Abstract
Background. Human metapneumovirus (HMPV) causes lower respiratory tract infections in young children. rHMPV-SHs is a recombinant HMPV (rHMPV) based on a biologically derived wild-type HMPV strain. We characterized its infectivity and immunogenicity in healthy adults to determine whether it would be suitable for use as the parent virus for the development of live attenuated rHMPV vaccines.
Methods. Twenty-one healthy adults were inoculated intranasally with 106 plaque-forming units of rHMPV-SHs. Respiratory symptoms and shedding of challenge virus were assessed. Neutralizing antibody responses, serum immunoglobulin G and A, and nasal wash specimen immunoglobulin A antibody responses to the HMPV F protein were also measured. Induction of nasal cytokines was assessed with electrochemiluminescence assays.
Results. Nine subjects (43%) were infected with challenge virus as determined by virus detection and/or ≥4-fold rise in serum antibody titers. Peak viral shedding occurred on days 7–9 after infection. Four weeks after inoculation, 35% of subjects had any antibody response. Six of 9 infected subjects had respiratory symptoms, and 3 had headache after inoculation. Cytokine patterns differed considerably between subjects with similar illness severity and viral shedding.
Conclusions. The rHMPV-SHs virus is infectious and is a suitable parent virus for development of live-attenuated HMPV vaccine candidates.
Clinical Trials Registration. NCT01109329.
Keywords: human metapneumovirus, HMPV, challenge
Human metapneumovirus (HMPV) was first described in 2001, though serologic studies have confirmed worldwide circulation for >50 years [1–7]. Information about the spectrum of HMPV-associated illnesses has been obtained primarily from epidemiologic studies; most persons have experienced ≥1 HMPV infection by 5 years of age [1, 7–9]. Healthy adults infected with HMPV may be asymptomatic or have upper respiratory tract illness (URTI) [10, 11]; older or asthmatic adults may be at increased risk for HMPV-associated lower respiratory tract illness (LRTI) and hospitalization [10–12]. In infants and young children, the spectrum of illness associated with HMPV is indistinguishable from that observed with respiratory syncytial virus (RSV), and can include URTI, otitis media, bronchiolitis, croup, pneumonia, and exacerbations of asthma [7, 13–16]. Children <2 years of age are most susceptible to severe HMPV illness, and HMPV is responsible for 4%–15% of pediatric hospitalizations for viral LRTI [7, 16, 17]. Thus, HMPV is a leading cause of viral LRTI in children [13, 16].
A vaccine against HMPV could have a substantial impact on the burden of respiratory illness in children [7, 18, 19]. The National Institute of Allergy and Infectious Diseases (NIAID) is working to develop a live-attenuated intranasally administered HMPV vaccine for use in infants [18, 19], which has the potential to reduce the burden of HMPV-associated LRTI significantly. In addition, intranasally administered live-attenuated vaccines induce humoral and cellular immunity and live-attenuated influenza, parainfluenza type 3, and RSV vaccines can replicate in infants in the presence of maternal antibodies [18–23].
The live-attenuated HMPV vaccine candidates that are being developed are derived from rHMPV-SHs, a recombinant HMPV (rHMPV) containing a codon-stabilized version of the small hydrophobic (SH) gene. Stabilization was desirable because wild-type (wt) HMPV tends to accumulate mutations in the SH gene during passage in vitro [24]. Assessment in adults of the replication competence of rHMPV-SHs, the kinetics of viral replication, and the innate and adaptive immune responses induced by this virus could serve as a useful guide for the design and conduct of studies of live-attenuated derivative HMPV strains in adults and children. For this reason, we conducted a challenge study of rHMPV-SHs in healthy adults.
MATERIALS AND METHODS
Study Objectives
This study had 3 primary objectives: (1) to determine the infectivity of rHMPV-SHs, (2) to describe the kinetics of viral replication, and (3) to determine the frequency and severity of respiratory illness. Secondary objectives were (1) to determine the magnitude, frequency, and duration of antibody responses induced by rHMPV-SHs; (2) to assess the correlation between viral shedding and clinical disease; and (3) to assess the nasal cytokine response after infection with rHMPV-SHs.
Challenge Virus
rHMPV-SHs was derived using the sequence of HMPV CAN97-83 [24]; HMPV CAN97-83 originally had been isolated from a patient with acute respiratory illness [25]. Because the SH gene of HMPV is not stable and accumulates mutations with in vitro passage [24], it was replaced by a stabilized version (SHs), as described elsewhere [24]. Stabilization did not change the amino acid assignments of the SH open reading frame (ORF). rHMPV-SHs was derived in Vero cells using a plasmid-only reverse genetics system consisting of 6 plasmids: (1) the full-length antigenomic sense rHMPV-SHs complementary DNA (cDNA) plasmid (pHMPV-SHs) containing the HMPV CAN97-83 strain sequence with its SH gene genetically stabilized; (2) 4 support plasmids, pTM(N), pTM(P), pTM(M2-1), and pTM(L), which supply the HMPV nucleocapsid protein (N), phosphoprotein (P), M2-1 protein, and large polymerase protein (L) [26]; and (3) pN3-T7 s, which contains the cytomegalovirus immediate early enhancer-promoter region driving expression of the T7 bacteriophage polymerase protein, the ORF of which had previously been optimized for eukaryotic expression. The T7 polymerase drives expression of each of the 5 cotransfected HMPV cDNA plasmids. The resulting viral components assemble into functional nucleocapsids and launch a productive infection that produces infectious rHMPV-SHs. After recovery from cDNA, rHMPV-SHs was biologically cloned in 2 limiting dilution steps.
The clinical lot, designated as rHMPV-SHs#102B, was manufactured at Charles River Laboratories. Briefly, seed virus was inoculated onto Vero cells, and supernatant was harvested after 6 days, clarified by centrifugation, treated with benzonase, diafiltered, and flash-frozen at −70°C ± 10°C, all according to good manufacturing practice. The titer was 7.9 log10 plaque-forming units (PFU)/mL. The genomic sequence of the clinical lot (passage 4 after limiting dilution) was confirmed by sequencing. Virus was diluted 1:40 in 1 × Leibovitz L-15 medium (Lonza) to achieve a dose of approximately 106 PFU/0.5 mL.
Study Population
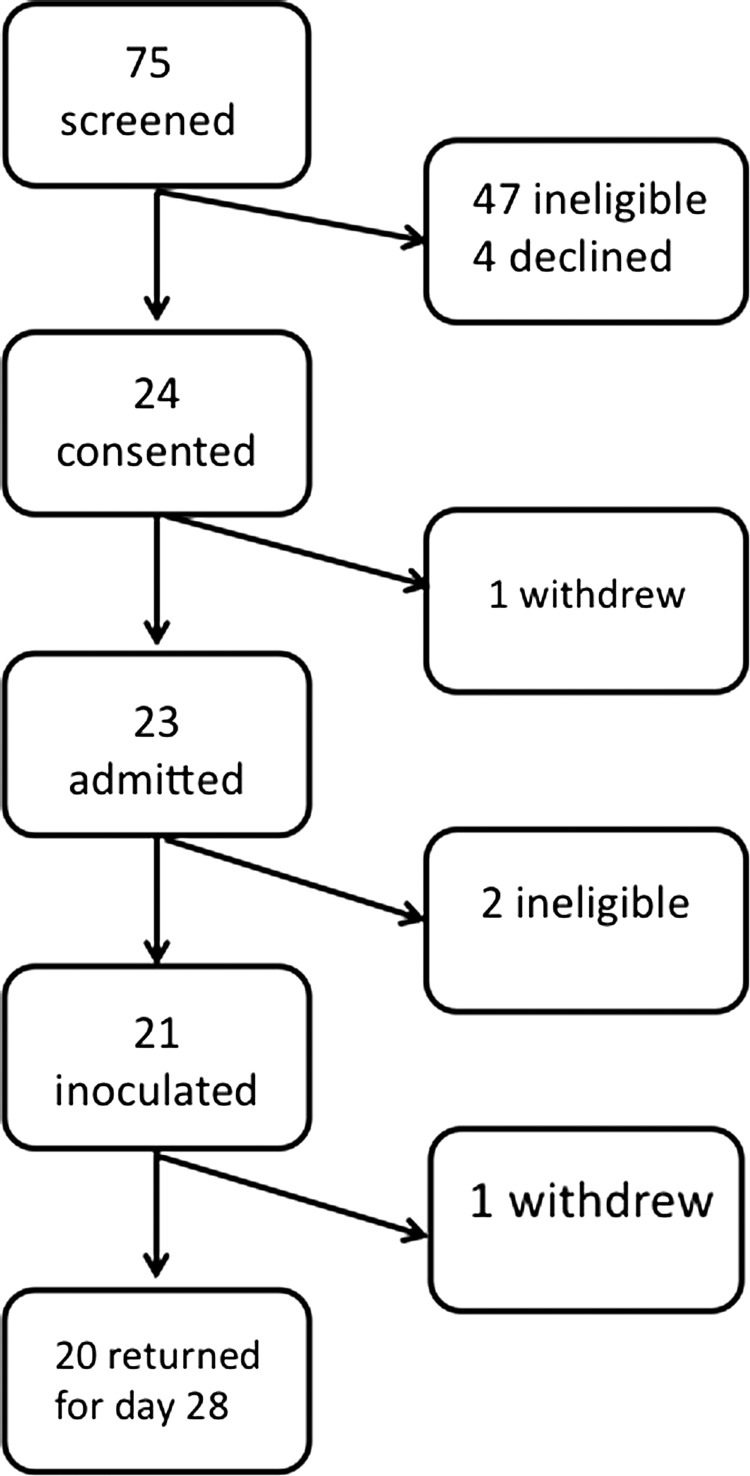
This phase 1 clinical trial (NCT01109329) was conducted at the Center for Immunization Research (CIR) isolation unit under BB-IND 14 323, sponsored by the Division of Clinical Research, NIAID. Twenty-one healthy adults from the Baltimore metropolitan area were recruited and enrolled in the summer of 2010 (Figure 1). The clinical protocol was approved by the Western Institutional Review Board. Informed, witnessed, written consent was obtained from each subject.
Figure 1.
Screening and participation in the challenge study. Of 75 potential subjects screened for the challenge study with rHMPV-SHs, 47 were ineligible because of high antibody titers to the HMPV F protein, medical reasons, or both. Informed consent was obtained from 24 subjects, 23 subjects were admitted, and 21 were challenged with rHMPV-SHs. The median age of the 21 inoculated subjects was 38 years (range, 20–44 years); 18 were male, 6 were white (2 self-identified as Hispanic), and 15 were black (1 self-identified as Hispanic). One subject (subject 4) withdrew after discharge from the inpatient unit for personal reasons.
Healthy adult men and nonpregnant women 18–49 years of age were enrolled if their serum and nasal wash (NW) specimen immunoglobulin A (IgA) titers to HMPV F were <1:50 and if they met other eligibility criteria [27] and were willing to remain on the isolation unit for the duration of the inpatient portion of the trial.
Isolation Unit and Staffing
The CIR Isolation Unit has been described elsewhere [27–31]. As a prerequisite for working on the inpatient portion of the study, each staff member was required to sign a written agreement intended to minimize the potential for transmission of challenge virus from participants to staff members and from staff members to others in the community.
Study Design and Procedures
This study was conducted as an open-label phase 1 inpatient trial with all subjects receiving 1 dose of challenge virus via nose drops. To establish the health of potential participants, CIR staff elicited medical histories, administered physical examinations, and obtained blood and urine samples for standard health screening assays [27–31]. Women were tested for pregnancy using urine and serum β-HCG assays and were counseled to avoid becoming pregnant during the study.
Participants were admitted to the isolation unit 2 days before vaccination for orientation and were monitored for signs or symptoms of acute illness that would preclude their participation in the study. Nasal wash specimens were obtained for viral culture and HMPV reverse-transcription polymerase chain reaction (RT-PCR).
On study day 0, each subject received 106 PFU of rHMPV-SHs delivered as nose drops (0.25 mL per nostril). Subjects were observed for 30 minutes after inoculation and were asked to report any symptoms occurring during their inpatient stay to the nursing staff.
For the duration of the inpatient portion of the study, symptoms were recorded and reported to the study physician. Vital signs were obtained twice daily and a targeted physical examination was performed daily. Fever, rhinorrhea, pharyngitis, cough, hoarseness, headache, otitis media, conjunctivitis, epistaxis, systemic illness (myalgia or chills), and LRTI were classified as reactogenicity events (solicited adverse events) and were defined as described elsewhere [28–31]. Nasal wash specimens were collected daily and tested for challenge virus by quantitative culture, plaque assay, and RT-PCR. In the event of a respiratory or febrile illness, NW specimens were tested for adventitious respiratory pathogens by multiplex real-time reverse transcription polymerase chain reaction (rRT-PCR) (Fast-track Diagnostics).
Subjects were discharged on or after study day 9 contingent on absence of vaccine virus in NW specimens on the day before discharge as detected with RT-PCR. Participants were asked to return for outpatient visits on study days 28, 120, and 180. At each visit, staff obtained vital signs, reviewed interim histories, and obtained blood and NW samples for antibody testing.
Antibody Assays
Serum and NW specimens obtained before and approximately 28 days after inoculation were tested for antibodies to HMPV. Serum neutralizing antibody to HMPV was measured using a modification of a 60% complement-enhanced plaque reduction neutralizing antibody assay used for RSV [32]. In brief, serial 4-fold dilutions of serum samples (starting at 1:10 dilution) were incubated with approximately 20–60 PFU of rHMPV-SHs, at 37°C for 60 minutes and then inoculated on newly confluent Vero cell monolayers in 24-well plates. Cells and serum samples were incubated at 37°C for 60 minutes, and then overlaid with 1% methylcellulose in Opti-MEM (Gibco). After 6 days incubation at 37°C 5% carbon dioxide, the methylcellulose was removed, plates were fixed with 80% methanol at 4°C for 1 hour. Plaques were stained using rabbit hyperimmune serum made against sucrose gradient purified HMPV CAN97-83) [26], peroxidase-conjugated mouse anti-rabbit immunoglobulin G (IgG; KPL), and TrueBlu substrate (KPL). Plaques were counted using an inverted microscope and 60% plaque reduction was calculated using an online tool (http://exon.niaid.nih.gov/plaquereduction/).
Serum IgA and IgG antibodies to HMPV F were measured by end-point titration in enzyme-linked immunosorbent assays. Nunc PolySorp 96-well plates (Fisher Scientific) were coated with histidine-tagged (His-tagged) HMPV F. To generate a His-tagged version of the F protein of strain CAN97-83, a C-terminal His6 epitope tag was added by PCR using a primer specific to the M-F intergenic region (Genbank accession No. NC004148, nucleotides 3034-58), and reverse primer F-His (5′-TTTGAATTCTTAATTAACTAatgatgatgatgatgatggccggcACTGTGTGGTATGAAGCCATTGTTTGTG-3′; His6 tag and linker sequence lower case). Green fluorescent protein–expressing rHMPV with F replaced by the His-tagged version was created. Lysates of infected cells were prepared using 1% octyl-b-D-glucopyranoside (Sigma), and the F protein was isolated using nickel agarose beads. The NW specimens were concentrated 10-fold, as described elsewhere [33, 34], and tested using rHMPV F to measure IgA; NW specimen IgA titers were adjusted for the presence of total IgA, also as described elsewhere [33].
Multiplex Cytokine Assay
The NW specimens from the 8 subjects who were positive for rHMPV-SHs at PCR or viral culture were tested for 12 cytokines and chemokines, using the Meso Scale Discovery (MSD) electrochemiluminescence assays; MSD plates were analyzed on the MS2400 imager (MSD), as described elsewhere [35].
Data Analysis
Infection was defined as shedding of HMPV as detected by culture and/or RT-PCR and/or a ≥4-fold rise in serum neutralizing antibody or serum IgG or IgA antibody to HMPV F protein. Antibody titers were expressed as reciprocal log2 values for calculation of geometric mean values.
RESULTS
Clinical Response to rHMPV-SHs Challenge
Ten (48%) of the 21 subjects experienced at least 1 reactogenicity event during the acute phase of the study (Tables 1, 2). No study-related severe adverse events occurred, and none of the subjects had fever or LRTI.
Table 1.
Individual Clinical, Virologic, and Antibody Responses of Healthy Adults to a Single Dose (106 PFU) of Live Recombinant HMPV (rHMPV-SHs)
| Viral Shedding |
Serum |
Serum Antibody Response to HMPV Fa |
NW Response to HMPV Fa |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Culture |
rRT-PCR |
Neutralizing Antibodya |
IgG |
IgA |
IgA |
Any Antibody Response | ||||||||||||
| Subject No. | Infectedb | Symptoms | Days | Peak Titerc | HMPV Days | Pre | 4 wk | 4-Fold Rise | Pre | 4 wk | 4- Fold Rise | Pre | 4 wk | 4-Fold Rise | Pre | 4 wk | 4- Fold Rise | |
| 1 | Yes | HA | … | 0.6 | 4 | 11.5 | 11.6 | No | 11.6 | 9.6 | No | 5.6 | 5.6 | No | 5.8 | 5.5 | No | No |
| 2 | No | … | … | 0.6 | … | 10.0 | 9.9 | No | 13.6 | 13.6 | No | 4.6 | 5.6 | No | 8.2 | 7.2 | No | No |
| 3 | Yes | HA, URTI | 4–10 | 3 | 5–10 | 6.3 | 10.6 | Yes | 7.6 | 13.6 | Yes | 4.3 | 9.6 | Yes | 4.2 | 8.1 | Yes | Yes |
| 4d | No | … | … | 0.6 | … | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 5 | No | … | … | 0.6 | … | 7.9 | 8.1 | No | 9.6 | 7.6 | No | 5.6 | 5.6 | No | 5.5 | 4.1 | No | No |
| 6 | Yes | URTI | 4–10 | 2.8 | 4–11 | 9.2 | 11.2 | Yes | 9.6 | 13.6 | Yes | 5.6 | 7.6 | Yes | 6.4 | 9.9 | Yes | Yes |
| 7 | No | … | … | 0.6 | … | 10.9 | 11.1 | No | 11.6 | 11.6 | No | 5.6 | 5.6 | No | 7.6 | 9.1 | No | No |
| 8 | Yes | … | 7 | 1.4 | 7 | 8.6 | 8.3 | No | 9.6 | 9.6 | No | 4.6 | 4.6 | No | 7.1 | 5.3 | No | No |
| 9e | No | HA, URTI | … | 0.6 | … | 7.9 | 7.1 | No | 9.6 | 9.6 | No | 5.6 | 5.6 | No | 5.6 | 5.5 | No | No |
| 10 | Yes | … | … | 0.6 | … | 9.1 | 9.4 | No | 9.6 | 9.6 | No | 5.6 | 7.6 | Yes | 7.0 | 8.3 | No | Yes |
| 11 | No | … | … | 0.6 | … | 10.9 | 11.3 | No | 11.6 | 11.6 | No | 5.6 | 5.6 | No | 6.9 | 7.9 | No | No |
| 12 | No | URTI | … | 0.6 | … | 9.5 | 9.5 | No | 11.6 | 9.6 | No | 7.6 | 7.6 | No | 11.6 | 7.9 | No | No |
| 13 | Yes | HA, URTI | 8 | 1.2 | … | 9.0 | 11.3 | Yes | 9.6 | 11.6 | Yes | 5.6 | 7.6 | Yes | 3.6 | 9.5 | Yes | Yes |
| 14 | Yes | URTI | 5, 6, 8, 9 | 1 | 6–9 | 9.3 | 10.6 | No | 9.6 | 9.6 | No | 5.6 | 5.6 | No | 6.3 | 6.5 | No | No |
| 15 | No | HA, URTI | … | 0.6 | … | 9.5 | 9.4 | No | 7.6 | 7.6 | No | 5.6 | 5.6 | No | 4.4 | 7.9 | Yes | Yes |
| 16 | No | … | … | 0.6 | … | 10.2 | 10.6 | No | 5.6 | 5.6 | No | 7.6 | 7.6 | No | 6.7 | 7.3 | No | No |
| 17 | Yes | URTI | … | 0.6 | 2–3 | 9.4 | 8.2 | No | 9.6 | 9.6 | No | 4.6 | 5.6 | No | 7.1 | 9.3 | Yes | Yes |
| 18 | Yes | HA, URTI | 7 | 1 | 7–8 | 12.7 | 12.6 | No | 9.6 | 9.6 | No | 4.6 | 4.6 | No | 5.6 | 5.2 | No | No |
| 19 | No | … | … | 0.6 | … | 11.8 | 10.7 | No | 9.6 | 9.6 | No | 4.6 | 4.6 | No | 7.6 | 5.7 | No | No |
| 20 | No | … | … | 0.6 | … | 12.7 | 12.3 | No | 9.6 | 9.6 | No | 4.6 | 4.6 | No | 8.9 | 9.2 | No | No |
| 21 | No | … | … | 0.6 | … | 13.1 | 13.3 | No | 9.6 | 9.6 | No | 5.6 | 5.6 | No | 4.5 | 9.8 | Yes | Yes |
| Total | 9/21 | 10/21 | 6/21 | … | 7/21 | … | … | 3/20 | … | … | 3/20 | … | … | 4/20 | … | … | 6/20 | 7/20 |
Abbreviations: used are as follows: HA, headache; HMPV, human metapneumovirus; IgA, immunoglobulin A; IgG, immunoglobulin G; NA, not applicable; NW, nasal wash; PFU, plaque-forming units; Pre, before vaccination; rRT-PCR, real-time reverse-transcription polymerase chain reaction; URTI, upper respiratory tract symptoms (rhinorrhea, nasal congestion, cough, hoarseness, or pharyngitis).
a Antibody titers are expressed as reciprocal log2.
b Infected with vaccine virus. Infection was defined as shedding of HMPV as detected by culture and/or rRT-PCR and/or a ≥4fold rise in serum neutralizing antibody or serum IgG or IgA antibody to HMPV F protein.
c Viral titers are expressed as log10 PFU/mL. For purposes of calculation, peak titers of 0.6 log10 PFU/mL were assigned to culture-negative samples.
d Subject 4 withdrew from the study after discharge. Immunology data is not available for this subject.
e Subject 9 shed Enterovirus on days 0–5.
Of the 10 symptomatic subjects, 7 (70%) had evidence of infection with rHMPV-SHs by culture, RT-PCR, or serum antibody response, compared with 1 of 11 asymptomatic subjects (9%; Table 1). Nine subjects (43%) were infected with challenge virus, as determined by virus detection and/or ≥4-fold rise in serum antibody titers. The following reactogenicity events were reported in these 9 infected subjects: headache (subjects 1, 3, 13, and 18), rhinorrhea (subjects 3, 6, 14, 17, and 18), nasal congestion (subjects 13, 14, and 18), pharyngitis (subject 18) cough (subject 14), and hoarseness (subject 18; Tables 1, 2); none of the subjects had fever or other systemic symptoms or LRTI. For most subjects, onset of symptoms occurred 4–9 days after inoculation. Other minor adverse events experienced by the infected subjects included cervical lymphadenopathy (subjects 10 and 18), fatigue (subject 1), recurrence of herpes labialis (subject 3), and transient elevation in blood pressure, lightheadedness, and dizziness (subject 18).
Table 2.
Clinical Response and Viral Shedding in Healthy Adults Following a Single Dose of 106 PFU of Live Recombinant Human Metapneumovirus rHMPV-SHs
| Viral Shedding |
No. (%) With Indicated Symptoms |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Subjects | % Infecteda | No. (%) Culture Positive | Peak Titer Mean (SD)b | No. (%) rRT-PCR+ | Fever | Systemic | Nasal symptomsc | Hoarseness | Pharyngitis | LRI | Cough | Headache | Any Reacto- genicity |
| 21 | 43 | 6 (29) | 1.55 (0.9) | 7 (33) | 0 | 0 | 8 (38) | 2 (10) | 1 (5) | 0 | 2 (10) | 6 (29) | 10 (48) |
Abbreviations used: No. = Number; SD= Standard Deviation; LRI= Lower respiratory infection
a Infection was defined as shedding of HMPV as detected by culture and/or rRT/PCR and/or a four fold or greater rise in serum neutralizing antibody, or serum IgG or IgA antibody to HMPV F protein.
b Of those that had culturable virus.
c Nasal symptoms include nasal congestion and rhinorrhea.
The following reactogenicity events were observed in the 3 symptomatic subjects who were not infected with the challenge virus: headache and rhinorrhea associated with enterovirus infection (subject 9), cough, hoarseness and right-sided pleuritic chest pain without detection of a respiratory pathogen (subject 12), and headache and nasal congestion without detection of a respiratory pathogen (subject 15).
On day 26 after inoculation, subject 2 was hospitalized with chest pain diagnosed as pericarditis and Wolff-Parkinson-White syndrome. This subject had no evidence of infection with rHMPV-SHs; virus was neither recovered by culture nor detected by RT-PCR, and he did not have an antibody response to HMPV detected by any assay. This serious adverse event was judged to be unrelated to receipt of the challenge virus.
Infectivity of the rHMPV-SHs Virus
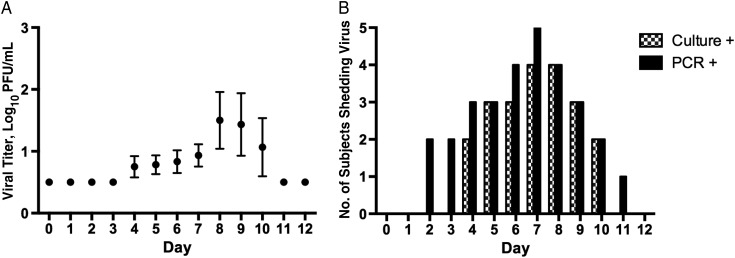
The patterns of viral shedding detected by culture and RT-PCR are shown in Figure 2. In general, shedding of infectious virus began later and persisted for longer than has been described in challenge studies of other paramyxoviruses [36, 37]. Three subjects had viral shedding after day 9, leading to late discharge on day 11 (2 subjects) or day 13 (1 subject; Figure 2B). Although the patterns of viral shedding detected by culture and by RT-PCR were highly concordant, RT-PCR detected the challenge virus both earlier and later than culture in selected individuals (Figure 2B). rHMPV-SHs was detected by viral culture in NW specimens obtained from 6 subjects (Tables 1, 2). The geometric mean peak titer of those who shed virus was 1.55 log10 PFU/mL of NW specimen (range, 1.0–3.0 log10 PFU/mL; Table 2, Figure 2A). Seven subjects had rHMPV-SHs detected by RT-PCR in NW after challenge (Table 2, Figure 2B). Interestingly, 5 of 6 subjects with cultivatable virus also had virus detected by RT-PCR, but 1 subject did not (subject 13; Table 1).
Figure 2.
A, Mean shedding of rHMPV-SHs by study day in the 6 subjects with virus detected at culture. The limit of detection was 1.0 log10 plaque-forming units (PFU)/mL; cultures below that limit were assigned a value of 0.6 log10 PFU/mL. Means are shown with standard errors of the mean. B, Number of subjects shedding virus by study day, by either culture or reverse-transcription polymerase chain reaction (RT-PCR). The nasal wash (NW) specimens were tested for the presence of challenge virus by quantitative viral culture on Vero cells; 100 μL of the NW specimen was added to each well of cells. Nucleic acid was extracted using the NucliSENS miniMAG system (BioMerieux), and specimens were tested for HMPV using the TaqMan 1-step RT-PCR kit and a custom TaqMan assay that amplified a portion of the HMPV P gene (both by Life Technologies, Applied Biosystems). One milliliter of the NW specimen was concentrated to 75 μL, and 5 μL was used for each assay; specimens were tested in triplicate. The primers and probes were specific for strain HMPV CAN97-83 (Genbank accession No. AY297749; primers, PHMPV+ [nucleotides 1432–54], PHMPV− [nucleotides 1491–72], and TaqMan MGB probe [nucleotides 1456–70]). The limit of detection for the RT-PCR is 0.4 PFU log10/mL.
Immune Responses to rHMPV-SHs
Serum and NW specimens were collected at the day 28 follow-up from 20 of 21 subjects. Three subjects (15%) had a ≥4-fold increase in serum neutralizing antibody titer, 3 (15%) had a ≥4-fold increase in serum HMPV F IgG, and 4 (20%) had a ≥4-fold increase in serum HMPV F IgA. In addition, 6 subjects (30%) had a ≥4-fold rise in IgA to the HMPV F protein in their NW specimens (Table 3). For 3 of these 6 subjects, increase in NW specimen IgA was the only rise in antibody titer observed (Table 1).
Table 3:
Summary of Immunological Responses of Adults to 106 PFU of Live Recombinant Human Metapneumovirus rHMPV-SHs
| Neutralizing Antibodyb |
Serum Antibody to HMPV Fb |
NW Antibody to HMPV Fb |
Any Antibody Response | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG |
IgA |
IgA |
||||||||||||
| No. of Subjects | % Infecteda | Pre Mean (SD) | 4 wk Post Mean (SD) | % ≥4-Fold Rise | Pre Mean (SD) | 4 wk Post Mean (SD) | % ≥4-Fold Rise | Pre Mean (SD) | 4 wk Post Mean (SD) | % ≥4-Fold Rise | Pre Mean (SD) | 4 wk Post Mean (SD) | % ≥4-Fold Rise | |
| 20 | 45 | 9.8 (1.8) | 10.2 (1.6) | 15 | 10.2 (1.3) | 10.6 (2.0) | 15 | 5.5 (0.9) | 6.4 (1.6) | 20 | 6.4 (2.1) | 7.3 (1.9) | 30 | 35 |
Abbreviations: No, Number; SD, Standard Deviation; wk, week; NW, Nasal Wash.
a Infection was defined as shedding of HMPV as detected by culture and/or rRT/PCR and/or a four fold or greater rise in serum neutralizing antibody, or serum IgG or IgA antibody to HMPV F protein.
b Antibody titters are expressed as the inverse log2.
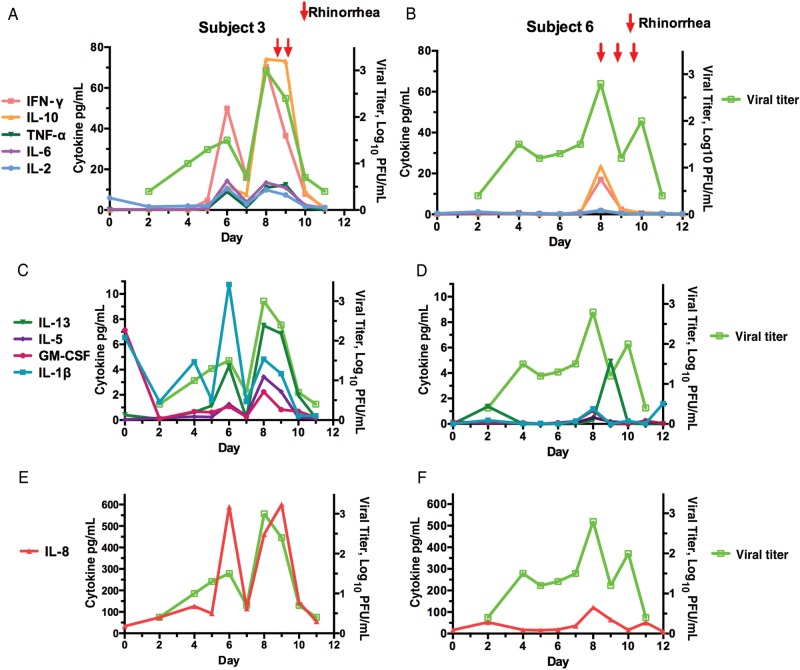
The NW samples from the 8 subjects who had virus recovered either by culture or RT-PCR were tested by electrochemiluminescence assay for the presence of 12 cytokines that may contribute to pathogenesis or to recovery from HMPV infection. Various subjects exhibited differences in timing and type of secreted cytokines; for example, subjects 17 and 18 had peak fold increases in granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL) 1β, 2, 5, 6, 8, 12p70, and 13 during the first 5 days after inoculation, whereas subject 3 had peak fold increases in a largely different set of cytokines occurring mostly after day 5, including interferon (IFN) γ, IL-4, IL-5, IL-6, IL-10, and tumor necrosis factor α (TNFα, data not shown). The viral shedding patterns by these subjects also differed (Table 1), but these patterns were not necessarily consistent with the cytokine expression; subject 17 shed virus early, on days 2–3, subject 18 shed virus on days 7–8, and subject 3 shed virus on days 4–10.
The relationship between viral titers, respiratory symptoms, and NW specimen cytokines are shown in Figure 3 for subjects 3 and 6, the 2 individuals with the greatest increases in cytokine levels and longest duration of viral shedding. In both of them, viral shedding was observed on days 4–10 and rhinorrhea occurred on days 8–10, at or slightly after the peak of shedding (Figure 3A and 3B). Subject 3 shed virus at a peak titer of 3 log10 PFU/mL. This subject was quite symptomatic, with headache, rhinorrhea, development of a herpetic oral lesion. She also had the greatest increases in serum and NW specimen antibodies to HMPV after challenge, with an 11–64-fold increase in serum and NW specimen antibodies. Subject 6 shed virus at a peak titer of 2.8 log10 PFU/mL and also had rhinorrhea (Figure 3B); this subject also had a vigorous HMPV-specific antibody response, with a 4–16-fold increase in antibody titers. Despite the similarities in duration of viral shedding, peak viral titers, and respiratory illness, subjects 3 and 6 exhibited considerable differences in the patterns of up-regulated cytokines (Figure 3A–F). In general, the peak of cytokine expression coincided with the peak of virus replication.
Figure 3.
Viral shedding, clinical symptoms (arrows), and nasal wash specimen cytokines in subjects 3 (A, C, E ) and 6 (B, D, F) by study day. Viral titer is shown in lime green in all panels. Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon γ; IL-1β, interleukin 1β; IL-2, interleukin 2; IL-5, interleukin 5; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; IL-13, interleukin 13; PFU, plaque-forming units; TNF-α, tumor necrosis factor α.
DISCUSSION
In this article, we describe the first human experimental infection with HMPV. This was done with a cDNA-derived virus that was designed from a wt biological strain and was modified to reduce adventitious mutations of the SH gene that can occur during passage in cell culture. Although all adults have experienced previous natural infection with HMPV and are therefore partially immune [10–12], we enrolled adults with relatively low levels of preexisting serum antibody to HMPV in an effort to maximize susceptibility to challenge virus infection. In our study, intranasal challenge with a single 106 PFU dose of the stabilized wt-like virus rHMPV-SHs was associated with viral shedding, as detected by culture in 6 subjects (29%) and by RT-PCR in 7 (33%); seven of 20 subjects with antibody data (35%) had a rise in serum and or nasal antibody to HMPV. In all, 9 subjects (43%) were infected with rHMPV-SHs, defined as viral shedding and/or a ≥4-fold rise in serum antibodies to HMPV. Replication of rHMPV-SHs was relatively restricted in this cohort: among the 6 subjects in whom virus was detected by culture, the geometric mean peak titer was 1.6 log10 PFU/mL.
These results raise the possibility that rHMPV-SHs is attenuated, despite being based on a clinical isolate isolated from an individual with acute respiratory tract illness [25]. The original rHMPV virus differs from the consensus sequence of the wt biological virus by only 4 nucleotide substitutions in the M-F intergenic region [26]. The stabilization of the SH gene involved another 31-nucleotide changes in the SH ORF that were silent with regard to amino acid coding [24]. It seems unlikely that these changes could confer attenuation, and this virus was not attenuated in African green monkeys [24, 38]. Alternatively, HMPV CAN 97-83, from which rHMPV-SHs is derived, may be a relatively attenuated strain despite its association with clinical disease, as has been described elsewhere for a wt human parainfluenza type 3 virus [36]. Attenuation may also have occurred during in vitro passage. It also is possible that rHMPV-SHs is not attenuated and that our results indicate the level of virulence of a wt HMPV strain in healthy adults under these conditions. Regardless, longitudinal assessment during our challenge study allowed us to make some preliminary observations about the temporal relationships between virus replication, clinical symptoms, and nasal cytokine expression, as detailed below.
Clinical symptoms and shedding of challenge virus seemed to coincide and generally occurred later than has been described for other paramyxoviruses. For example, in a recent challenge study with RSV, shedding began as early as day 2 after challenge and peaked at days 5-6 [37]. In contrast, shedding of rHMPV-SHs peaked at days 8-9 by culture and at days 6–8 by RT-PCR (Figure 2), and 2 subjects shed virus as late as 10 or 11 days after challenge. Among those who had virus detected by culture, the mean incubation period was 5.8 days. The longer incubation period is consistent with growth characteristics of HMPV in vitro and with that seen during a nosocomial outbreak of HMPV in Korea, where the estimated incubation period was 7–9 days for a symptomatic case [39]. Other studies have estimated the incubation period to be 3–6 days [40]. The clinical symptoms that occurred in these subjects consisted primarily of URTI and were not distinguishable from those observed after challenge with wt RSV [37]. In general, subjects who shed the highest viral titers were also the most symptomatic, although 2 subjects with peak titers of 1.4 and 1.2 log10 PFU/mL had no symptoms temporally related to their viral shedding. It may be that the limited level of viral replication observed in this study was insufficient to induce symptoms in some subjects and that a more consistent correlation between shedding and symptoms would have been observed with greater viral replication.
This study also allowed us to make some preliminary observations about nasal cytokines in adults infected with a wt-like HMPV. As shown in Figure 3, increases in IL-10 and IFN-γ tended to coincide with peak viral replication and clinical symptoms. Elevations in other cytokines, including IL-2, IL-4, IL-5, IL-8, IL-13, and granulocyte-macrophage colony-stimulating factor occurred in some subjects and tended to peak before maximum virus replication but were more variable in magnitude and onset. Elevations in IL-4 and in IFN-γ are consistent with findings of an HMPV challenge study in mice, which showed that these increases generally peaked later than day 5 after infection [41]. In a clinical study of young infants hospitalized with respiratory virus infections, those with HMPV infection (n = 22) were reported to have lower levels of IL-2, IL-12, IL-10, IL-6, tumor necrosis factor α, and IL-1β in NW specimens than infants with RSV or influenza [42]. Levels of IL-10, IL-12, and IL-8 seemed substantially lower in that study than those observed in infected individuals in our study. In addition, in our study there was remarkable subject-to-subject heterogeneity in cytokine pattern, with respect to the specific cytokines that were induced, their level of expression, and in some cases the temporal pattern. Further evaluation is needed to assess the spectrum of cytokine responses in adults infected with wt HMPV.
In summary, we describe the first challenge study of adult subjects with a wt-like HMPV virus, designated rHMPV-SHs. Although this virus may be partially attenuated for adults, approximately half of the subjects shed virus or had a serum antibody response to the challenge strain. Compared with infection with wt RSV [37], the incubation period is relatively long, with peak viral shedding and symptoms occurring about 6–9 days after inoculation. rHMPV-SHs is appropriate for use as the backbone for a live-attenuated HMPV vaccine candidates, although care should be taken to avoid overattenuation.
Notes
Acknowledgments. We thank Kimberly Boucher for her clinical research expertise.
Financial support. This research was supported in part by the Intramural Research Program of the NIAID, (National Institutes of Health contract N01-AI-15444) and a Collaborative Research and Development Agreement between NIAID, the National Institutes of Health, and MedImmune.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esper F, Boucher D, Weibel C, Martinello RA, Kahn JS. Human metapneumovirus infection in the United States: clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics. 2003;111:1407–10. doi: 10.1542/peds.111.6.1407. [DOI] [PubMed] [Google Scholar]
- 3.Freymouth F, Vabret A, Legrand L, et al. Presence of the new human metapneumovirus in French children with bronchiolitis. Pediatr Infect Dis J. 2003;22:92–4. doi: 10.1097/00006454-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Stockton J, Stephenson I, Fleming D, Zambon M. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg Infect Dis. 2002;8:897–901. doi: 10.3201/eid0809.020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peiris JS, Tang WH, Chan KH, et al. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9:628–33. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madhi SA, Ludewick H, Kuwanda L, van Niekerk N, Cutland C, Klugman KP. Seasonality, incidence, and repeat human metapneumovirus lower respiratory tract infections in an area with a high prevalence of human immunodeficiency virus type-1 infection. Pediatr Infect Dis J. 2007;26:693–9. doi: 10.1097/INF.0b013e3180621192. [DOI] [PubMed] [Google Scholar]
- 7.Kroll JL, Weinberg A. Human metapneumovirus. Semin Respir Crit Care Med. 2011;32:447–53. doi: 10.1055/s-0031-1283284. [DOI] [PubMed] [Google Scholar]
- 8.Ebihara T, Endo R, Kikuta H, Ishiguro N, Ishiko H, Kobayashi K. Comparison of the seroprevalence of human metapneumovirus and human respiratory syncytial virus. J Med Virol. 2004;72:304–6. doi: 10.1002/jmv.10572. [DOI] [PubMed] [Google Scholar]
- 9.Wolf DG, Zakay-Rones Z, Fadeela A, Greenberg D, Dagan R. High seroprevalence of human metapneumovirus among young children in Israel. J Infect Dis. 2003;188:1865–7. doi: 10.1086/380100. [DOI] [PubMed] [Google Scholar]
- 10.Falsey AR. Human metapneumovirus infection in adults. Pediatr Infect Dis J. 2008;27:S80–3. doi: 10.1097/INF.0b013e3181684dac. [DOI] [PubMed] [Google Scholar]
- 11.Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168:2489–96. doi: 10.1001/archinte.168.22.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams JV, Crowe JE, Jr., Enriquez R, et al. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J Infect Dis. 2005;192:1149–53. doi: 10.1086/444392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deffrasnes C, Hamelin ME, Boivin G. Human metapneumovirus. Semin Respir Crit Care Med. 2007;28:213–21. doi: 10.1055/s-2007-976493. [DOI] [PubMed] [Google Scholar]
- 14.Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–50. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esper F, Martinello RA, Boucher D, et al. A 1-year experience with human metapneumovirus in children aged <5 years. J Infect Dis. 2004;189:1388–96. doi: 10.1086/382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn JS. Epidemiology of human metapneumovirus. Clin Microbiol Rev. 2006;19:546–57. doi: 10.1128/CMR.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaunt ER, Harvala H, McIntyre C, Templeton KE, Simmonds P. Disease burden of the most commonly detected respiratory viruses in hospitalized patients calculated using the disability adjusted life year (DALY) model. J Clin Virol. 2011;52:215–21. doi: 10.1016/j.jcv.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchholz UJ, Nagashima K, Murphy BR, Collins PL. Live vaccines for human metapneumovirus designed by reverse genetics. Expert Rev Vaccines. 2006;5:695–706. doi: 10.1586/14760584.5.5.695. [DOI] [PubMed] [Google Scholar]
- 19.Herfst S, Fouchier RA. Vaccination approaches to combat human metapneumovirus lower respiratory tract infections. J Clin Virol. 2008;41:49–52. doi: 10.1016/j.jcv.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Karron RA, Wright PF, Belshe RB, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191:1093–104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 21.Wright PF, Karron RA, Belshe RB, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182:1331–42. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg DP, Walker RE, Lee MS, et al. A bovine parainfluenza virus type 3 vaccine is safe and immunogenic in early infancy. J Infect Dis. 2005;191:1116–22. doi: 10.1086/428092. [DOI] [PubMed] [Google Scholar]
- 23.Karron RA, Steinhoff MC, Subbarao EK, et al. Safety and immunogenicity of a cold-adapted influenza A (H1N1) reassortant virus vaccine administered to infants less than six months of age. Pediatr Infect Dis J. 1995;14:10–6. doi: 10.1097/00006454-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Biacchesi S, Murphy BR, Collins PL, Buchholz UJ. Frequent frameshift and point mutations in the SH gene of human metapneumovirus passaged in vitro. J Virol. 2007;81:6057–67. doi: 10.1128/JVI.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peret TC, Boivin G, Li Y, et al. Characterization of human metapneumoviruses isolated from patients in North America. J Infect Dis. 2002;185:1660–3. doi: 10.1086/340518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biacchesi S, Skiadopoulos MH, Tran KC, Murphy BR, Collins PL, Buchholz UJ. Recovery of human metapneumovirus from cDNA: optimization of growth in vitro and expression of additional genes. Virology. 2004;321:247–59. doi: 10.1016/j.virol.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Karron RA, Callahan K, Luke C, et al. A Live Attenuated H9N2 influenza vaccine is well tolerated and immunogenic in healthy adults. J Infect Dis. 2009;199:711–6. doi: 10.1086/596558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karron RA, Talaat K, Luke C, et al. Evaluation of two live attenuated cold-adapted H5N1 influenza virus vaccines in healthy adults. Vaccine. 2009;27:4953–60. doi: 10.1016/j.vaccine.2009.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talaat KR, Karron RA, Callahan KA, et al. A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a phase I trial in healthy adults. Vaccine. 2009;27:3744–53. doi: 10.1016/j.vaccine.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talaat KR, Karron RA, Liang PH, et al. An open-label phase I trial of a live attenuated H2N2 influenza virus vaccine in healthy adults. Influenza Other Respi Viruses. 2013;7:66–73. doi: 10.1111/j.1750-2659.2012.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talaat KR, Karron RA, Luke CJ, et al. An open label phase I trial of a live attenuated H6N1 influenza virus vaccine in healthy adults. Vaccine. 2011;29:3144–8. doi: 10.1016/j.vaccine.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coates HV, Alling DW, Chanock RM. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol. 1966;83:299–313. doi: 10.1093/oxfordjournals.aje.a120586. [DOI] [PubMed] [Google Scholar]
- 33.Richman DD, Murphy BR, Tierney EL, Chanock RM. Specificity of the local secretory antibody to influenza A virus infection. J Immunol. 1974;113:1654–6. [PubMed] [Google Scholar]
- 34.Murphy BR, Nelson DL, Wright PF, Tierney EL, Phelan MA, Chanock RM. Secretory and systemic immunological response in children infected with live attenuated influenza A virus vaccines. Infect Immun. 1982;36:1102–8. doi: 10.1128/iai.36.3.1102-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dabitao D, Margolick JB, Lopez J, Bream JH. Multiplex measurement of proinflammatory cytokines in human serum: comparison of the Meso Scale Discovery electrochemiluminescence assay and the cytometric bead array. J Immunol Methods. 2011;372:71–7. doi: 10.1016/j.jim.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clements ML, Belshe RB, King J, et al. Evaluation of bovine, cold-adapted human, and wild-type human parainfluenza type 3 viruses in adult volunteers and in chimpanzees. J Clin Microbiol. 1991;29:1175–82. doi: 10.1128/jcm.29.6.1175-1182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee FE, Walsh EE, Falsey AR, Betts RF, Treanor JJ. Experimental infection of humans with A2 respiratory syncytial virus. Antiviral Res. 2004;63:191–6. doi: 10.1016/j.antiviral.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Biacchesi S, Pham QN, Skiadopoulos MH, Murphy BR, Collins PL, Buchholz UJ. Infection of nonhuman primates with recombinant human metapneumovirus lacking the SH, G, or M2–2 protein categorizes each as a nonessential accessory protein and identifies vaccine candidates. J Virol. 2005;79:12608–13. doi: 10.1128/JVI.79.19.12608-12613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S, Sung H, Im HJ, Hong SJ, Kim MN. Molecular epidemiological investigation of a nosocomial outbreak of human metapneumovirus infection in a pediatric hemato-oncology patient population. J Clin Microbiol. 2009;47:1221–4. doi: 10.1128/JCM.01959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Te Wierik MJ, Nguyen DT, Beersma MF, Thijsen SF, Heemstra KA. An outbreak of severe respiratory tract infection caused by human metapneumovirus in a residential care facility for elderly in Utrecht, the Netherlands, January to March 2010. Euro Surveill. 2012;17:20132 [PubMed] [Google Scholar]
- 41.Darniot M, Petrella T, Aho S, Pothier P, Manoha C. Immune response and alteration of pulmonary function after primary human metapneumovirus (hMPV) infection of BALB/c mice. Vaccine. 2005;23:4473–80. doi: 10.1016/j.vaccine.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 42.Laham FR, Israele V, Casellas JM, et al. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. J Infect Dis. 2004;189:2047–56. doi: 10.1086/383350. [DOI] [PubMed] [Google Scholar]