Abstract
Introduction
The BEACON study evaluated the efficacy and safety of QVA149, a once-daily dual bronchodilator containing a fixed-dose combination of the long-acting β2-agonist (LABA) indacaterol and long-acting muscarinic antagonist (LAMA) glycopyrronium (NVA237), in development for the treatment of patients with chronic obstructive pulmonary disease (COPD), compared with the free-dose concurrent administration of indacaterol plus glycopyrronium (IND+GLY).
Methods
In this multicenter, double-blind, parallel group study, patients with stage II or stage III COPD (Global initiative for chronic Obstructive Lung Disease [GOLD] 2010) were randomized (1:1) to once-daily QVA149 (110 μg indacaterol/50 μg glycopyrronium) or concurrent administration of indacaterol (150 μg) and glycopyrronium (50 μg) via the Breezhaler® device (Novartis AG, Basel, Switzerland) for 4 weeks. The primary endpoint was to evaluate the noninferiority of QVA149 as compared with concurrent administration of IND+GLY, for trough forced expiratory volume in 1 second (FEV1) after 4 weeks of treatment. The other assessments included FEV1 area under the curve from 0 to 4 hours (AUC0–4 hours) at day 1 and week 4, symptom scores, rescue medication use, safety, and tolerability over the 4-week study period.
Results
Of 193 patients randomized, 187 (96.9%) completed the study. Trough FEV1 at week 4 for QVA149 and IND+GLY was 1.46 L ± 0.02 and 1.46 L ± 0.18, respectively. The FEV1 AUC0–4 hours at day 1 and week 4 were similar between the two treatment groups. Both treatment groups had a similar reduction in symptom scores and rescue medication use for the 4-week treatment period. Overall, 25.6% of patients in QVA149 group and 25.2% in the IND+GLY group experienced an adverse event, with the majority being mild-to-moderate in severity. No deaths were reported during the study or during the 30 days follow-up period.
Conclusion
The BEACON study demonstrated that once-daily QVA149 provides an efficacy and safety profile similar to the concurrent administration of its monocomponents indacaterol and glycopyrronium.
Keywords: COPD, LABA, LAMA, FEV1 AUC0–4 hours, rescue medication
Introduction
Current guidelines for the effective management of moderate-to-severe chronic obstructive pulmonary disease (COPD) recommend the addition of a second long-acting bronchodilator class to the existing bronchodilator regimen, thus combining a long-acting β2-agonist (LABA) with a long-acting muscarinic antagonist (LAMA).1 Moreover, β2-agonists and muscarinic antagonists have a complementary mechanism of action, and their combination has been shown to improve bronchodilation as compared with their respective monotherapies.2–8
The addition of a second bronchodilator has been seen to provide additional benefits in terms of enhanced broncho-dilation, inspiratory capacity, improved dyspnea, quality of life, and a decline in rescue medication use.3,9 This concept of dual bronchodilation can currently be achieved with a free combination of a LABA and LAMA. However, the free LABA/LAMA combination requires the administration of the medications separately in different devices, and this would potentially affect patient convenience and compliance.10 Thus, a fixed-dose LABA/LAMA combination would be a better treatment option.
Currently, there is no fixed-dose combination of LABA/LAMA available for patients with COPD. QVA149, in development for the treatment of patients with COPD, is a once-daily dual bronchodilator containing a fixed-dose combination of the LABA indacaterol and the LAMA glyco-pyrronium (NVA237). Both indacaterol and glycopyrronium have established safety and efficacy profiles,11–16 and are approved as once-daily monotherapies for the maintenance treatment of COPD. In addition, they both offer the benefit of being administered in the same Breezhaler® device (Novartis AG, Basel, Switzerland). Previous Phase III studies have demonstrated that QVA149 is safe and provides superior and clinically meaningful improvements in lung function compared with its monocomponents indacaterol 150 μg and glycopyrronium 50 μg, as well as tiotropium 18 μg and salmeterol/fluticasone 50/500 μg. These improvements in lung function were translated into concomitant improvements in patient-reported outcomes (health status via St George’s Respiratory Questionnaire [SGRQ] and breathlessness via transition dyspnea index [TDI]) and a reduction in exacerbations.17–21 QVA149 is thus expected to provide similar efficacy and safety as compared with the concurrent administration of its monocomponents.
In addition, the fixed-dose combination will offer a simplified and more convenient dosing regimen, compared with a free combination with the potential to improve patient compliance.
In the BEACON study, the efficacy and safety of once-daily QVA149 was evaluated in comparison with the concurrent administration of its monocomponents – indacaterol and glycopyrronium – over a treatment period of 4 weeks.
Methods
Study design and treatment
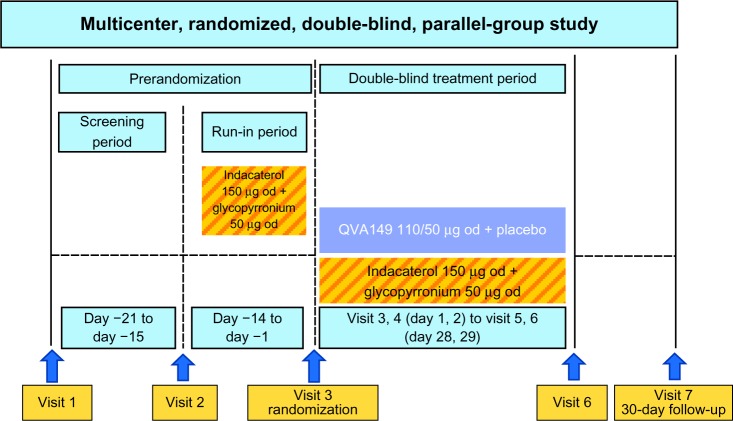
This was a multicenter, double-blind, parallel-group, active-controlled, noninferiority design to compare the efficacy of QVA149 110/50 μg versus concurrent administration of its monocomponents indacaterol (150 μg) and glycopyr-ronium (50 μg) in patients with moderate-to-severe COPD (Figure 1).22 The study consisted of a prerandomization period, which included screening/washout (7 days) and run-in period (14 days). To stabilize patients and standardize baseline lung function, open-label, 150 μg of indacaterol and 50 μg of glycopyrronium were administered during the 14-day run-in period. Patients were then randomized (1:1) to QVA149 and placebo or concurrent administration of indacaterol (IND) plus (+) glycopyrronium (GLY); all were administered via the Breezhaler® device.
Figure 1.
BEACON study design.
Abbreviation: od, once daily.
To ensure that the fine-particle dose of indacaterol delivered to the lung from QVA149 matched that of the monotherapy, the dose of indacaterol was reduced from 150 μg to 110 μg when given in the QVA149 fixed-dose combination. Pharmacokinetic data from previous indacaterol studies demonstrated the similarity of the steady-state systemic exposures of 150 μg of indacaterol and 50 μg of glycopyrronium to QVA149 (110/50 μg). Patients took their study medication (two capsules) concurrently, once daily, between 8–11 am.
Salbutamol (albuterol) was allowed as rescue medication. Inhaled corticosteroids (ICS) were allowed if the patients were on the stable ICS dose for at least 30 days prior to visit 1 and during the study. The protocol and procedures were approved by relevant institutional review boards and ethics committees. All participants provided written informed consent, and the study was designed in accordance with the International Conference on Harmonisation (ICH) Harmonised Tripartite Guidelines for Good Clinical Practice and with the ethical principles of the World Medical Association’s Declaration of Helsinki.
Patients
According to the Global initiative for chronic Obstructive Lung Disease (GOLD) 2010 guidelines, men and women were enrolled if they: were ≥40 years of age with moderate-to-severe COPD (stage II or stage III); had post-bronchodilator forced expiratory volume in 1 second (FEV1) ≥30% and <80% of the predicted normal; post-bronchodilator FEV1/forced vital capacity (FVC) <0.70; were current or ex-smokers, having a smoking history of at least ten pack-years. In addition, all patients enrolled were symptomatic (coughing, wheezing, sputum production, breathlessness) according to daily electronic diary data, with a total symptom score of 1 or more (on a scale of 0 to 3) on at least 3 days prior to visit 3.
Key exclusion criteria included: patients with type I or uncontrolled type II diabetes; history of long QT syndrome or prolonged QTc; patients who had a COPD exacerbation that required treatment with antibiotics and/or oral corticosteroids and/or hospitalization in the 6 weeks prior to visit 1; patients who had a respiratory tract infection within 4 weeks prior to visit 1; and patients who had a clinically significant electrocardiogram abnormality.
End points and assessments
The primary endpoint was to evaluate the noninferiority of QVA149 compared to concurrent administration of indacaterol plus glycopyrronium for trough FEV1 (mean of 23 hours, 15 minutes and 23 hours, 45 minutes postdose) after 4 weeks of treatment in patients with moderate-to-severe COPD. The other assessments included FEV1 AUC0–4 hours at day 1 and week 4, daily number of puffs of rescue medication use, e-diary data with recording of daily clinical symptoms on the scale of 0–3 (where 0 refers to no symptoms) over the study period of 4 weeks, and safety and tolerability (adverse events, laboratory tests, electrocardiograms, and vital signs).
Statistical method and analysis
The treatment difference between QVA149 and concurrent administration of indacaterol and glycopyrronium for trough FEV1 was estimated to be 0 mL after 4 weeks of treatment. Based on the treatment difference of 226 mL between QVA149 300/50 μg and placebo (lower limit of the 95% confidence interval [CI] of 192 mL) in mean trough FEV1 in the QVA149A2204 study,23 the noninferiority margin of 100 mL was taken to be approximately one-half of this. Noninferiority was considered to be achieved if the lower bound values of two-sided 95% CI were greater than −100 mL.
A total of 154 evaluable patients would achieve 90% power with a one-sided noninferiority test at a significance level of 2.5%. Approximately 184 randomized patients were required to adjust for an estimated combined attrition rate of 17%. The per protocol set was the primary analysis data set for this non-inferiority study and included all patients in the full analysis set without any major protocol deviations.
The primary analysis was performed using a mixed model, with treatment as a fixed effect. Trough FEV1 measurement at baseline and FEV1 measurement prior and post to inhalation of short-acting bronchodilator were taken as covariates. This model also included smoking status (current/ex-smoker), history of inhaled corticosteroids at baseline as fixed effects, and center as random effects. Secondary analyses in terms of FEV1 AUC0–4 hours at day 1 and week 4, daily number of puffs of rescue medication, and diary symptoms were performed using a similar mixed model (as per the primary endpoint) with the associated 95% CI. Secondary analyses were not statistically adjusted for multiplicity.
The safety set included all patients who received at least one dose of the study drug. The number (%) of patients with notable values of pulse rate of <40 and >90 bpm, systolic blood pressure of <90 and >140 mmHg, and diastolic blood pressure of <50 and >90 mmHg was summarized. Notable QTc values and changes from baseline were summarized. A notable value was defined as a QTc interval of greater than 450 milliseconds for both sexes at baseline and the number of newly occurring or worsening notable QTc values postbaseline time points.
Results
Patients
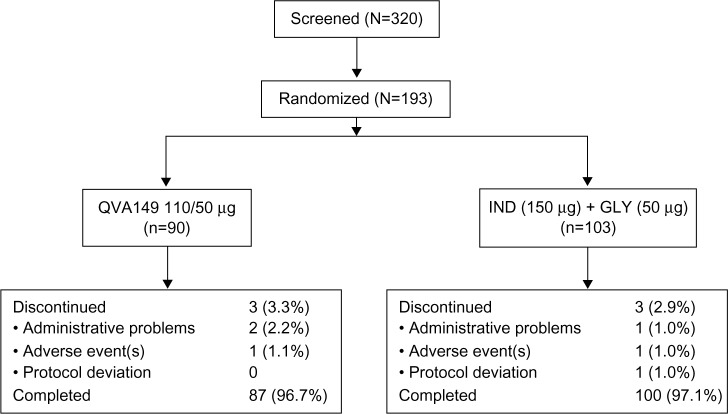
Of the total 320 patients screened, 193 were randomized and 187 (96.9%) completed the study (QVA149: N=87, IND+GLY: N=100) (Figure 2). The patient demographics and baseline characteristics were numerically comparable between the two treatment groups (Table 1).
Figure 2.
Patient disposition.
Abbreviation: IND+GLY, indacaterol and glycopyrronium.
Table 1.
Demographics and clinical characteristics of patients at baseline
| QVA149 (110/50 μg) N = 90 | IND (150 μg) + GLY (50 μg) N = 103 | Total N = 193 | |
|---|---|---|---|
| Age (years) | 65.6 (7.28) | 64.2 (7.40) | 64.9 (7.36) |
| Sex, n (%) | |||
| Male | 58 (64.4) | 59 (57.3) | 117 (60.6) |
| Female | 32 (35.6) | 44 (42.7) | 76 (39.4) |
| Race, n (%) | |||
| Caucasian | 90 (100) | 103 (100) | 193 (100) |
| Pre-bronchodilator FEV1 (% predicted) | 45.1 (12.88) | 44.4 (12.95) | 44.7 (12.89) |
| Post-bronchodilator FEV1 (% predicted) | 54.1 (12.27) | 53.8 (12.85) | 54.0 (12.55) |
| FEV1 reversibility (% increase) | 22.7 (16.78) | 24.4 (19.47) | 23.6 (18.24) |
| Duration of COPD (years) | 7.3 (5.03) | 6.8 (4.83) | 7.0 (4.92) |
| Severity of COPD (GOLD 2010), n (%) | |||
| Moderate | 55 (61.1) | 60 (58.3) | 115 (59.6) |
| Severe | 35 (38.9) | 43 (41.7) | 78 (40.4) |
| ICS use, n (%) | |||
| No | 29 (32.2) | 39 (37.9) | 68 (35.2) |
| Yes | 61 (67.8) | 64 (62.1) | 125 (64.8) |
| Smoking history, n (%) | |||
| Ex-smoker | 53 (58.9) | 62 (60.2) | 115 (59.6) |
| Current smoker | 37 (41.1) | 41 (39.8) | 78 (40.4) |
| Number of pack-years | 41.6 (17.84) | 39.2 (20.22) | 40.3 (19.14) |
Notes: All values in mean (SD) unless specified. FEV1 reversibility is calculated as % increase of FEV1 value after inhalation of bronchodilator. Pack-year is calculated by packs smoked per day × number of years as a smoker.
Abbreviations: IND+GLY, indacaterol and glycopyrronium; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; COPD, chronic obstructive pulmonary disease; GOLD, Global initiative for chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; SD, standard deviation.
Efficacy
Patients who were stable on the concurrent administration of indacaterol and glycopyrronium during the run-in period continued to be stable when randomized to receive QVA149 along with the free-dose concurrent administration of indacaterol and glycopyrronium, with similar maintenance of their lung function from day 1 to week 4 without any significant difference. The least squares mean ± standard error (LSM ± SE) trough FEV1 at week 4 for QVA149 and for the free-dose combination of IND+GLY was 1.5 L ± 0.02 and 1.5 L ± 0.18, respectively (Figure 3).
Figure 3.
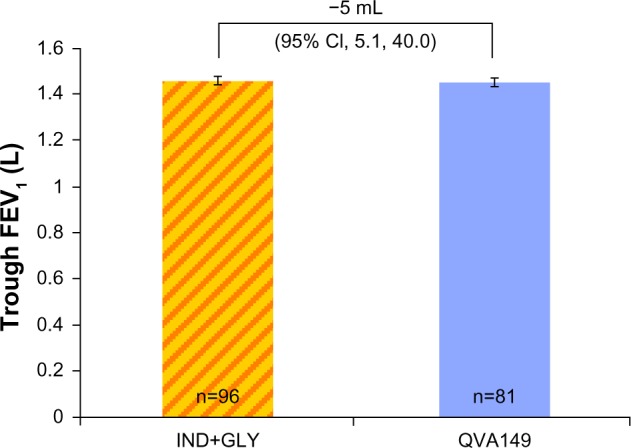
Trough FEV1 (L) at week 4.
Abbreviations: IND+GLY, indacaterol and glycopyrronium; FEV1, forced expiratory volume in 1 second; CI, confidence interval.
The FEV1 AUC0–4 hours at day 1 and week 4 were similar for QVA149 in comparison with IND+GLY (Figure 4). Also, the FEV1 5 minutes to 4 hours postdose spirometry measurements at week 4 were similar between the two groups (Figure 5). Likewise, both groups had a similar reduction in symptom scores and rescue medication use from baseline for more than 4 weeks of treatment (Table 2).
Figure 4.
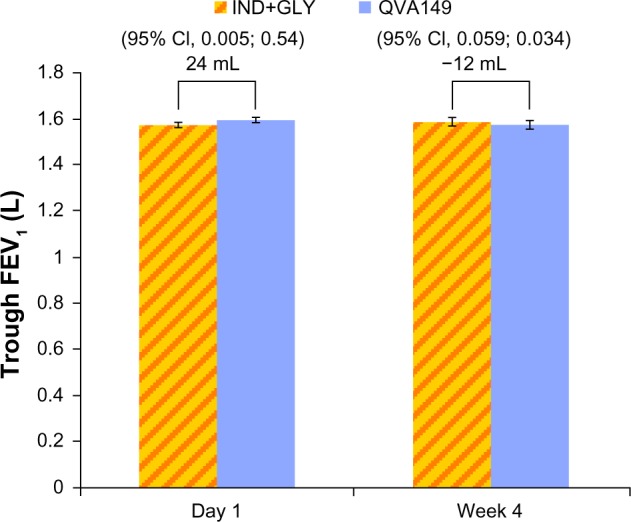
FEV1 AUC0–4 hours at day 1 and week 4.
Abbreviations: FEV1, forced expiratory volume in 1 second; AUC, area under curve; IND+GLY, indacaterol and glycopyrronium; CI, confidence interval.
Figure 5.
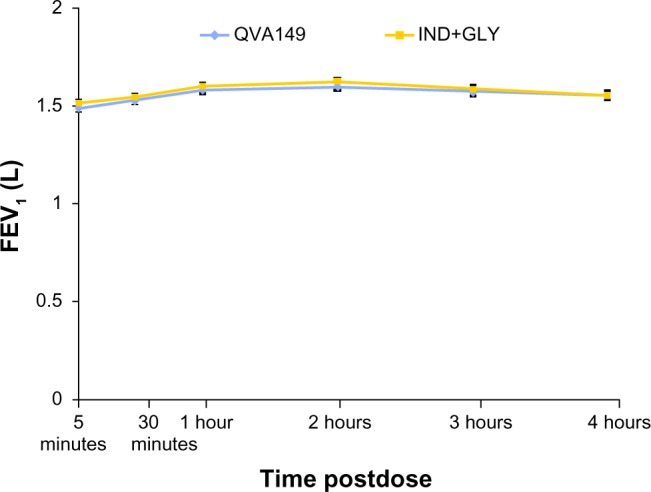
FEV1 for the first 4 hours at week 4.
Abbreviations: IND+GLY, indacaterol and glycopyrronium; FEV1, forced expiratory volume in 1 second.
Table 2.
Daily total symptom score and rescue medication use over 4 weeks
| Treatment | n | Baseline mean (SE) | LS mean | SE | Comparison | Treatment difference
|
||
|---|---|---|---|---|---|---|---|---|
| LS mean | SE | 95% CI | ||||||
| Change from baseline in the daily total symptom score | ||||||||
| QVA149 (110/50 μg) (N = 84) | 84 | 5.25 (0.275) | −0.42 | 0.140 | QVA149 – | 0.07 | 0.161 | (−0.24, 0.39) |
| IND (150 μg) + GLY (50 μg) (N = 97) | 95 | 5.74 (0.295) | −0.49 | 0.130 | (IND+GLY) | |||
| Change from baseline in the mean daily number of puffs of rescue medication | ||||||||
| QVA149 (110/50 μg) (N = 84) | 84 | 2.24 (0.266) | −0.45 | 0.124 | QVA149 – | −0.04 | 0.159 | (−0.35, 0.28) |
| IND (150 μg) + GLY (50 μg) (N = 97) | 95 | 2.04 (0.265) | −0.42 | 0.113 | (IND+GLY) | |||
Abbreviations: IND+GLY, indacaterol and glycopyrronium; LS mean, least squares mean; SE, standard error; CI, confidence interval.
The overall incidence of adverse events (AEs) was similar in both treatment groups, with 25.6% of patients experiencing an AE in the QVA149 group and 25.2% in the IND+GLY treatment group, with the majority of AEs being mild-to-moderate in severity. Nasopharyngitis and COPD worsening were the most frequently occurring AEs in both treatment groups (Table 3).
Table 3.
Most frequent AEs (at least 1% in any treatment group) by preferred term of patients
| QVA149 (110/50 μg) N = 90 n (%) | IND (150 μg) + GLY (50 μg) N = 103 n (%) | |
|---|---|---|
| Patients with any AE(s) | 23 (25.6) | 26 (25.2) |
| Preferred term | ||
| Nasopharyngitis | 7 (7.8) | 6 (5.8) |
| COPD worsening | 4 (4.4) | 2 (1.9) |
| Cough | 4 (4.4) | 2 (1.9) |
| Chest discomfort | 2 (2.2) | 0 |
| Influenza | 2 (2.2) | 1 (1.0) |
| Myalgia | 2 (2.2) | 0 |
| Pneumonia | 2 (2.2) | 0 |
| Aortic aneurysm | 1 (1.1) | 0 |
| Breast pain | 1 (1.1) | 0 |
| Depression | 1 (1.1) | 0 |
| Diarrhea | 1 (1.1) | 1 (1.0) |
| Dysphonia | 1 (1.1) | 1 (1.0) |
| Dyspnea | 1 (1.1) | 2 (1.9) |
| Headache | 1 (1.1) | 1 (1.0) |
| Ligament injury | 1 (1.1) | 0 |
Abbreviations: IND+GLY, indacaterol and glycopyrronium; AEs, adverse events; COPD, chronic obstructive pulmonary disease.
A similar number of patients had serious AEs in the IND+GLY treatment group (5.8%) compared with QVA149 group (4.4%). No patient discontinued the study medication due to a serious adverse event (SAE). There were no cardio- and cerebrovascular SAEs in either treatment group.
Four patients in the IND+GLY group had notably increased QTc levels, compared with one patient in the QVA149 treatment group (Table 4). A total of six patients (6.7%) in the QVA149 group had AEs that were suspected to be study drug-related, compared with two patients (1.9%) in the indacaterol and glycopyrronium group. No deaths were reported in either treatment group during the study or during the 30-day follow-up period.
Table 4.
Number (%) of patients with newly occurring or worsening notable QTc values (according to Fridericia’s formula) and maximum increase from baseline on study treatment
| According to Fridericia’s formula | QVA149 (110/50 μg) N = 90 n/N (%) | IND (150 μg) + GLY (50 μg) N = 103 n/N (%) |
|---|---|---|
| QTc > 450 ms | 1/89 (1.1) | 4/102 (3.9) |
| QTc > 480 ms | 0/89 | 0/102 |
| QTc > 500 ms | 0/89 | 0/102 |
| Maximum increase from baseline | ||
| 30–60 ms | 0/84 | 4/101 (4.0) |
| >60 ms | 0/84 | 0/101 |
Abbreviations: IND+GLY, indacaterol and glycopyrronium; ms, milliseconds.
Discussion
The BEACON study directly compared the efficacy and safety of the fixed and the free combination of a LABA with a LAMA. The study met its primary objective to prove QVA149 as noninferior to the concurrent administration of indacaterol and glycopyrronium for its effect on trough FEV1 after 4 weeks of treatment. QVA149 also improved other clinical endpoints and had a safety and tolerability profile that was similar to the concurrent administration of its monocomponents indacaterol and glycopyrronium.
There have been studies demonstrating the combination of different classes of bronchodilators to improve efficacy without compromising safety in patients whose symptoms are not adequately controlled by bronchodilator monotherapy.3,6,7,24 This is due to the fact that LABAs and LAMAs are known to have a complementary mode of action, especially in terms of bronchodilation. LABAs augment the bronchodilatory effect produced by LAMAs by decreasing the release of acetylcholine via modulation of cholinergic neurotransmission. Moreover, LAMAs antagonize the effects of acetylcholine, amplifying the bronchodilation caused by LABAs. It has also been observed in previous studies that concurrent administration of a LABA with a LAMA produces superior bronchodilation in comparison with the monotherapies, with additional benefits of reductions in dyspnea, dynamic hyperinflation, improved symptoms, and exercise endurance. QVA149 has been found to be efficacious and well-tolerated in comparison with indacaterol, glycopyrronium, tiotropium, salmeterol/fluticasone, and placebo in previous Phase III clinical trials.4,7,25 In the present study, QVA149 provided similar efficacy in terms of trough FEV1 and FEV1 AUC0–4 hours as compared with IND+GLY, along with providing the advantage of dual bronchodilation in a single inhaler device. Also, peak FEV1 values revealed that the maximum improvements seen with the lung function were similar in both treatment groups. The symptomatic improvements achieved by patients in the QVA149 treatment group reinforced the association between improvements in lung function with other clinical outcomes.5,26
ICS are recommended for patients with severe COPD and recurrent exacerbations. However, in the real world setting, patients with COPD are often inappropriately treated with ICS in combination with a LABA, irrespective of their GOLD classification.27 In the present study, 65% of the study population (60% had moderate and 40% had severe COPD) were on ICS at baseline. The BEACON study targeted this segment of patients with moderate-to-severe COPD for achieving more effective bronchodilation, symptom control, and reduced requirements for rescue medication with a fixed-dose LABA/LAMA combination.
Although safety was not a primary objective of the study, it was important to demonstrate that there was no difference in the safety profile between the fixed-dose combination QVA149 and the free combination therapy with indacaterol plus glycopyrronium. Overall, the incidence of AEs reported was numerically similar in both of the treatment groups, with no additional safety concerns for either the fixed- or free-dose combination. The results were consistent with the previous clinical studies that assessed the cardiac safety of QVA149,17–20,28 confirming that no significant cardiovascular safety concerns were observed with QVA149.
Patients with COPD have several comorbidities and are likely to be on other medications.29 Also, physical decon-ditioning due to hyperinflation and cognitive impairment would make the use of multiple inhalers difficult for these patients.10 In comparison to the free-dose combinations, QVA149 provides the advantage of delivering the LABA/LAMA combination in a single inhaler device, and thus aims to simplify the treatment regimen, improving patient adherence.10
Conclusion
The BEACON study demonstrated that once-daily QVA149 improves lung function and symptom scores, reduces rescue medication use, and has a safety and tolerability profile similar to the free combination of its monocomponents ie, indacaterol and glycopyrronium. This study provides reassurance that the LABA/LAMA fixed-dose combination QVA149 provides benefits of dual bronchodilation in a single device, making it a more convenient treatment option for patients with COPD.
Acknowledgments
The authors thank the patients who participated and the staff at the participating clinical centers. The study was funded by Novartis Pharma AG, Basel, Switzerland. The authors were assisted in the preparation of the manuscript by Mohit Joshi and Mark J Fedele (Novartis).
Footnotes
Disclosure
In the past 3 years, Professor Dahl has received compensation for consulting with Boehringer Ingelheim, Novartis, Vectura, Roche, Elevation Pharmaceuticals, Inc, and Norpharma. He has also undertaken research funded by AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, ALK-Abello, and Stallergenes and has participated in educational activities sponsored by AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, ALK-Abello, Novartis, and Almirall. Dalal Jadayel, Vijay KT Alagappan, Hungta Chen, and Donald Banerji are employees of Novartis. The authors report no other conflicts of interest in this work.
References
- 1.Global initiative for chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2010 update Global Initiative for Chronic Obstructive Lung Disease, Inc; 2010Available from: http://www.goldcopd.org/uploads/users/files/GOLDReport_April112011.pdfAccessed on July 20, 2012 [Google Scholar]
- 2.Karner C, Cates CJ. Long-acting beta(2)-agonist in addition to tiotro-pium versus either tiotropium or long-acting beta(2)-agonist alone for chronic obstructive pulmonary disease [review] Cochrane Database Syst Rev. 2012;4:CD008989. doi: 10.1002/14651858.CD008989.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahler DA, D’Urzo A, Bateman ED, et al. INTRUST-1 and INTRUST-2 study investigators Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double-blind comparison. Thorax. 2012;67(9):781–788. doi: 10.1136/thoraxjnl-2011-201140. [DOI] [PubMed] [Google Scholar]
- 4.Rabe KF, Timmer W, Sagkriotis A, Viel K. Comparison of a combination of tiotropium plus formoterol to salmeterol plus fluticasone in moderate COPD. Chest. 2008;134(2):255–262. doi: 10.1378/chest.07-2138. [DOI] [PubMed] [Google Scholar]
- 5.Tashkin DP, Celli BR, Decramer M, Lystig T, Liu D, Kesten S. Efficacy of tiotropium in COPD patients with FEV1 ≥ 60% participating in the UPLIFT® trial. COPD. 2012;9(3):289–296. doi: 10.3109/15412555.2012.656211. [DOI] [PubMed] [Google Scholar]
- 6.van Noord JA, Aumann JL, Janssens E, et al. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J. 2005;26(2):214–222. doi: 10.1183/09031936.05.00140404. [DOI] [PubMed] [Google Scholar]
- 7.van Noord JA, Aumann JL, Janssens E, et al. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest. 2006;129(3):509–517. doi: 10.1378/chest.129.3.509. [DOI] [PubMed] [Google Scholar]
- 8.van Noord JA, Buhl R, Laforce C, et al. QVA149 demonstrates superior bronchodilation compared with indacaterol or placebo in patients with chronic obstructive pulmonary disease. Thorax. 2010;65(12):1086–1091. doi: 10.1136/thx.2010.139113. [DOI] [PubMed] [Google Scholar]
- 9.Szmidt M. The influence of treatment with formoterol, formoterol with tiotropium, formoterol with inhaled glucocorticosteroid and tiotropium on lung functions, tolerance of exercise and simple, morning everyday activities in patients with chronic obstructive pulmonary disease (COPD) Pneumonol Alergol Pol. 2012;80(3):255–262. Polish [with English abstract] [PubMed] [Google Scholar]
- 10.Yu AP, Guérin A, Ponce de Leon D, et al. Therapy persistence and adherence in patients with chronic obstructive pulmonary disease: multiple versus single long-acting maintenance inhalers. J Med Econ. 2011;14(4):486–496. doi: 10.3111/13696998.2011.594123. [DOI] [PubMed] [Google Scholar]
- 11.Beeh KM, Singh D, Di Scala L, Drollmann A. Once-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trial. Int J Chron Obstruct Pulmon Dis. 2012;7:503–513. doi: 10.2147/COPD.S32451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res. 2011;12:156. doi: 10.1186/1465-9921-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahl R, Chung KF, Buhl R, et al. INVOLVE (INdacaterol: Value in COPD: Longer Term Validation of Efficacy and Safety) Study Investigators Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax. 2010;65:473–479. doi: 10.1136/thx.2009.125435. [DOI] [PubMed] [Google Scholar]
- 14.Feldman G, Siler T, Prasad N, et al. INLIGHT 1 study group Efficacy and safety of indacaterol 150 microg once-daily in COPD: a double-blind, randomised, 12-week study. BMC Pulm Med. 2010;10:11. doi: 10.1186/1471-2466-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerwin E, Hébert J, Gallagher N, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study. Eur Respir J. 2012;40(5):1106–1114. doi: 10.1183/09031936.00040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kornmann O, Dahl R, Centanni S, et al. INLIGHT-2 (Indacaterol Efficacy Evaluation Using 150-μg Doses with COPD Patients) study investigators Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J. 2011;37(2):273–279. doi: 10.1183/09031936.00045810. [DOI] [PubMed] [Google Scholar]
- 17.Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013 May 30; doi: 10.1183/09031936.00200212. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahl R, Chapman KR, Rudolf M, et al. Safety and efficacy of dual bronchodilation with QVA149 in COPD patients: the ENLIGHTEN study. Respir Med. 2013;107(10):1558–1567. doi: 10.1016/j.rmed.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol – fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Resp Med. 2013;1(1):51–60. doi: 10.1016/S2213-2600(12)70052-8. [DOI] [PubMed] [Google Scholar]
- 20.Wedzicha JA, Decramer M, Ficker JH, Sandstorm T, Taylor AF. Dual bronchodilator treatment for the prevention of exacerbations of chronic obstructive pulmonary disease: the SPARK Study. Lancet Resp Med. 2013;1:199–209. doi: 10.1016/S2213-2600(13)70052-3. [DOI] [PubMed] [Google Scholar]
- 21.Mahler DA, Decramer M, D’Urzo A, et al. Superior lung function with once-daily QVA149 translates into improvements in patient-reported breathlessness compared with placebo and tiotropium in COPD patients: the BLAZE study. Am J Respir Crit Care Med. 2013;187:A6070. [Google Scholar]
- 22.Novartis Pharmaceuticals Comparison of safety and efficacy of the combination product QVA149 A against the concurrent administration of the individual components, QAB149 and NVA237, in patients with chronic obstructive pulmonary disease (COPD) (BEACON) Available from: http://clinicaltrials.gov/ct2/show/NCT01529632. NLM identifier: NCT01529632Accessed August 23, 2013
- 23.Novartis Pharmaceuticals Efficacy and safety of QVA149 in patients with chronic obstructive pulmonary disease (COPD) Available from: http://www.clinicaltrials.gov/ct2/show/NCT00570778?term=NCT00570778&rank=1. NLM identifier: NCT00570778Accessed February 25, 2013
- 24.Cazzola M, Tashkin DP. Combination of formoterol and tiotro-pium in the treatment of COPD: effects on lung function. COPD. 2009;6(5):404–415. doi: 10.1080/15412550903156333. [DOI] [PubMed] [Google Scholar]
- 25.Berton DC, Reis M, Siqueira AC, et al. Effects of tiotropium and for-moterol on dynamic hyperinflation and exercise endurance in COPD. Respir Med. 2010;104(9):1288–1296. doi: 10.1016/j.rmed.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Jones PW, Donohue JF, Nedelman J, Pascoe S, Pinault G, Lassen C. Correlating changes in lung function with patient outcomes in chronic obstructive pulmonary disease: a pooled analysis. Respir Res. 2011;12:161. doi: 10.1186/1465-9921-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antón E. How and when to use inhaled corticosteroids in chronic obstructive pulmonary disease? Expert Rev Respir Med. 2013;7(Suppl 2):25–32. doi: 10.1586/ers.13.14. [DOI] [PubMed] [Google Scholar]
- 28.Van de Maele B, Fabbri LM, Martin C, Horton R, Dolker M, Overend T. Cardiovascular safety of QVA149, a combination of Indacaterol and NVA237, in COPD patients. COPD. 2010;7(6):418–427. doi: 10.3109/15412555.2010.528812. [DOI] [PubMed] [Google Scholar]
- 29.Carreiro A, Santos J, Rodrigues F. Impact of comorbidities in pulmonary rehabilitation outcomes in patients with chronic obstructive pulmonary disease. Rev Port Pneumol. 2013;19(3):106–113. doi: 10.1016/j.rppneu.2012.12.004. Portuguese [with English abstract] [DOI] [PubMed] [Google Scholar]