Abstract
Background. The incidence and clinical impact of coronavirus (CoV) infection in elderly persons and those with underlying cardiopulmonary disease over a long duration is not well described. We determined the incidence and clinical impact of 229E and OC43 CoV in this population during 4 consecutive winters, and compared illnesses to influenza A, respiratory syncytial virus, and human metapneumovirus.
Methods. CoV 229E and OC43 were detected by reverse transcription polymerase chain reaction and serology in 4 adult populations under surveillance for acute respiratory illness during the winters of 1999–2003. Cohorts included healthy young adults, healthy elderly adults, high-risk adults with underlying cardiopulmonary disease, and a hospitalized group.
Results. Three hundred ninety-eight CoV infections were identified, with annual infection rates ranging from 2.8% to 26% in prospective cohorts, and prevalence ranging from 3.3% to 11.1% in the hospitalized cohort. The incidence of infections with each strain was similar, although asymptomatic infection and viral coinfection was significantly more common with 229E than OC43 infection. Although the incidence and clinical manifestations were similar for each strain, OC43-infected subjects tended to seek more medical care, as OC43 was twice as common as 229E among the hospitalized cohort.
Conclusions. CoV infections in the elderly are frequent, likely causing substantial medical disease burden.
Keywords: coronavirus, adults, elderly
Coronaviruses (CoVs) are enveloped, nonsegmented positive strand RNA viruses of the family Coronaviridae and cause a wide spectrum of illness including respiratory and gastrointestinal disease in a variety of mammalian species including humans [1]. CoVs are divided into 4 genera and are capable on occasion of interspecies transmission, as postulated for the severe acute respiratory syndrome (SARS) coronavirus that emerged briefly in 2003 and a very recently identified lethal novel coronavirus (MERS-CoV) in the Middle East [2, 3]. Since the 1960s, CoV strains 229E and OC43, classified as alpha- and betacoronaviruses, respectively, have been recognized as frequent causes of the “common cold” [4]. In the past decade, CoV NL63 (an alphacoronavirus) and HKU1 (a betacoronavirus) were discovered as causes of respiratory illnesses and together with 229E and OC43 continue to circulate widely in humans during the winter months in temperate climates [5–7]. In addition to upper respiratory tract illness, these latter 4 strains are also associated with bronchitis, acute exacerbation of chronic obstructive pulmonary disease (AECOPD), and pneumonia in all age groups [8–11]. Infection rates range widely from 2% to 20% with marked variation in the predominant circulating strain. Coronaviruses are rarely isolated in routine tissue culture and without molecular diagnostic tests the majority of infections go unrecognized. Most descriptions of the epidemiology and clinical impact of CoV are in infants and young children, with only a few small long-term studies in adults, and none that evaluated illness in outpatient and hospitalized populations simultaneously. With the exception of SARS and CoV-EMC, there is little information regarding the relative virulence among coronavirus strains.
In this report we describe and compare the incidence, clinical characteristics, and outcome of infection with CoV strains 229E and OC43 in 4 large adult cohorts with a variety of underlying medical conditions over 4 consecutive winter seasons. In addition, we contrast the relative clinical impact of these CoV strains to influenza A, respiratory syncytial virus (RSV), and human metapneumovirus (hMPV).
METHODS
Subjects
The 4 adult populations included in this study have previously been described in detail [12, 13]. Coronavirus infections were identified using archived serum and nasal respiratory secretions previously collected during respiratory illness surveillance encompassing 4 consecutive winters (15 November—15 April) of 1999–2003 in Rochester, New York. Three prospective cohorts (healthy persons ≥65 years of age, young adults 19–40 years of age, and high-risk adults with underlying chronic obstructive pulmonary disease [COPD] or congestive heart failure) were enrolled in the late summer/early fall of each year and participated for a maximum of 2 winters. Approximately half of the subjects in the prospective cohorts were replaced each year using a rolling enrollment strategy. Upon enrollment, demographics, medical history, and directed respiratory examination were recorded and a serum specimen was obtained. Prospective subjects were asked to notify study staff of acute respiratory symptoms (cough, sore throat, increased or new sputum, dyspnea, wheezing with or without fever), at which time the signs and symptoms of illness were recorded and nose and throat swabs and acute serum specimen were collected. Four to 6 weeks later, a follow-up assessment was performed and convalescent serum samples were collected. At the conclusion of each season, subjects were queried about any other respiratory illnesses during the season, and a serum sample was obtained.
Simultaneously, a hospital cohort was recruited from among persons admitted to Rochester General Hospital with acute respiratory symptoms. Patients with acute coronary syndromes, documented pulmonary embolism, or aspiration pneumonia were excluded [12]. Acute illness and follow-up evaluations were similar to the prospective cohorts. The University of Rochester and Rochester General Hospital institutional review boards approved the study, and all participants or guardians provided written informed consent.
Laboratory Testing
Serum samples and nose and throat swab specimens were stored at −80°C for 3–6 years before testing for CoV. Nose and throat swabs were analyzed for human coronavirus 229E and OC43 RNA in single runs using real-time multiplex reverse transcription polymerase chain reaction (RT-PCR) with primers and probes targeting the polymerase gene. RNA was extracted into 12 µL of water from 250 µL of stored respiratory specimens. For reverse transcription, 5.25 µL of RNA was incubated at 42°C for 30 minutes with 10 U of avian myeloblastosis virus reverse transcriptase, 10 U of RNasin, 200 µM dNTPs, and primers specific for strain OC43 (5′AATACCTTTCTTGGCTCGAGTAAT) and strain 229E (5′AATACCTTTCTTTGCTCTAGTAAT) at a concentration of 200 nM each. The amplification mixture contained 5 µL of complementary DNA, 5 mM MgCl2, 400 µM dNTP (dUTP replacing dTTP), 5 U of Taq polymerase, and 1 U of uracil-DNA glycosylase, 200 nM of OC43 forward primer, 229E forward primer, a common reverse primer (5′GTGGATTCTGCTCAAG), and strain-specific probes (Sigma-Genosys); OC43 (5′FAM-TACAGAATGCGCTGTTTCTGCAGTCTGTGAAT-BHQ1); 229E (5′-TR-ACAGGCATGAGCAGTATCTGATGTCTGTGCGA-BHQ2). Amplification was performed on a Bio-Rad iCycler for 42 cycles of 95°C for 5 seconds, 42°C for 40 seconds, and 68°C for 10 seconds. The cycle threshold was defined when the relative fluorescent units (RFUs) exceeded 15% of the highest RFU value for the positive control. Strain-specific specificity of the assay was confirmed using CoV 229E and OC43 obtained from the American Type Culture Collection (ATCC; VR 740 and VR 759, respectively).
Serologic testing for CoV 229E and OC43 was performed by enzyme immunoassay using serum samples spanning each winter season (fall through spring) separately. CoV 229E from ATCC was propagated in MRC-5 cells (Viromed Laboratories) until cytopathic effect was extensive. Cells were scraped into the medium and sonicated followed by low-speed clarification. The supernatant fluid was pelleted in a Beckman SW27 rotor at 20 000 rpm for 90 minutes, resuspended in TNE buffer (50 mM Tris/140 mM NaCl/10 mM EDTA, pH 7.5), and sonicated, and the virus was purified on a 20%/60% discontinuous sucrose gradient (32 000 rpm in a SW41 rotor for 100 minutes). The virus band was diluted to 3.5 mL in TNE buffer and stored at −80°C until use. OC43 (ATCC VR 759) was grown in HRT-18G cells (obtained from ATCC, CRL-11663) until cytopathic effect was extensive, at which time cells were scraped into the supernatant and clarified [14]. The pellet was resuspended into phosphate-buffered saline/0.5% NP40, placed on ice, sonicated 4 times for 15 seconds, and then clarified. The supernatant was stored at −80°C until use. CoV antigens coated onto enzyme immunoassay plates and control plates were coated with uninfected cell preparations. Serial 2-fold dilutions of sera were incubated in antigen-coated plates and developed by addition of alkaline phosphatase–conjugated goat antihuman immunoglobulin G (IgG) followed by substrate. The usual convention of a ≥4-fold rise in serum IgG specific for the CoV antigens was considered evidence of infection.
Infection with other viruses were also identified by culture, RT-PCR, or serology (influenza A and B, RSV, hMPV) or culture alone (parainfluenza virus) in the same samples as previously described [12, 13].
Definition of Infection
Symptomatic CoV infection was defined as an illness with upper or lower respiratory symptoms associated with a positive RT-PCR at the time of symptoms and/or a ≥4-fold rise in CoV-specific IgG between acute and convalescent serum samples. Incompletely evaluated CoV illnesses were those for which participants failed to report symptoms during the winter surveillance, or were out of town at the time of the illness, but were reported to study staff at the final spring interview and demonstrated a significant increase in CoV-specific serum IgG. Asymptomatic infection was defined as a ≥4-fold increase in CoV-specific serum IgG bracketing a period when no respiratory symptoms were reported.
Statistical Analysis
Differences between groups were analyzed using the χ2 test of independence for dichotomous variables and unpaired, 2-tailed t tests for continuous variables. P values were corrected for multiple comparisons using the Bonferroni correction method.
RESULTS
Populations Studied
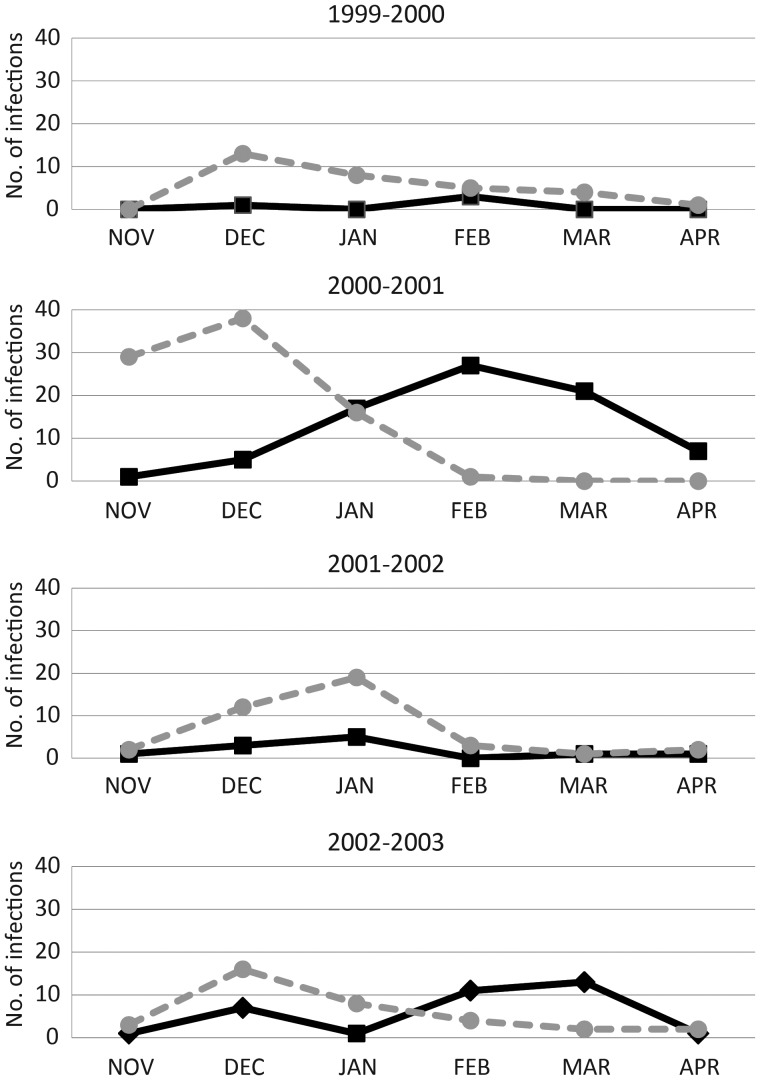
The prospective cohorts included 611 healthy elderly adults, 537 high-risk adults with underlying cardiopulmonary disease, and 291 healthy young adults, and the hospital cohort numbered 1388 (Table 1). These cohorts have previously been described in detail [12, 13]. The young adult cohort was approximately 40 years younger on average than the other cohorts, and had a substantially greater frequency of living with or exposure to children. Compared to the healthy elderly cohort, the high-risk and hospital cohorts had a relatively high prevalence of cardiac and pulmonary disease and greater impairment in the Instruments of Activities of Daily Living (IADL) scores. During the 4 winters, 398 coronavirus infections in 394 subjects were identified (4 subjects had a dual OC43/229E infection). In all years, OC43 was more common than 229E early in the surveillance period, and in years 2 and 4, 229E peaked later (Figure 1).
Table 1.
Demographic and Clinical Characteristics of Study Groups
| Characteristic | Healthy Elderly (n = 611) | High-Risk (n = 537) | Young Adults (n = 291) | Hospital (n = 1388) |
|---|---|---|---|---|
| Age, mean ± SD | 75 ± 6 | 70 ± 11 | 33 ± 5 | 75 ± 12 |
| Male, % | 42.4 | 54.4 | 16.2 | 44.7 |
| Race, % | ||||
| White | 97.7 | 93.7 | 82.1 | 84.9 |
| Black | 2.1 | 5.4 | 11.0 | 9.6 |
| Hispanic | 0.2 | 0.9 | 5.2 | 5.5 |
| Live with children, % | 2.2 | 6.0 | 88 | 7.4 |
| Exposed to childrena, % | 58 | 65 | 100 | 52 |
| Chronic illnesses, % | ||||
| Lung disease | 2.1 | 64.6 | 10.0 | 58.6 |
| Cardiac disease | 16.5 | 47.9 | 1.4 | 54.6 |
| Smoking (ever), % | 55.3 | 81.9 | 38.1 | 78.1 |
| IADLb score, mean ± SD | 0.31 ± 1.1 | 1.2 ± 2.2 | 0.03 ± 0.4 | 3.8 ± 4.1 |
| Use of home oxygen, % | 0 | 18 | 0 | 24 |
| No. of illnesses | 525 | 519 | 314 | 1471 |
a Defined as exposure at least once per month.
b Instruments of Activities of Daily Living (6 items scored from 0 to 2, with a maximum of 12 indicating severe disability).
Figure 1.
Incidence of symptomatic 229E (solid black line) and OC43 (dashed gray line) coronavirus infections in the combined prospective cohorts and the hospital cohort for each of the 4 winters.
Incidence of Coronavirus Infections in the Prospective Cohorts
A total of 302 CoV infections occurred in the prospective cohorts, with slightly more OC43 than 229E infections (Table 2). Overall, there were 113 CoV infections in the healthy elderly cohort (54 229E, 59 OC43), 112 in the high-risk group (57 229E, 55 OC43), and 77 in the young cohort (36 229E, 41 OC43). The percentage of subjects infected with coronaviruses varied considerably from year to year, with infection rates for 229E ranging from 0.5% to 15.0% and OC43 from 2.3% to 11.4%. Overall, infection rates were greatest during the winter of year 2 (2000–2001), when 23%–26% of subjects in each of the prospective cohorts were infected with 1 of the 2 strains.
Table 2.
Incidence of 229E and OC43 Coronavirus Infections During 4 Winters
| Year | Healthy Elderly | High-Risk | Young Adults | Hospital |
|---|---|---|---|---|
| 1999–2000 | ||||
| No. in cohort | 212 | 206 | 103 | 274 |
| No. of illnesses | 123 | 146 | 78 | 289 |
| No. of 229E infections (% of cohort) | 1 (0.5%) | 2 (0.9%) | 2 (1.9%) | 3 (1.1%) |
| No. asymptomatic 229E infections | 1 | 2 | 1 | 0 |
| No. of OC43 infections (% of cohort) | 5 (2.3%) | 11 (5.3%) | 10 (9.7%) | 6 (2.2%) |
| No. asymptomatic OC43 infections | 1 | 0 | 0 | 0 |
| 2000–2001 | ||||
| No. in cohort | 280 | 271 | 180 | 296 |
| No. of illnesses | 159 | 160 | 113 | 307 |
| No. of 229E infections (% of cohort) | 37 (13.2%) | 36 (13.3%) | 27 (15.0%) | 11 (3.7%) |
| No. asymptomatic 229E infections | 9 | 10 | 14 | 0 |
| No. of OC43 infections (% of cohort) | 28 (10.0%) | 31 (11.4%) | 20 (11.1%) | 24 (8.1%) |
| No. of asymptomatic OC43 infections | 6 | 10 | 3 | 0 |
| 2001–2002 | ||||
| No. in cohort | 180 | 195 | 164 | 434 |
| No. of illnesses | 102 | 115 | 101 | 165 |
| No. of 229E infections (% of cohort) | 1 (0.6%) | 6 (3.1%) | 1 (0.6%) | 5 (1.2%) |
| No. asymptomatic 229E infections | 0 | 2 | 0 | 0 |
| No. of OC43 infections (% of cohort) | 9 (5.0%) | 7 (3.6%) | 7 (4.3%) | 18 (4.1%) |
| No. asymptomatic OC43 infections | 0 | 1 | 1 | 0 |
| 2002–2003 | ||||
| No. in cohort | 295 | 210 | 90 | 384 |
| No. of illnesses | 135 | 103 | 37 | 410 |
| No. of 229E infections (% of cohort) | 15 (5.1%) | 13 (6.2%) | 6 (6.7%) | 15 (3.9%) |
| No. asymptomatic 229E infections | 9 | 5 | 1 | 0 |
| No. of OC43 infections (% of cohort) | 17 (5.8%) | 6 (2.9%) | 4 (4.4%) | 14 (3.6%) |
| No. asymptomatic OC43 infections | 1 | 2 | 3 | 0 |
| All years combined | ||||
| No. of 229E infections | 54 | 57 | 36 | 34 |
| % asymptomatic | 35.1% | 33.3% | 44.4% | NA |
| No. of OC43 infections | 59 | 55 | 41 | 62 |
| % asymptomatic | 13.6% | 23.6% | 17.1% | NA |
Of note, asymptomatic infection in the prospective cohorts (defined as a serologic response without a reported illness) was relatively common, and was significantly greater for 229E than for OC43 infections (36.7% vs 18.1%; P = .0003) when the 3 cohorts are considered together. Mixed viral infections were also significantly more frequent with symptomatic 229E (16.1%) than OC43 infection (6.3%, P = .03). For 229E-infected persons, coinfecting viruses were hMPV (6), RSV (5), influenza A and B (1 each), and OC43 (1). For OC43-infected persons, coinfecting viruses were RSV (3), hMPV (2), influenza A and B (1 each), and 229E (1).
Clinical Characteristics and Outcome in Prospective Cohorts
To compare clinical characteristics and outcomes associated with 229E and OC43 infections in the prospective cohorts, we analyzed fully evaluated symptomatic illnesses not associated with other viruses (Table 3). Upper respiratory symptoms were relatively common in all 3 cohorts, and nasal congestion and rhinorrhea were nearly universal regardless of infecting strain. Other upper respiratory symptoms were less common, with 30%–63% of subjects complaining of sore throat and 25%–44% complaining of hoarseness. Cough was more common in OC43 infections when all 3 cohorts were considered together, although lower respiratory signs and symptoms were similar for both strains.
Table 3.
Clinical Characteristics of 229E and OC43 Coronavirus Infections in the Prospective Cohortsa
| Characteristic | Healthy Elderly |
High-Risk |
Young Adults |
|||
|---|---|---|---|---|---|---|
| 229E (n = 27) | OC43 (n = 42) | 229E (n = 20) | OC43 (n = 34) | 229E (n = 15) | OC43 (n = 32) | |
| Symptoms, No. (%) | ||||||
| Congestion | 27 (100) | 41 (98) | 20 (100) | 34 (100) | 15 (100) | 32 (100) |
| Sore throat | 9 (33) | 17 (40) | 6 (30) | 15 (44) | 7 (47) | 20 (63) |
| Hoarseness | 9 (33) | 17 (40) | 5 (25) | 17 (50) | 5 (33) | 14 (44) |
| Coughb | 14 (52) | 30 (71) | 10 (50) | 27 (79) | 6 (40) | 20 (63) |
| Sputum | 6 (22) | 14 (33) | 7 (35) | 20 (59) | 3 (20) | 6 (19) |
| Dyspnea | 1 (4) | 6 (14) | 9 (44) | 18 (53) | 5 (33) | 7 (19) |
| Wheezing | 2 (7) | 6 (14) | 5 (25) | 10 (29) | 2 (13) | 5 (16) |
| Constitutional | 20 (74) | 25 (60) | 14 (70) | 21 (62) | 8 (53) | 19 (59) |
| Feverishness | 7 (26) | 8 (19) | 4 (20) | 11 (32) | 6 (40) | 7 (22) |
| Signs | ||||||
| Rhinorrhea | 27 (100) | 37 (88) | 19 (95) | 32 (94) | 13 (87) | 29 (91) |
| Wheezing | 0 | 3 (7) | 2 (10) | 5 (15) | 0 | 2 (6) |
| Rales | 0 | 1 (2) | 1 (5) | 5 (15) | 0 | 0 |
| Rhonchi | 0 | 0 | 0 | 0 | 0 | 0 |
| Temperature, °C, mean ± SD | 36.4 ± 0.4 | 36.5 ± 0.6 | 36.8 ± 0.4 | 36.7 ± 0.9 | 36.8 ± 0.4 | 36.6 ± 0.6 |
| SaO2 on room air | 96.6 ± 2.1 | 95.6 ± 4.0 | 95.8 ± 1.9 | 95.2 ± 3.4 | 98.3 ± 1.4 | 98.3 ± 1.2 |
Abbreviation: SaO2, saturation of arterial oxygen.
a Dual virus infections excluded; only fully evaluated illnesses are included in this analysis.
b P = .065 comparing OC43 to 229E when all groups are combined.
The outcome of coronavirus illness in these same subjects is shown in Table 4. For each cohort, there was a trend toward longer duration of illness with OC43 infection. In aggregate, OC43-infected subjects used significantly more cough suppressants and bronchodilators. In addition, there was a trend toward more health service use, such as physician or emergency room visits in the healthy elderly and high-risk groups during OC43 infection.
Table 4.
Outcome of Coronavirus Infections in the Prospective Cohortsa
| Outcome | Healthy Elderly |
High-Risk |
Young Adults |
|||
|---|---|---|---|---|---|---|
| 229E (n = 27) | OC43 (n = 42) | 229E (n = 20) | OC43 (n = 34) | 229E (n = 15) | OC43 (n = 32) | |
| Days of illness, mean (range) | 10.0 ± 5.2 | 12.7 ± 8.1 | 11.1 ± 6.6 | 12.4 ± 10.8 | 6.7 ± 4.2 | 12.0 ± 9.5 |
| Days house bound, mean ± SD | 0.9 ± 2.1 | 0.8 ± 1.5 | 1.2 ± 1.8 | 2.1 ± 5.2 | 0.1 ± 0.5 | 0.3 ± 0.8 |
| Called MD, No. (%) | 0 | 6 (14) | 3 (15) | 8 (26) | 0 | 1 (3) |
| Visited MD, No. (%) | 1 (4) | 7 (17) | 4 (20) | 11 (36) | 0 | 0 |
| Visited ED, No. (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Hospitalized, No. (%) | 0 | 0 | 0 | 1 (3) | 0 | 0 |
| Medications | ||||||
| Antipyretics | 14 (52) | 18 (43) | 10 (50) | 13 (41) | 12 (80) | 21 (68) |
| Cough suppressantsb | 5 (19) | 19 (45) | 1 (5) | 10 (31) | 4 (27) | 11 (35) |
| Bronchodilators | 0 | 2 (5) | 1 (5) | 6 (18) | 0 | 3 (10) |
| Systemic steroids | 0 | 3 (7) | 0 | 6 (18) | 0 | 1 (3) |
| Antibiotics | 1(4) | 4 (10) | 2 (10) | 10 (29) | 1 (7) | 0 |
Abbreviations: ED, emergency department; MD, physician.
a Dual virus infections were excluded, and only fully evaluated illnesses are included in this analysis.
b P = .02 for differences between 229E and OC43 when all 3 prospective groups combined.
Prevalence and Clinical Impact of Coronavirus Infection in the Hospital Cohort
The hospital cohort included 1388 subjects admitted for 1471 illnesses (Table 1). Subjects were similar in age to the healthy elderly group and slightly older than the high-risk group. Rates of underlying cardiopulmonary disease and tobacco use were also similar to the high-risk group, whereas home oxygen use was significantly greater in the hospital cohort (24% vs 18%, P = .003). During the 4 winters, 96 coronavirus infections were identified in the 1388 subjects in the hospital cohort. Of these, 34 (2.4%) were 229E and 62 (4.5%) OC43, with 2 subjects having both 229E and OC43 identified (Table 2). Similar to the prospective cohorts, the highest incidence of coronavirus infections occurred in year 2 (11.8%) and ranged from 3.3% to 7.5% in other years.
Because all subjects had respiratory symptoms, none were classified as asymptomatic. However, overall, 25% of CoV infections in the hospital cohort were associated with a second virus. As in the prospective cohorts, a significantly greater percentage of dual virus infection was noted in 229E-infected subjects than in OC43-infected subjects (16/34 [47%] vs 7/62 [11%]; P = .0002). For the 229E-infected persons, the coinfecting viruses were RSV (7), hMPV (9), OC43 (2), and influenza A and B (1 each). For the OC43-infected persons, the viruses were RSV (2), 229E (2), hMPV (1), parainfluenza virus (1), and influenza A (1). Admission diagnoses were similar for 229E and OC43, with pneumonia (32% and 35%, respectively) and AECOPD (36% and 41%, respectively) most common. The clinical presentation of the 2 strains in the hospital cohort was similar, although there was a trend toward longer hospital stay, and greater need for intensive care unit care and ventilatory support in OC43-infected subjects (Table 5). The only significant difference between strains was that OC43-infected subjects were more likely to have pulmonary infiltrates noted on chest radiographs.
Table 5.
Clinical Characteristics of Coronavirus Infections in the Hospital Cohort
| Characteristic | 229E (n = 18) | OC43 (n = 53) |
|---|---|---|
| Symptoms, No. (%) | ||
| Congestion | 6 (33) | 29 (55) |
| Sore throat | 4 (18) | 15 (28) |
| Hoarseness | 3 (19) | 17 (32) |
| Cough | 15 (83) | 51 (96) |
| Sputum | 11 (65) | 36 (68) |
| Dyspnea | 18 (100) | 51 (96) |
| Wheezing | 10 (63) | 35 (66) |
| Constitutional | 6 (35) | 23 (43) |
| Feverishness | 8 (44) | 23 (43) |
| Signs | ||
| Rhinorrhea | 3 (17) | 5 (9) |
| Wheezing | 12 (67) | 40 (76) |
| Rales | 12 (67) | 31 (60) |
| Rhonchi | 7 (41) | 20 (38) |
| Temperature, °C, mean (SD) | 37.6 ± 1.0 | 37. 5 ± 1.0 |
| SaO2 on room air | 87.9 ± 5.8 | 89.3 ± 6.9 |
| Outcome | ||
| Days in hospital, mean ± SD | 5.9 ± 4.7 | 8.8 ± 9.6 |
| ICU care, No. (%) | 1 (3) | 9 (15) |
| Ventilatory support | 2 (6) | 8 (15) |
| Infiltrate on chest radiography | 4 (12) | 19 (37)a |
| Death | 1 (3) | 2 (4) |
Dual virus infections not included.
Abbreviations: ICU, intensive care unit; SaO2, saturation of arterial oxygen.
a P = .05 compared to 229E.
Laboratory Diagnosis of 229E and OC43 Infections
For both strains, the combination of serology and RT-PCR greatly enhanced the diagnosis of infection. Results from symptomatic subjects in whom both tests were available indicate that a significantly greater proportion of OC43-infected subjects were RT-PCR positive (109/158 [69%]) than 229E-infected subjects (52/108 [48%], P = .001). For both strains, approximately 30% were positive by both RT-PCR and serology.
Relative Impact of Coronavirus Infection Compared to Other Respiratory Viruses
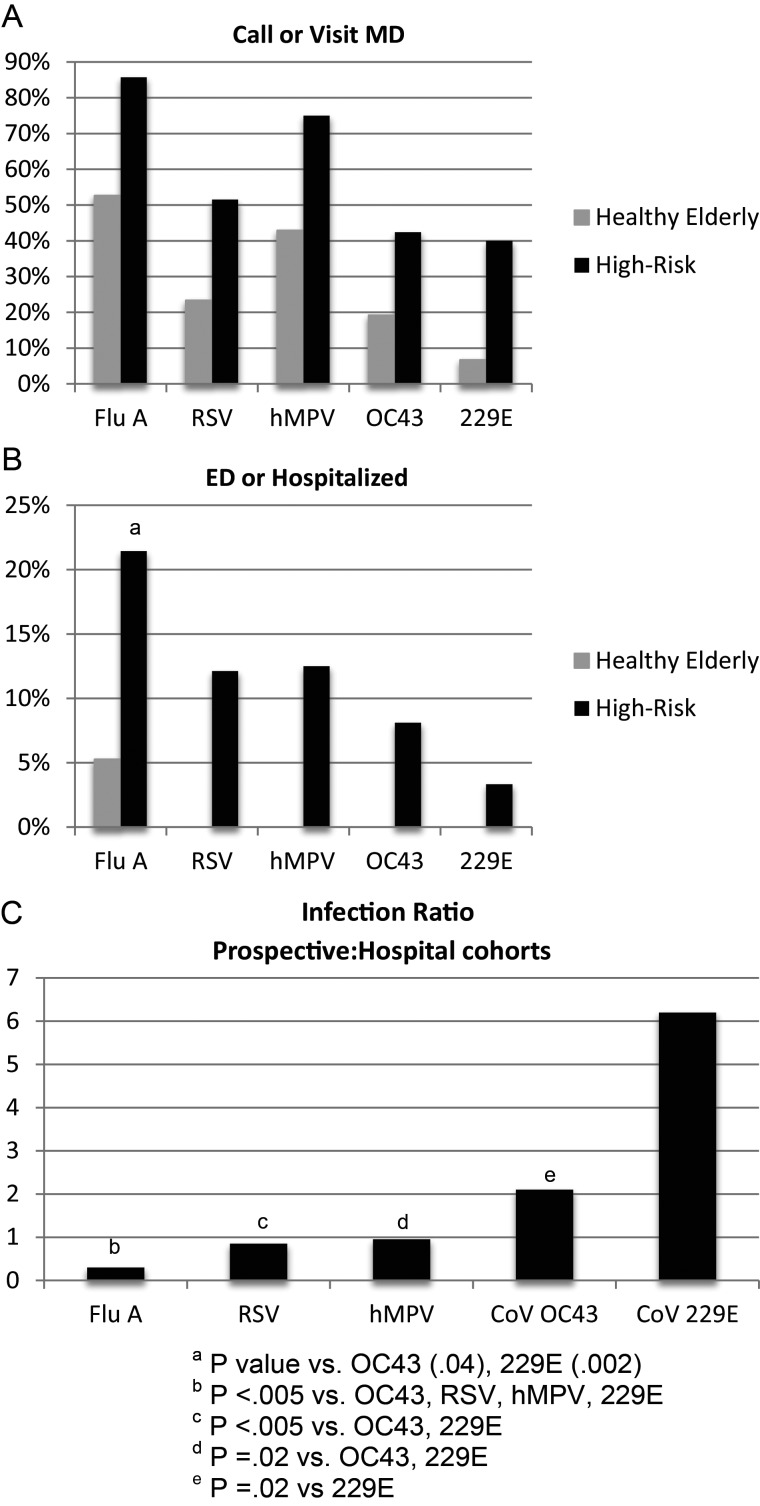
We had previously reported the incidence and clinical outcomes of several other viruses in these same cohorts, thus providing an opportunity to compare 229E and OC43 to influenza A, RSV, and hMPV [12, 13]. Figure 2A and 2B shows the virus-specific medical care utilization for the 2 older prospective cohorts in subjects with symptomatic illness not associated with dual virus infection. Influenza A infection, followed by hMPV and RSV, had the greatest impact and 229E the least. It should be noted that the majority of influenza A illnesses occurred when H3N2 strains were the dominant circulating viruses.
Figure 2.
Comparative clinical impact of coronavirus (CoV) 229E and OC43 to influenza A (Flu A), respiratory syncytial virus (RSV), and human metapneumovirus (hMPV) in the healthy elderly and high-risk cohorts. A and B, Percentage of subjects infected with each virus who called or visited their physician (MD; A) or sought care in an emergency department (ED) or were admitted to the hospital (B). Data used include symptomatic fully evaluated infections, excluding viral coinfections. Healthy elderly cohort (gray bars), high-risk cohort (black bars). C, Ratio of infections in combined healthy elderly and high-risk cohorts to the number of infections in the hospital cohort. Data for this analysis include asymptomatic and viral coinfections in the prospective cohorts.
We next compared the incidence of each virus in the prospective cohorts to the incidence in the hospital cohort. This should provide a reasonable gauge of the relative virulence of each virus, with a low ratio indicating that fewer outpatient infections are required to produce similar numbers of hospitalizations compared to a virus with a high ratio. For this analysis, asymptomatic infections and dual virus infections are included in the prospective cohorts. Figure 2C shows that influenza A has the lowest ratio (0.30), followed in ascending order by RSV (0.85), hMPV (0.95), OC43 (2.1), and 229E (6.2).
DISCUSSION
Noninfluenza respiratory viruses are increasingly recognized as significant pathogens in adult populations, especially in elderly adults. However, unlike influenza, the impact and importance of other respiratory viruses are less well documented in this age group. Although coronaviruses have been identified in elderly adults and high-risk populations such as those with COPD, cystic fibrosis, or immune deficiencies, only a few studies have included extended periods or directly compared results to other viruses [10, 11, 15–19]. Our data indicate that depending upon the year, 3%–26% of prospectively enrolled outpatients were infected with OC43 and 229E CoV strains. More importantly, among the hospital cohort the incidence of coronavirus infection varied from 3.3% to 11.8%, with an average of 6.8% per year. These findings are consistent with reports from other investigators. In a 1-year analysis of 2215 patients with COPD enrolled in an influenza vaccine efficacy study, Gorse et al identified coronaviruses in 14% of acute influenza-like illnesses (defined as ≥3 respiratory symptoms or fever plus 2 respiratory symptoms), a rate similar to that of influenza [10]. In that study, OC43 was approximately 3 times more common than 229E; however, asymptomatic infection was not sought and thus the true number of infections was likely greater. In our study, we used less stringent criteria for evaluation and thus we likely detected a broader spectrum of illness. In contrast to our study, Gorse et al found that coronavirus and influenza infections were equally associated with the need for hospitalization, although only influenza was temporally linked to impairment of pulmonary function. In a community-based study from the Netherlands, Graat et al detected coronaviruses by RT-PCR in 19% of 97 elderly subjects during a 1-year period, none of whom required hospitalization [20]. In studies from Houston, Texas, Greenberg identified OC43 or 229E in 16 of 62 COPD patients during 30 months of surveillance, with the majority of infections caused by OC43, whereas Glezen et al diagnosed serologically confirmed 229E or OC43 infection in 3.4% of 417 hospitalized adults during a 4-year period [11, 15]. In an analysis of a large number of outpatient respiratory illnesses in the United Kingdom, Gaunt identified CoV in approximately 1.1% of older adults over 3 years [8]. In that study, lower respiratory tract illness was noted in 43% of OC43 infections, but in no 229E infections.
Our data also suggest that OC43 has greater clinical impact than 229E in adults. OC43 infection was significantly less likely to be asymptomatic or associated with mixed viral infection, the latter a common finding also noted by other investigators. We defined asymptomatic infection by a positive serologic rise during an asymptomatic interval rather than by a positive PCR in asymptomatic persons. The latter may represent “colonization” whereas the former likely represents true infection. The true frequency of coinfections is likely to be considerably greater, as we did not assess subjects for rhinovirus and we only performed culture for parainfluenza viruses. The most frequent coinfecting viruses, RSV and hMPV, have previously been shown by us and others to account for significant morbidity in elderly adults [12, 13, 21–23]. Thus, the observed illness in coinfected subjects may primarily be attributable to the coinfecting virus, a suggestion made by Prill et al in a study of CoV infection in infants [24]. Perhaps most significant is the lower ratio of outpatient infections to inpatient infections for OC43 compared to 229E, suggesting a virulence difference between strains. Our results imply that in elderly adults with a high incidence of underlying cardiopulmonary disease, it would require approximately 3-fold more outpatient 229E infections than OC43 infections to result in an equivalent number of hospitalizations. Although another group of investigators also simultaneously measured the incidence of infection in outpatient and hospitalized adults in the same community with a similar trend, they did not differentiate the coronavirus or influenza strains in the outpatient groups [11, 15].
In a similar fashion, we were also able to compare the relative impact of both coronaviruses to that of other previously characterized respiratory viruses in these same cohorts. Not surprisingly, the results indicate that the clinical impact is greatest for influenza A followed by RSV, hMPV, OC43, and 229E in order of decreasing importance. Although the relative impact of coronavirus infection appears to be less than the other viruses, the overall burden, particularly for OC43, is not insignificant. Less virulent organisms, such as rhinoviruses, have also been shown to have significant overall disease burden in the elderly due to their relatively high prevalence [25]. Using our coronavirus data in relation to influenza A during the 4-year period of surveillance and eliminating viral coinfections, the hospital burden of these 2 coronaviruses combined was approximately 47% of the influenza A.
Our study has several important limitations. The first is that we did not test for NL63 and HKU1 infections, and thus are unable to provide a complete picture of the clinical impact of coronavirus infection in adults. In some studies, NL63 or HKU1 has been more frequently identified than OC43 and 229E, although the majority reported OC43 to be the most common coronavirus strain identified [8, 9, 17–19, 24, 26]. It is possible that some of our subjects diagnosed with OC43 or 229E by serology alone could include HKU1 or NL63 infections due to antigenic cross-reactivity within coronavirus groups [27]. In addition, we also did not include an asymptomatic control group for the hospital cohort to assess illness causality, as done in one pediatric study [24]. Futhermore, viral coinfections were likely underestimated, and thus the attributable impact of CoV infection may be overestimated. Finally, our study was limited to winter seasons, and although coronaviruses tend to be most active in the winter, we likely missed some CoV infections, especially in year 2 when OC43 was highly active at the start of our surveillance period. Because we performed serology for each winter season separately, we would not have detected summer illnesses.
In conclusion, the overall incidence and impact of combined coronavirus 229E and OC43 infections in diverse adult populations over 4 consecutive winters is relatively high and the overall medical burden of disease is substantial.
Notes
Acknowledgments. We thank Patricia Hennessey, RN, and Mary Criddle RN, for assistance with clinical evaluations of study subjects, and Maryanne Formica and Gloria Andolina for technical assistance in performing laboratory assays.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant numbers RO1-AI-45969, RO1-AI-045465).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011–33. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peiris JS, Lai ST, Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–25. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh K, Kapikian AZ, Turner HC, Hartley JW, Parrott RH, Chanock RM. Seroepidemiologic studies of coronavirus infection in adults and children. Am J Epidemiol. 1970;91:585–92. doi: 10.1093/oxfordjournals.aje.a121171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus. Nat Med. 2004;10:368–73. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fouchier RA, Hartwig NG, Bestebroer TM, et al. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci U S A. 2004;101:6212–6. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo WP, Doan T, Herd KA, Netter HJ, Tindle RW. Hepatitis B surface antigen vector delivers protective cytotoxic T-lymphocyte responses to disease-relevant foreign epitopes. J Virol. 2006;80:3975–84. doi: 10.1128/JVI.80.8.3975-3984.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaunt ER, Hardie A, Claas EC, Simmonds P, Templeton KE. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol. 2010;48:2940–7. doi: 10.1128/JCM.00636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talbot HK, Shepherd BE, Crowe JE, Jr, et al. The pediatric burden of human coronaviruses evaluated for twenty years. Pediatr Infect Dis J. 2009;28:682–7. doi: 10.1097/INF.0b013e31819d0d27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorse GJ, O'Connor TZ, Hall SL, Vitale JN, Nichol KL. Human coronavirus and acute respiratory illness in older adults with chronic obstructive pulmonary disease. J Infect Dis. 2009;199:847–57. doi: 10.1086/597122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283:499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 12.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–59. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 13.Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168:2489–96. doi: 10.1001/archinte.168.22.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mounir S, Talbot PJ. Sequence analysis of the protein gene of human coronavirus OC43 and evidence for O-glycosylation. J Gen Virol. 1992;73:273–6. doi: 10.1099/0022-1317-73-10-2731. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg SB, Allen M, Wilson J, Atmar RL. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:167–73. doi: 10.1164/ajrccm.162.1.9911019. [DOI] [PubMed] [Google Scholar]
- 16.da Silva Filho LV, Zerbinati RM, Tateno AF, et al. The differential clinical impact of human coronavirus species in children with cystic fibrosis. J Infect Dis. 2012;206:384–8. doi: 10.1093/infdis/jis274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garbino J, Crespo S, Aubert JD, et al. A prospective hospital-based study of the clinical impact of non-severe acute respiratory syndrome (non-SARS)-related human coronavirus infection. Clin Infect Dis. 2006;43:1009–15. doi: 10.1086/507898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu X, Lu R, Wang Z, et al. Etiology and clinical characterization of respiratory virus infections in adult patients attending an emergency department in Beijing. PLoS One. 2012;7:e32174. doi: 10.1371/journal.pone.0032174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau SK, Woo PC, Yip CC, et al. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44:2063–71. doi: 10.1128/JCM.02614-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graat JM, Schouten EG, Heijnen ML, et al. A prospective, community-based study on virologic assessment among elderly people with and without symptoms of acute respiratory infection. J Clin Epidemiol. 2003;56:1218–23. doi: 10.1016/S0895-4356(03)00171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowell SF, Anderson LJ, Gary HE, Jr, et al. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis. 1996;174:456–62. doi: 10.1093/infdis/174.3.456. [DOI] [PubMed] [Google Scholar]
- 22.van Asten L, van den Wijngaard C, van Pelt W, et al. Mortality attributable to 9 common infections: significant effect of influenza A, respiratory syncytial virus, influenza B, norovirus, and parainfluenza in elderly persons. J Infect Dis. 2012;206:628–39. doi: 10.1093/infdis/jis415. [DOI] [PubMed] [Google Scholar]
- 23.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 24.Prill MM, Iwane MK, Edwards KM, et al. Human coronavirus in young children hospitalized for acute respiratory illness and asymptomatic controls. Pediatr Infect Dis J. 2012;31:235–40. doi: 10.1097/INF.0b013e31823e07fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson KG, Kent J, Hammersley V, Cancio E. Risk factors for lower respiratory complications of rhinovirus infections in elderly people living in the community: prospective cohort study. Br Med J. 1996;313:1119–23. doi: 10.1136/bmj.313.7065.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dijkman R, Jebbink MF, Gaunt E, et al. The dominance of human coronavirus OC43 and NL63 infections in infants. J Clin Virol. 2012;53:135–9. doi: 10.1016/j.jcv.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan KH, Cheng VC, Woo PC, et al. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229E, OC43, and NL63. Clin Diagn Lab Immunol. 2005;12:1317–21. doi: 10.1128/CDLI.12.11.1317-1321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]