Abstract
Background. In treatment-naive, human immunodeficiency virus (HIV)–infected persons, combination antiretroviral therapy (cART) incorporating raltegravir (RAL) is highly effective for virologic suppression, but characteristics of immunologic recovery have not been described.
Methods. We performed a 48-week substudy of 15 patients, median age 40 years, within a phase 2 randomized trial of RAL-cART in treatment-naive patients with chronic HIV infection.
Results. Plasma viral load decreased from 5.2 ± 5.3 log10 HIV RNA copies/mL to 2.2 ± 2.4 log10 copies/mL at week 4, reaching <50 copies/mL at week 8 in 13 of 15 patients. Total CD4 T cells increased at week 4, as did central memory CD4 T cells in association with reduction of the immune activation markers HLA-DR and CD38 and immune exhaustion marker PD1 in CD4 and CD8 T cells. Naive CD4 T cells increased at week 24 with appearance of HIV gag–specific interleukin 2, interferon-γ, and CD107a responses in CD4 and CD8 T cells at week 48. Plasma lipopolysaccharide and soluble CD14 decreased, but at week 48 were elevated as compared to healthy volunteers. Altogether, the week 48 immune profile was more favorable in patients taking RAL-cART than in patients treated with non–RAL-cART.
Conclusions. RAL in first-line treatment regimens results in rapid immune reconstitution with residual low-level microbial translocation.
Keywords: HIV, raltegravir, immune reconstitution, immune activation, immune exhaustion, RAL-cART
Rapid gains in CD4 T-cell numbers associated with combination antiretroviral therapy (cART) incorporating raltegravir (RAL) have been described in treatment-naive patients with chronic human immunodeficiency virus (HIV) infection [1, 2]. Raltegravir belongs to a novel class of antiretroviral drugs that inhibit the HIV type 1 (HIV-1) integrase, a crucial enzyme involved in the life cycle of HIV [3], and was approved by the US Food and Drug Administration for treatment-naive HIV-1–infected patients in 2009 [4]. RAL monotherapy with multiple doses for 10 days showed superior virologic control compared to placebo in treatment-naive individuals with chronic HIV infection [2]. Administered with a nucleoside backbone, RAL results in rapid suppression of HIV replication to <50 HIV RNA copies/mL within 4 weeks [2, 5], and first-line RAL-cART regimens have proven durable efficacy and safety records [5–7]. Although RAL-cART leads to rapid gain in CD4 T-cell numbers [1, 2], the resulting immunologic recovery in chronic HIV infection has not been fully characterized.
Chronic HIV infection is characterized by depletion of CD4 T cells and immune activation that often persists even after durable virus suppression with cART. In such situations, the immune activation is negatively correlated with immune reconstitution [8, 9] and is attributed to gut microbial translocation [10]; plasma levels of soluble CD14 (sCD14) are increased and independently predict mortality in cART-treated HIV-infected individuals [11]. The objective of the present study was to evaluate the impact of RAL in first-line therapy on quantitative and qualitative immune reconstitution and on immune activation.
MATERIALS AND METHODS
Study Groups and Controls
This immunology substudy was performed in patient samples collected during 2009–2011 from participants in A009 (Clinicaltrials.gov: NCT00654147), a 48-week prospective, randomized, open-label pilot study of RAL-cART in treatment-naive chronically HIV infected patients with plasma HIV-1 RNA levels ≥5000 copies/mL. RAL 400 mg twice daily was administered in combination with either lopinavir 400 mg/ritonavir 100 mg twice daily (Kaletra) or emtricitabine 200 mg/tenofovir disoproxil fumarate 300 mg once a day. Participants were evaluated clinically, for CD4 counts, immunologic assessment, and virus load determinations at study entry (week 0) and at weeks 4, 8, 24, and 48. Fifteen patients, with a median age of 40 years (range, 21–53 years) with plasma HIV RNA levels 5.2 ± 5.3 log10 copies/mL and mean absolute CD4 T cell counts 360.3 ± 201.5 cells/µL completed the immunology substudy for which both treatment groups were combined for data analysis. Peripheral blood mononuclear cells (PBMCs) and plasma samples were cryopreserved as per approved guidelines [12]. Because A009 did not have a randomized control arm, we utilized similarly processed and cryopreserved PBMCs and plasma samples collected during 2007–2008 from patients who had also achieved HIV-RNA suppression to <50 copies/mL at week 48 following treatment with either efavirenz or atazanavir sulfate and ritonavir in combination with 2 nonnucleoside reverse transcriptase inhibitors (non–RAL-cART). Participants in the RAL-cART and non–RAL-cART groups were similar in regard to age and immunologic and virologic characteristics prior to starting cART (Table 1). Eight HIV-seronegative volunteers served as healthy controls. This study was approved by the University of Miami Institutional Review Board, and all participants provided written informed consent.
Table 1.
Virologic and Immunologic Measures of Patients Taking Combination Antiretroviral Therapy (cART) Incorporating Raltegravir (RAL) and Non–RAL-cART Control Patients at Entry
| Measure | RAL-cART (n = 15) | Non–RAL-cART (n = 15) |
|---|---|---|
| Plasma VL (copies/mL) | 5.2 ± 5.3 log10 | 4.69 ± 4.42 log10 |
| Mean absolute CD4 (cells/µL) | 360.3 ± 201.5 | 376.8 ± 196.64 |
| Mean CD4+CD38+HLA-DR+ (%) | 7.1 ± 3.5 | 6.7 ± 2.9 |
| Mean CD8+CD38+HLA-DR+ (%) | 17.6 ± 7.6 | 18.1 ± 5.6 |
| Mean CD4+PD1+ (%) | 19.2 ± 5.5 | 16.6 ± 9.5 |
| Mean CD8+PD1+ (%) | 24.2. ± 8.6 | 26.8 ± 10.8 |
| Mean plasma sCD14 (pg/mL) | 1 268 000 ± 346 634 | 1 129 845 ± 308069.5 |
| Mean plasma LPS (pg/mL) | 221.3 ± 51.7 | 195.0 ± 38.1 |
Abbreviations: LPS, lipopolysaccharide; PD1, programmed death receptor 1; RAL-cART, combination antiretroviral therapy incorporating raltegravir; sCD14, soluble CD14; VL, viral load.
Analysis of Phenotypic Markers by Flow Cytometry
Thawed cryopreserved PBMCs were rested overnight, stained with cell surface markers against CD3, CD4, CD8, CD45RO, CD27, CCR7, HLA-DR, CD38, PD1, and CD25 (BD Biosciences) at room temperature, and were washed, fixed, and permeabilized for intracellular staining for Ki67. FoxP3 staining was performed using BD FoxP3 staining buffer as per the manufacturer's instructions. Violet fixable live/dead amine dye-PacBlue (ViViD, Molecular Probes) and appropriate isotype controls were included in staining panels for exclusion of dead cells and nonspecific staining [13]. Cells were acquired on a BD LSRII Flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar 8.8.6). Frequencies of desired subsets were determined in gated live (ViViD−) cell populations. CD4 and CD8 T cells were gated to identify maturation subsets: naive (TN; CD27+CD45RO−CCR7+), central memory (TCM; CD27+CD45RO+CCR7+), effector memory (TEM; CD27−CD45RO+CCR7−), and effector (TE; CD45RO−CD27−CCR7−) T-cell subsets, peripheral T follicular helper cells (pTfh; CD4+CD45RO+CXCR5+ [13]), and regulatory T cells (Tregs; CD4+CD25brightFoxP3+ [14]). Immune activation markers (HLA-DR and CD38) and immune exhaustion marker PD1 were determined on CD4 and CD8 T cells and their maturation subsets [15].
Intracytoplasmic Cytokine Production
PBMCs (1 × 106 cells) were incubated with 1 µg/mL each of anti-CD28 and anti-CD49d monoclonal antibodies (mAbs) and cultured alone (negative control) or with pooled overlapping HIV gag peptides (2 µg/mL each) or 50 ng/mL PMA (phorbol 12-myristate 13-acetate) plus 1 µg/mL ionomycin (positive control). Anti-CD107a antibody was added to the cells before stimulation [16]. Cultures were incubated for 6 hours at 37°C in 5% CO2 in the presence of the secretion inhibitor monensin (0.7 µL/mL) and brefeldin A (10 µg/mL). PBMCs were washed; surface stained with mAbs for CD3, CD4, CD8, and ViViD; washed; permeabilized; and stained with mAbs specific for interferon gamma (IFN-γ), interleukin 2 (IL-2), interleukin 17 (IL-17), and interleukin 21 (IL-21) (BD Biosciences) for 30 minutes at room temperature. Total CD4 and CD8 T cells were analyzed for degranulation marker CD107a and intracytoplasmic cytokines. Boolean gate analysis was performed using the FlowJo platform to identify functional combinations of 1 or more functions.
Plasma Lipopolysaccharide Analysis
Lipopolysaccharide (LPS) levels were measured in ethylenediaminetetraacetic acid plasma by the use of the limulus amebocyte lysate chromogenic endpoint assay (Lonza Group Ltd). Ten microliters of plasma was diluted 1:10 in endotoxin-free water and heat-inactivated at 85°C for 15 minutes to inactivate inhibitory plasma proteins, and the assay was performed as per the manufacturer's instructions [9] together with the Escherichia coli endotoxin standard provided with the kit. Results were calculated after background subtraction to determine the endotoxin units (EU) per milliliter, and converted to picograms per milliliter using the formula 1 EU = 100 pg.
Plasma sCD14 Analysis
Plasma levels of sCD14 were quantified by Human sCD14 Immunoassay (R&D Systems). A 400-fold dilution of plasma was used for assay, and the results were expressed in picograms per milliliter [9].
Statistical Analysis
A general linear mixed models procedure to perform a repeated measures analysis of variance with 1 between-subjects factor (groups) and 1 within-subjects factor (time). Contrasts were used to test for significant differences. Correlations between 2 variables were done using the Spearman correlation. Graphs were plotted using GraphPad Prism (version 6.02). P values <.05 were considered significant.
RESULTS
Plasma Viral Load Decreases and Absolute Numbers of CD4 T Cells Increase Within 4 Weeks After RAL-cART
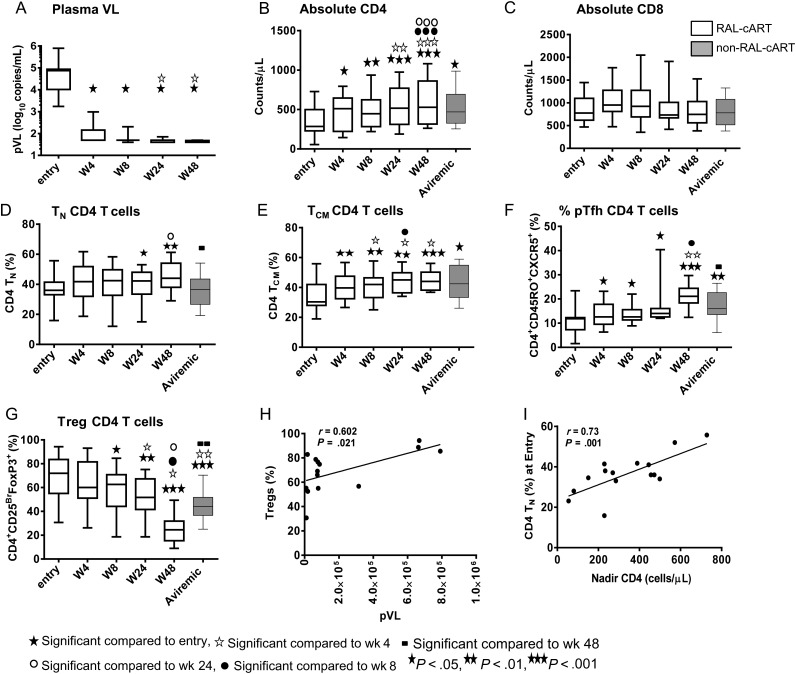
At entry, plasma viral load (pVL) was inversely correlated with absolute numbers of CD4 T cells (r = 0.54; P = .04). A rapid 3 log reduction in pVL was observed within 4 weeks of RAL-cART in the study groups, decreasing from 5.2 ± 5.3 log10 copies/mL at baseline to 2.2 ± 2.4 log10 copies/mL at week 4 after therapy (Figure 1A). Decline in pVL was associated with increase in absolute number of CD4 T cells within 4 weeks of therapy with continued increases through week 48 (P = .0005; Figure 1B). Although mean absolute numbers of CD4 T cells at entry were similar among both treatment groups, the increase of CD4 T cells at 48 weeks was higher in the RAL-cART (258.8 cells/µL) than in the non–RAL-cART (168.6 cells/µL) group. Absolute CD4 T cells at week 48 were correlated with nadir CD4 T cells (r = 0.911; P < .0001). Absolute numbers of CD8 T cells did not change (Figure 1C). Correlation analyses of various markers at entry and week 48 after RAL-cART are shown in Table 2.
Figure 1.
Immune reconstitution after combination antiretroviral therapy incorporating raltegravir (RAL-cART) in treatment-naive patients with chronic human immunodeficiency virus (HIV) infection: plasma HIV RNA levels were detected by real-time polymerase chain reaction. Frequencies of CD4 and CD8 T cells and their subsets were analyzed by flow cytometry and the results were compared with those of aviremic non-RAL-cART controls (Aviremic). T-cell subsets were identified as naive (TN; CD45RO−CD27+CCR7+) cells, central memory (TCM; CD45RO+CD27+CCR7+) cells, peripheral blood T follicular helper cells (pTfh; CD4+CD45RO+CXCR5+), and regulatory T cells (Tregs; CD4+CD25brightFoxP3+). Plasma viral load (pVL) (A) decreases, absolute numbers of CD4 T cells (B) and CD8 T cells (C), percentage of CD4 TN (D), CD4TCM (E), and pTfh (F) cells increase and Tregs (G) decrease after starting RAL-cART. Box plots represent median with 25th and 75th percentile borders; error bars represent 10th and 90th percentile mean. Linear correlation was observed between pVL and frequencies of Tregs (H), and of nadir CD4 with percentage of CD4 TN cells at entry (I).
Table 2.
Correlation Analyses of Various Markers at Entry and Week 48 After Combination Antiretroviral Therapy Incorporating Raltegravir
| Correlations | At Entry | Week 48 |
|---|---|---|
| pVL vs absolute CD4 | r = −0.53, P = .040 | r = −0.47, P = .10 |
| pVL vs Tregs (%) | r = 0.60, P = .021 | r = 0.52, P = .041 |
| pVL vs CD4+HLA-DR+CD38+ (%) | r = 0.79, P = .0006 | r = 0.75, P = .001 |
| pVL vs CD8+HLA-DR+CD38+ (%) | r = 0.86, P < .0001 | r = 0.68, P = .001 |
| pVL vs CD4+PD1+ (%) | r = 0.68, P = .007 | r = 0.36, P = .242 |
| pVL vs CD8+PD1+ (%) | r = 0.62, P = .017 | r = 0.12, P = .70 |
| pVL vs LPS (pg/mL) | r = 0.39, P = .163 | r = 0.17, P = .539 |
| pVL vs sCD14 (pg/mL) | r = 0.81, P = .0004 | r = 0.66, P = .008 |
| Nadir CD4 vs absolute CD4 | … | r = 0.91; P < .0001 |
| Nadir CD4 vs Tregs | r = −0.32, P = .26 | r = −0.22, P = .482 |
| Nadir CD4 vs TN CD4 (%) | r = 0.73, P = .001 | r = 0.26, P = .334 |
| Nadir CD4 vs TCM CD4 (%) | r = −0.28, P = .33 | r = −0.02, P = .93 |
| Nadir CD4 vs CD4+HLA-DR+CD38+ (%) | r = −0.66, P = .006 | r = −0.52, P = .04 |
| Nadir CD4 vs CD8+HLA-DR+CD38+ (%) | r = −0.56, P = .034 | r = −0.31, P = .26 |
| Nadir CD4 vs CD4 + PD1 + (%) | r = −0.64, P = .013 | r = −0.60, P = .036 |
| Nadir CD4 vs CD8 + PD1 + (%) | r = −0.34, P = .223 | r = −0.37, P = .23 |
| Nadir CD4 vs LPS (pg/mL) | r = −0.71, P = .003 | r = −0.15, P = .592 |
| Nadir CD4 vs sCD14 (pg/mL) | r = −0.62, P = .016 | r = −0.57, P = .032 |
| LPS vs sCD14 | r = 0.64, P = .013 | r = 0.56, P = .033 |
| LPS vs CD4+HLA-DR+CD38+ | r = 0.75, P = .006 | r = 0.50, P = .023 |
| LPS vs CD8+HLA-DR+CD38+ | r = 0.61, P = .011 | r = 0.76, P = .001 |
| LPS vs CD4+PD1+ | r = 0.26, P = .35 | r = 0.15, P = .62 |
| LPS vs CD8+PD1+ | r = 0.39, P = .15 | r = 0.18, P = .59 |
| sCD14 vs CD4+HLA-DR+CD38+ | r = 0.68 P = .007 | r = 0.56, P = .023 |
| sCD14 vs CD8+HLA-DR+CD38+ | r = 0.43 P = .072 | r = 0.63, P = .014 |
| sCD14 vs CD4+PD1+ | r = 0.64, P = .013 | r = 0.10, P = .613 |
| sCD14 vs CD8+PD1+ | r = 0.57, P = .032 | r = 0.32, P = .422 |
Abbreviations: LPS, lipopolysaccharide; PD1, programmed death receptor 1; pVL, plasma viral load; RAL-cART, combination antiretroviral therapy incorporating raltegravir; sCD14, soluble CD14; TCM CD4, central memory CD4 T cells; TN CD4, naive CD4 T cells; Tregs, regulatory T cells.
Frequencies of TN and TCM CD4 T Cells Increase Whereas Frequencies of Tregs Decrease After RAL-cART
The rate of recovery of CD4 TN cells was slower than that of CD4 TCM cells. Whereas CD4 TN cells increase was noted only at 24 weeks (Figure 1D), CD4 TCM cells increases occurred within 4 weeks of treatment initiation (Figure 1E). Both subsets continued to increase through week 48 and their frequencies at week 48 were higher than in the non–RAL-cART group. No changes were noted for CD4 TEM and CD4 TE subsets (not shown). Frequencies of circulating pTfh cells increased from week 4 through week 48, at which time the percentage of pTfh was higher in the RAL-cART group (Figure 1F). A decrease in the percentage of Tregs was noted at week 8 and these frequencies persisted at week 24 and week 48 (Figure 1G), and were significantly lower than in the non–RAL-cART patient group at week 48. At entry, pVL correlated with frequencies of Tregs (Figure 1H), and nadir CD4 T cells correlated with frequencies of naive CD4 T cells (Figure 1I).
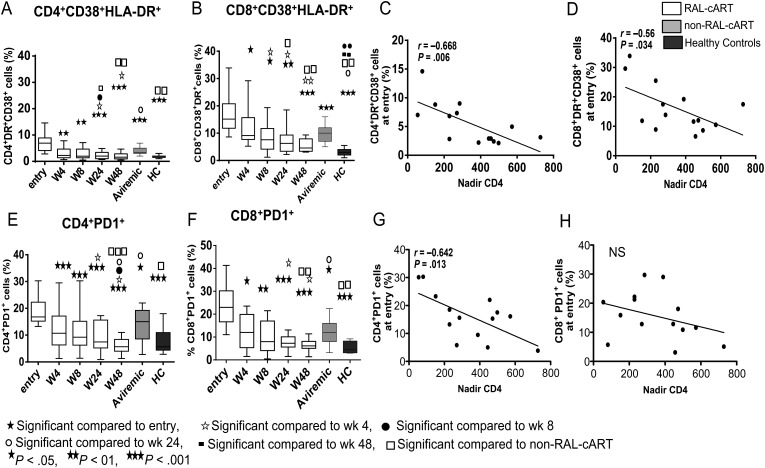
Immune Activation and Exhaustion Decrease Rapidly After Therapy Initiation
The percentages of HLA-DR+CD38+CD4 (Figure 2A) and CD8 (Figure 2B) T cells were elevated at entry and were correlated with pVL (Table 2) and inversely with nadir CD4 count (Figures 2C and 2D, respectively). Activated CD8 T cells at entry were also inversely correlated with absolute CD4 T cells at week 48 (r = −0.76; P = .0034). Percentages of both activated CD4 and CD8 T cells decreased within 4 weeks of RAL-cART initiation, and continued to decrease through week 48. Median fluorescence intensity (MFI) of HLA-DR and CD38 in CD4 and CD8 T cells also decreased within 8 weeks of therapy (not shown). At week 48, HLA-DR+CD38+ CD4 and CD8 T cells were lower in the RAL-cART than in the non–RAL-cART patient groups, but activated CD8 T cells remained higher in comparison to healthy controls. At entry, frequencies of CD4 and CD8 T cells expressing immune exhaustion marker PD1 were elevated and decreased within 4 weeks of treatment (Figure 2E and 2F), with both subsets decreasing through week 48 to healthy control values, but in the non–RAL-cART group, their frequencies at week 48 were significantly higher. PD1 expression on CD4 and CD8 T cell correlated with pVL (Table 2), and PD1+ CD4 T cells were correlated inversely with nadir CD4 counts (Figure 2G). The MFI of PD1 expression also followed a similar pattern (not shown).
Figure 2.
Changes in markers of immune activation and PD1 expression on CD4 and CD8 T cells following combination antiretroviral therapy incorporating raltegravir (RAL-cART): CD4 and CD8 T cells were analyzed for expression of immune activation markers CD38+HLA-DR+ and immune exhaustion marker PD1 and compared the results with that of aviremic non–RAL-cART controls (Aviremic) and healthy controls. Expression of CD38+HLA-DR+ on CD4 T cells (A) and CD8 T cells (B). Nadir CD4 counts inversely correlated with percentage of CD4+HLA-DR+CD38+ cells (C) and CD8+HLA-DR+CD38+ (D) cells. Expression of PD1 on CD4 T cells (E) and CD8 T cells (F). Nadir CD4 counts were inversely correlated with percentage of CD4+PD1+ cells (G) and CD8+PD1+ cells (H) at entry. Box plots represent median with 25th and 75th percentile borders; error bars represent 10th and 90th percentile means.
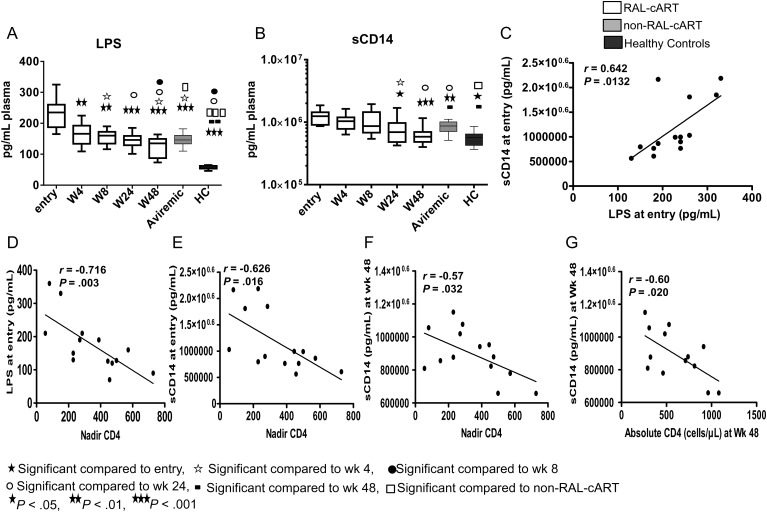
Microbial Translocation Markers Decrease Significantly After RAL-cART
LPS and sCD14 levels were elevated at entry compared to healthy controls (Figure 3A and 3B, respectively). Entry sCD14 levels were directly correlated with entry LPS levels (Figure 3C). LPS and sCD14 were inversely correlated with CD4 T-cell counts at entry (Figure 3D and 3E). LPS levels decreased rapidly within 4 weeks of treatment (Figure 3A), but the decrease in sCD14 was evident only at week 24 and continued through week 48 (Figure 3B). At 48 weeks, levels of LPS and sCD14 were lower in RAL-cART–treated patients than in the non–RAL-cART group. Despite the rapid initial decline, LPS levels remained higher than in healthy controls at 48 weeks whereas sCD14 levels normalized. Levels of sCD14 at week 48 were inversely correlated with absolute numbers of CD4 T cells at entry (Figure 3F) and at week 48 (Figure 3G). LPS levels were directly correlated with corresponding levels of activated CD8 T cells (HLA-DR+CD38+) at week 48 (Table 2).
Figure 3.
Markers of gut microbial translocation decrease starting from 8 weeks after combination antiretroviral therapy incorporating raltegravir (RAL-cART). Plasma lipopolysaccharide (LPS) and soluble CD14 (sCD14) levels were measured longitudinally and results were compared with those of non–RAL-cART controls (Aviremic) and healthy controls. LPS levels in plasma were quantified by limulus amebocyte assay and plasma sCD14 by ELISA. Plasma levels of LPS (A) and sCD14 (B) in RAL-cART patients and controls. Plasma levels of sCD14 correlate with plasma LPS levels at entry (C). Inverse correlations between nadir CD4 with plasma LPS (D) and sCD14 (E) at entry and sCD14 levels (F) at week 48. Absolute CD4 at week 48 was inversely correlated with sCD14 at week 48 (G). Box plots represent median with 25th and 75th percentile borders; error bars represent 10th and 90th percentile means.
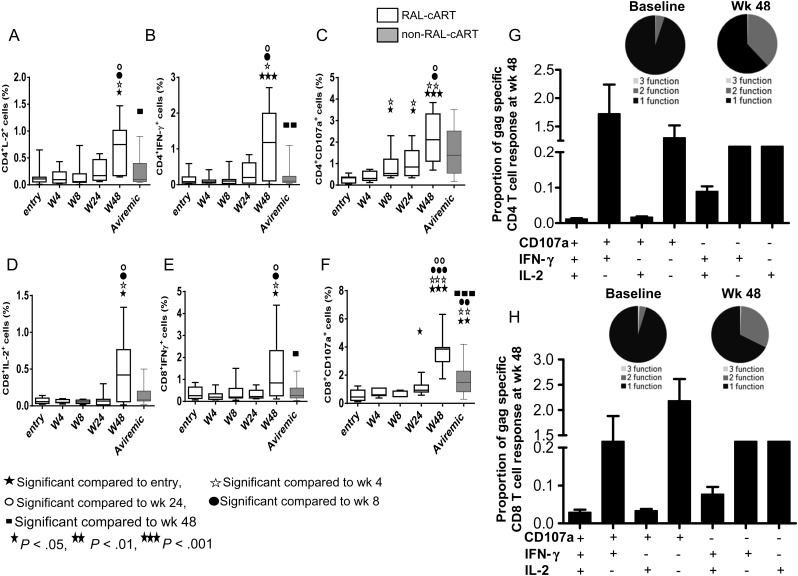
Improvement in CD4 and CD8 T-Cell Function at Week 48
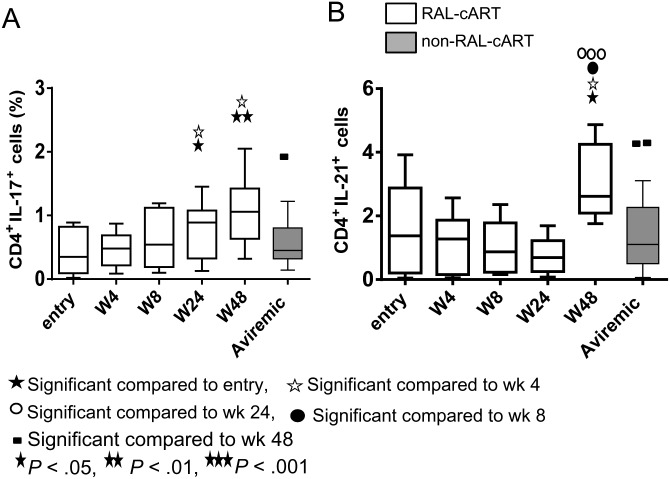
Improvement in HIV gag-specific CD4 and CD8 T cell function was noted in the RAL-cART group only at week 48, as evidenced by increase in IL-2, IFN-γ, and CD107a in CD4 (Figure 4A–C) and CD8 T cells (Figure 4D–F) that were higher than in the non–RAL-cART group. The cytokine profile increased from single function and low frequencies of polyfunctional cells to 2 or more functions, predominantly CD107a+IFNγ+ CD4 and CD8 T cells at week 48 (pie charts and bar graphs shown in Figure 4G and 4H), whereas single function CD4 and CD8 T cells dominated in the non–RAL-cART group (not shown). IL-17+ CD4 T cells were evident only with the more potent PMA + ionomycin stimulation but not with HIV gag stimulation, and increases noted at week 24 were sustained through week 48 and were higher compared to non–RAL-cART–treated patients (Figure 5A). Similar to the observation for Th17 cells, frequencies of IL-21–producing CD4 T cells also increased at week 48 preferentially in the RAL-cART group (Figure 5B). These results indicate an overall improvement of T-cell functions following initiation of RAL-cART.
Figure 4.
Improvement in gag-specific function (interleukin 2 [IL-2], interferon [IFN]-γ) and degranulation (CD107a) in CD4 and CD8 T cells at week 48 of therapy. Thawed peripheral blood mononuclear cells were stimulated with human immunodeficiency virus (HIV) gag pool (2 mg/mL) for 6 hours in the presence of monensin and brefeldin A and CD107a antibody. Medium alone was used as negative control. Cells were stained for CD3, CD4, and CD8 along with ViViD, and fixed, permeabilized, and stained intracellularly for IL-2, IFN-γ, and CD107a. CD4 and CD8 T cells were gated from live CD3+ cells and analyzed for the expression of different cytokines along with degranulation marker CD107a and the results were compared with those of aviremic controls not taking combination antiretroviral therapy incorporating raltegravir (RAL-cART) (Aviremic). A–F, Frequencies of HIV gag–specific CD4 T cells producing IL-2 (A), IFN-γ (B), and CD107a (C) and CD8 T cells producing IL-2 (D), IFN-γ (E), and CD107a (F). Box plots represent median with 25th and 75th percentile borders, error bars represent 10th and 90th percentile means. G and H, Functional combinations in CD4 (G) and CD8 (H) T cells were identified after Boolean gating. Pie chart represents 1, 2, and 3 functions and bar chart shows 7 possible functional combinations of CD4 and CD8 T cells at week 48 after therapy.
Figure 5.
Frequencies of interleukin 17 (IL-17) and interleukin 21 (IL-21)–producing CD4 T cells increase at 48 weeks after therapy. Thawed peripheral blood mononuclear cells were stimulated with PMA (100 ng/mL) + ionomycin (1 µg/mL) for 6 hours in the presence of brefeldin A (10 mg/mL). Medium alone was included as a negative control. Cells were stained and analyzed for the intracellular expression of IL-21 and IL-17 by flow cytometry and results were compared with those of aviremic controls not taking combination antiretroviral therapy incorporating raltegravir (RAL-cART) (Aviremic). A and B, Box plot showing frequencies of CD4 T cells producing IL-17 (A) and IL-21 (B). Box plots represent median with 25th and 75th percentile borders; error bars represent 10th and 90th percentile means.
DISCUSSION
Our data indicate that early pVL decay following RAL-cART is associated with significant improvement in the immune profile of chronically HIV-infected patients. As expected, an early indicator of immune reconstitution after antiretroviral therapy was the rapid increase [1, 2] in the absolute CD4 T-cell numbers at week 4 of therapy. The increase in CD4 cell numbers occurred in parallel with increase in TCM cells, important indicators of immune reconstitution [17, 18], that preceded the gains in CD4 TN cells. Other findings of relevance are the significant role of RAL-cART in reversing HIV-associated damage by changing an unfavorable immune cell phenotype to a favorable one that was characterized by decrease in immune activation of T cells and in microbial translocation, concurrently with gains in CD4 T-cell numbers and function over the 48 weeks of the study. Qualitative improvement in T-cell function for CD4 and CD8 T cells was evidenced by increase in HIV gag–specific production of cytokines and degranulation, and of PMA-stimulated IL-21– and IL-17–producing CD4 T cells. Interestingly even within the small cohort, we could identify negative indicators of immune reconstitution, noting an activated immune phenotype of CD8 T cells, higher sCD14 and LPS, and lower nadir CD4 counts, adding to the accumulating evidence in favor of early treatment initiation and importance of immune status prior to treatment initiation.
The early recovery of CD4 T cells with RAL regimens may increase the ability to improve the second phase of immune recovery [19, 20]. Although we cannot differentiate between thymic output and extrathymic expansion of cells, increasing numbers of CD4 TN cells is suggestive of improved immune regenerative capacity. Unlike TCM cells, which increased rapidly, significant increase in CD4 TN cells were observed only after 24 weeks of therapy, and are in agreement with earlier studies that have demonstrated slower increase in phenotypically naive lymphocytes in successfully treated HIV-infected adults in comparison with HIV-1 infected children [21, 22]. In this study, we also evaluated the impact of RAL-cART on phenotypically distinct T-cell subsets that mark different functional attributes. Within the TCM CD4 T cells, an expansion of CXCR5+ cells was observed. In recent studies, we and others have identified these CXCR5+TCM CD4 T cells as a pTfh subset that is endowed with helper function for enabling B cells to produce antibodies following antigen stimulation or polyclonal activation [13, 23]. B-cell function was not evaluated in this study but reconstitution of the pTfh cells bodes well for humoral immune function, for example, responsiveness to vaccines such as influenza [13]. Similarly, a decrease in Tregs following RAL-cART is a favorable response to treatment, as increased frequencies of Tregs occur in patients with chronic HIV infection [24, 25] and are associated with reduced T-cell function. Consistent with previous studies [26], we observed a direct association between pVL and Treg frequencies in these patients at study entry.
An important feature of HIV infection is chronic immune activation that is negatively associated with immune recovery and positively associated with progression to AIDS [27, 28] and increased morbidity and mortality among individuals treated with cART [29, 30]. We examined HLA-DR and CD38 as markers of immune activation on CD4 and CD8 T cells. In our cohort, immune activation was evident at study entry, and RAL-cART resulted in a significant decrease in immune activation of CD4 and CD8 T cells within 4 weeks of therapy and continued to decrease through the course of the study. Notably, levels of activated CD4 and CD8 T cells at week 48 after therapy were lower in RAL-cART patients than in the non–RAL-cART group with normalization of activated CD4 T cells to levels in healthy controls. Reversal of CD4 immune activation is a significant achievement and is likely to favorably influence reconstitution of cell-mediated immune responses [13, 31] and to restore helper function for humoral immune responses [13].
We also examined PD1, a marker for both immune exhaustion and immune activation [15, 32]. Elevated PD1 expression in HIV-specific CD8 T cells [33, 34] and on HIV-specific CD4 T cells [35, 36] has been correlated with disease progression, viral loads, and inversely with CD4 counts. In longitudinal studies, control of viremia has been associated with reduced levels of PD1 on HIV-specific CD8 T cells, [33, 34]. Similar to immune activation, expression of PD1 decreased as early as 4 weeks of therapy initiation, emphasizing the rapid effects of RAL-cART on altering immune status. Interestingly, the decrease in PD1 expression on both CD4 and CD8 T cells at week 48 to levels comparable to healthy controls suggests that RAL-cART alleviates exhaustion of CD4 and CD8 T cells, with restoration of immune function.
Despite marked decrease in comparison to baseline, CD8 immune activation remained elevated in comparison to healthy controls at 48 weeks after treatment initiation. Several lines of evidence indicate that gut microbial translocation is a key contributor to immune activation and disease progression during HIV infection (reviewed in [10]). Levels of LPS in HIV-infected individuals have been shown to correlate with sCD14, a marker of monocyte response to LPS and indicative of activation of both the adaptive and innate immune systems [37]. Plasma LPS and sCD14 levels decreased significantly after treatment with RAL-cART. At week 48, however, LPS levels were higher in our study cohort than in healthy controls, suggesting that despite evidence of immune restoration, gut healing requires a longer time. The low-level circulating LPS at week 48 could explain the persistence of CD8 T-cell immune activation observed in these patients. Gut-associated Th17 cells play an important role in the preservation of gut integrity [38, 39], and peripheral Th17 cells are negatively associated with HIV plasma viremia [40] but may not be reflective of gut Th17 cells. In our study, cellular analysis showed an increase in the IL-17–producing CD4 T cells of PBMCs at week 24, but the status of gut Th17 cells was not investigated.
A goal of antiretroviral therapy is to reverse immunologic damage caused by HIV infection, and identification of barriers to immune recovery remains an important objective in this context. In this study, entry criteria requiring low-level viremia with no restrictions for entry CD4 counts favored early treatment initiation. However, the actual range for both CD4 counts and viral load varied considerably among patients. Entry CD4 was found to be correlated with frequencies of TN CD4 T cells and had an inverse relationship to immune activation, immune exhaustion, and levels of LPS and sCD14 in plasma, thus reinforcing importance of early institution of cART in HIV infection. Importantly, LPS and sCD14 at entry as well as nadir CD4 counts were inversely correlated with CD4 counts at week 48, consistent with prior observations that the relationship of efficacy of cART correlates with nadir CD4 counts [19, 41, 42]. Another important consideration of cART is its impact on viral reservoirs. Although treatment in primary HIV infection has been shown to reduce HIV reservoirs [43, 44], HIV reservoirs are relatively stable during chronic HIV infection with a very slow decay rate [45]. Treatment intensification with RAL in chronic HIV infection has led to conflicting results, and the role of primary therapy with RAL on reservoirs is unknown [46, 47]. Based on emerging evidence showing PD1 to be a marker of latently infected cells [48, 49], one can speculate that the decrease in frequencies of PD1–expressing CD4 T cells may be associated with a decrease in HIV reservoirs in CD4 T cells. Studies are needed to understand the effect of RAL-cART in chronic infection on virus reservoirs, residual viremia, and virus replication in tissues and other lymphoid organs.
A limitation of this study is that the A009 did not have a randomized control arm, and thus we sought samples collected 2 years prior from a group of non–RAL-cART patients. Although not ideal, the 2 groups were matched for absolute CD4 T cells and plasma virus load at study entry (Table 1). The samples from the non–RAL-cART group were older by about 2 years, but it is unlikely that the measurements of cell function and marker expression were affected, as we followed strictly regulated conditions for cryopreservation, with cell viabilities and recovery >80%, which is considered optimal by the Division of AIDS Immunology Quality Assessment program [12, 50]. Regardless of the limitations of the comparator group, the findings in the RAL-cART group independently offer important insight into the dynamics of immune recovery after potent cART that incorporates an integrase inhibitor. We found rapid reconstitution of immune parameters with a RAL regimen in a first-line treatment. It is possible that synergistic RAL effect on cART in pVL decay kinetics may be an important contributor to the immune reconstitution in these patients. The persistence of low levels of immune activation at week 48 suggests that collateral damage inflicted by HIV is not completely ameliorated in 48 weeks, underscoring the need for continued investigation of the etiology and management of immune activation and of mechanisms by which immune activation imparts quantitative and qualitative deficiency of the immune system.
Notes
Acknowledgments. We acknowledge the support for flow cytometry facilities from the Miami Center for AIDS Research, which was funded by a grant (P30AI073961) from the National Institutes of Health (NIH). We thank Drs Sudheesh Pilakka Kanthikeel, Varghese K. George, and Margaret Roach of the Department of Microbiology and Immunology for helping with microbial translocation assays; Dr Kristopher L. Arheart, Department of Epidemiology and Public Health, University of Miami Miller School of Medicine, for statistical analysis; Dr Rajendra Pahwa for his help with the study design; and patients for their participation. The gag derived overlapping peptide pool (15-mers with 11 overlap) was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases.
Financial support. This work was supported by the Merck Investigator-Initiated Studies Program (IISP ID number 36262).
Potential conflicts of interest. All authors: No potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374:796–806. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- 2.Markowitz M, Nguyen BY, Gotuzzo E, et al. Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46:125–33. doi: 10.1097/QAI.0b013e318157131c. [DOI] [PubMed] [Google Scholar]
- 3.Hazuda DJ, Felock P, Witmer M, et al. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science. 2000;287:646–50. doi: 10.1126/science.287.5453.646. [DOI] [PubMed] [Google Scholar]
- 4.Pandey KK. Raltegravir in HIV-1 infection: safety and efficacy in treatment-naive patients. Clin Med Rev Ther. 2011;2012:13–30. doi: 10.4137/CMRT.S5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynes J, Trinh R, Pulido F, et al. Lopinavir/ritonavir (LPV/r) combined with raltegravir (RAL) or tenofovir/emtricitabine (TDF/FTC) in antiretroviral (ARV)-naive subjects: 96-week results of the PROGRESS Study. AIDS Res Hum Retroviruses. 2013;29:256–65. doi: 10.1089/aid.2011.0275. [DOI] [PubMed] [Google Scholar]
- 6.Charpentier C, Weiss L. Extended use of raltegravir in the treatment of HIV-1 infection: optimizing therapy. Infect Drug Resist. 2010;3:103–14. doi: 10.2147/IDR.S8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynes J, Lawal A, Pulido F, et al. Examination of noninferiority, safety, and tolerability of lopinavir/ritonavir and raltegravir compared with lopinavir/ritonavir and tenofovir/ emtricitabine in antiretroviral-naive subjects: the progress study, 48-week results. HIV Clin Trials. 2011;12:255–67. doi: 10.1310/hct1205-255. [DOI] [PubMed] [Google Scholar]
- 8.Anselmi A, Vendrame D, Rampon O, Giaquinto C, Zanchetta M, De Rossi A. Immune reconstitution in human immunodeficiency virus type 1-infected children with different virological responses to anti-retroviral therapy. Clin Exp Immunol. 2007;150:442–50. doi: 10.1111/j.1365-2249.2007.03526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilakka-Kanthikeel S, Huang S, Fenton T, Borkowsky W, Cunningham CK, Pahwa S. Increased gut microbial translocation in HIV-infected children persists in virologic responders and virologic failures after antiretroviral therapy. Pediatr Infect Dis J. 2012;31:583–91. doi: 10.1097/INF.0b013e31824da0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10:655–66. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- 11.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.hanc: HIV/AIDS network cordination. Cross-Network PBMC Processing SOP. https://www.hanc.info/labs/labresources/procedures/Pages/pbmcSop.aspx.V4.0. Accessed 5 March 2012.
- 13.Pallikkuth S, Parmigiani A, Silva SY, et al. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood. 2012;120:985–93. doi: 10.1182/blood-2011-12-396648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pahwa R, Jaggaiahgari S, Pahwa S, Inverardi L, Tzakis A, Ricordi C. Isolation and expansion of human natural T regulatory cells for cellular therapy. J Immunol Methods. 2010;363:67–79. doi: 10.1016/j.jim.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachdeva M, Fischl MA, Pahwa R, Sachdeva N, Pahwa S. Immune exhaustion occurs concomitantly with immune activation and decrease in regulatory T cells in viremic chronically HIV-1-infected patients. J Acquir Immune Defic Syndr. 2010;54:447–54. doi: 10.1097/QAI.0b013e3181e0c7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pallikkuth S, Rogers K, Villinger F, et al. Interleukin-21 administration to rhesus macaques chronically infected with simian immunodeficiency virus increases cytotoxic effector molecules in T cells and NK cells and enhances B cell function without increasing immune activation or viral replication. Vaccine. 2011;29:9229–38. doi: 10.1016/j.vaccine.2011.09.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua W, Jiao Y, Zhang H, et al. Central memory CD4 cells are an early indicator of immune reconstitution in HIV/AIDS patients with anti-retroviral treatment. Immunol Invest. 2012;41:1–14. doi: 10.3109/08820139.2011.576739. [DOI] [PubMed] [Google Scholar]
- 18.Murdoch DM, Suchard MS, Venter WD, et al. Polychromatic immunophenotypic characterization of T cell profiles among HIV-infected patients experiencing immune reconstitution inflammatory syndrome (IRIS) AIDS Res Ther. 2009;6:16. doi: 10.1186/1742-6405-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lange CG, Valdez H, Medvik K, Asaad R, Lederman MM. CD4+ T-lymphocyte nadir and the effect of highly active antiretroviral therapy on phenotypic and functional immune restoration in HIV-1 infection. Clin Immunol. 2002;102:154–61. doi: 10.1006/clim.2001.5164. [DOI] [PubMed] [Google Scholar]
- 20.Goicoechea M, Smith DM, Liu L, et al. Determinants of CD4+ T cell recovery during suppressive antiretroviral therapy: association of immune activation, T cell maturation markers, and cellular HIV-1 DNA. J Infect Dis. 2006;194:29–37. doi: 10.1086/504718. [DOI] [PubMed] [Google Scholar]
- 21.Connick E, Lederman MM, Kotzin BL, et al. Immune reconstitution in the first year of potent antiretroviral therapy and its relationship to virologic response. J Infect Dis. 2000;181:358–63. doi: 10.1086/315171. [DOI] [PubMed] [Google Scholar]
- 22.Lederman MM, McKinnis R, Kelleher D, et al. Cellular restoration in HIV infected persons treated with abacavir and a protease inhibitor: age inversely predicts naive CD4 cell count increase. AIDS. 2000;14:2635–42. doi: 10.1097/00002030-200012010-00002. [DOI] [PubMed] [Google Scholar]
- 23.Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–21. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiao Y, Fu J, Xing S, et al. The decrease of regulatory T cells correlates with excessive activation and apoptosis of CD8+ T cells in HIV-1-infected typical progressors, but not in long-term non-progressors. Immunology. 2009;128:e366–75. doi: 10.1111/j.1365-2567.2008.02978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montes M, Sanchez C, Lewis DE, et al. Normalization of FoxP3(+) regulatory T cells in response to effective antiretroviral therapy. J Infect Dis. 2011;203:496–9. doi: 10.1093/infdis/jiq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suchard MS, Mayne E, Green VA, et al. FOXP3 expression is upregulated in CD4 T cells in progressive HIV-1 infection and is a marker of disease severity. PLoS One. 2010;5:e11762. doi: 10.1371/journal.pone.0011762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–70. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 28.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 29.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ledwaba L, Tavel JA, Khabo P, et al. Pre-ART levels of inflammation and coagulation markers are strong predictors of death in a South African cohort with advanced HIV disease. PLoS One. 2012;7:e24243. doi: 10.1371/journal.pone.0024243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nayak JL, Fitzgerald TF, Richards KA, Yang H, Treanor JJ, Sant AJ. CD4+ T-cell expansion predicts neutralizing antibody responses to monovalent, inactivated 2009 pandemic influenza A(H1N1) virus subtype H1N1 vaccine. J Infect Dis. 2013;207:297–305. doi: 10.1093/infdis/jis684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piconi S, Trabattoni D, Gori A, et al. Immune activation, apoptosis, and Treg activity are associated with persistently reduced CD4+ T-cell counts during antiretroviral therapy. AIDS. 2010;24:1991–2000. doi: 10.1097/QAD.0b013e32833c93ce. [DOI] [PubMed] [Google Scholar]
- 33.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 34.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 35.D'Souza M, Fontenot AP, Mack DG, et al. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179:1979–87. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 36.Kaufmann DE, Kavanagh DG, Pereyra F, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–54. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 37.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 38.Klatt NR, Estes JD, Sun X, et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol. 2012;5:646–57. doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–35. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ndhlovu LC, Chapman JM, Jha AR, et al. Suppression of HIV-1 plasma viral load below detection preserves IL-17 producing T cells in HIV-1 infection. AIDS. 2008;22:990–2. doi: 10.1097/QAD.0b013e3282ff884e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boulassel MR, Chomont N, Pai NP, Gilmore N, Sekaly RP, Routy JP. CD4 T cell nadir independently predicts the magnitude of the HIV reservoir after prolonged suppressive antiretroviral therapy. J Clin Virol. 2012;53:29–32. doi: 10.1016/j.jcv.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Lederman MM, Calabrese L, Funderburg NT, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–26. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chun TW, Murray D, Justement JS, et al. Relationship between residual plasma viremia and the size of HIV proviral DNA reservoirs in infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2011;204:135–8. doi: 10.1093/infdis/jir208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe D, Ibe S, Uehira T, et al. Cellular HIV-1 DNA levels in patients receiving antiretroviral therapy strongly correlate with therapy initiation timing but not with therapy duration. BMC Infect Dis. 2011;11:146. doi: 10.1186/1471-2334-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–7. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 46.Buzon MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16:460–5. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 47.Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000321. doi:10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DaFonseca S, Chomont N, EL Phar M, Boulassel R, Routy J, Sekaly RP. Purging the HIV-1 reservoir through the disruption of the PD-1 pathway. J Int AIDS Soc. 2010;13:O15. [Google Scholar]
- 49.Perreau M, Savoye AL, De Crignis E, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210:143–56. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinberg A, Louzao R, Mussi-Pinhata MM, et al. Quality assurance program for peripheral blood mononuclear cell cryopreservation. Clin Vaccine Immunol. 2007;14:1242–4. doi: 10.1128/CVI.00187-07. [DOI] [PMC free article] [PubMed] [Google Scholar]