus (HIV) seroprevalence and correlates with tissue damage on chest radiographs. PIIINP concentrations were analyzed in a second cohort recruited in Durban, South Africa, of mixed HIV seroprevalence. (A) Plasma PIIINP was significantly increased in patients with tuberculosis. (B) Plasma PIIICP concentration showed a trend towards increase in tuberculosis that did not achieve statistical significance. (C) Plasma PIIINP concentration was elevated in patients with an abnormal CXR compared to those with a normal radiograph. (D) Patients with cavitary pulmonary tuberculosis assessed by chest radiography had higher plasma PIIINP concentrations than those without cavities.
Plasma MMPs Are Elevated in Patients With Tuberculosis
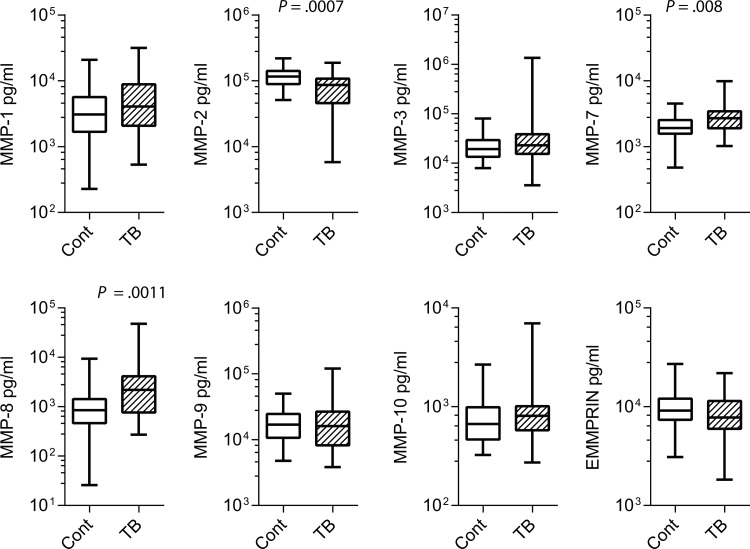
Next, we investigated plasma MMPs in the Durban cohort as we have previously demonstrated that several MMPs are elevated in the induced sputum of patients with tuberculosis [13, 17], but we have not investigated their potential as peripheral markers of pathology. MMP-8 (neutrophil collagenase) and MMP-7 (matrilysin) concentrations were most significantly increased in patients with tuberculosis. Mean MMP-8 concentrations 3.3–fold higher and mean MMP-7 concentrations were 1.4–fold higher (Figure 5). In contrast, plasma MMP-2 concentrations were suppressed in patients with tuberculosis (Figure 5). Plasma MMP-1 concentrations were not significantly different between groups.
Figure 5.
Plasma MMP-7 and -8 are elevated, while MMP-2 is suppressed, in patients with tuberculosis (TB). Plasma MMP and extracellular matrix metalloproteinase inducer (EMMPRIN) concentrations were analyzed by luminex array. In patients with pulmonary tuberculosis, plasma MMP-2 concentrations were suppressed, while MMP-7 and MMP-8 were increased in tuberculosis. No other MMPs analyzed differed significantly.
Matrix Degradation Products Are Pathological Markers of Pulmonary Tuberculosis
Finally, we created a statistical model to determine whether a matrix-based diagnostic algorithm might have potential to identify patients with pulmonary tuberculosis by analysis of plasma samples. For the univariate logistic regression, the laboratory variables were rescaled by dividing the original values of PIIINP, PIIICP, MMP-2, MMP-7, and MMP-8 by a factor of 100, and MMP-2 by a factor of 1000, to generate an equivalent range for each variable. Body mass index (BMI) was included in the modeling as it was significantly lower in patients with tuberculosis. These factors were studied in a simple logistic regression, and all except PIIICP had a statistically significant relationship with tuberculosis status (Supplementary Table 2). For each 100 units increase in PIIINP, the odds of tuberculosis increased by 1.011 times, with 95% confidence interval (CI) (1.004, 1.017, P = .001). For each 1 unit increase in BMI, the odds of having tuberculosis changed by 0.917 times, therefore reduced by 8.3%, with 95% CI (1.2%, 15.0%, P = .023). A multivariate logistic model was then developed simultaneously fitting all 6 factors, with the primary factor as PIIINP. Each of the other 5 factors became non-statistically significant after adjusting for all other variables.
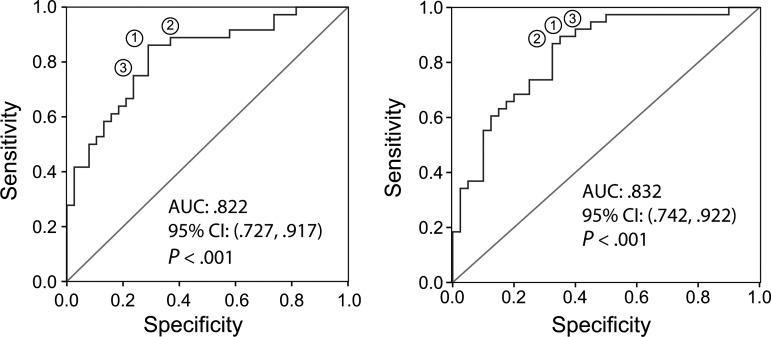
Next, a receiver operating characteristic (ROC) curve was generated based on a model incorporating all 6 variables (Figure 6A). The area under the curve was 0.822 (P < .001), with the optimal cut point providing a sensitivity of 86.1% and a specificity of 71.1%. A backward elimination strategy was then applied to this model to iteratively remove all nonsignificant factor(s) that had exceeded the 5% significance level. Since PIIINP was the main predictor, it was fixed in the model. After the elimination strategy, PIIINP (P = .002) and MMP-8 (P = .027) remained the only predictive markers. ROC curve of this reduced model provided an optimal sensitivity of 89.5% and specificity of 65.0% (Figure 6B, P < .001 and Table 1).
Figure 6.
Receiver Operating Characteristic analysis of matrix-turnover products in plasma from patients with pulmonary tuberculosis (TB) compared to controls. (A) Variables included in the initial model were plasma PIIINP, PIIICP, MMP-2, MMP-7 and MMP-8, and the patients' BMI. (B) Variables in the final model were plasma PIIINP and MMP-8. Matrix turnover products may be incorporated into a multi-analyte panel to identify patients with pulmonary tuberculosis. Abbreviations: AUC, area under the curve; BMI, body mass index.
Table 1.
Optimal Cut Points of the Final Model for Outcome Tuberculosis Against No TB
| Option | Factors in the model | Optimal cut point | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| (1) | PIIINP, MMP-8 | −0.6188 | 89.5% | 65.0% | 70.8% | 86.7% |
| (2) | PIIINP, MMP-8 | −0.5809 | 86.8% | 67.5% | 71.7% | 84.4% |
| (3) | PIIINP, MMP-8 | −0.7339 | 92.1% | 60.0% | 68.6% | 88.9% |
Abbreviations: NPV, negative predictive value PPV, positive predictive value.
DISCUSSION
We investigated matrix degradation products (MDPs) generated by MMP activity in tuberculosis and demonstrated that both collagen and elastin fragments are elevated in tuberculosis patients compared to controls. PIIINP, released from both the synthesis and degradation of type III collagen, is elevated in plasma of tuberculosis patients, and this finding was confirmed in a second larger independent cohort recruited in Durban. Plasma PIIINP concentrations were elevated in patients with radiological manifestations of tissue destruction. Matrix degradation products correlate with MMP-1, which cleaves fibrillar collagen, and MMP-9, which degrades elastin, indicating that they are peripheral markers of immunopathology at the site of disease.
To our knowledge, this is the first study of MDPs in tuberculosis, a disease characterized by extensive and rapid extracellular matrix remodeling [3]. We identified PIIINP as a peripheral marker of matrix turnover, which is consistent with other pathologies characterized by aberrant extracellular matrix turnover such as sarcoidosis [24], lung fibrosis [25], liver fibrosis [27], wound repair [28], and atherosclerosis [18]. The significant elevation of PIIINP is in contrast to relatively unchanged concentrations of plasma cytokines in tuberculosis [8, 29], which have not been found to be consistently elevated in pulmonary tuberculosis despite extensive investigation [5]. Interestingly, a previous report has found that elevated markers of matrix turnover at HIV presentation was associated with a more rapid progression to AIDS and death [30]. To fully define the utility of MDPs as markers of pathology in tuberculosis, analysis of a wider patient cohort is necessary, including diverse inflammatory conditions characterized by destruction of the extracellular matrix such as bronchiectasis, cigarette smoke-associated lung disease, asthma, and systemic inflammatory conditions. Analysis of such a cohort will permit calculation of the specificity of elevated MDPs in tuberculosis infection and the potential confounding effect of other inflammatory conditions.
Matrix degradation products have not been identified by proteomic approaches, potentially because of the molecular weight of the molecules that we identified. Desmosine is a single amino acid (MW 526 Da) while PIIINP is a relatively large molecule (42 kDa). Mass spectrometry approaches such as Surface Enhanced Laser Desorption Ionization Time of Flight (SELDI-ToF) are optimal for molecules in the molecular weight range 5–15 kDa and so will not identify elevated PIIINP and desmosine in tuberculosis due to their molecular weight [31]. Gene expression profiling studies, which have been a major focus of tuberculosis research recently [32, 33], will not identify MDPs in tuberculosis because MDPs are produced by the proteolytic destruction of the extracellular matrix, as opposed to resulting from changes in gene expression. One such study of whole blood transcript signature identified MMP-9 as a divergently expressed gene in tuberculosis [33], but plasma MMP-9 was not elevated at a protein level in our study.
Induced sputum concentrations of MMP-1 correlated most closely with PIIINP in both the sputum and plasma, supporting a central role for this collagenase driving lung collagen destruction in tuberculosis [13, 34]. Analysis of plasma MMPs demonstrated increased MMP-8 (neutrophil collagenase), consistent with an emerging role of neutrophil activity in pulmonary tuberculosis [33, 35]. MMP-1 appears to correlate most closely with lung destruction but was not elevated in plasma, and so analysis of plasma samples may not reflect the immunopathological events taking place in the lung interstitium. Sputum desmosine concentrations correlated closely with MMP-8 and -9 concentrations, which are both released by neutrophils on degranulation, and MMP-9 is an elastolytic MMP [36]. Taken together, these data suggest a cascade of proteolytic activity may drive the extensive lung matrix destruction that occurs in tuberculosis.
MDPs have the potential to be used to monitor immune-mediated tissue damage in tuberculosis and to be incorporated into a multiplex diagnostic panel to identify infectious patients with cavitary lung disease who drive the pandemic [37]. MDPs may also be developed as markers to monitor potential immunopathology caused by therapeutic vaccination or immunomodulatory therapies [38], as they represent peripheral markers of pulmonary tissue damage. Population screening for tuberculosis has remained difficult to implement due to the lack of an appropriate diagnostic test, which would require no infrastructure, minimal reagents, no training, and no electricity [9]. Peptide-based diagnostics may be developed into a lateral flow assay [9], and so MDPs may be useful components of near patient tests. However, although we demonstrated that PIIINP and desmosine were significantly increased in tuberculosis, there was overlap between the non-tuberculosis and tuberculosis groups, and so MDPs will need to be part of a multi-analyate panel to diagnose tuberculosis.
In summary, we demonstrate for the first time that immune-mediated tissue damage in tuberculosis drives the generation of MDPs, which are markers of pulmonary pathology. MDPs are elevated in the plasma and so have potential as peripheral markers of immune-mediated lung destruction. MDPs may be used to monitor the effects of new treatments in tuberculosis, such as therapeutic vaccination [39] or immunomodulatory approaches [40], and may also have potential to identify infectious patients with pulmonary disease as part of a multianalyate panel.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgements. We are grateful to Rene Goliath, Tolu Oni, Relebohile Tsekela, Yekiwe Hlombe, and all the staff and patients at the Ubuntu HIV/TB clinic in Cape Town, and Virginia de Azevedo, Giovanni Perez, and Provincial Government Western Cape staff for their assistance with this study. We thank all patients and staff at McCord Hospital, Durban, for taking part in the study. We thank Lynnette Roux, Afton Dorasamy, and Zinhle Mgaga at the Kwazulu-Natal Research Institute for Tuberculosis and-HIV (K-RITH) laboratories for excellent technical support.
Financial support. This work was supported by a Howard Hughes Medical Institute K-RITH travelling scholar award (PE). P. E. and J. S. F. are grateful for support from the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) funding scheme at Imperial College. J. S. and N. W. were funded by the BRC and the Imperial College Wellcome Trust Centre for Clinical Tropical Medicine, and N. W. holds a Wellcome Trust Clinical Research Training Fellowship in Public Health and Tropical Medicine (094000). V. K. is funded by a Harvard University CFAR grant (P30 AI060354). G. M. was funded by the Wellcome Trust and a Fogarty International Center South Africa TB/AIDS Training Award (National Institute for Health/FIC U2R TW007373-01A1 and U2R TW007370-01A1). R. J. W. receives support from the Wellcome Trust (081667, 084323, 088316) and Medical Research Council (UK) (U1175.02.002.00014.01).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328:856–61. doi: 10.1126/science.1185449. [DOI] [PubMed] [Google Scholar]
- 2.Fennelly KP, Jones-Lopez EC, Ayakaka I, et al. Variability of infectious aerosols produced during coughing by patients with pulmonary tuberculosis. Am J Respir Crit Care Med. 2012;186:450–7. doi: 10.1164/rccm.201203-0444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elkington PT, D'Armiento JM, Friedland JS. Tuberculosis immunopathology: the neglected role of extracellular matrix destruction. Sci Transl Med. 2011;3:71ps6. doi: 10.1126/scitranslmed.3001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362:887–99. doi: 10.1016/S0140-6736(03)14333-4. [DOI] [PubMed] [Google Scholar]
- 5.Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nat Rev Immunol. 2011;11:343–54. doi: 10.1038/nri2960. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Jiang J, Cao Z, Yang B, Zhang J, Cheng X. Diagnostic performance of multiplex cytokine and chemokine assay for tuberculosis. Tuberculosis (Edinb) 2012;92:513–20. doi: 10.1016/j.tube.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Chegou NN, Essone PN, Loxton AG, et al. Potential of host markers produced by infection phase-dependent antigen-stimulated cells for the diagnosis of tuberculosis in a highly endemic area. PLoS ONE. 2012;7:e38501. doi: 10.1371/journal.pone.0038501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djoba Siawaya JF, Chegou NN, van den Heuvel MM, et al. Differential cytokine/chemokines and KL-6 profiles in patients with different forms of tuberculosis. Cytokine. 2009;47:132–6. doi: 10.1016/j.cyto.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 9.McNerney R, Daley P. Towards a point-of-care test for active tuberculosis: obstacles and opportunities. Nat Rev Microbiol. 2011;9:204–13. doi: 10.1038/nrmicro2521. [DOI] [PubMed] [Google Scholar]
- 10.Brust B, Lecoufle M, Tuaillon E, et al. Mycobacterium tuberculosis lipolytic enzymes as potential biomarkers for the diagnosis of active tuberculosis. PLoS ONE. 2011;6:e25078. doi: 10.1371/journal.pone.0025078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawn SD, Edwards DJ, Kranzer K, Vogt M, Bekker LG, Wood R. Urine lipoarabinomannan assay for tuberculosis screening before antiretroviral therapy diagnostic yield and association with immune reconstitution disease. AIDS. 2009;23:1875–80. doi: 10.1097/qad.0b013e32832e05c8. [DOI] [PubMed] [Google Scholar]
- 12.Dorman SE, Chihota VN, Lewis JJ, et al. Performance characteristics of the Cepheid Xpert MTB/RIF test in a tuberculosis prevalence survey. PLoS ONE. 2012;7:e43307. doi: 10.1371/journal.pone.0043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elkington P, Shiomi T, Breen R, et al. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest. 2011;121:1827–33. doi: 10.1172/JCI45666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim M, Martinez T, Jablons D, et al. Tumor-derived EMMPRIN (extracellular matrix metalloproteinase inducer) stimulates collagenase transcription through MAPK p38. FEBS Lett. 1998;441:88–92. doi: 10.1016/s0014-5793(98)01474-4. [DOI] [PubMed] [Google Scholar]
- 16.Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev. 2011;24:351–76. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker NF, Clark SO, Oni T, et al. Doxycycline and HIV infection suppress tuberculosis-induced matrix metalloproteinases. Am J Respir Crit Care Med. 2012;185:989–97. doi: 10.1164/rccm.201110-1769OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez B, Gonzalez A, Diez J. Circulating biomarkers of collagen metabolism in cardiac diseases. Circulation. 2010;121:1645–54. doi: 10.1161/CIRCULATIONAHA.109.912774. [DOI] [PubMed] [Google Scholar]
- 19.Ma S, Lieberman S, Turino GM, Lin YY. The detection and quantitation of free desmosine and isodesmosine in human urine and their peptide-bound forms in sputum. Proc Natl Acad Sci U S A. 2003;100:12941–3. doi: 10.1073/pnas.2235344100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828–34. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turino GM, Ma S, Lin YY, Cantor JO, Luisetti M. Matrix elastin: a promising biomarker for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184:637–41. doi: 10.1164/rccm.201103-0450PP. [DOI] [PubMed] [Google Scholar]
- 22.Stone PJ, Gottlieb DJ, O'Connor GT, et al. Elastin and collagen degradation products in urine of smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;151:952–9. doi: 10.1164/ajrccm.151.4.7697272. [DOI] [PubMed] [Google Scholar]
- 23.Meduri GU, Tolley EA, Chinn A, Stentz F, Postlethwaite A. Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med. 1998;158:1432–41. doi: 10.1164/ajrccm.158.5.9801107. [DOI] [PubMed] [Google Scholar]
- 24.Lammi L, Kinnula V, Lahde S, et al. Propeptide levels of type III and type I procollagen in the serum and bronchoalveolar lavage fluid of patients with pulmonary sarcoidosis. Eur Respir J. 1997;10:2725–30. doi: 10.1183/09031936.97.10122725. [DOI] [PubMed] [Google Scholar]
- 25.Lammi L, Ryhanen L, Lakari E, et al. Type III and type I procollagen markers in fibrosing alveolitis. Am J Respir Crit Care Med. 1999;159:818–23. doi: 10.1164/ajrccm.159.3.9805060. [DOI] [PubMed] [Google Scholar]
- 26.Mannello F. Serum or plasma samples? The “Cinderella” role of blood collection procedures: preanalytical methodological issues influence the release and activity of circulating matrix metalloproteinases and their tissue inhibitors, hampering diagnostic trueness and leading to misinterpretation. Arterioscler Thromb Vasc Biol. 2008;28:611–4. doi: 10.1161/ATVBAHA.107.159608. [DOI] [PubMed] [Google Scholar]
- 27.Leroy V, Monier F, Bottari S, et al. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: comparison with PIIINP and hyaluronic acid. Am J Gastroenterol. 2004;99:271–9. doi: 10.1111/j.1572-0241.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- 28.Haukipuro K, Risteli L, Kairaluoma MI, Risteli J. Aminoterminal propeptide of type III procollagen in serum during wound healing in human beings. Surgery. 1990;107:381–8. [PubMed] [Google Scholar]
- 29.Riou C, Perez Peixoto B, Roberts L, et al. Effect of standard tuberculosis treatment on plasma cytokine levels in patients with active pulmonary tuberculosis. PLoS ONE. 2012;7:e36886. doi: 10.1371/journal.pone.0036886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boulware DR, Hullsiek KH, Puronen CE, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203:1637–46. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandhu G, Battaglia F, Ely BK, et al. Discriminating active from latent tuberculosis in patients presenting to community clinics. PLoS ONE. 2012;7:e38080. doi: 10.1371/journal.pone.0038080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maertzdorf J, Ota M, Repsilber D, et al. Functional correlations of pathogenesis-driven gene expression signatures in tuberculosis. PLoS ONE. 2011;6:e26938. doi: 10.1371/journal.pone.0026938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berry MP, Graham CM, McNab FW, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–7. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salgame P. MMPs in tuberculosis: granuloma creators and tissue destroyers. J Clin Invest. 2011;121:1686–8. doi: 10.1172/JCI57423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208:2251–62. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foronjy RF, Nkyimbeng T, Wallace AM, et al. The transgenic expression of matrix metalloproteinase-9 causes adult-onset emphysema in mice associated with the loss of alveolar elastin. Am J Physiol Lung Cell Mol Physiol. 2008;294:1149–57. doi: 10.1152/ajplung.00481.2007. [DOI] [PubMed] [Google Scholar]
- 37.Yoder MA, Lamichhane G, Bishai WR. Cavitary pulmonary tuberculosis: The Holey Grail of disease transmission. Current Sci. 2004;86:74–81. [Google Scholar]
- 38.Churchyard GJ, Kaplan G, Fallows D, Wallis RS, Onyebujoh P, Rook GA. Advances in immunotherapy for tuberculosis treatment. Clin Chest Med. 2009;30:769–82. doi: 10.1016/j.ccm.2009.08.009. , ix. [DOI] [PubMed] [Google Scholar]
- 39.Sander CR, Pathan AA, Beveridge NE, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals. Am J Respir Crit Care Med. 2009;179:724–33. doi: 10.1164/rccm.200809-1486OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uhlin M, Andersson J, Zumla A, Maeurer M. Adjunct immunotherapies for tuberculosis. J Infect Dis. 2012;205(Suppl 2):S325–34. doi: 10.1093/infdis/jis197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.