Abstract
Tropomyosin (TM) plays a central role in calcium mediated striated muscle contraction. There are three muscle TM isoforms: α-TM, β-TM, and γ-TM. α-TM is the predominant cardiac and skeletal muscle isoform. β-TM is expressed in skeletal and embryonic cardiac muscle. γ-TM is expressed in slow-twitch musculature, but is not found in the heart. Our previous work established that muscle TM isoforms confer different physiological properties to the cardiac sarcomere. To determine whether one of these isoforms is dominant in dictating its functional properties, we generated single and double transgenic mice expressing β-TM and/or γ-TM in the heart, in addition to the endogenously expressed α-TM. Results show significant TM protein expression in the βγ-DTG hearts: α-TM: 36%, β-TM: 32%, and γ-TM: 32%. These βγ-DTG mice do not develop pathological abnormalities; however, they exhibit a hyper contractile phenotype with decreased myofilament calcium sensitivity, similar to γ-TM transgenic hearts. Biophysical studies indicate that γ-TM is more rigid than either α-TM or β-TM. This is the first report showing that with approximately equivalent levels of expression within the same tissue, there is a functional dominance of γ-TM over α-TM or β-TM in regulating physiological performance of the striated muscle sarcomere. In addition to the effect expression of γ-TM has on Ca2+ activation of the cardiac myofilaments, our data demonstrates an effect on cooperative activation of the thin filament by strongly bound rigor cross-bridges. This is significant in relation to current ideas on the control mechanism of the steep relation between Ca2+ and tension.
Keywords: Tropomyosin isoforms, Thin filament regulation, Calcium sensitivity, Muscle contraction, Transgenic mice
Introduction
Cardiac muscle contraction is dependent upon the precise assembly of thick and thin filaments of the sarcomere. Tropomyosin (TM) a component of the thin filament, interacts with actin and the troponin (Tn) complex to regulate calcium mediated muscle contraction. TM is encoded by four distinct genes (TPM1, TPM2, TPM3, and TPM4) in vertebrates which utilize alternative splicing and multiple promoters to encode cytoskeletal, smooth and striated muscle isoforms (Vrhovski et al. 2008). The three primary striated muscle isoforms, α-TM, β-TM and γ-TM, are alternatively spliced products of the TPM1, TPM2, and TPM3 genes, respectively. Also, they are highly homologous but exhibit distinct physiological properties (Muthuchamy et al. 1995, 1997; Pieples et al. 2002; Jagatheesan et al. 2010). α-TM is the predominant isoform in both cardiac and skeletal musculature, whereas β-TM is only expressed at moderate levels during cardiogenesis, but is highly expressed in slow-twitch muscles. γ-TM is primarily expressed in slow-twitch musculature (Muthuchamy et al. 1993; Pieples and Wieczorek 2000).
Skeletal musculature, with its diverse array of fast and slow muscle fibers, possesses a myriad of different sarcomeric protein isoforms. Because of this complexity, we have focused on TM isoform functional analysis using a more uniform system, namely the murine heart. As mentioned, the primary striated muscle TM isoform in adult myocardium is α-TM, with β-TM cardiac expression occurring prenatally and during cardiac hypertrophy. However, simultaneous co-expression of α-, β-, and γ-TM does occur in skeletal muscle. Expression of β-TM in transgenic mouse hearts shows that with increased β-TM expression, there is diastolic dysfunction, coupled with an increase in the sensitivity of cardiac myofilaments to calcium (Muthuchamy et al. 1995; Palmiter et al. 1996; Patel et al. 2001). However, expression of γ-TM in transgenic mouse hearts leads to decreased myofilament calcium sensitivity and results in hyper contractile performance (Pieples et al. 2002).
Though α-, β-, and γ-TM are highly homologous, there are significant amino acid differences between the three isoforms which may play a crucial role in conferring divergent physiological functions. Interactions of TM with actin and/or Tn may be involved in these differential functions. Previous studies employing chimeric TM molecules clearly established that the putative internal and carboxyl terminal TnT binding domains of α- and β-TM impart different functions with respect to myocardial performance (Jagatheesan et al. 2003, 2004, 2009). The physiological parameters that are most dramatically affected are associated with the rates of contraction and relaxation, time to peak left ventricular pressure development, duration of time for left ventricular relaxation, and calcium sensitivity of the myofilament.
Studies on mammalian hearts have demonstrated the importance of different protein isoform expression during both development and the diseased state. For example, during fetal development, the murine heart principally expresses the β-myosin heavy chain (β-MHC) isoform. At birth, with the increase in T3 circulating hormone, α-MHC replaces β-MHC expression in the adult heart. Similarly, β-TM expression gradually decreases its expression during murine fetal development of the heart from 20% at embryonic day 11 to less than 2% in adult hearts (Muthuchamy et al. 1993). Interestingly, adult human hearts principally express α-TM, with lower levels of β-TM and α-TMκ, an isoform not expressed in mice (Rajan et al. 2010). The simultaneous expression of multiple TM isoforms in striated muscle makes it imperative to understand the physiological function of the distinct TM isoforms and how they influence sarcomeric performance when they are co-expressed in the heart.
To extend our studies on TM isoform expression and determine whether one of the striated muscle TM isoforms is dominant in imparting its physiological properties, we generated double transgenic mice (βγ-DTG) where α-, β-, and γ-TM isoforms were equivalently expressed in the heart. Results show these mice have a normal life span with no overt cardiac pathology. Also, these mice exhibit near normal cardiac physiology with the exception of a slight increase in the rate of contraction. The βγ-DTG myofilaments do exhibit a decrease in myofilament calcium sensitivity, thus implying that γ-TM may be more dominant in exerting its functional properties over α- and β-TM. This is the first study to examine the physiological effects on cardiac function and sarcomeric performance of simultaneous co-expression of the three major striated muscle TM isoforms. These results have potential therapeutic implications for cardiomyopathic disease, such as patients with familial hypertrophic cardiomyopathy who exhibit increased myofilament calcium sensitivity.
Experimental procedures
All animal procedures were conducted in conformance with the “Guiding Principles for Research Involving Animals and Human Beings” of the American Physiological Society.
Generation of βγ-DTG mice
To generate transgenic mice where both the striated β-TM and γ-TM proteins are specifically expressed in the heart, we crossed β-TM TG mice (line 12, transgene copy number 4) (Muthuchamy et al. 1995) and γ-TM TG mice (line 65, transgene copy number 5) (Pieples et al. 2002). The β-TM and γ-TM TG constructs used to generate the parental TG mice are shown in Fig. 1a, b, respectively. The TM cDNA sequences were ligated to the 3′ end to the murine α-myosin heavy chain (α-MHC) promoter with the 5′-UTR, which confers cardiac-specific expression. An SV40 polyadenylation and termination cassette is located 3′ of the β-TM cDNA and the human growth hormone (HGH) poly A site is located 3′ of γ-TM cDNA to ensure correct transcript processing. Nucleotide sequencing and comparisons with published sequences verified accuracy in TM sequences for these transgenes. All mice are of the FVB/N background. Polymerase chain reaction (PCR) and genomic Southern blot analysis were used to identify the different genotypes (NTG, β-TM TG, γ-TM TG and βγ-DTG mice) and to determine their respective transgene DNA copy numbers.
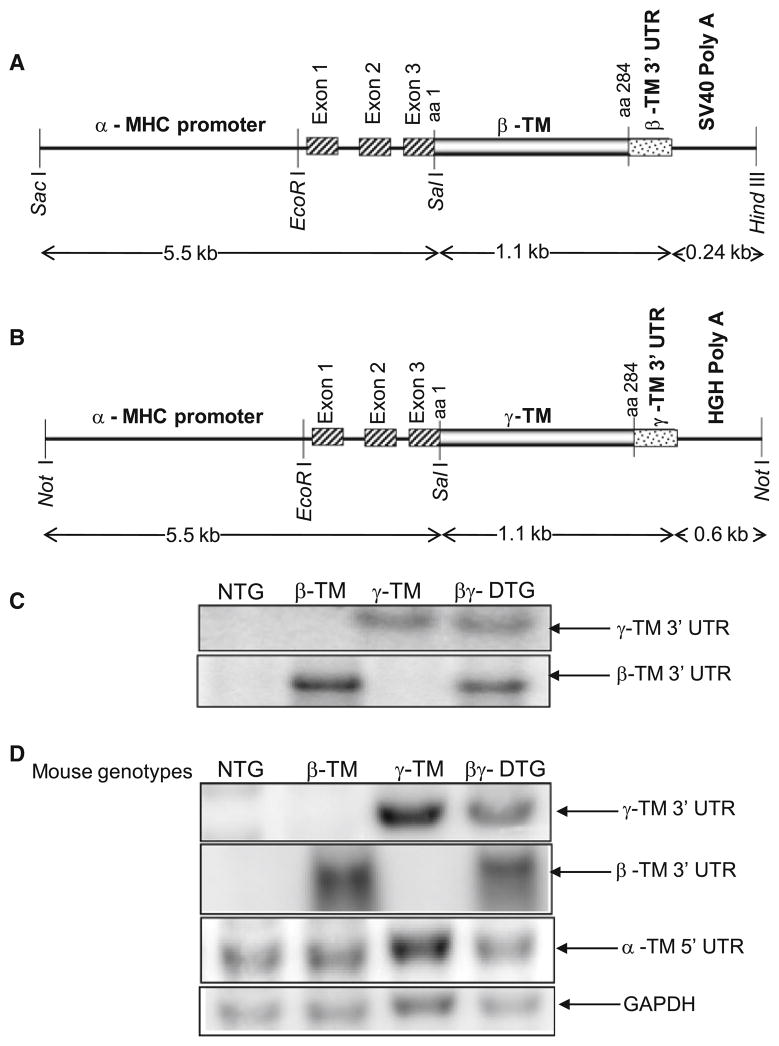
Fig. 1.
a Cardiac specific β-TM construct. β-TM cDNA coding for amino acids (aa) 1–284 and the 3′ untranslated region (UTR) of β-TM was linked to the α-myosin heavy chain promoter at the 5′ end and to the SV40 polyadenylation cassette at the 3′ end. b Cardiac specific γ-TM construct. γ-TM cDNA coding for amino acids (aa) 1–284 and the 3′ untranslated region (UTR) of γ-TM was linked to the α-myosin heavy chain promoter at the 5′ end and to the HGH polyadenylation cassette at the 3′ end. c Genomic Southern blot analysis of NTG, β-TM, γ-TM and βγ-DTG mice. Mouse-tail genomic DNA was digested with EcoRI, Southern blotted, and hybridized to 32P-radiolabeled β-TM or γ-TM 3′-UTR probes. d RNA expression in transgenic mice. Ten micrograms of total RNA (pooled from five mice for each line) were electrophoresed, transferred to nitrocellulose membrane, and probed with 32P-radiolabeled β-TM 3′-UTR, γ-TM 3′-UTR, α-TM 5′-UTR, or GAPDH
RNA and protein analyses
RNA was isolated from murine hearts using Trizol (Invitrogen, Carlsbad, CA). Total RNA (10 μg) from TG and NTG mouse ventricles was subject to Northern blot analyses with hybridization to 32P-radiolabeled β-TM 3′-UTR and γ-TM 3′-UTR cDNAs corresponding to the transgene RNAs, and α-TM 5′-UTR cDNA corresponding to the endogenous α-TM RNA. GAPDH served as an internal control to normalize TM message levels. Whole tissue and myofibrillar proteins were prepared from ventricles as described (Pieples et al. 2002; Muthuchamy et al. 1995; Jagatheesan et al. 2003).
Histological analysis
Hearts were removed from 3 to 12 month old mice and immediately fixed in 10% neutral buffered formalin for 48 h. The hearts were dehydrated through a gradient of alcohols and xylene, followed by embedding in paraffin. Sections were cut at 5 μm thickness and stained with either hematoxylin and eosin, or trichrome stain. The heart/body weight ratios were calculated for 3 and 12 month old hearts from all 4 genotypes. A 2-way ANOVA statistical analysis was conducted using age and genotype as factors. The criterion for statistical significance was set at P < 0.05. Experimental values are expressed as means ± SD.
Isolated anterograde perfused heart preparation
Age matched (4-month-old) NTG control, β-TM TG, γ-TM TG and βγ-DTG mice were subject to the work performing heart analysis as described (Jagatheesan et al. 2007, 2009). Data are presented as means ± SEM. Parameters of cardiac performance were compared between the NTG and TG groups using a 1-way ANOVA with Bonferroni multiple comparison tests. The criterion for statistical significance was set at P <0.05. The responses of isolated work-performing hearts to β-adrenergic stimulation were studied using the same hearts. Adrenergic responsiveness tests were carried out to evaluate possible alterations in cardiac intrinsic regulatory mechanisms and functional responsiveness to a sympathetic agonist. The β-adrenoagonist, isoproterenol was added to the Krebs-Henseleit solution in varying concentrations (10−11–10−7 mol/l) and entered the heart using a microperfusion pump as described (Jagatheesan et al. 2003, 2004). Dose–response curves were tested by a 2-way repeated measurement ANOVA and a post hoc Bonferonni test. P values of <0.05 were considered significant.
Fiber bundle preparation and force measurements
We measured force developed by bundles of detergent-extracted fibers obtained from 4-month-old papillary muscle as described (Jagatheesan et al. 2003, 2004, 2009; Wolska et al. 1999). Data in which measurements were made in the same fiber bundle at different sarcomere lengths were analyzed individually using Student’s paired t-test. When three or more groups were analyzed, a one-way repeated measure ANOVA was used with a Newman-Keuls multiple comparison test. The criterion for statistical significance was set at P < 0.05. Experimental values are expressed as means ± SEM.
Bacterial recombinant protein expression
Recombinant tropomyosin was expressed and purified using the Champion pET SUMO Expression System (Invitrogen). Wild type α-TM, β-TM and γ-TM cDNA constructs were designed to include an N-terminal Ala-Ser dipeptide to functionally compensate for lack of acetylation of bacterially expressed tropomyosin (Monteiro et al. 1994). The coding sequences of the expression plasmids were confirmed by DNA sequencing.
Circular dichroism measurements
Thermal stability measurements were made by following the ellipticity (θ) of TM at 222 nm as a function of temperature, beginning at 5°C using an Aviv model 215 spectropolarimeter. Data were obtained at 2°C intervals with a protein concentration of 3 μmol/l. The apparent melting temperature and the thermodynamic parameters for TM unfolding was calculated based on the assumption that the unfolding could be fit by up to three independent helix-coil transitions with dissociation accompanying the helix-coil transition at the highest temperature, as previously described (Conway and Parry 1990).
Results
Generation of βγ-DTG mice
To explore whether there is a dominant effect of one of the three major striated muscle TM isoforms on cardiac physiology, we generated double transgenic mice with cardiac specific expression of β-TM and γ-TM (βγ-DTG). This expression occurs in a background of endogenous α-TM. Four different genotypes of mice were identified by PCR and confirmed by Southern blot analysis. Southern blot analysis with a 32P radiolabeled β-TM 3′-UTR probe shows hybridization to the transgene band (6.8 kb) in the β-TM and βγ-DTG DNAs (Fig. 1c); the endogenous genomic band size is 4.2 kb. Similarly, hybridization of a transgene band (3.4 kb) with the γ-TM 3′-UTR probe is observed only in the γ-TM and βγ-DTG genomic DNAs; the endogenous genomic band is 3.8 kb. These results demonstrate that the βγ-DTG mice have both sets of transgenes with copy numbers similar to the original parental mice (4 copies of β-TM and 5 copies of γ-TM transgenes). β-TM and γ-TM transcript and protein expression in TG mice
We measured TM expression using Northern blot analysis following RNA isolation from hearts of TG and NTG littermates. RNA from each genotype was isolated and probed with 32P-labeled TM isoform specific probes from β-TM 3′-UTR, γ-TM 3′-UTR, α-TM 5′-UTR, or GAPDH to assess expression from the transgenes, endogenous α-TM, or control RNAs, respectively. As seen in Fig. 1d, RNA from the β-TM transgene was abundantly expressed in β-TM TG and βγ-DTG mouse hearts. Transcripts from the γ-TM transgene were expressed in both γ-TM TG and βγ-DTG mouse hearts and endogenous α-TM is expressed in all hearts. For quantification, TM levels are normalized to GAPDH expression to account for loading differences. A PhosphorImager quantitative analysis shows that endogenous α-TM mRNA is expressed in equivalent levels in NTG and TG hearts (data not shown).
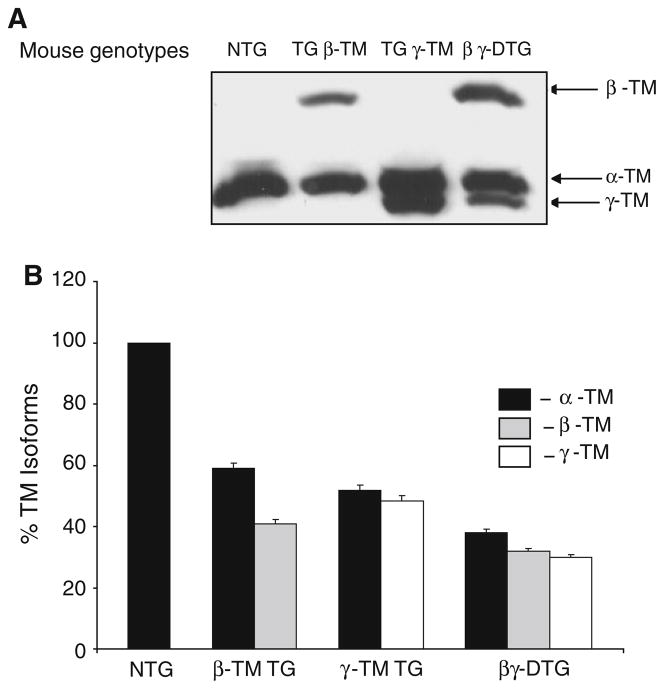
To examine TM protein production, we prepared total cardiac lysates and isolated myofibrillar proteins from the hearts of TG and NTG mice. The various TM species were resolved in 3.4 M urea and 8% SDS-PAGE gels. Western blot analyses show the amount of TM in myofilament preparations (Fig. 2a) and total cardiac lysates (data not shown) is constant. Coomassie Blue staining of myofibrillar proteins shows there are no gross differences in the expression of other cardiac contractile proteins (including actin, TnI, MLC 1 and 2) between the TG and NTG mice (data not shown). Quantification of myofilament TM content shows that while NTG myofilaments express only α-TM, β-TM TG myofilaments have a ratio of α-TM to β-TM as 60:40, and γ-TM TG myofilaments have a ratio of α-TM to γ-TM as 55:45 (Fig. 2). Interestingly, βγ-DTG myofilaments have a ratio of α-TM to β-TM to γ-TM as 36:32:32, respectively (Fig. 2b). Unfortunately, we are unable to resolve TM dimer formation (i.e. homodimers and heterodimers) in the βγ-DTG hearts due to limitations in biochemical techniques.
Fig. 2.
a Western blot analysis of total TM in the cardiac myofilaments of NTG and TG mice. Proteins were probed with a striated TM specific monoclonal antibody. b Quantitative analysis of the %TM isoforms in the hearts of NTG and TG mice. The signal intensity of the TM isoform distribution in the NTG, β-TM TG, γ-TM TG and βγ-DTG cardiac myofilaments was quantified with ImageQuant version 5.1. Levels within each line were set at 100% and the relative % distribution was calculated for each TM isoform. Four sets of experiments were averaged to calculate the % distribution
To negate the possibility that an unexpected mutation or deletion of the β-TM or γ-TM cDNA sequences occurred during transgenesis, we conducted PCR analysis of TG mouse tail DNA. With oligonucleotide primers corresponding to the α-MHC and the β-TM or γ-TM cDNA 3′-UTR sequences, PCR fragments were generated, amplified, cloned, and sequenced. Results show there are no mutations or deletions in the β-TM or γ-TM cDNA nucleotide sequences (or their corresponding amino acid sequences) in the integrated transgene constructs from any of the TG mice.
Histological analysis
To determine whether histological or pathological alterations occur after expression of the TM isoforms in the mouse hearts, we examined mice from all four genotypes ranging in age from 3 to 12 months. NTG and TG hearts were isolated, weighed, sectioned, and stained with hematoxylin/eosin or trichrome. Results demonstrate that at 3, 6, 8, and 12 months of age, there are no gross cardiac phenotypic alterations in any of the mice, nor any indication of fibrosis, necrosis, inflammation, or cardiomyocyte hypertrophy. Also, there are no significant differences in the percentage heart weight-to-body weight ratios in TG and βγ-DTG mice at 3 months or 1 year of age when compared with age and sex matched controls. At 3 months, the values (±SD) are: NTG: 4.6 ± 0.4; β-TM TG: 4.5 ± 0.5; γ-TM TG: 5.2 ± 0.4; βγ-DTG: 5.2 ± 0.5; at 5 months, the values are: NTG: 5.0 ± 0.5; β-TM TG: 4.6 ± 0.3; γ-TM TG: 5.7 ± 0.5; βγ-DTG: 5.1 ± 0.6. These results agree with previous studies on β-TM and γ-TM TG mice (Pieples et al. 2002; Palmiter et al. 1996). Thus, co-expression at significant levels of the three major striated muscle TM protein isoforms in the hearts of the βγ-DTG mice does not lead to morphological or pathological alterations.
Physiological analyses of βγ-DTG mice
To obtain a better functional understanding of simultaneous expression of all three TM isoforms in murine hearts, we conducted analyses of cardiac performance utilizing isolated working-heart preparations and skinned fiber bundles. The work-performing heart model was used to obtain an ex vivo assessment of cardiac performance. These measurements were conducted on NTG, β-TM TG, γ-TM TG and βγ-DTG mice. Results show a decrease in the maximum rate of pressure development for contraction (+dP/dt) from 3335 ± 142 mmHg/s in the wild-type hearts to 2612 ± 150 mmHg/s in the β-TM TG hearts (P < 0.01) (Table 1). Coupled with the decreased contractility, the maximal rate of pressure decline (−dP/dt) also decreased from 3097 ± 242 mmHg/s in wild type to 2247 ± 84 mmHg/s in the β-TM TG hearts (P < 0.05). These results demonstrate that incorporation of β-TM into the cardiac sarcomere leads to decreased rates of contraction and relaxation.
Table 1.
Parameters describing isolated work-performing NTG and TG mouse hearts
| Parameters | NTG, n = 6 | β-TM TG, n = 5 | γ-TM TG, n = 5 | βγ-DTG, n = 5 |
|---|---|---|---|---|
| Systolic pressure (mmHg) | 124 ± 4.8 | 89.9 ± 2.3** | 118.4 ± 5.7 | 120.3 ± 5.9 |
| Diastolic pressure (mmHg) | −9.9 ± 3.0 | −1.9 ± 1.8* | −5.62 ± 1.4 | −6.6 ± 2.3 |
| End diastolic pressure (mmHg) | 3.4 ± 0.84 | 3.6 ± 0.2 | 3.8 ± 1.3 | 4.6 ± 1.9 |
| Maximal rate of pressure development (+dP/dt) (mmHg/s) | 3335 ± 142 | 2612 ± 150** | 3792 ± 101* | 3779 ± 53** |
| Maximal rate of pressure decline (−dP/dt) (mmHg/s) | 3097 ± 242 | 2247 ± 84** | 3707 ± 94* | 3485 ± 161 |
| Time to peak pressure (TPP) (ms/mmHg) | 0.45 ± 0.019 | 0.58 ± 0.018** | 0.37 ± 0.022* | 0.43 ± 0.027 |
| Half time to relaxation (RT½) (ms/mmHg) | 0.6 ± 0.022 | 0.76 ± 0.025** | 0.55 ± 0.024 | 0.58 ± 0.026 |
| Heart rate (beats/min) | 262 ± 21 | 290 ± 13 | 325 ± 16 | 304 ± 13 |
P < 0.05,
P < 0.01, NTG vs. TG
A physiological assessment of γ-TM TG hearts demonstrated results contrary to those observed with the β-TM TG hearts. Results show an increase in the maximum rate of pressure development for contraction (+dP/dt) from 3335 ± 142 mmHg/s in the wild-type hearts to 3792 ± 101 mmHg/s in the γ-TM TG hearts (P < 0.05) (Table 1). Coupled with this increased contractility, the maximal rate of pressure decline (−dP/dt) is also increased from 3097 ± 242 mmHg/s in wild type to 3707 ± 94 mmHg/s in the γ-TM TG hearts (P < 0.05). Thus, incorporation of γ-TM into the murine heart leads to a hyperdynamic physiological performance.
To determine whether there is a functional dominance of a specific TM isoform, we analyzed the physiological performance of the βγ-DTG mouse hearts. Results for the βγ-DTG mice are interesting in that they recapitulate the physiological properties of the γ-TM TG hearts in spite of the presence of β-TM protein in the heart. There was an increase in the maximum rate of pressure development for contraction (+dP/dt) from 3335 ± 142 mmHg/s in the NTG hearts to 3779 ± 53 mmHg/s in the βγ-DTG TG hearts (P <0.01) (Table 1). Although not statistically significant, the maximal rate of pressure decline (−dP/dt) showed an increased tendency from 3097 ± 242 mmHg/s in NTG to 3485 ± 161 mmHg/s in the βγ-DTG hearts. The time to peak pressure (TPP) and half time to relaxation (RT½) exhibit a slight, but not significant, decrease in the βγ-DTG mouse hearts which is similar to these functional parameters in the γ-TM mice (Table 1).
Response to β-adrenergic stimulation
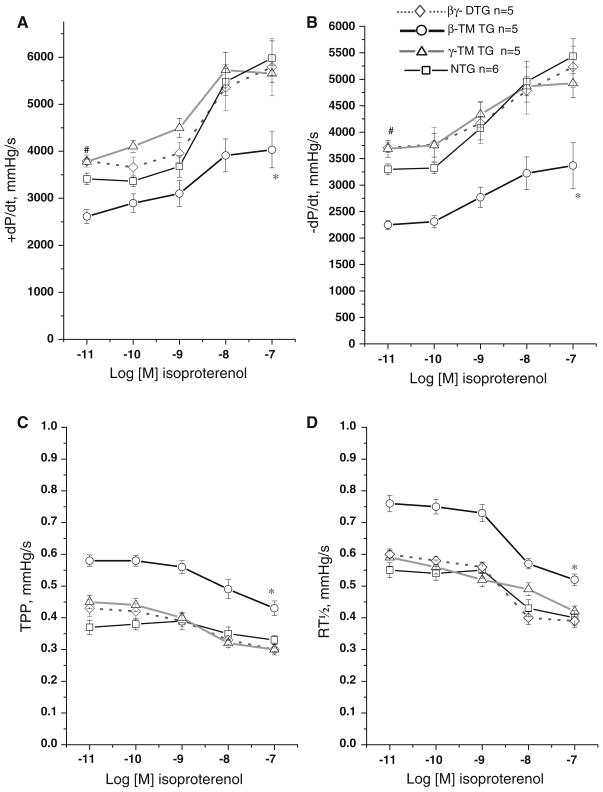
Since the βγ-DTG hearts exhibit a significant increase in the rates of both contraction and relaxation, we measured the isoproterenol response in cardiac performance to determine whether these physiological alterations may be due to maximal activation of the β-adrenergic response. Isoproterenol, a β-adrenergic agonist, augments muscle contraction and relaxation through cAMP/PKA-dependent phosphorylation by increasing the rate of Ca2+ cycling. Results show the βγ-DTG hearts respond positively to isoproterenol, similar to control and γ-TM TG rates of contraction and relaxation at all concentrations of isoproterenol (Fig. 3). However, the β-TM TG mouse hearts show a blunted response and were unable to fully respond, even at high concentrations of isoproterenol. We hypothesize the presence of γ-TM (and α-TM) in the βγ-DTG cardiac myofilaments compensates for the physiological blunting effect of the β-TM protein in the cardiac myofilaments, and these results indicate that the increased performance of the βγ-DTG hearts is not due to an altered adrenergic response mechanism.
Fig. 3.
Isoproterenol dose response curves in NTG and TG mouse hearts. Mouse hearts from β-TM TG (n = 5), γ-TM TG (n = 5), βγ-DTG (n = 5) and NTG controls (n = 6) at 4 months of age were subject to isolated heart analyses with increasing concentrations of isoproterenol (10−11–10−7 mol/l). Administration of the β-adrenergic agonist isoproterenol elicits a robust positive inotropic response in γ-TM TG, βγ-DTG and NTG controls but a partially attenuated response in β-TM TG hearts. a Plot of increasing concentrations of isoproterenol versus maximum rate of pressure development; b plot of increasing concentrations of isoproterenol versus maximum rate of pressure decline; c plot of increasing concentrations of isoproterenol versus time to peak pressure; d plot of increasing concentrations of isoproterenol versus half time to relaxation. “*” Indicates the entire β-TM curve is statistically different from NTG; “#” indicates NTG at this specific concentration is statistically different from γ-TM and βγ-DTG at P < 0.05
Comparison of tension activation in skinned fiber bundles
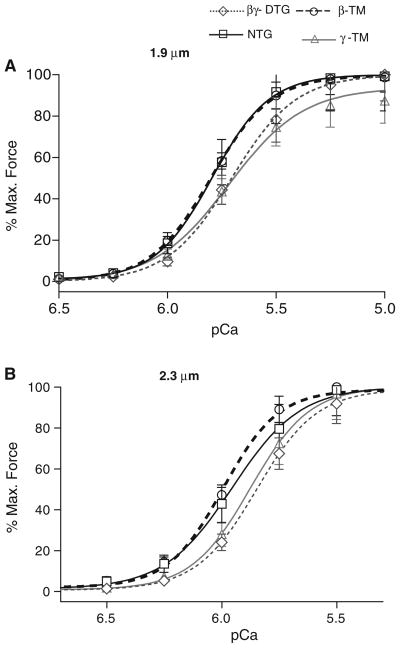
To examine the correlation between physiological results from the whole heart and co-expression of the TM isoforms at the sarcomere level, we conducted a series of experiments using detergent-extracted (skinned) fiber bundles. One series of experiments compared the relation between Ca2+ and tension generation at both short (1.9 μm) and long (2.3 μm) sarcomeric lengths (SL) to investigate potential changes in length dependent activation. As illustrated in Fig. 4 and summarized in Table 2, all of the muscle preparations demonstrate length dependent activation with a significant increase in Ca2+ sensitivity (lower pCa50) associated with an increase in SL. β-TM TG myofilaments exhibit increased (leftward shift) Ca2+ sensitivity (higher pCa50) only at a 1.9 μm SL. The γ-TM TG myofilaments exhibit a significant decrease in their Ca2+ sensitivity at both SLs. Interestingly, the βγ-DTG myofilaments also exhibit a decrease in their myofilament Ca2+ sensitivity at both sarcomeric lengths, and this decrease was relatively large at the 2.3 μm SL. In addition to the significant decrease in Ca2+ sensitivity between NTG and βγ-DTG myofilaments at 2.3 μm, there is also a difference between β-TM TG and βγ-DTG myofilaments at 2.3 μm. Thus, it is apparent that γ-TM protein exerts a strong influence over the α-TM and β-TM proteins with respect to myofilament calcium sensitivity. There is a significant difference in the maximum tension developed between NTG myofilaments and the other three genotypes at either 1.9 or 2.3 μm SL (Table 2). It is noteworthy that βγ-DTG myofilaments develop decreased tension at both sarcomeric lengths: 20.71 ± 2.57 mN/mm2 at SL 1.9 μm and 23.15 ± 2.56 mN/mm2 at SL 2.3 μm compared to NTG: 27.37 ± 4.51 mN/mm2 at SL 1.9 μm and 28.79 ± 3.66 mN/mm2 at SL 2.3 μm, P < 0.05 (Table 2). The Hill slopes summarized in Table 2 demonstrate no significant changes in the steepness of the force-pCa relation in γ-TM TG myofilaments compared to the NTG and other TG myofilaments.
Fig. 4.
a pCa—% maximum force relations of NTG, β-TM TG, γ-TM TG, βγ-DTG skinned fiber bundles at sarcomere length 1.9 μm. βγ-DTG myofilaments exhibit a significant decrease in their calcium sensitivity which is similar to γ-TM when compared to NTG myofilaments. b pCa—% maximum force relations of NTG, β-TM TG, γ-TM TG, βγ-DTG skinned fiber bundles at sarcomere length 2.3 μm. βγ-DTG TG mouse myofilaments exhibit a very significant decrease in their calcium sensitivity similar to γ-TM TG myofilaments when compared to NTG myofilaments
Table 2.
Parameters describing Ca2+-dependent activation of tension in skinned fiber bundles from NTG and TG hearts
| Mouse model sarcomere length (μm) | pCa50 | Δ pCa | nH | Tension (mN/mm2) | n |
|---|---|---|---|---|---|
| NTG 1.9 | 5.79 ± 0.004* | 0.14 ± 0.02 | 3.31 ± 0.10 | 27.37 ± 4.51# | 9 |
| NTG 2.3 | 5.95 ± 0.01 | 2.94 ± 0.17 | 28.79 ± 3.66 | ||
| γ-TM TG 1.9 | 5.72 ± 0.03*† | 0.17 ± 0.01 | 2.53 ± 0.34 | 33.74 ± 4.06# | 8 |
| γ-TM TG 2.3 | 5.88 ± 0.01† | 3.37 ± 0.14 | 34.93 ± 4.33 | ||
| β-TM TG 1.9 | 5.82 ± 0.003* | 0.18 ± 0.03 | 3.13 ± 0.06 | 27.35 ± 1.84# | 11 |
| β-TM TG 2.3 | 5.99 ± 0.01 | 3.62 ± 0.37 | 29.00 ± 2.66 | ||
| βγ-TM 1.9 | 5.70 ± 0.01*† | 0.12 ± 0.03 | 2.91 ± 0.37 | 20.71 ± 2.57**# | 8 |
| βγ-TM 2.3 | 5.85 ± 0.01† | 3.31 ± 0.14 | 23.15 ± 2.56** |
P < 0.05 between SL 1.9 and 2.3 μm in all groups
P < 0.05 for pCa50 compared to NTG and β-TM TG at the both SL
P < 0.05 between βγ-TM maximum tension at SL 1.9 and 2.3 μm compared with the rest of the groups at the same SL
P < 0.05 tension for between SL 1.9 and 2.3 μm
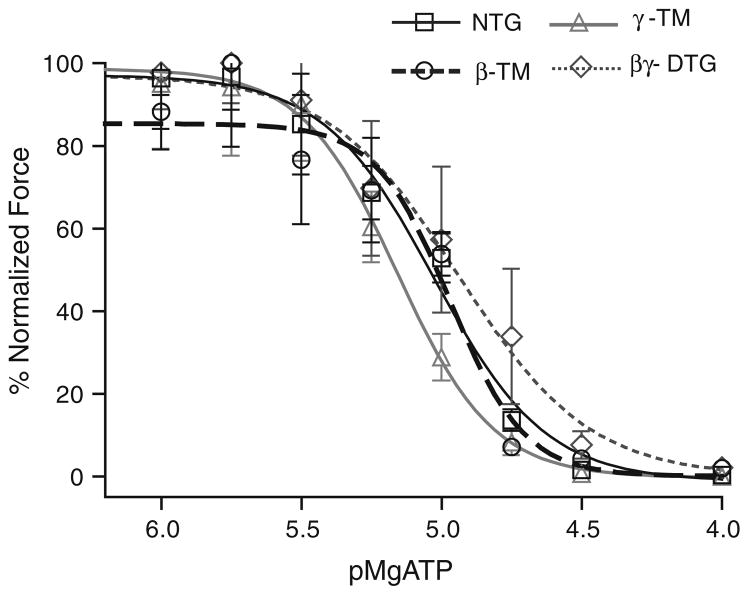
To determine whether the presence of both β-TM and γ-TM in the cardiac myofilaments along with endogenous α-TM affects cooperative activation of the thin filament by strongly bound cross-bridges, we controlled the population of strongly bound rigor cross-bridges in the skinned fiber bundles by varying the MgATP concentration at pCa 9. Our results (Fig. 5; Table 3) indicate that there is no significant difference in pMgATP50 for activation of relative tension by the rigor cross-bridges among the various skinned fiber preparations. The similarity in pMgATP50 among all the myofilaments indicates that the number of rigor cross-bridges is the same in these preparations. Yet there was a significant depression of maximum tension developed at the lowest MgATP concentration between γ-TM myofilaments and the other preparations indicating that recruitment of force generating cross-bridges by rigor linkages is depressed in fibers expressing γ-TM. However, this effect of γ-TM is not present in fiber bundles also expressing β-TM. Thus, in contrast to the case with Ca2+ dependent activation of tension, expression of β-TM is able to dominate effects of γ-TM in the DTG myofilaments activated by strongly bound cross-bridges (Solaro 2009; Sun et al. 2009). This result supports the hypothesis that activation of the myofilaments by thin filament related processes reflects a mechanism different from activation by rigor cross bridges. These data also indicate that the effects of TM isoform switching may have different functional effects under conditions of ischemia where rigor cross bridges are likely to be present.
Fig. 5.
pMg ATP—% normalized force relations of NTG, β-TM TG, γ-TM TG, and βγ-DTG skinned fiber bundles at a sarcomere length of 1.9 μm. NTG, γ-TM TG and βγ-DTG skinned fiber bundles exhibit no significant differences in their developed tension at various pMgATP concentrations; β-TM TG myofilaments develop significantly less tension
Table 3.
Parameters describing effects of reducing MgATP on strong cross-bridge-dependent activation of tension in skinned fiber bundles from NTG and TG hearts
| Mouse model | pMgATP50 | Tension (mN/mm2) | n |
|---|---|---|---|
| NTG | 5.03 ± 0.03 | 43.76 ± 7.93* | 7 |
| γ-TM TG | 4.94 ± 0.01 | 14.68 ± 2.69*∞ | 5 |
| β-TM TG | 4.97 ± 0.05** | 27.48 ± 1.45*∞ | 5 |
| βγ-TM | 5.16 ± 0.03 | 37.62 ± 3.55∞ | 7 |
P < 0.05 between β-TM TG and βγ-TM
P < 0.05 between NTG and β-TM TG; NTG and γ-TM TG
P < 0.05 between βγ-TM and β-TM TG; βγ-TM and γ-TM TG
Analysis of thermal stability of TM isoforms
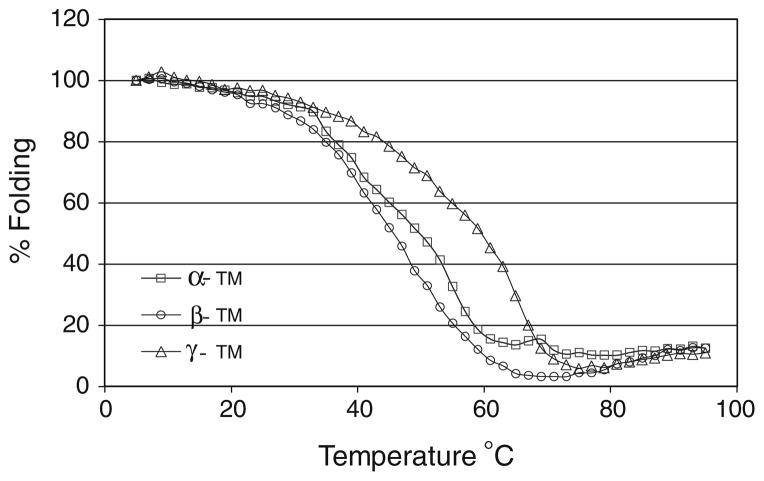
To investigate whether the decreased calcium sensitivity and associated physiological alterations observed in βγ-DTG hearts is due to the differences in the amino acid sequences and protein structure of these TM isoforms, we used circular dichroism to study the thermal stability of homodimeric TM proteins. The bacterially-expressed TMs have an N-terminal AlaSer tag to simulate acetylation and promote polymerization (Monteiro et al. 1994). Thermal stability measurements were made following the ellipticity (θ) of TMs at 222 nm as a function of temperature. Results show that homodimers of β-TM exhibit a downward shift at intermediate temperatures when compared with either α-TM or γ-TM proteins (Fig. 6) and homodimers of γ-TM exhibit an upward shift at intermediate temperatures when compared with either α-TM or β-TM proteins. At physiological temperatures, it appears that β-TM homodimers are less stable and γγ-TM homodimers demonstrate increased stability. This indicates that β-TM protein is more flexible than the other two isoforms and γ-TM is more rigid. This feature of γ-TM may contribute to the decreased calcium sensitivity seen in both the γ-TM TG and βγ-DTG myofilaments. The biophysical properties of γ-TM may also influence the changes observed with respect to myofilament tension development, crossbridge activation and physiological changes seen in βγ-DTG hearts.
Fig. 6.
Thermal denaturation profiles of recombinant α-TM, β-TM and γ-TM TG homodimeric proteins assessed by circular dichroism analysis. The % folding of the proteins were plotted against the increasing temperature (°C). Fraction of the unfolded protein was determined based on the ellipticity at wavelength 222 nm. Results indicate the γ-TM TG homodimer is more stable than the α-TM homodimer, whereas β-TM is more flexible
Discussion
Data reported here significantly extend our understanding of the functional significance of variations in the isoform population of TM. We examined the functional significance of the three predominant striated muscle TM isoforms: α-TM, β-TM, and γ-TM, which are all alternatively spliced proteins produced from three distinct genes. This study shows that there are different functional properties for TM homo- and heterodimers (i.e. αα-TM vs. αβ-TM or αγ-TM). In the case of functional effects and Ca2+ dependent activation of the myofilaments, we determined that when these proteins are simultaneously expressed at approximately equivalent levels within the same tissue, there is a functional dominance of γ-TM over α-TM and β-TM in regulating physiological performance of the striated muscle sarcomere. Defining the functional significance of contractile protein isoforms is essential for a complete understanding of muscle physiology. Sarcomeric thin filament proteins play an essential role in muscle contraction and relaxation by regulating the interaction of myosin heads with actin binding sites through the tropomyosintroponin complex. The generation of multiple contractile protein isoforms, specifically tropomyosin and troponin T, through multigene families, alternative splicing, and differential promoter and 3′ polyadenylation sites is well documented. These multiple isoforms increases the plasticity of striated muscle which is essential for a terminally differentiated cell that needs to adapt to a vast array of mechanical, hormonal, and nutritional signals in the process of maintaining its physiological function.
The three principle TM isoforms share a high degree of amino acid homology: α-TM and β-TM are 87% identical, α-TM and γ-TM are 91% identical, and β-TM and γ-TM are 86% identical (Fig. 7) (Muthuchamy et al. 1997). The differences in the 284 amino acids are scattered throughout the TM molecule. Our previous work has established that physiological differences reside in the putative TnT binding regions of α- and β-TM (amino acids 175–190 and 258–284) (Palmiter et al. 1996; Jagatheesan et al. 2003, 2004). It should be noted that there is only 1 conserved amino acid difference between α- and γ-TM in the carboxyl region (E271D) which would not be expected to confer a significant change in the physiological function of this region. Our previous studies also demonstrated that there are functional differences among α-TM, β-TM, and γ-TM (Pieples et al. 2002; Muthuchamy et al. 1995; Jagatheesan et al. 2010; Pieples and Wieczorek 2000); the current investigation extends this work and shows that with simultaneous expression of the three TM isoforms, the physiological effects on sarcomeric and cardiac performance that are associated with γ-TM expression are predominant with simultaneous expression of all three TMs. This observed physiological phenotype (increased rates of contraction and relaxation, coupled with a decrease in myofilament Ca2+ sensitivity) in the βγ-DTG mouse hearts shows that γ-TM elicits its associated functional effects even though it is expressed at ~1/3 of the total TM protein level. Interestingly, 9 of the 25 amino acid changes between α-TM and γ-TM occur between amino acids 40–80; this region is associated with mutations that cause dilated cardiomyopathy and often exhibit a decrease in myofilament Ca2+ sensitivity (Wieczorek et al. 2008). As such, it appears that amino acids 40–80 play a significant role in sarcomeric function, perhaps through the conduction of force transmission through the thin filament (Rajan et al. 2007; Olson et al. 2001).
Fig. 7.
Amino acid sequence alignment of the mouse striated muscle TM isoforms. α-TM was compared to β-TM, γ-TM, and α-TMκ using the ClustalW2 alignment program for proteins. “*” Indicates amino acid identity; “:” indicates a conserved amino acid substitution; “.” represents a semi-conserved amino acid substitution. Accession numbers of the M. musculus striated muscle TM sequences used for the analysis are the following: α-TM, X64831; β-TM, M81086; γ-TM, AF317223 and human α-TMκ, AY640414. The human α-TMκ sequence is used because α-TMκ is not expressed endogenously in mouse (Rajan et al. 2010)
As mentioned, γ-TM has a significant number of amino acid differences from α-TM in the amino acid 40–80 region, whereas there is only 1 amino acid difference in the 258–284 region. The cardiac specific α-TMκ isoform is identical to the striated muscle α-TM isoform, with the exception of exon 2 (amino acids 40–80) which is normally associated with the smooth muscle α-TM protein (Fig. 7) (Rajan et al. 2010; Denz et al. 2004). Interestingly, transgenic mice that express α-TMκ exhibit a decrease in myofilament Ca2+ sensitivity (Rajan et al. 2010) which is similar to the γ-TM and βγ-DTG mice. However, the α-TMκ mice have decreased rates of contraction and relaxation which is opposite to the rates produced in γ-TM or βγ-DTG mice. These results illustrate that although there may be specific TM regions that contribute significantly to influencing a particular biophysical parameter, there do not appear to be any TM regions that are solely responsible for a given physiological trait. Thus, it appears that the entire sequence and length of TM contributes to its performance through transmission of force, cooperativity with adjacent TM molecules, and interactions with actin and the troponin complex (TnI and TnT).
In this study, we have used the heart as the experimental system to examine the physiological contribution of the TM isoforms to sarcomeric contraction and relaxation. In the mouse under normal in vivo conditions, α-TM, β-TM, and γ-TM are co-expressed in skeletal musculature, with the level of expression dependent upon the specific muscle being examined. Usually, β-TM and γ-TM are expressed in greater quantities in slow twitch musculature (Pieples and Wieczorek 2000). This diversity of TM expression allows for increased flexibility in sarcomeric performance depending upon functional needs. In the heart, α-TM is the predominant isoform, with β-TM levels being primarily restricted to less than 20% during fetal development (Jagatheesan et al. 2010). Expression of β-TM is minimal (<1–2%) in adult musculature. There is no expression of γ-TM in the heart (Pieples and Wieczorek 2000). Thus, the adult mouse heart is an excellent experimental system to employ because there is no significant background expression of β-TM or γ-TM, and expression of TM transgenes do not alter the expression of other contractile proteins (Pieples et al. 2002; Muthuchamy et al. 1995; Palmiter et al. 1996; Jagatheesan et al. 2003, 2004, 2009). However, it is difficult to quantitatively assess the exact proportion of α-TM, β-TM, and γ-TM homo- and heterodimers in the βγ-DTG mouse hearts. Our previous work demonstrated a preference for αβ-TM heterodimers in the β-TM TG mice (Muthuchamy et al. 1995); this result agrees with numerous studies showing preferential formation of αβ-TM heterodimers versus αα- or ββ-homodimers (Gimona 2008; Brown and Schachat 1985; Lehrer et al. 1989). Also, Corbett et al. (2005) show that all possible TM homo- and heterodimers species have the ability to form in vitro, but quantification is difficult.
Integrating the data from the skinned fiber bundles with the work-performing heart analysis yields an intriguing observation. Muscle fibers from the βγ-DTG hearts exhibit decreases in both Ca2+ sensitivity and tension, yet an increased rate of contraction (+dP/dt) and a tendency for increased rates of relaxation (–dP/dt) in the whole heart. These increased rates may compensate for the decreased myofilament tension to insure adequate peripheral blood supply. This conclusion is supported by the fact that there is a lack of cardiac pathologic abnormalities in the βγ-DTG hearts. As such, these hearts increase our understanding of muscle plasticity and may assist in the design of future therapeutic approaches to cardiac disease.
A well-established observation is that most patients and experimental model systems associated with familial hypertrophic cardiomyopathy (FHC) exhibit increased myofilament Ca2+ sensitivity. Several studies have shown in transgenic mice and cultured cardiomyocyte systems that modification of calcium handling in these systems can reverse the FHC phenotype by improving cardiac and sarcomeric function (Jagatheesan et al. 2007; Coutu et al. 2004; Pena et al. 2003; del Monte and Hajjar 2003). For example, co-expression of a chimeric TM molecule that demonstrates decreased myofilament sensitivity to Ca2+ in a mouse FHC model improves cardiac function and allows for the survival of the lethal phenotype (Jagatheesan et al. 2007). Similarly, this current study shows that co-expression of β-TM and γ-TM proteins in βγ-DTG mice results in improved cardiac function over β-TM TG mouse hearts. As such, a potential therapeutic approach to diseases associated with mutations in contractile proteins may be through gene therapy using transgenes that exhibit compensatory physiological functions.
In addition to the effect of expression γ-TM has on Ca2+ activation of the cardiac myofilaments, our data demonstrate an effect on cooperative activation of the thin filament by strongly bound rigor cross-bridges. This is a finding of significance in relation to current ideas on the mechanism of control of the steep relation between Ca2+ and tension, which is present despite a single regulatory Ca2+ binding site on cardiac troponin C. How these relations are maintained in skeletal muscle is the focus of future investigations.
Acknowledgments
We thank Jon Neumann for production of the transgenic mice and Maureen Bender for her care of the animals. This work was supported in part by National Institutes of Health Grants HL71952 and HL081680 awarded to DFW, HL22231 and HL 62426 awarded to RJS, K01 HL67709 awarded to GMA.
Contributor Information
Ganapathy Jagatheesan, Department of Molecular Genetics, Biochemistry, and Microbiology, University of Cincinnati College of Medicine, 231 Albert B. Sabin Way, Cincinnati, OH 45267-0524, USA.
Sudarsan Rajan, Department of Molecular Genetics, Biochemistry, and Microbiology, University of Cincinnati College of Medicine, 231 Albert B. Sabin Way, Cincinnati, OH 45267-0524, USA.
Rafeeq P. H. Ahmed, Department of Molecular Genetics, Biochemistry, and Microbiology, University of Cincinnati College of Medicine, 231 Albert B. Sabin Way, Cincinnati, OH 45267-0524, USA
Natalia Petrashevskaya, Department of Medicine, University of Maryland, Baltimore, MD 21201, USA.
Greg Boivin, Department of Pathology and Laboratory Medicine, University of Cincinnati College of Medicine, Cincinnati, OH 45267-0529, USA.
Grace M. Arteaga, Department of Physiology and Biophysics, University of Illinois, Chicago College of Medicine, Chicago, IL 60612-7342, USA
Stephen B. Liggett, Department of Medicine, University of Maryland, Baltimore, MD 21201, USA
R. John Solaro, Department of Physiology and Biophysics, University of Illinois, Chicago College of Medicine, Chicago, IL 60612-7342, USA.
David F. Wieczorek, Email: David.Wieczorek@uc.edu, Department of Molecular Genetics, Biochemistry, and Microbiology, University of Cincinnati College of Medicine, 231 Albert B. Sabin Way, Cincinnati, OH 45267-0524, USA
References
- Brown HR, Schachat FH. Renaturation of skeletal muscle tropomyosin: implications for in vivo assembly. Proc Natl Acad Sci USA. 1985;82:2359–2363. doi: 10.1073/pnas.82.8.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JF, Parry DA. Structural features in the heptad substructure and longer range repeats of two-stranded alpha-fibrous proteins. Int J Biol Macromol. 1990;12(5):328–334. doi: 10.1016/0141-8130(90)90023-4. [DOI] [PubMed] [Google Scholar]
- Corbett MA, Akkari A, Domazetovska A, Cooper ST, North KN, Laing NG, Gunning PW, Hardeman EC. An atropomysoin mutation alters dimer preference in nemaline myopathy. Ann Neurol. 2005;57:42–49. doi: 10.1002/ana.20305. [DOI] [PubMed] [Google Scholar]
- Coutu P, Bennett CN, Favre EG, Day SM, Metzger JM. Parvalbumin corrects slowed relaxation in adult cardiac myocytes expressing hypertrophic cardiomyopathy-linked alpha-tropomyosin mutations. Circ Res. 2004;94(9):1235–1241. doi: 10.1161/01.RES.0000126923.46786.FD. [DOI] [PubMed] [Google Scholar]
- del Monte F, Hajjar RJ. Targeting calcium cycling proteins in heart failure through gene transfer. J Physiol. 2003;546(Pt 1):49–61. doi: 10.1113/jphysiol.2002.026732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denz CR, Narshi A, Zajdel RW, Dube DK. Expression of a novel cardiac-specific tropomyosin isoform in humans. Biochem Biophys Res Commun. 2004;320(4):1291–1297. doi: 10.1016/j.bbrc.2004.06.084. [DOI] [PubMed] [Google Scholar]
- Gimona M. Dimerization of tropomyosins. Adv Exp Med Biol. 2008;644:73–84. doi: 10.1007/978-0-387-85766-4_6. [DOI] [PubMed] [Google Scholar]
- Jagatheesan G, Rajan S, Petrashevskaya N, Schwartz A, Boivin G, Vahebi S, DeTombe P, Solaro RJ, Labitzke E, Hilliard G, Wieczorek DF. Functional importance of the carboxyl-terminal region of striated muscle tropomyosin. J Biol Chem. 2003;278(25):23204–23211. doi: 10.1074/jbc.M303073200. [DOI] [PubMed] [Google Scholar]
- Jagatheesan G, Rajan S, Petrashevskaya N, Schwartz A, Boivin G, Arteaga G, de Tombe PP, Solaro RJ, Wieczorek DF. Physiological significance of troponin t binding domains in striated muscle tropomyosin. Am J Physiol Heart Circ Physiol. 2004;287(4):H1484–H1494. doi: 10.1152/ajpheart.01112.2003. [DOI] [PubMed] [Google Scholar]
- Jagatheesan G, Rajan S, Petrashevskaya N, Schwartz A, Boivin G, Arteaga GM, Solaro RJ, Liggett SB, Wieczorek DF. Rescue of tropomyosin-induced familial hypertrophic cardiomyopathy mice by transgenesis. Am J Physiol Heart Circ Physiol. 2007;293(2):H949–H958. doi: 10.1152/ajpheart.01341.2006. [DOI] [PubMed] [Google Scholar]
- Jagatheesan G, Rajan S, Schulz EM, Ahmed RP, Petrashevskaya N, Schwartz A, Boivin GP, Arteaga GM, Wang T, Wang YG, Ashraf M, Liggett SB, Lorenz J, Solaro RJ, Wieczorek DF. An internal domain of beta-tropomyosin increases myofilament Ca2+ sensitivity. Am J Physiol Heart Circ Physiol. 2009;297(1):H181–H190. doi: 10.1152/ajpheart.00329.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagatheesan G, Rajan S, Wieczorek DF. Investigations into tropomyosin function using mouse models. J Mol Cell Cardiol. 2010;48(5):893–898. doi: 10.1016/j.yjmcc.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer SS, Qian Y, Hvidt S. Assembly of the native heterodimer of Rana esculanta tropomyosin by chain exchange. Science. 1989;246:926–928. doi: 10.1126/science.2814515. [DOI] [PubMed] [Google Scholar]
- Monteiro PB, Lataro RC, Ferro JA, de Castro Reinach F. Functional alpha-tropomyosin produced in escherichia coli. A dipeptide extension can substitute the amino-terminal acetyl group. J Biol Chem. 1994;269(14):10461–10466. [PubMed] [Google Scholar]
- Muthuchamy M, Pajak L, Howles P, Doetschman T, Wieczorek DF. Developmental analysis of tropomyosin gene expression in embryonic stem cells and mouse embryos. Mol Cell Biol. 1993;13(6):3311–3323. doi: 10.1128/mcb.13.6.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuchamy M, Grupp IL, Grupp G, O’Toole BA, Kier AB, Boivin GP, Neumann J, Wieczorek DF. Molecular and physiological effects of overexpressing striated muscle beta-tropomyosin in the adult murine heart. J Biol Chem. 1995;270(51):30593–30603. doi: 10.1074/jbc.270.51.30593. [DOI] [PubMed] [Google Scholar]
- Muthuchamy M, Rethinasamy P, Wieczorek DF. Tropomyosin structure and function: new insights. Trends Cardiovasc Med. 1997;7:124–128. doi: 10.1016/S1050-1738(97)00004-2. [DOI] [PubMed] [Google Scholar]
- Olson TM, Kishimoto NY, Whitby FG, Michels VV. Mutations that alter the surface charge of alpha-tropomyosin are associated with dilated cardiomyopathy. J Mol Cell Cardiol. 2001;33(4):723–732. doi: 10.1006/jmcc.2000.1339. [DOI] [PubMed] [Google Scholar]
- Palmiter KA, Kitada Y, Muthuchamy M, Wieczorek DF, Solaro RJ. Exchange of beta- for alpha-tropomyosin in hearts of transgenic mice induces changes in thin filament response to Ca2+, strong cross-bridge binding, and protein phosphorylation. J Biol Chem. 1996;271(20):11611–11614. doi: 10.1074/jbc.271.20.11611. [DOI] [PubMed] [Google Scholar]
- Patel JR, Fitzsimons DP, Buck SH, Muthuchamy M, Wieczorek DF, Moss RL. Pka accelerates rate of force development in murine skinned myocardium expressing alpha- or beta-tropomyosin. Am J Physiol Heart Circ Physiol. 2001;280(6):H2732–H2739. doi: 10.1152/ajpheart.2001.280.6.H2732. [DOI] [PubMed] [Google Scholar]
- Pena JR, Goldspink PH, Prabhakar R, del Monte F, Hajjar RJ, Wieczorek DF, Wolska BM. Neonatal gene transfer of serca2a improves the response to β-adrenergic stimulation in the α-tropomyosin (glu180gly) mouse model of familial hypertrophic cardiomyopathy. Scientific conference on molecular mechanisms of growth, death, and regeneration in the myocardium: basic biology and insights into ischemic heart disease and heart failure; Snowbird Conference Center, Snowbird, Utah. August 14, 2003; American Heart Association’s Council on Basic Cardiovascular Sciences; 2003. [Google Scholar]
- Pieples K, Wieczorek DF. Tropomyosin 3 increases striated muscle isoform diversity. Biochemistry. 2000;39(28):8291–8297. doi: 10.1021/bi000047x. [DOI] [PubMed] [Google Scholar]
- Pieples K, Arteaga G, Solaro RJ, Grupp I, Lorenz JN, Boivin GP, Jagatheesan G, Labitzke E, DeTombe PP, Konhilas JP, Irving TC, Wieczorek DF. Tropomyosin 3 expression leads to hypercontractility and attenuates myofilament length-dependent Ca(2+) activation. Am J Physiol Heart Circ Physiol. 2002;283(4):H1344–H1353. doi: 10.1152/ajpheart.00351.2002. [DOI] [PubMed] [Google Scholar]
- Rajan S, Ahmed RP, Jagatheesan G, Petrashevskaya N, Boivin GP, Urboniene D, Arteaga GM, Wolska BM, Solaro RJ, Liggett SB, Wieczorek DF. Dilated cardiomyopathy mutant tropomyosin mice develop cardiac dysfunction with significantly decreased fractional shortening and myofilament calcium sensitivity. Circ Res. 2007;101(2):205–214. doi: 10.1161/CIRCRESAHA.107.148379. [DOI] [PubMed] [Google Scholar]
- Rajan S, Jagatheesan G, Karam CN, Alves ML, Bodi I, Schwartz A, Bulcao CF, D’Souza KM, Akhter SA, Boivin GP, Dube DK, Petrashevskaya N, Herr AB, Hullin R, Liggett SB, Wolska BM, Solaro RJ, Wieczorek DF. Molecular and functional characterization of a novel cardiac-specific human tropomyosin isoform. Circulation. 2010;121(3):410–418. doi: 10.1161/CIRCULATIONAHA.109.889725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro RJ. Maintaining cooperation among cardiac myofilament proteins through thick and thin. J Physiol. 2009;587(Pt 1):3. doi: 10.1113/jphysiol.2008.166751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YB, Lou F, Irving M. Calcium- and myosin-dependent changes in troponin structure during activation of heart muscle. J Physiol. 2009;587(Pt 1):155–163. doi: 10.1113/jphysiol.2008.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrhovski B, Theze N, Thiebaud P. Structure and evolution of tropomyosin genes. Adv Exp Med Biol. 2008;644:6–26. doi: 10.1007/978-0-387-85766-4_2. [DOI] [PubMed] [Google Scholar]
- Wieczorek DF, Jagatheesan G, Rajan S. The role of tropomyosin in heart disease. Adv Exp Med Biol. 2008;644:132–142. doi: 10.1007/978-0-387-85766-4_11. [DOI] [PubMed] [Google Scholar]
- Wolska BM, Keller RS, Evans CC, Palmiter KA, Phillips RM, Muthuchamy M, Oehlenschlager J, Wieczorek DF, de Tombe PP, Solaro RJ. Correlation between myofilament response to Ca2+ and altered dynamics of contraction and relaxation in transgenic cardiac cells that express beta-tropomyosin. Circ Res. 1999;84(7):745–751. doi: 10.1161/01.res.84.7.745. [DOI] [PubMed] [Google Scholar]