Abstract
Plasmodium falciparum is an intracellular protozoan parasite that infects erythrocytes and hepatocytes. The blood stage of its life cycle causes substantial morbidity and mortality associated with millions of infections each year, motivating an intensive search for potential components of a multi-subunit vaccine. In this study, we present data showing that antibodies from natural infections can recognize a recombinant form of the relatively conserved merozoite surface antigen, PfRH5. Furthermore, we performed invasion inhibition assays on clinical isolates and laboratory strains of P. falciparum in the presence of affinity purified antibodies to RH5 and show that these antibodies can inhibit invasion in vitro.
Keywords: RH2, RH5, malaria, vaccine candidate, invasion, Plasmodium falciparum, recombinant protein expression
Malaria accounts for 1–3 million deaths a year, mainly in children aged <5 years and pregnant women, with the preponderance of the disease burden in sub-Saharan Africa [1–3]. The high parasitemia generated during the blood stage accounts for the clinical manifestations of the disease, including cerebral malaria and severe anemia [4]. Substantial recent progress in understanding the basic biology of malaria focuses attention on the molecular mechanisms of interaction of Plasmodium falciparum with the human erythrocyte and hepatocyte [5].
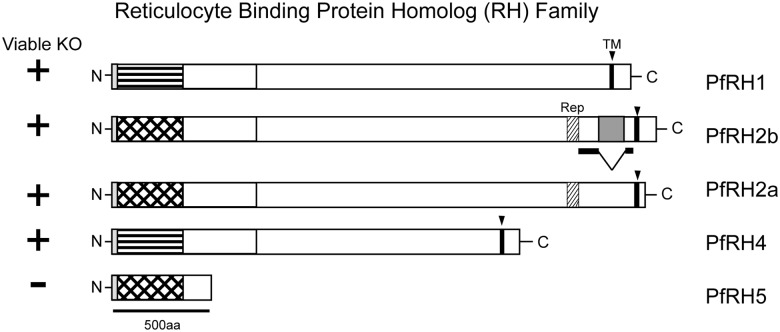
Parasite invasion of erythrocytes depends on binding of parasite surface ligands to specific erythrocyte receptors. Molecular and structural details are known only for PfAMA1(apical membrane antigen 1) [6, 7] and PfEBA-175 (erythrocyte binding antigen) [8]. The latter, proposed to interact with sialic acid residues on glycophorin A, is thought to participate in the irreversible tight junction step of invasion. Members of the RBL (reticulocyte binding ligand) family of proteins have also been implicated in the formation of the tight junction between parasite and erythrocyte, and homologs in other Plasmodium species appear to be expressed in the sporozoite [9], the form that invades hepatocytes. The RBL proteins of P. falciparum, known as the RH (reticulocyte binding protein homolog) family of proteins (Figure 1), have no evident homology to other known invasion ligands. Both PfRH2b and PfRH5 have been implicated in the sialic acid–independent pathway of erythrocyte invasion [10, 11]. Alignment of the amino acid sequences of the PfRH proteins indicates a small but significant degree of sequence conservation (approximately 20%) among members [5] and thus the likelihood of a common fold. PfRH1, 2a, 2b, and 4 are large proteins (approximately 300 kDa); PfRH5 is much smaller (approximately 58 kDa) and may represent a module that is repeated in the other RH family members (Figure 1, Supplementary Figure 1B). PfRH2a and PfRH2b, which may have arisen by gene duplication [12], are identical for the first 84% of the gene, and differ in the last 500 amino acids. Efforts to knock out PfRH5 have not succeeded, and it appears to be critical for parasite survival in vitro [13, 14]. In contrast, PfRh2b can be deleted in vitro, but it cannot be knocked out in strains that lack PfRH2a [15].
Figure 1.
Schematic of RH family and domain architecture in P. falciparum. PfRH5 is a compact single domain which may represent the characteristic fold of the larger multidomain RHs. Cross-hatch stripes indicate the N-terminal portion of PfRH2a/b, which are homologous to PfRH5. Horizontal stripes indicate the N-terminal portion of PfRH1 and 4 with a lower level of sequence conservation. PfRH5 lacks a transmembrane domain and is refractory to gene deletion.
The receptor for PfRH5 has been identified as basigin, also known as extracellular matrix metalloproteinase inducer (EMMPRIN) or cluster of differentiation 147 (CD147) [16]. A soluble form of this receptor can inhibit invasion of all lab-adapted strains and clinical isolates of P. falciparum tested to date [16]. This result implies that the basigin-PfRH5 interaction may be a good blood-stage vaccine target, if antibodies that bind PfRH5 also inhibit invasion. Recently, 2 groups have shown that rabbit polyclonal antibodies against PfRH5 can inhibit invasion by lab-adapted strains of P. falciparum [17–19], a first step in evaluating PfRH5 as a vaccine candidate.
In mouse models of malaria, natural infection or vaccination can result in complete protection against challenge [20, 21]. In contrast, human vaccines show only minimal protective efficacy. Preliminary data from the RTS,S vaccine phase 3 trials suggest that the vaccine is approximately 50% effective at preventing a first malaria episode [22], but other estimates from a more rigorous, intention-to-treat analysis put the figure closer to 34% [23]. These findings are a promising start, but they are far from the ultimate goal of sterile, protective immunity.
Recently, there has been a renewed interest in a blood-stage vaccine for malaria [24, 25]. Because development of allele-specific immunity has been observed with some blood-stage vaccine trials [26, 27], such a vaccine may need to target multiple antigens [28]. A proper understanding of the impact of antigenic diversity on protective immunity in humans will also be essential.
To study the interaction of PfRH5 with the humoral arm of the human immune system, we have expressed stable, soluble forms of PfRH5 and the homologous portion of PfRH2 (PfRH2D1), in substantial quantities. We have characterized the recombinant proteins biochemically, and we have studied their antigenicity to patient sera from high and low endemicity areas of Senegal. We show that affinity-purified antibodies from endemic sera against these recombinant domains can inhibit erythrocyte invasion in vitro by both laboratory-adapted strains and ex vivo clinical isolates of P. falciparum.
MATERIALS AND METHODS
Expression of Recombinant RH2D1 and RH5 Proteins
Codon-optimized expression constructs were synthesized for PfRH2 (RH2D1) (amino acids 79–473) and PfRH5 (amino acids 96–518) (Geneart, AG); amino acid residue numbering is with respect to the full-length protein, including the native signal peptide. These inserts were cloned into a modified version of the pFastbac1 (Invitrogen) vector containing an insect Kozak sequence, honeybee melittin signal peptide [29], and a C-terminal Streptag II [30] (Supplementary Figure 1A). Baculovirus DNA was produced using DH10Bac Escherichia coli, and transfected into Sf9 cells using Cellfectin II (Invitrogen). P2 virus was used to infect Sf9 cells grown in SFM-900 II (Invitrogen); leupeptin, pepstatin, aprotinin, and phenylmethyl sulfonyl fluoride (PMSF) were added 1 day postinfection, and recombinant protein was collected after 3 days. Cells were spun down and the supernatant was concentrated using a Cogent-M tangential flow concentrator (Millipore) fitted with 10 kDa cutoff membrane. The proteins were purified using Strep-Tactin Sepharose (IBA), and eluted with 2.5 mM desthiobiotin in 10 mM Tris pH8, 500 mM sodium chloride, concentrated, and further purified by size exclusion chromatography on Superdex200 16/60 (GE Life Sciences). The size exclusion column was calibrated (Bio-Rad, catalog No. 151–1901). Purified proteins were concentrated to 10 mg/mL, snap-frozen in liquid nitrogen, and stored at −80°C. The approximate yield after purification was 0.3 mg/L insect media for PfRH2, and 0.15 mg/L for PfRH5.
Study Sites and Samples
This study was approved by the Institutional Review Board of the Harvard School of Public Health and by the Ethics Committee of the Ministry of Health in Senegal. Plasma was collected from venous blood draws of consenting Senegalese patients with uncomplicated malaria during the transmission season (September to December). Patient samples were collected from Thies (2009–2011), a low-endemic area of Senegal (entomological inoculation rate [EIR] = 1–10), and Velingara (2004–2005), a region with higher malaria endemicity (EIR >100). Affinity purification of antibodies from single sera were carried out with uninfected Senegalese adult blood units from the National Blood Bank in Dakar, Senegal.
Enzyme-Linked Immunosorbent Assay
Enzyme-linked immunosorbent assay (ELISA) microtiter plates (Immulon 4B) were coated with 30 ng/well of recombinant protein in phosphate-buffered saline (PBS) at 4°C overnight. We chose the coating concentration based on a checkerboard approach to minimize noise due to excess protein coat and/or high levels of secondary antibody. Plates were blocked with 1% nonfat milk in PBST (1× PBS + 0.05% Tween-20) for 1 hour at room temperature and washed 3 times with PBST. Individual plasma samples (Thies n = 100, Velingara n = 108) were added in duplicate at 1:800 dilution and incubated for 2 hours at room temperature. After washing 3 times with PBST, goat antihuman total immunoglobulin G (IgG) horseradish peroxidase–conjugated secondary antibody (Southern Biotech) was added at 1:8000 dilution and incubated at room temperature for 2 hours. Plates were washed 5 times with PBST, developed using Sureblue TMB one-component substrate (KPL) and the reaction stopped with 1N hydrogen chloride. ELISA plates were read at 450 nm. Positive cutoffs for each antigen was determined as an optical density (OD) 3 times the standard deviation of the mean of 72 Boston unexposed sera. For the age-dependent antibody acquisition analysis, samples were stratified by median age of the samples from each endemic site. For the IgG subclass analysis, ELISAs were performed as above with subclass-specific antibodies (Southern Biotech), and positive cutoffs for each subclass were determined as an OD 3 times the standard deviation of the mean of 72 Boston unexposed sera. The denominator to calculate percentage positives of IgG subtypes is less than the total number of samples tested as 3σ cutoff selects for strong responders with high confidence and excludes samples that are weakly positive for multiple IgG subclasses.
Antibody Affinity Purification From Pooled Endemic Sera
We generated affinity-purified antibodies to PfRH5 and PfRH2D1 by separately coupling the recombinant proteins to cyanogen bromide–activated sepharose (Sigma) and running pooled sera from highly endemic Velingara over these affinity columns. After extensive washing, specific antibodies were eluted using 0.1 M glycine pH 2.5 buffer. Elution wells contained 100 µL of 1 M Tris pH8 to minimize the exposure of antibody to low pH. Elution fractions were pooled and concentrated using 100 kDa cutoff Amicon Ultra-15 Centrifugal Filters (Millipore), and buffer exchange was performed with 1× PBS. Antibody stocks were made highly concentrated in 1× PBS and effective stocks were prepared in supplemented Roswell Park Memorial Institute (RPMI) medium.
Antibody Invasion Inhibition Assay
Ring-stage parasites from patient peripheral blood were diluted to 1% parasitemia and treated with neuraminidase, trypsin, and chymotrypsin to prevent reinvasion. These parasitized cells were mixed 50:50 with either untreated (RPMI medium only) acceptor cells or neuraminidase-treated acceptor cells and cultured in a 96-well tissue culture plate at final plating parasitemia of between 0.35% and 0.5% and a final volume of 25 µL. Affinity-purified antibodies against PfRH5 and PfRH2D1 were added at final concentration of 50 µg/mL [31]. Control affinity-purified plasma were the pooled plasma (Vel pool) from which antibodies were purified, the void from the RH5 or RH2 column (RH5 Void, RH2 Void), and pooled plasma from 72 Boston unexposed donors (Bos pool), all added at 50 µg/mL. Samples were plated in duplicate and incubated at 37°C until reinvasion. For the inhibition titration curve, samples were plated in triplicate.
Harvesting of inhibition assays by flow cytometry was performed as previously described [32]. In brief, cultures were pelleted via centrifugation (1200 rpm, 5 minutes) and washed twice in 100 µL 1× PBS + 0.5% bovine serum albumin (BSA). Cells were incubated with 75 µL of 1:1000 SYBR Green I (Molecular Probes) for 20 minutes at 25°C with agitation. Cells were washed twice in 1× PBS + 0.5% BSA and resuspended in 200 µL PBS. Flow cytometry data were collected using a FACSCalibur (Becton Dickinson); 100 000 events were acquired per sample. Initial gating was with unstained, uninfected erythrocytes to account for erythrocyte autofluorescence. Data were analyzed with FlowJo (Tree Star). For ex vivo clinical isolates, the criteria for a successful assay was a parasite multiplication ratio [33] ≥1. We control for growth effects by performing a single cycle experiment. In addition, invasion rates are presented as a ratio to nonimmune (Boston control) sera to remove the known growth enhancement effect of nonspecific antibodies and plasma proteins. Although we cannot fully exclude growth inhibitory effects, they are not likely to be significant, as the fluorescence-activated cell sorting plots do not show an accumulation of schizonts (or earlier stages) in inhibited samples.
RESULTS
Characterization of Recombinant Proteins
By alignment with homologous genes from closely related species and secondary structure prediction, we have defined a candidate domain of PfRH5 and the likely homologous portion of PfRH2 (Figure 1, Supplementary Figure 1B and 1D). Our domain boundaries are slightly different than those in some prior publications (see Methods) [13, 34, 35]. We generated baculovirus expression vectors for these 2 proteins, using codon optimized sequences, and purified the products (Figure 2A and 2C). Size exclusion chromatography showed that both products had elution volumes expected for monomers of the corresponding domains (Figure 2B and 2D).
Figure 2.
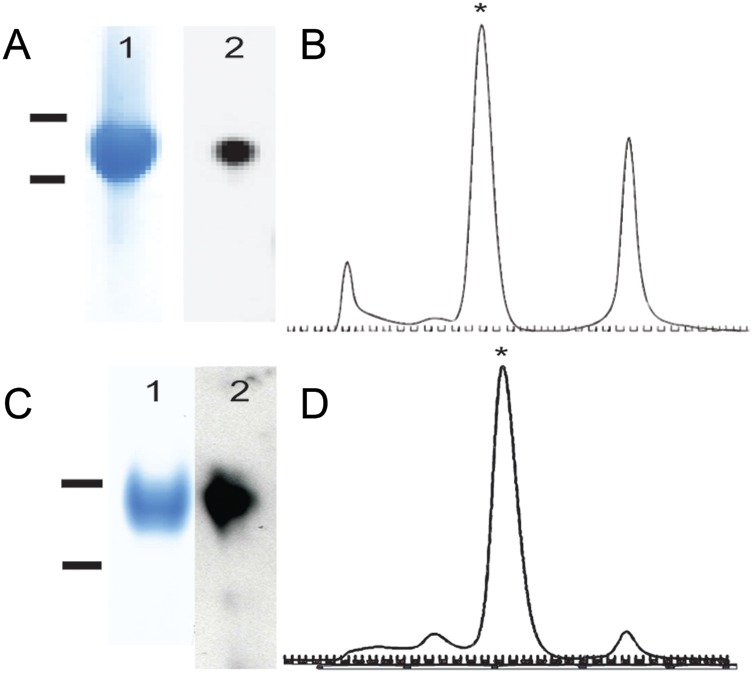
Biochemical characterization of recombinant PfRH2D1 and PfRH5. A, Gel of purified PfRH5 protein after size exclusion chromatography: 1 = PfRH5 Coomassie (bars 80 kDa/60 kDa), 2 = α-StrepII tag western. B, Elution profile on Superdex 200 column. Monomeric RH5 is starred. C, Gel of purified PfRH2D1 protein after size exclusion chromatography. 1 = PfRH2D1 Coomassie (bars 60 kDa/50 kDa), 2 = α-Streptag II western. D, Elution profile on Superdex 200 column. Starred peak is monomeric.
Immune Reactivity of Patient Sera
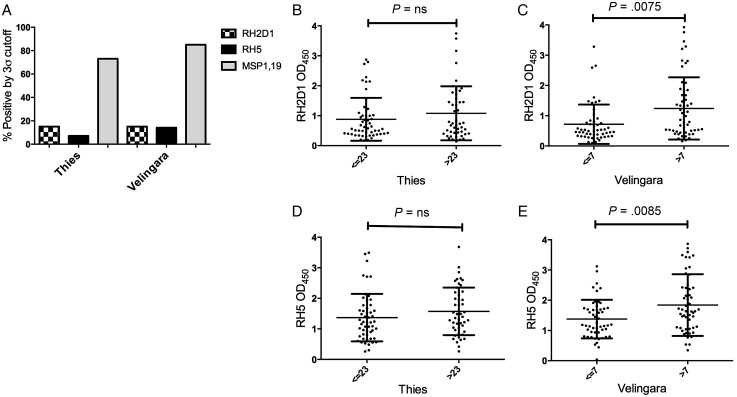
To determine whether these recombinant invasion ligand domains induce a humoral immune response following natural exposure, we analyzed sets of patient sera from low (Thies) and high (Velingara) endemicity areas in Senegal by ELISA. About 15% of the sera from both low and high endemicity areas of Senegal were PfRH2D1 ELISA positive; PfRH5 reactivity was higher in the latter group (7% vs 14%, respectively). For comparison, ELISAs with MSP 1–19, an abundant merozoite surface protein, were 60% positive in the same bank of sera (Figure 3A); this frequency is comparable to that seen in other endemic areas [36, 37]. Glycosylation did not appear to play a significant role in ELISA reactivity to RH5 as glycosylated insect cell–produced RH5 had similar OD values to mammalian cell–produced RH5 (which has all 4 N-linked sites mutated away; Supplementary Figure 2D and 2E); RH2 was not significantly glycosylated (Supplementary Figure 2E). Stratification of PfRH2D1 and PfRH5 positives by median age demonstrates a statistically significant age-dependent acquisition of antibodies in the high-endemicity area (Velingara) with a Mann–Whitney U equality-of-populations rank test (P = .0075 and P = .0085, respectively; Figure 3C and 3E). Age-dependent acquisition was also significant when ELISA positives were compared by Fisher exact test (P = .041 and P = .008, respectively). These results suggest that repeated exposure ultimately leads to production of antibodies that recognize even the conserved, PfRH2D1 and PfRH5 that are relatively nonimmunogenic compared to MSP-1. Age stratification of PfRH2D1 and PfRH5 positives failed to reach statistical significance in the low-endemicity area (Thies; Figure 3B and 3D).
Figure 3.
Immune reactivity in malaria exposed sera. A, Immunoglobulin G (IgG) responses against recombinant PfRH2D1, PfRH5, and MSP 1–19 were assessed in low (Thies) and high (Velingara) endemicity plasma by enzyme-linked immunosorbent assay. Percentage positive responses are shown based on 3σ cutoff to Boston controls. B, Stratification of PfRH2D1 optical density (OD) by median age in low-endemicity samples (Thies). C, Stratification of PfRH2D1 OD by median age in high-endemicity samples (Velingara). D, Stratification of PfRH5 OD by median age in low-endemicity samples (Thies). E, Stratification of PfRH5 OD by median age in high-endemicity samples (Velingara).
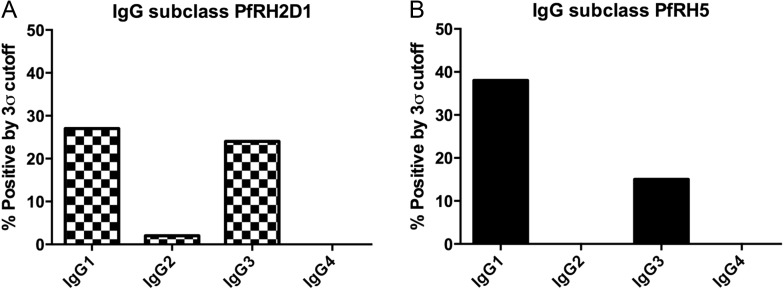
IgG subtype analysis of sera that reacted positively with PfRH5 showed that the antibodies were either IgG1 (69%: 13/19) or IgG3 (31%: 6/19); for PfRH2D1, the results were similar—either IgG1 (50%: 11/22) or IgG3 (45%: 10/22), with only 1 sample IgG2 (Figure 4).
Figure 4.
Immunoglobulin G (IgG) subclass analysis of ELISA positives. A, PfRH2D1 IgG subclass positives, by 3σ cutoff to Boston controls. B, PfRH5 IgG subclass positives, by 3σ cutoff to Boston controls.
Inhibition Assays
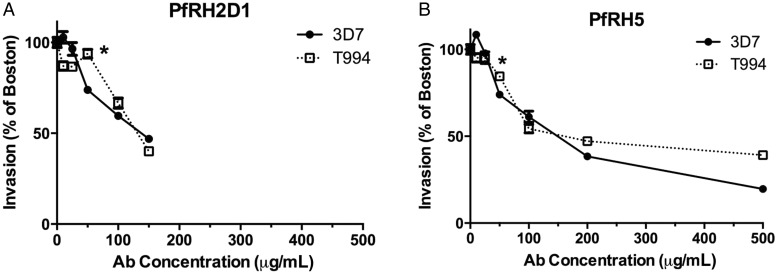
Because we could detect a significant humoral immune response from human sera, we next tested whether the antibodies generated by natural infection were inhibitory. Specific antibodies were enriched by affinity purification from Senegalese plasma 25- and 625-fold for PfRH5 and PfRH2, respectively. The PfRH2 affinity-purified antibodies were also enriched in anti-PfRH5 activity (Supplementary Figure 2A). We determined that the enrichment was probably due to cross-reactivity between RH2 and RH5 and not to reactivity with the Streptag II affinity tag. We tested RH2- and RH5-affinity purified antibodies from a single plasma sample on ELISA plates coated either with StreptagII-RH5 or with CD4-RH5 [16] and found that the RH2 and RH5 signals were independent of both tags (Supplementary Figure 2B). We also showed that our affinity purification did not co-purify antibodies to other abundant merozoite antigens by performing an ELISA to MSP 1–19 on the affinity-purified anti-RH2 and anti-RH5 antibodies (Supplementary Figure 2D). The affinity-purified RH5 antibodies gave 1.3 ELISA OD units/µg protein, whereas the Velingara pool had 0.02 ELISA OD units/µg protein. Individually, positive samples ranged from 0.15 to 0.35 ELISA OD units/µg protein. We performed invasion-inhibition assays with sialic acid–dependent (3D7) and –independent (T994) lab strains of P. falciparum [38] in the presence of affinity-purified antibodies. Both PfRH5- and PfRH2D1-affinity purified antibodies inhibited invasion of these laboratory strains (Figure 5). The Inhibitory Concentration of 50% (IC50) is approximately 120 µg/mL for anti-RH2, and 110 µg/mL for anti-RH5. The reproducibility of these assays is ±5%, so we would note that the inhibition becomes statistically significant at the starred level in Figure 5 (50 µg/mL).
Figure 5.
Antibody invasion inhibition of laboratory-adapted Plasmodium falciparum using affinity-purified antibodies. A, Inhibition by affinity-purified antibodies against PfRH2D1 of red blood cell invasion by P. falciparum lab lines, as a ratio to invasion in the presence of unexposed control plasma (“Boston”). B, Inhibition by affinity-purified antibodies against PfRH5 of red blood cell invasion by P. falciparum lab lines, as a ratio to invasion in the presence of unexposed control Boston plasma. *Indicates 50 µg/mL antibody concentration used in experiments with clinical isolates.
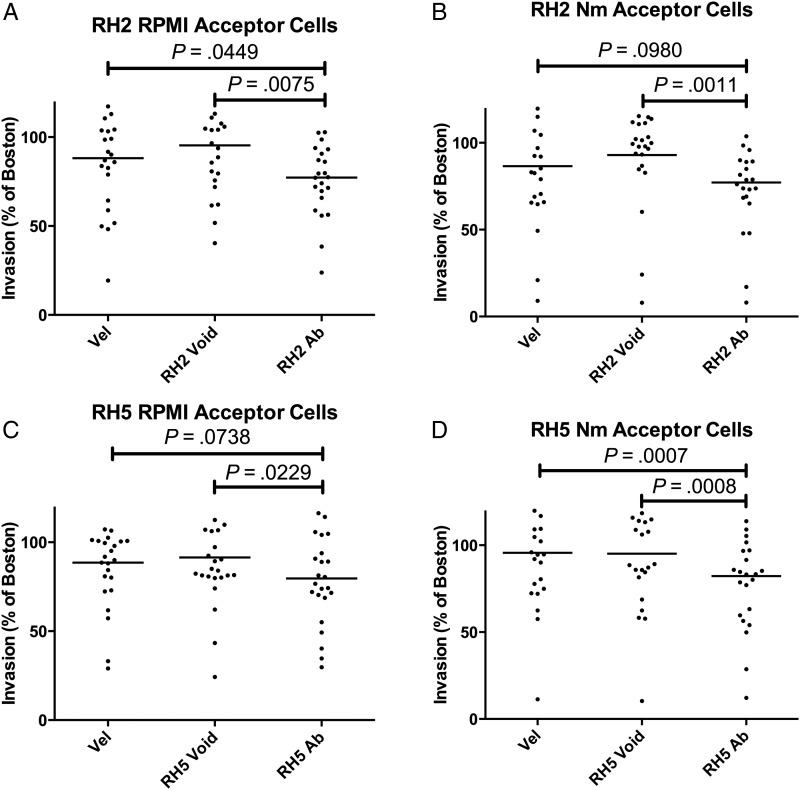
To see if the inhibitory capacity of affinity-purified antibodies was maintained against genetically diverse clinical isolates, we performed ex vivo invasion inhibition assays on clinical isolates of P. falciparum (n = 23) from Thies, Senegal (Figure 6), at an antibody concentration of 50 µg/mL. Because of limitations in the amount of affinity-purified antibody, we chose to test many clinical isolates at this single concentration rather than testing fewer isolates at a range of higher concentrations, at which we expect that the inhibitory effects would have been more pronounced as observed for the laboratory strains (Figure 5). We saw a statistically significant inhibition of invasion for α-PfRH2D1 antibodies relative to the void or the pool (P = .0075, P = .0449, mean inhibition = 22.3%; Wilcoxon matched-pairs test; Figure 6A), which was also observed when acceptor cells were treated with neuraminidase to remove the contribution of sialic acid–dependent invasion pathways (P = .0011, P = .0980, mean inhibition = 22.8%; Figure 6B). α-PfRH5 antibodies showed a similar ability to inhibit invasion (P = .0229, P = .0738, mean inhibition 20.4%; Figure 6C), which persisted when neuraminidase-treated acceptor cells were used (P = .0008, P = .0007, mean inhibition = 17.7%; Figure 6D). When invasion inhibition by α-PfRH2D1 and α-PfRH5 antibodies was compared for RPMI- and neuraminidase-treated cells, the difference was not statistically significant, implying that the inhibition observed is not enhanced when sialic acid is limited.
Figure 6.
Antibody (Ab) inhibition of clinical isolates of Plasmodium falciparum. All antibodies were tested at 50 µg/mL. Invasion is presented as a ratio to Boston control sera. A, α-PfRH2D1 antibodies, Roswell Park Memorial Institute (RPMI)–treated acceptor cells. B, α-PfRH2D1 antibodies, neuraminidase (Nm)–treated acceptor cells. C, α-PfRH5 antibodies, RPMI-treated acceptor cells. D, α-PfRH5 antibodies, neuraminidase-treated acceptor cells. RH void = invasion of acceptor cells treated with the portion of Velingara (Vel) sera that did not bind to the PfRH2D1 or PfRH5 affinity column, respectively. Bars indicate mean level of inhibition. RH Ab = invasion of acceptor cells treated with the portion of Velingara sera that bound specifically to the PfRH2D1 or PfRH5 affinity column, respectively.
DISCUSSION
Our recombinant PfRH5 and PfRH2D1 proteins are well-characterized, soluble, monomeric species. The stability of our expressed protein, and its activity in binding inhibitory antibodies from endemic sera, gives us confidence in the domain assignment. Although there are descriptions in the literature for the production of PfRH5 by refolding in E. coli [11, 13], or as a fusion to the CD4 tag in a mammalian expression system [16], there are no previous reports of expression and characterization of well-behaved, soluble protein that bears only a small affinity tag.
We showed that PfRH5 and PfRH2D1 elicit humoral responses in endemic sera. Although not directly comparable to other studies, it is interesting to note that the PfRH2D1 that we produced is ELISA positive in a significantly lower percentage of endemic sera [35]. There are several possible explanations for the difference. The assay conditions are not strictly comparable, as the prior work [35] has used a significantly higher concentration of protein for coating the ELISA plates (1–2 µg/mL compared to 300ng/mL in our studies). Moreover, our recombinant protein has been produced in a eukaryotic system, whereas prior studies have used E. coli–produced protein with small differences in domain boundaries. Disulfide-rich domains are often misfolded when overexpressed in E. coli, resulting in presentation of epitopes that may not be relevant in vivo. Finally, there may be differences in the invasion pathway used by the parasite in the 2 geographic areas, Papua New Guinea vs Senegal. In particular, Papua New Guinea parasites may present higher levels of the RH2 and RH5 invasion ligands, giving greater exposure to the humoral immune system and resulting in higher titers of antibody to these proteins. Another group has also shown modest levels of α-RH5 antibodies in sera from a small cohort of Kenyan children tested against mammalian cell–expressed RH5, but lack of statistical treatment makes it impossible to draw further conclusions [17].
The ELISA experiments show that the humoral response to PfRH2D1 is the same in high- and low-endemicity areas whereas the response to PfRH5 is not. One possible implication is that there is a selection of antibodies with strong binding to PfRH5; these antibodies would then develop upon repeated exposure to the antigen. Recently adapted clinical isolates suggest that there are very few coding polymorphisms in RH5 [19], and repeated exposure is therefore possible. Further studies will be required to test this hypothesis.
Affinity-purified antibodies against PfRH2D1 and PfRH5 from endemic sera inhibit invasion by both clinical and laboratory isolates of P. falciparum. The equivalent level of inhibition in 3D7 and T994 at higher concentrations of antibody suggest cross-reactivity of the affinity-purified antibodies, as T994 has been previously shown not to express significant levels of PfRH2a or PfRH2b. Our ELISA data indeed indicate that RH2D1 affinity-purified antibodies are enriched in PfRH5 reactivity (Supplementary Figure 2A). This cross-reactivity is not surprising, as we selected the RH2 domain for its strong similarity (31%) and likely homology to RH5. In addition, Western blots on supernatants from T994 show that there is a low level of RH2a and RH2b expression in this strain (Supplementary Figure 2C). We cannot exclude the possibility that this low level of expression is essential for invasion and that this residual expression is what the affinity-purified antibodies are targeting.
The lack of inhibition in some of the clinical isolates may be due to the effects of the limited polymorphisms in RH5 (Supplementary Figure 3A). It is interesting to note that one prevalent polymorphism C203Y results in an unpaired cysteine; as previously noted [13], this may have implications on the structure. Additionally, these parasites may be using alternative invasion pathways, relying less on RH5 and the sialic acid–independent pathway. One other possibility is that glycosylation may play a role in the humoral response. It has recently been appreciated that Plasmodium species may perform a rudimentary form of N-linked glycosylation [39]. Because insect cell produced protein has more complex N-linked glycosylation, it may not present the relevant sugar epitopes of native Plasmodium glycosylation, and thus we would not have enriched for such antibodies in our affinity purification.
Beyond the experimental possibilities above for low levels of neutralizing antibodies, there are also several interesting structural and immunological explanations. One possibility is PfRH5 is not very accessible to the immune system during the process of invasion. Another is that RH5 is accessible, but neutralizing epitopes are not, or nonneutralizing epitopes are dominant, with neutralizing ones subdominant. A final consideration is that the PfRH5 interacting protein (PfRIPR) [40] may play a role in the modulating the humoral response to PfRH5. Identification and characterization of human monoclonal antibodies against RH5 should help distinguish among these alternative hypotheses.
Our demonstration of the presence of inhibitory antibodies to PfRH5 in vivo has important implications for the use of this protein in a multiple-antigen vaccine strategy. A soluble domain of the PfRH5 receptor, basigin, can inhibit invasion in vitro of all clinical and lab strains of P. falciparum tested [16], and rabbit polyclonal antibodies to RH5 can also inhibit invasion [17–19]. Our data are the next step in the evaluation of PfRH5 as a vaccine candidate: they demonstrate that natural infection in humans elicits invasion-inhibiting antibodies. Future experiments should investigate age-dependent acquisition of protective antibodies, the role of RH5 polymorphism on invasion, and potential synergy between RH family and EBA family human invasion inhibitory antibodies as it relates to invasion pathway. For rational design of a malaria vaccine, we will need to understand the host–pathogen interactions that contribute to infection, the parasite proteins and polymorphisms under immune selection, and the mechanisms that govern productive immune effector functions in humans.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Julian Rayner for the rabbit RH2a and RH2b antibodies, and Gavin Wright for the RH5CD4 expression construct; the members of the sample collection team in Senegal (D. Ndiaye, J. Daily, L. Ndiaye, Y. Diedhiou, O. Ly, P. D. Sene, A. Mbaye, and D. Diop); Selasi Dankwa for assistance with invasion assays; the patients who agreed to participate in these studies; and Dr Jeffrey Dvorin for comments on the manuscript. S. D. P. would also like to acknowledge Wewe Utajiju, MD.
Financial support. S. D. P. was supported by the National Institutes of Health (NIH) (grants K12-HD00850 and 5K12-HD052896), the Boston Children's Hospital Office of Faculty Development, and the Shore Fellowship. A. K. B. was supported by the Centers for Disease Control and Prevention (grant R36 CK000119-01) and an Epidemiology of Infectious Disease and Biodefense Training Grant (2T32 AI007535-12). M. T. D. was supported by the NIH (grant R03 TW008053). S. C. H. is an Investigator in the Howard Hughes Medical Institute.
Potential conflicts of interest. All authors: No potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Snow RW, Craig M, Newton CR, Steketee RW . The public health burden of Plasmodium falciparum malaria in Africa: deriving the numbers. Working paper number 11; Bethesda, MD: Fogarty International Center, National Institutes of Health; 2003. [Google Scholar]
- 2.Murphy SC, Breman JG. Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. Am J Trop Med Hyg. 2001;64:57–67. doi: 10.4269/ajtmh.2001.64.57. [DOI] [PubMed] [Google Scholar]
- 3.Craig MH, Snow RW, le Sueur D. A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol Today. 1999;15:105–11. doi: 10.1016/s0169-4758(99)01396-4. [DOI] [PubMed] [Google Scholar]
- 4.Greenwood BM, Bojang K, Whitty CJ, Targett GA. Malaria. Lancet. 2005;365:1487–98. doi: 10.1016/S0140-6736(05)66420-3. [DOI] [PubMed] [Google Scholar]
- 5.Rayner JC. The merozoite has landed: reticulocyte-binding-like ligands and the specificity of erythrocyte recognition. Trends Parasitol. 2009;25:104–6. doi: 10.1016/j.pt.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Tonkin ML, Roques M, Lamarque MH, et al. Host cell invasion by apicomplexan parasites: insights from the co-structure of AMA1 with a RON2 peptide. Science. 2011;333:463–7. doi: 10.1126/science.1204988. [DOI] [PubMed] [Google Scholar]
- 7.Nair M, Hinds MG, Coley AM, et al. Structure of domain III of the blood-stage malaria vaccine candidate, Plasmodium falciparum apical membrane antigen 1 (AMA1) J Mol Biol. 2002;322:741–53. doi: 10.1016/s0022-2836(02)00806-9. [DOI] [PubMed] [Google Scholar]
- 8.Tolia NH, Enemark EJ, Sim BK, Joshua-Tor L. Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum. Cell. 2005;122:183–93. doi: 10.1016/j.cell.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 9.Preiser PR, Khan S, Costa FT, et al. Stage-specific transcription of distinct repertoires of a multigene family during Plasmodium life cycle. Science. 2002;295:342–5. doi: 10.1126/science.1064938. [DOI] [PubMed] [Google Scholar]
- 10.Baum J, Maier AG, Good RT, Simpson KM, Cowman AF. Invasion by P. falciparum merozoites suggests a hierarchy of molecular interactions. PLoS Pathog. 2005;1:e37. doi: 10.1371/journal.ppat.0010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez M, Lustigman S, Montero E, Oksov Y, Lobo CA. PfRH5: a novel reticulocyte-binding family homolog of Plasmodium falciparum that binds to the erythrocyte, and an investigation of its receptor. PLoS One. 2008;3:e3300. doi: 10.1371/journal.pone.0003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dvorin JD, Bei AK, Coleman BI, Duraisingh MT. Functional diversification between two related Plasmodium falciparum merozoite invasion ligands is determined by changes in the cytoplasmic domain. Mol Microbiol. 2010;75:990–1006. doi: 10.1111/j.1365-2958.2009.07040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baum J, Chen L, Healer J, et al. Reticulocyte-binding protein homologue 5—an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int J Parasitol. 2009;39:371–80. doi: 10.1016/j.ijpara.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–66. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Duraisingh MT, Triglia T, Ralph SA, et al. Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. EMBO J. 2003;22:1047–57. doi: 10.1093/emboj/cdg096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crosnier C, Bustamante LY, Bartholdson SJ, et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–7. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas AD, Williams AR, Illingworth JJ, et al. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat Commun. 2011;2:601. doi: 10.1038/ncomms1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams AR, Douglas AD, Miura K, et al. Enhancing blockade of Plasmodium falciparum erythrocyte invasion: assessing combinations of antibodies against PfRH5 and other merozoite antigens. PLoS Pathog. 2012;8:e1002991. doi: 10.1371/journal.ppat.1002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bustamante LY, Bartholdson SJ, Crosnier C, et al. A full-length recombinant Plasmodium falciparum PfRH5 protein induces inhibitory antibodies that are effective across common PfRH5 genetic variants. Vaccine. 2013;31:373–9. doi: 10.1016/j.vaccine.2012.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anders RF, Crewther PE, Edwards S, et al. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine. 1998;16:240–7. doi: 10.1016/s0264-410x(97)88331-4. [DOI] [PubMed] [Google Scholar]
- 21.Cohen S. Progress in malaria vaccine development. Br Med Bull. 1982;38:161–5. doi: 10.1093/oxfordjournals.bmb.a071753. [DOI] [PubMed] [Google Scholar]
- 22.Asante KP, Abdulla S, Agnandji S, et al. Safety and efficacy of the RTS,S/AS01E candidate malaria vaccine given with expanded-programme-on-immunisation vaccines: 19 month follow-up of a randomised, open-label, phase 2 trial. Lancet Infect Dis. 2011;11:741–9. doi: 10.1016/S1473-3099(11)70100-1. [DOI] [PubMed] [Google Scholar]
- 23.Duncan CJ, Hill AV. What is the efficacy of the RTS, S malaria vaccine? BMJ. 2011;343:d7728. doi: 10.1136/bmj.d7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crabb BS, Beeson JG. Promising functional readouts of immunity in a blood-stage malaria vaccine trial. PLoS Med. 2005;2:e380. doi: 10.1371/journal.pmed.0020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards JS, Beeson JG. The future for blood-stage vaccines against malaria. Immunol Cell Biol. 2009;87:377–90. doi: 10.1038/icb.2009.27. [DOI] [PubMed] [Google Scholar]
- 26.Spring MD, Cummings JF, Ockenhouse CF, et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS One. 2009;4:e5254. doi: 10.1371/journal.pone.0005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genton B, Betuela I, Felger I, et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1–2b trial in Papua New Guinea. J Infect Dis. 2002;185:820–7. doi: 10.1086/339342. [DOI] [PubMed] [Google Scholar]
- 28.Lopaticki S, Maier AG, Thompson J, et al. Reticulocyte and erythrocyte binding-like proteins function cooperatively in invasion of human erythrocytes by malaria parasites. Infect Immun. 2011;79:1107–17. doi: 10.1128/IAI.01021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tessier DC, Thomas DY, Khouri HE, Laliberte F, Vernet T. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin signal peptide. Gene. 1991;98:177–83. doi: 10.1016/0378-1119(91)90171-7. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt TG, Skerra A. The random peptide library-assisted engineering of a C-terminal affinity peptide, useful for the detection and purification of a functional Ig Fv fragment. Protein Eng. 1993;6:109–22. doi: 10.1093/protein/6.1.109. [DOI] [PubMed] [Google Scholar]
- 31.Egan AF, Burghaus P, Druilhe P, Holder AA, Riley EM. Human antibodies to the 19 kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 1999;21:133–9. doi: 10.1046/j.1365-3024.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- 32.Bei AK, Desimone TM, Badiane AS, et al. A flow cytometry-based assay for measuring invasion of red blood cells by Plasmodium falciparum. Am J Hematol. 2010;85:234–7. doi: 10.1002/ajh.21642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chotivanich K, Udomsangpetch R, Simpson JA, et al. Parasite multiplication potential and the severity of falciparum malaria. J Infect Dis. 2000;181:1206–9. doi: 10.1086/315353. [DOI] [PubMed] [Google Scholar]
- 34.Sahar T, Reddy KS, Bharadwaj M, et al. Plasmodium falciparum reticulocyte binding-like homologue protein 2 (PfRH2) is a key adhesive molecule involved in erythrocyte invasion. PLoS One. 2011;6:e17102. doi: 10.1371/journal.pone.0017102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiling L, Richards JS, Fowkes FJ, et al. Evidence that the erythrocyte invasion ligand PfRh2 is a target of protective immunity against Plasmodium falciparum malaria. J Immunol. 2010;185:6157–67. doi: 10.4049/jimmunol.1001555. [DOI] [PubMed] [Google Scholar]
- 36.Okech BA, Corran PH, Todd J, et al. Fine specificity of serum antibodies to Plasmodium falciparum merozoite surface protein, PfMSP-1(19), predicts protection from malaria infection and high-density parasitemia. Infect Immun. 2004;72:1557–67. doi: 10.1128/IAI.72.3.1557-1567.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corran PH, O'Donnell RA, Todd J, et al. The fine specificity, but not the invasion inhibitory activity, of 19-kilodalton merozoite surface protein 1-specific antibodies is associated with resistance to malarial parasitemia in a cross-sectional survey in The Gambia. Infect Immun. 2004;72:6185–9. doi: 10.1128/IAI.72.10.6185-6189.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Triglia T, Duraisingh MT, Good RT, Cowman AF. Reticulocyte-binding protein homologue 1 is required for sialic acid-dependent invasion into human erythrocytes by Plasmodium falciparum. Mol Microbiol. 2005;55:162–74. doi: 10.1111/j.1365-2958.2004.04388.x. [DOI] [PubMed] [Google Scholar]
- 39.Bushkin GG, Ratner DM, Cui J, et al. Suggestive evidence for Darwinian selection against asparagine-linked glycans of Plasmodium falciparum and Toxoplasma gondii. Eukaryot Cell. 2010;9:228–41. doi: 10.1128/EC.00197-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, Lopaticki S, Riglar DT, et al. An EGF-like protein forms a complex with PfRh5 and is required for invasion of human erythrocytes by Plasmodium falciparum. PLoS Pathog. 2011;7:e1002199. doi: 10.1371/journal.ppat.1002199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.