Abstract
Human APOBEC3F is an anti-retroviral single strand DNA cytosine deaminase, susceptible to degradation by the HIV-1 protein Vif. In this study the crystal structure of the HIV Vif binding, catalytically active, C-terminal domain of APOBEC3F (A3F-CTD) was determined. The A3F-CTD shares structural motifs with portions of APOBEC3G-CTD, APOBEC3C and APOBEC2. Residues identified to be critical for Vif-dependent degradation of APOBEC3F all fit within a predominantly negatively charged contiguous region on the surface of A3F-CTD. Specific sequence motifs, previously shown to play a role in Vif susceptibility and virion encapsidation, are conserved across APOBEC3’s and between APOBEC3’s and HIV-1 Vif. In this structure these motifs pack against each other at intermolecular interfaces, providing potential insights both into APOBEC3 oligomerization and Vif interactions.
Introduction
Members of the AID/APOBEC family of polynucleotide cytosine deaminases are robust DNA mutators that not only play diverse immunological roles (Biasin et al., 2010; Hamilton et al., 2010) but are also implicated in genome-wide mutation events with significant repercussions for fundamental biological phenomena as cancer, epigenetic remodeling and evolution (Burns et al., 2013; Conticello, 2012; Guo et al., 2011; Nik-Zainal et al., 2012; Roberts et al., 2012). All seven members of the human APOBEC3 subfamily likely participate to varying degrees in restricting retroviruses, inhibiting mobility of active retroelements, and mediating clearance of foreign DNA (Stenglein et al., 2010). The mammalian APOBEC3 proteins coordinate zinc and have been classified into Z1, Z2 and Z3 domains (LaRue et al., 2009). Each of the human APOBEC3 domains contains a single Z-signature sequence (LaRue et al., 2009). According to this classification, both APOBEC3F domains are Z2, as is the single-domain APOBEC3C (A3C), whereas APOBEC3G (A3G) N-terminal domain is Z2 and the catalytically active A3G C-terminal domain is Z1 (Supplementary Figure S1A). These signature sequences emphasize the evolutionary relationships across the various APOBEC3 proteins with deeper implications for structure and function (LaRue et al., 2008; LaRue et al., 2010).
At least two APOBEC3 proteins, A3G and A3F, are central to the innate immune response against HIV-1 (Bishop et al., 2004; Harris et al., 2003; Lecossier et al., 2003; Liddament et al., 2004; Mangeat et al., 2003; Sheehy et al., 2002; Wiegand et al., 2004; Zhang et al., 2003; Zheng et al., 2004). Both proteins are incorporated into budding virions and exert an antiviral effect upon subsequent infection by introducing point mutations in the newly reverse transcribed viral ssDNA (Harris et al., 2012). This deamination-dependent mode of inhibition leads to G-to-A mutations in the viral genome (Browne et al., 2009; Harris et al., 2003; Miyagi et al., 2007; Schumacher et al., 2008). A deamination-independent mode may also suppress viral replication by directly interfering with reverse transcription (Holmes et al., 2007a; Holmes et al., 2007b; Newman et al., 2005; Strebel, 2005).
During HIV-1 infection, the viral protein virion infectivity factor (Vif) counters this restriction by forming a complex with CBFβ and the Cul5-E3 ubiquitin ligase that targets APOBEC3 proteins for polyubiquitination and subsequent proteasomal degradation (Jäger et al., 2011; Yu et al., 2003; Zhang et al., 2011). Vif may also counteract the antiviral effect by non-degradation mediated mechanisms such as inhibiting the translation of cellular APOBEC3 mRNA or by inhibiting APOBEC3 encapsidation during viral budding (Chiu and Greene, 2007). The structural basis of Vif-APOBEC3 interaction for proteasomal targeting is still poorly understood, complicated by Vif being an intrinsically disordered protein (Auclair et al., 2007; Reingewertz et al., 2009; Reingewertz et al., 2010) that interacts with more than one APOBEC3 family member (Smith and Pathak, 2010). Until now no atomic structure of an APOBEC3 - Vif complex has been determined, thwarting a true elucidation of this key molecular recognition event.
Recently the crystal and NMR structures of three APOBEC family members – APOBEC2, the C-terminal catalytic domain of APOBEC3G (A3G-CTD) and APOBEC3C (A3C) – have helped identify critical determinants of substrate single strand DNA sequence specificity and affinity (Chen et al., 2008; Holden et al., 2008; Kitamura et al., 2012; Prochnow et al., 2007; Shandilya et al., 2010). In the analysis of A3C structure (Kitamura et al., 2012), residues involved in Vif-dependent degradation were identified, even though unlike A3F, A3C does not normally restrict HIV (Figure 2 in (Zheng et al., 2004)). This analysis highlighted the evolutionarily conserved regions in the two proteins that are required for Vif-mediated proteasomal degradation. In conjunction with previous studies (Albin et al., 2010b; Smith and Pathak, 2010) that identified other residues in A3F required for Vif-mediated degradation, the mutational data from (Kitamura et al., 2012) suggest that a more extensive protein surface is responsible for Vif-dependent degradation in A3F than in A3C. The absence of an anti-retroviral effect in the case of A3C, coupled with a comparatively smaller set of Vif-interacting residues (Kitamura et al., 2012) leave open the possibility that Vif evolved to mitigate the anti-HIV threat from A3F and A3G (Albin and Harris, 2010), rather than A3C. In the current study we present the crystal structure A3F-CTD, an HIV-1 restricting, Vif-binding protein. This structure complements our previous structure of A3G-CTD (Shandilya et al., 2010) to provide key insights into APOBEC3-Vif host-pathogen interaction with potential implications for anti-retroviral drug discovery.
Fig. 2. Crystallographic Asymmetric unit, crystallographic binding and potential oligomerization interfaces.
(A): The A3F-CTD crystal structure asymmetric unit comprises of four chains, illustrated in identical colors schematically and in the ribbon representation. Chains A/B and C/D form the heterologous interface and chains B/D form the isologous interface. The active-site catalytic zinc atoms are illustrated as blue spheres.
(B): The A3F-CTD has a canonical cytosine deaminase fold with five β-strands and six α-helices. The conformational plasticity in β1-β2 loop region is highlighted (inset).
(C): The isologous interface, formed between chains B (cyan) and D (yellow), is the largest interface in terms of surface area, burying a total of 657 Å2.
(D): The isologous interface is splayed open to show interfacial residues (red/orange), with chains B and D turned by +90°/−90° respectively. Residues from the two chains B and D that are major contributors to this interface are labeled.
(C): The heterologous interface formed between chains A (green) and B (cyan), and identical to the interface between chains C and D, is the second largest interface in terms of surface area, burying a total of 569 Å2.
(D): The heterologous interface splayed open to show interfacial residues (orange/red), with chain A turned by +180°. Residues from the two chains A and B that are major contributors to this interface are labeled.
Results
Derivation of an active A3F variant for structural studies
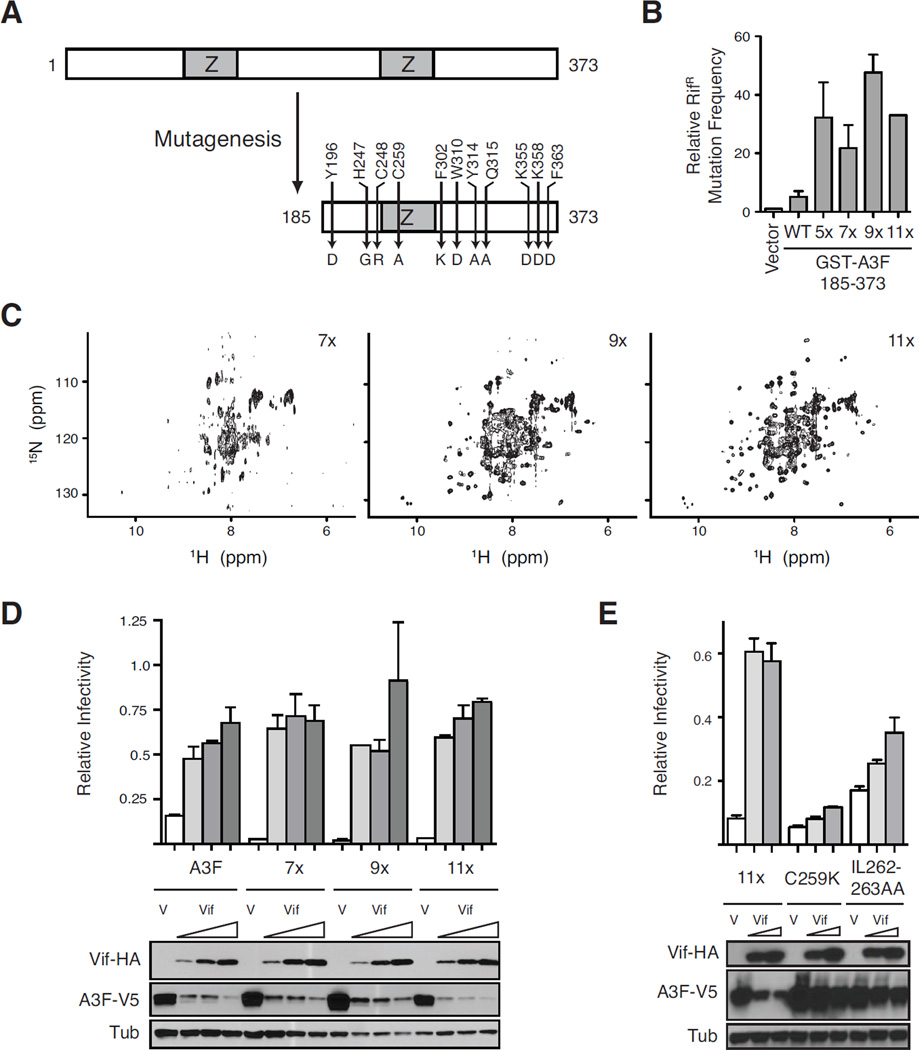
The wildtype human APOBEC3F catalytic C-terminal domain was insoluble and unsuitable for structural studies. In a manner analogous to engineering a soluble construct of the APOBEC3G catalytic domain (A3G191–384-2K3A) (Chen et al., 2008; Harjes et al., 2009), a systematic mutagenesis approach was taken to render the protein soluble and simultaneously maintain key activities. A series of deletion mutants identified a minimal CTD construct, A3F185–373, which was 5-fold more active than the full-length protein in an E. coli mutation assay (Figure 1A, 1B). This step was followed by multiple rounds of mutagenesis yielding a construct with 11 amino acid substitutions, A3F185–373-11X (A3F-CTD), that was amenable to purification and structural studies (Figure 1). Key intermediate constructs illustrate enhancement of catalytic activity accompanied by improvements in solubility as assessed by 2D heteronuclear correlation NMR spectroscopy experiments (Figure 1B, 1C). Importantly, A3F185–373-11X had a better-than-wildtype capacity to restrict Vif-deficient HIV-1 and was still fully susceptible to Vif-dependent degradation (Figure 1D). Recently identified mutations conferring resistance to Vif-dependent proteasomal degradation in human A3C and A3F (Kitamura et al., 2012) also rendered A3F185–373-11X less susceptible to Vif (Figure 1E).
Fig. 1. Derivation of an active A3F variant for structural studies.
(A) Schematic of the N-terminal deletion and amino acid substitutions rendered on wildtype A3F to yield the soluble variant A3F185-373-11x.
(B) Relative capacity of GST-A3F185-373 (WT) and indicated derivatives to trigger RifR mutation in E. coli. A3F185-373-5x has C259A, F302K, W310K, Y314A, and Q315A, 7x is 5x plus Y196D and F363D, 9x is 7x plus K355D and K358D and W310D instead of W310K, and 11x is 9x plus H247G and C248R. Data are reported as the mean ± SEM of the median mutation frequencies acquired from multiple independent experiments (relative to each experiment’s vector-only control set to 1).
(C) NMR spectra of 1H, 15N-labeled A3F-CTD 7x (~30 µM), 9x (~30 µM), and 11x (~100 µM). HSQC spectra were recorded at 298 K on a Bruker Avance 700 MHz instrument.
(D & E) Single-cycle HIV-1 infectivity data. Histogram graphs showing the infectivity of Vif-deficient HIVIIIB produced in the presence of a fixed amount of each indicated full-length A3F-V5 construct and vector control (V) or increasing amounts of Vif-HA. Each bar represents the mean ± SD of duplicate infections normalized to the corresponding infectivity of virus produced in the absence of A3F. The 11x used in panel (D) has F363, whereas the 11x in panel (E) has the same set of mutations (D363) as those in the final crystallized construct depicted in panel (A) however this change does not affect restriction or Vif sensitivity.
The crystal structure of A3F-CTD
The crystal structure of this HIV-1 restricting, Vif-susceptible A3F-CTD (A3F185–373-11X) construct was determined. After extensive screening of crystallization conditions (see Methods) diffracting crystals were obtained. The structure was solved in the P1 spacegroup to a resolution of 2.75 Å (Figure 2A and Table 1) with four molecules in the asymmetric unit. The overall RMSD across the C-α backbone for the four independent molecules is 0.77 Å, reflecting the conformational plasticity of this enzyme (Figure 2B).
TABLE 1.
Crystallographic statistics for the A3F185–373-11X crystal structure (PDB ID: 4IOU)
| A3HS5–373-1IX (A3F-CTD) | ||
|---|---|---|
| Resolution | 2.75 Å | |
| Temperature | Cryogenic (−80 °C) | |
| Space Croup | P1 | |
| Cell dimensions | ||
| a | 50 .85 Å | |
| b | 67.04Å | |
| c | 75,77 Å | |
| α | 110.9° | |
| β | 94.3° | |
| γ | 108.8° | |
| Nmber of Molecules in AU | 4 | |
| Completeness | 98.2%(98.l% (last shell 2.85–2.75 Å) | |
| Total Reflections | 22096 | |
| Unique Reflections | 22560(21432) | |
| I/σ | 13.5 | |
| Average Redundancy | 2.0 | |
| Rmergc % | 6.3 | |
| RMSD | ||
| Bonds | 0.0086 Å | |
| Angles | 1.473° | |
| R factor % | 18.61 | |
| R free % | 24.49 | |
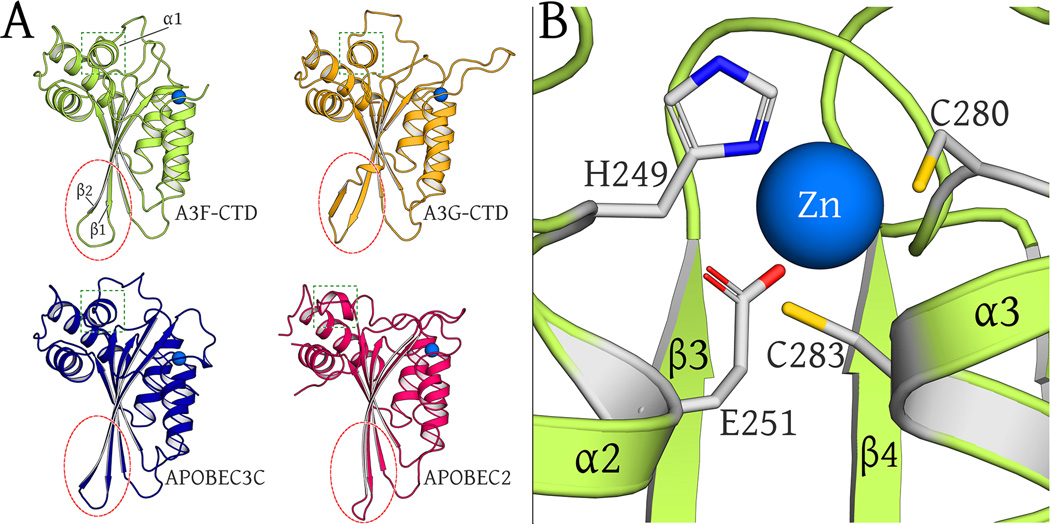
A3F-CTD has a canonical DNA cytosine deaminase fold, composed of five β-strands, six α-helices and the catalytic zinc-binding site (Figure 2B and Figure 3A). The zinc atom is coordinated by direct interactions with H249, C280, C283 and indirectly, via a water molecule, with the catalytic glutamate, E251 (Figure 3B). The zinc-coordinating residue C283 and the catalytic residue E251 are located on helices α2 and α3 and define the catalytic pocket.
Fig. 3. Structural Alignment of APOBEC family members.
(A): Comparison of ribbon representations of A3F-CTD, A3G-CTD (3V4K), A3C (3VOW) and APOBEC2 (2NYT) crystal structures (chain A of all). The β2/ β2-β2’ region is circled in red to highlight similarities across all proteins except A3G-CTD. The α1 helix region is boxed in green to highlight similarities across all proteins except APOBEC2.
(B): The catalytic zinc (blue sphere) is coordinated at the active site by H249, C280, C283 and indirectly via a water molecule (view occluded by zinc atom) with E251.
Intermolecular Interfaces
With four molecules in the asymmetric unit, unique intermolecular interfaces exist within the unit cell. In particular, two extensive interfaces (calculated with PISA (Krissinel and Henrick, 2007)) are observed. The largest interface is a quasi-2-fold-symmetric/isologous interface formed across identical regions of the two chains B and D (Figure 2A, 2C, 2D), and buries a total of 657Å2 surface area. On both of these chains, the interface involves the β1-β2-loop (residues H227-H228), α2-β3-loop region (residues N266 and N268), α3-β4-loop region (residues L291, S295 and N298) and the α4-β5-loop (residue G325). The second interface, a non-symmetric or heterologous interface, is observed twice in the asymmetric unit and buries a total of 569Å2 (Figures 2A, 2E, 2F). This heterologous interface, formed both between chains A and B and between chains C and D (Figures 2A, 2E, 2F), involves the enzyme active site and a domain specific R305-L306 motif (part of the Z2 domain fingerprint) in chains B and D. Twelve residues contributed by chain B (or D) form a contiguous surface (A210, R213, S216, H249, W277, C280, P281, Y307-F309, D311 and A314) comprising one half of the interface. The complementary half of this interface is formed of ten surface contiguous residues (E223, V225, H227-P230, N268, E270, S295 and N298) contributed by chain A (or C), with H227-H228 inserted nearest the active-site catalytic zinc in the first subunit (chain B (or D)). Interestingly, the β1-β2-loop region, involved in the formation of both interfaces, exists in two distinct conformations depending on the interface (Figure 2B, inset).
A distinguishing feature of double-domain APOBEC3 proteins is the ability to form catalytically inactive high molecular mass complexes in cells (Kreisberg et al., 2006). A3F was also shown to localize to the virion core in this form and a dynamic conversion between high molecular mass and low molecular mass forms may determine differences in activity (Wang et al., 2007). In our investigations the A3F-CTD forms complexes of high molecular mass as observed by dynamic light scattering (Figure S2). These complexes do not persist in analytical gel filtration chromatography and are thus not likely to be stable aggregates but rather transient occurrences in solution (Figures S2A, S2B). A variant of A3F-CTD in which the VKHH (225–228) motif within the β1-β2-loop is replaced with MHND, as in A3G-CTD, does not form the high molecular mass complexes (Figure S2), suggesting this region mediates favorable intermolecular interactions.
Structural comparison between APOBEC family members
The fold and structure of A3F-CTD is similar to structures of other APOBEC family members APOBEC2 (PDB: 2NYT), A3G-CTD (PDB: 2KEM, 3IR2, 3V4K) and A3C (PDB: 3VM8, 3VOW) (Harjes et al., 2009; Kitamura et al., 2012; Li et al., 2012; Prochnow et al., 2007; Shandilya et al., 2010) (Figure 3A). The structural similarity parallels the sequence identity of 25%, 50% and 77% with APOBEC2, A3G-CTD and A3C, respectively (Figure S1B). Structural analysis reveals specific differences amongst these deaminase family members. Two changes are due to deletions/insertions in the sequence. Specifically, the loop region between β2-strand and α2-helix, although disordered in the electron density, is five residues shorter in A3F-CTD compared to A3G-CTD. In addition, sequence alignment reveals that the secondary zinc coordination site observed in A3G β2-α2-loop crystal structure (Shandilya et al., 2010), that bridges two molecules in the asymmetric unit, does not exist in the A3F-CTD structure (Figure S1B) nor is this site conserved across APOBEC3 family members. Another two distinct structural features make A3F-CTD a hybrid between the other structures. The A3F β2-strand is extended, as in APOBEC2 and A3C, but not like the A3G-CTD with a discontinuous β2-β2’-strand. However, α1-helix in A3F-CTD is in the same conformation as in A3G-CTD and A3C, whereas α1-helix in APOBEC2 is oriented perpendicular to this conformation (Figure 3A). This helix and the following loop region are structurally conserved in A3G-CTD and A3C (but not APOBEC2) despite low sequence similarity, indicating this feature to be a conserved motif of APOBEC3 proteins.
The SWSPC motif, spanning residues S276-C280 in A3F-CTD is a common feature between A3F-CTD and other APOBEC3 proteins that contain either Z1 or Z2 domains (Figure S1A) (LaRue et al., 2009), analogous to the SSSPC motif in APOBEC2 proteins. These motifs may contribute to substrate specificity, potentially forming a trough in which the polynucleotide (ssDNA) strand might be positioned (Furukawa et al., 2009; Harjes et al., 2009). A second loop region playing a role in substrate recognition lies between β4-strand and α4-helix (Carpenter et al., 2010; Conticello, 2008; Kohli et al., 2009; Wang et al., 2010). The C-termini of β3- and β4-strands are two residues shorter in A3F-CTD and A3G-CTD compared to APOBEC2. A combination of these structural and sequence differences are likely responsible for modulating the differential specificity of these APOBEC proteins.
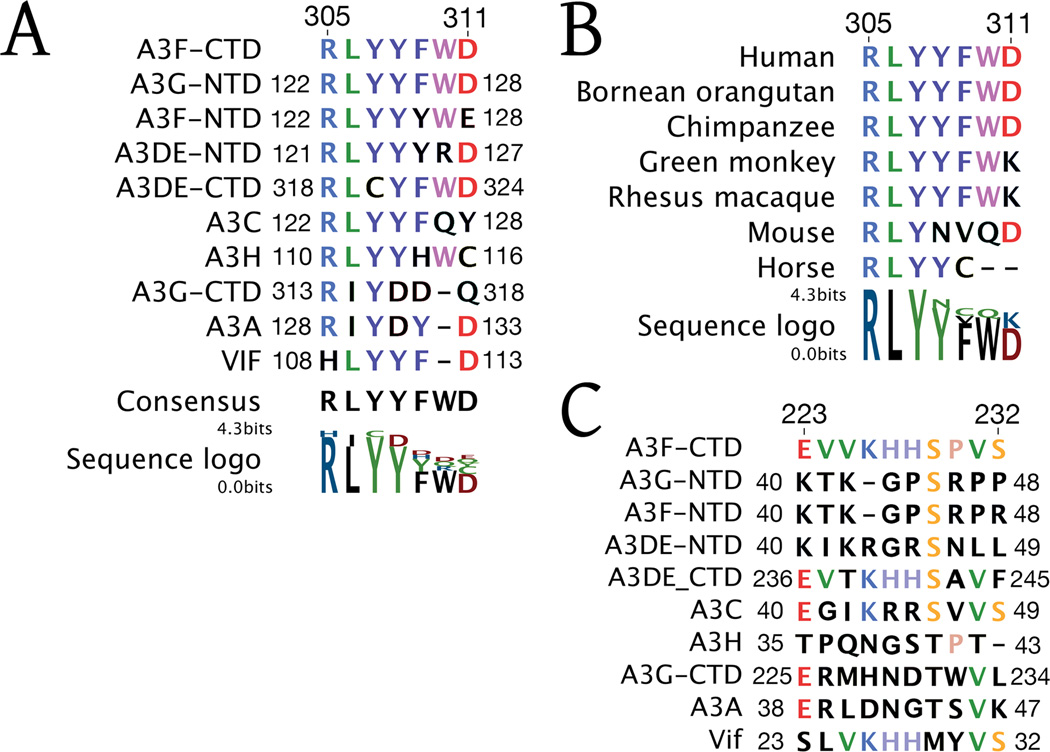
A highly conserved sequence motif across the various human APOBEC3’s is 305-RLYYFWD-311 in A3F-CTD, which is a part of the Z2 domain specific fingerprint (LaRue et al., 2009) (Figure 4A). Sequence alignment also reveals this motif to be strongly conserved in APOBEC3’s across mammalian species infected by lentiviruses (Figure 4B). Within the motif, residues R305-Y308 are most strongly conserved, whereas larger sequence variation is observed in residues F309-D311. Structurally, in the A3F-CTD crystal, this region participates in the heterologous binding interface (307–311) (Figures 2A, 2E, 2F). Residues R305 and L306 are not involved in intermolecular interactions but impart structural integrity to the β4-α4 loop. F309 in A3F-CTD, as Y128 in A3C, assumes a conformation that places the aromatic side chain in a structurally conserved position. Similarly, D311 in A3F, as D317 in A3G, also assumes a conserved conformation. However, the Y308 in the LYYF (306–309) motif in A3F and A3C is not structurally conserved with Y315 in A3G, potentially distinguishing the Z1 from Z2 family members. Thus, this motif may likely regulate complex formation across APOBEC3 proteins.
Fig. 4. Sequence Conservation of A3F loops with regions of HIV-1 Vif.
(A): The 305-RLYYFWD-311 motif of the A3F-CTD β4-α4 loop is conserved across human
APOBEC3 protein domains and reappears in HIV-1 Vif.
(B): Sequence alignment of the 305-RLYYFWD-311 motif of the A3-CTD β4-α4 loop across a wide array of mammalian APOBEC3 proteins.
(C): The 223-EVVKHHSPVS-232 motif of the A3F-CTD β1-β2 loop is conserved in HIV-1 Vif.
In contrast to the conserved motifs, the sequence of β1-β2-loop region involved in forming both intermolecular interfaces is not conserved across APOBEC3 family members, with the exception of APOBE3DE-CTD (Figure 4C). Although the sequences in this region between family members often include basic residues, their composition and even length varies extensively. Thus this region, which bridges key crystal interfaces, may serve as an A3F specific oligomerization determinant.
Discussion
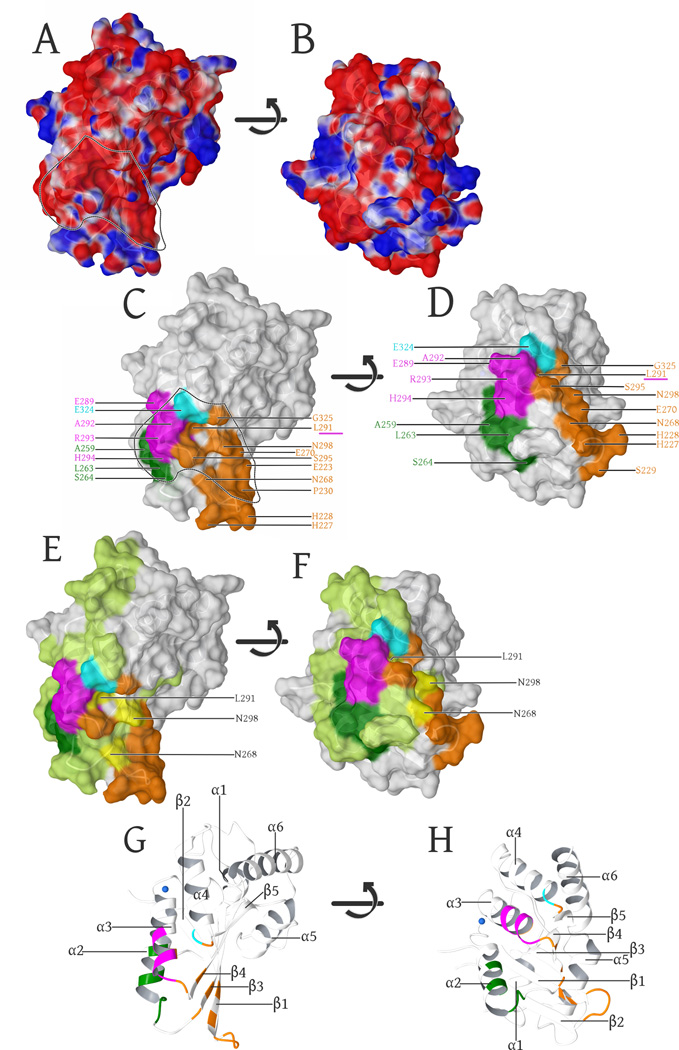
In this study we present the first, high-resolution crystal structure of the A3F catalytic domain, elucidating the first HIV1 Vif-binding surface, amongst all APOBEC3’s that exert a demonstrable anti-HIV effect (Albin and Harris, 2010). The majority of A3F-CTD molecular surface is strongly negatively charged (Figure 5A, 5B). Within this negatively charged surface previous studies have identified Vif-binding regions (Figure 5C, 5D) to include separate portions of α3-helix (E289-H294, magenta) (Smith and Pathak, 2010), α4-helix (E324, cyan) (Albin et al., 2010b), β3-strand (Y269) along with part of the α2-helix and the adjacent loop region (L255, F258, C259, I262-S264, green) (Kitamura et al., 2012) (Figure 5C, 5D). The negative surface of these regions includes portions of secondary structure: α3- α4-helices/the β4 strand and the α2-α3-helices/β3 strand (Figure 5G, 5H) and the negative surface extends into our crystal contact region within the β1-β2-loop region (Figure 5C, 5D, orange). Most of this additional negatively charged surface was not probed by the previous mutational scans of A3C (light green Figure 5E, 5F) (Kitamura et al., 2012). In fact only three residues overlap between the A3C scan and the β1-β2-loop region: A3C are K85, L108 and N115, the analogous residues in A3F were not tested and include: N268, L291 and N298 (Figure 5E, 5F yellow) (Kitamura et al., 2012). However, L291 (α3) was previously identified as a part of the Vif-binding region (Smith and Pathak, 2010) (Figure 5C/D underlined in magenta). Overall the large contiguous negatively charged binding surface is electrostatically complementary to HIV-1 Vif, as Vif is an extremely positively charged, intrinsically disordered protein, with an isoelectric point of 10.4. The presence of a negatively charged contiguous surface suggests that the A3F-Vif binding interface may extend further along this surface.
Fig. 5. Surface representation of the A3F-CTD structure.
(A): Surface representation, colored by electrostatic potential (blue is positive, red is negative: − 0.05 -to- +0.05 kcal/mol (Maestro 9/Schrodinger, LLC)). The negatively charged groove region, electrostatically favored to bind the positively charged Vif is delineated by the dashed line and highlights the overlap with known Vif-binding regions in (C).
(B). Same representation as in (A), with the surface rotated +90° (bottom facing out) along the horizontal axis in the image plane.
(C): α3-helix (289–294, magenta) (Smith and Pathak, 2010), the α4 (324, cyan (Albin et al., 2010b), β3-strand (Y269) including part of the α2-helix and the adjacent loop region (L255, F258, C259, I262-S264, dark green) (Kitamura et al., 2012) are known Vif-binding regions that adjoin the β1-β2 (E223, H227-P230), β3(N268, E270), α3(L291, S295), β4(N298) and the α4-β5 (G325) regions (orange) involved in forming the interface. The α3 (L291) residue involved in our intermolecular interface as well as the previously characterized Vif-binding region (Smith and Pathak, 2010) is underlined in magenta.
(D). Same coloring and labels as in (C), with the surface rotated +90° (bottom facing out) along the horizontal axis in the image plane.
(E): Highlights of the complete mutational scan (Kitamura et al., 2012) of A3C (light green) projected onto our A3F-CTD structure. The regions that have were shown to be important for HIV-1 Vif binding are highlighted as above in (C). Yellow highlights and labels show the three residues that overlap between our interface and the A3C mutational scan not tested in A3F by (Kitamura et al., 2012), However L291 was independently probed (Smith and Pathak, 2010)) and found to be important in A3F binding to Vif.
(F). Same coloring and labels as in (E), with the surface rotated +90° (bottom facing out) along the horizontal axis in the image plane.
(G): Secondary structure of these views labeled for reference orientation, with the highlights colored as per (C).
(H). Same coloring and labels as in (G), with the structure rotated +90° (bottom facing out) along the horizontal axis in the image plane.
Packaging of A3F and A3G into budding virions is essential to permit the antiviral activity of both A3F and A3G (Huthoff and Malim, 2007; Kao et al., 2003). In our structure of A3F-CTD, the crystallographic contacts may be potential oligomerization surfaces (Figure 2) predominantly involving two regions in the A3F-CTD sequence, the highly conserved 305-RLYYFWD-311, which includes the β4-α4 loop, and the less conserved 223-EVVKHHSPVS-232, which includes the β1-β2-loop (Figure 2 and Figure 4). L306, which forms part of the heterologous interface (Figures 1A, 1E, 1F), along with W126 in the analogous portion of A3F-NTD, are involved in localizing A3F to the virion core (Han et al., 2008; Song et al., 2012). Both of these residues are within the conserved sequence motif, suggesting this region may be conserved to facilitate encapsidation of both A3F and A3G into virions. Specific mutations in the region homologous to A3F 305–311 in human A3G (125YFW127 to 125–DY127), drastically alter the stability of A3G in presence of HIV-1 Vif, while not inhibiting the ability of Vif to bind A3G (Gooch and Cullen, 2008). The other less conserved sequence at the interface including the β1-β2-loop in A3F is not conserved in A3G. In this heterologous interface H228 packs into the active site of the corresponding molecule in the crystallographic asymmetric unit (Figure 2). Comparing A3G-CTD with a construct where this loop was swapped with the corresponding sequence from A3G dynamic light scattering data showed (Figure S2C) the loop swap variant disrupted oligomer formation. Thus the combination of crystal packing and solution scattering implicating this interface as potentially auto-regulating the oligomerization mechanism for A3F.
Intriguingly, both A3F sequences (305-RLYYFWD-311 and 223-EVVKHHSPVS-232) potentially involved in oligomerization, align closely with sequences within HIV-1 Vif: 108-HLYYF*D-113 and 23-SLVKHHMYVS-32 (Figures 4A, 4C). BLAST (Altschul et al., 1997) searches of human and HIV proteins verified these matches: the first pair matched well for a short sequence - the A3F sequence to Vif (A3F:305–311/Vif:108–113, score: 18.5 and e-value:395) and the second query was even significantly stronger matching the A3F sequence to Vif (A3F:223–232/Vif:23–32 score: 19.7 and e-value:0.4). This suggests that perhaps HIV-1 Vif evolved to mimic portions of A3F. Substitution mutations in the region of Vif, replacing 111YF112 with AA, have been shown to reduce viral infectivity by up to 93% (Simon et al., 1999). This region has also been previously implicated in Vif oligomerization (Auclair et al., 2007; Yang et al., 2003), perhaps suggesting a mechanism underlying regulation of A3F activity. This mechanism may explain the reduced anti-HIV activity of A3F even with rates of higher virion packaging when compared to A3G (Holmes et al., 2007a; Song et al., 2012; Zennou and Bieniasz, 2006). While the regions of A3F have not been directly implicated in HIV-1 Vif binding, the molecular mimicry of Vif could play a role in modulating oligomerization or another molecular recognition event.
Targeting the APOBEC3-Vif interaction for therapeutic intervention has been an inspired goal since the discovery of antiviral activity in this class of enzymes. The advances that we and others have made on the structural biology of APOBEC enzymes in the last couple of years provide us with an understanding of the overall architecture of a catalytic domain (Prochnow et al., 2007), determinants for substrate interaction of the most prominent family member APOBEC3G (Chen et al., 2008), the modus operandi of a small molecule inhibitor (Li et al., 2012) and, recently, determinants for degradation-dependent Vif-susceptibility of APOBEC3C (Kitamura et al., 2012). With this crystal structure of A3F-CTD, for the first time, we elucidate Vif-susceptibility determinants based on structure and identify potential oligomerization interfaces that might help elucidate the anti-viral regulatory role of high molecular mass complexes of active APOBEC3 proteins. This structure provides the scaffold to elucidate the APOBEC3 polyubiquitination complex and gives structural insights that will establish APOBEC3F as a structural target for novel anti-retroviral strategies.
Materials and Methods
E. coli-based deaminase activity assays
An E. coli-based RifR mutation assay used previously to measure DNA cytosine deaminase activity (Chen et al., 2008; Harjes et al., 2009; Harris et al., 2002) was employed to determine the intrinsic deaminase activity of A3F variants as outlined in the supplementary material.
NMR HSQC Spectra
The experimental methods are as reported previously (Chen et al., 2008).
HIV-1 infectivity assays
Single-cycle infectivity and Vif susceptibility were determined as previously described (Albin et al., 2010a) and outlined in the supplementary material.
Expression, Crystallization and Structural data analysis
Samples for crystallographic and biophysical characterization (gel filtration and dynamic light scattering) were prepared and analyzed as described in the supplementary material.
Supplementary Material
Highlights.
Crystal structure of APOBEC3F domain reveals binding surface of HIV-1 Vif
Sequences of crystallographic interfaces conserved across APOBEC3 family
Crystallographic interfaces maybe functional oligomerization interfaces of APOBEC3F
HIV-1 Vif has homologous sequences suggesting potential evolution of bio-mimicry
Acknowledgments
This work was supported by National Institute of Health Institute of General Medical Sciences P01 GM091743. Structure factors and coordinates have been deposited in the Protein Data Bank (www.rcsb.org) with accession code 4IOU. Drs. Nese KurtYilmaz and William Royer are thanked for their editorial and crystallographic support, respectively. Dr. Gang Han is thanked for the generous use of the Zetasizer Nano instrument to collect DLS data. X-ray diffraction data were collected at the Advanced Photon Source at sector 23-ID-B GM/CA-CAT, supported by National Cancer Institute (Y1-CO-1020) and the National Institute of General Medical Sciences (Y1-GM-1104).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin JS, Hache G, Hultquist JF, Brown WL, Harris RS. Long-term restriction by APOBEC3F selects human immunodeficiency virus type 1 variants with restored Vif function. J Virol. 2010a;84:10209–10219. doi: 10.1128/JVI.00632-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin JS, Harris RS. Interactions of host APOBEC3 restriction factors with HIV-1 in vivo: implications for therapeutics. Expert reviews in molecular medicine. 2010;12:e4. doi: 10.1017/S1462399409001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin JS, LaRue RS, Weaver Ja, Brown WL, Shindo K, Harjes E, Matsuo H, Harris RS. A single amino acid in human APOBEC3F alters susceptibility to HIV-1 Vif. The Journal of biological chemistry. 2010b;285:40785–40792. doi: 10.1074/jbc.M110.173161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair JR, Green KM, Shandilya S, Evans JE, Somasundaran M, Schiffer CA. Mass spectrometry analysis of HIV-1 Vif reveals an increase in ordered structure upon oligomerization in regions necessary for viral infectivity. Proteins. 2007;69:270–284. doi: 10.1002/prot.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasin M, Clerici M, Piacentini L. Innate immunity in resistance to HIV infection. The Journal of infectious diseases. 2010;202(Suppl):S361–S365. doi: 10.1086/655965. [DOI] [PubMed] [Google Scholar]
- Bishop KN, Holmes RK, Sheehy AM, Davidson NO, Cho SJ, Malim MH. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr Biol. 2004;14:1392–1396. doi: 10.1016/j.cub.2004.06.057. [DOI] [PubMed] [Google Scholar]
- Browne EP, Allers C, Landau NR. Restriction of HIV-1 by APOBEC3G is cytidine deaminase-dependent. Virology. 2009;387:313–321. doi: 10.1016/j.virol.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns MB, Lackey L, Carpenter MA, Rathore A, Land AM, Leonard B, Refsland EW, Kotandeniya D, Tretyakova N, Nikas JB, et al. APOBEC3B is an enzymatic source of mutation in breast cancer. Nature. 2013;494:366–370. doi: 10.1038/nature11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MA, Rajagurubandara E, Wijesinghe P, Bhagwat AS. Determinants of sequence-specificity within human AID and APOBEC3G. DNA Repair (Amst) 2010;9:579–587. doi: 10.1016/j.dnarep.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K-MM, Harjes E, Gross PJ, Fahmy A, Lu Y, Shindo K, Harris RS, Matsuo H. Structure of the DNA deaminase domain of the HIV-1 restriction factor APOBEC3G. Nature. 2008;452:116–119. doi: 10.1038/nature06638. [DOI] [PubMed] [Google Scholar]
- Chiu YL, Greene WC. The APOBEC3 cytidine deaminases: an innate defensive network opposing exogenous retroviruses and endogenous retroelements. Annual Review of Immunology. 2007:317–353. doi: 10.1146/annurev.immunol.26.021607.090350. in press. [DOI] [PubMed] [Google Scholar]
- Conticello SG. The AID/APOBEC family of nucleic acid mutators. Genome Biol. 2008;9:229. doi: 10.1186/gb-2008-9-6-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conticello SG. Creative deaminases, self-inflicted damage, and genome evolution. Ann N Y Acad Sci. 2012;1267:79–85. doi: 10.1111/j.1749-6632.2012.06614.x. [DOI] [PubMed] [Google Scholar]
- Furukawa A, Nagata T, Matsugami A, Habu Y, Sugiyama R, Hayashi F, Kobayashi N, Yokoyama S, Takaku H, Katahira M. Structure, interaction and real-time monitoring of the enzymatic reaction of wild-type APOBEC3G. EMBO Journal. 2009;28:440–451. doi: 10.1038/emboj.2008.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooch BD, Cullen BR. Functional domain organization of human APOBEC3G. Virology. 2008;379:118–124. doi: 10.1016/j.virol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Su Y, Zhong C, Ming G-L, Song H. Hydroxylation of 5-Methylcytosine by TET1 Promotes Active DNA Demethylation in the Adult Brain. Cell. 2011:423–434. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton CE, Papavasiliou FN, Rosenberg BR. Diverse functions for DNA and RNA editing in the immune system. RNA biology. 2010;7:220–228. doi: 10.4161/rna.7.2.11344. [DOI] [PubMed] [Google Scholar]
- Han Y, Wang X, Dang Y, Zheng YH. APOBEC3G and APOBEC3F require an endogenous cofactor to block HIV-1 replication. PLoS Pathog. 2008;4:e1000095. doi: 10.1371/journal.ppat.1000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harjes E, Gross PJ, Chen K-MM, Lu Y, Shindo K, Nowarski R, Gross JD, Kotler M, Harris RS, Matsuo H. An extended structure of the APOBEC3G catalytic domain suggests a unique holoenzyme model. Journal of Molecular Biology. 2009;389:819–832. doi: 10.1016/j.jmb.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS, Malim MH. DNA Deamination Mediates Innate Immunity to Retroviral Infection. Cell. 2003;113:803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- Harris RS, Hultquist JF, Evans DT. The restriction factors of human immunodeficiency virus. J Biol Chem. 2012;287:40875–40883. doi: 10.1074/jbc.R112.416925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RS, Petersen-Mahrt SK, Neuberger MS. RNA editing enzyme APOBEC1 and some of its homologs can act as DNA mutators. Mol Cell. 2002;10:1247–1253. doi: 10.1016/s1097-2765(02)00742-6. [DOI] [PubMed] [Google Scholar]
- Holden LG, Prochnow C, Chang YP, Bransteitter R, Chelico L, Sen U, Stevens RC, Goodman MF, Chen XS. Crystal structure of the anti-viral APOBEC3G catalytic domain and functional implications. Nature. 2008;456:121–124. doi: 10.1038/nature07357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes RK, Koning FA, Bishop KN, Malim MH. APOBEC3F can inhibit the accumulation of HIV-1 reverse transcription products in the absence of hypermutation. Comparisons with APOBEC3G. J Biol Chem. 2007a;282:2587–2595. doi: 10.1074/jbc.M607298200. [DOI] [PubMed] [Google Scholar]
- Holmes RK, Malim MH, Bishop KN. APOBEC-mediated viral restriction: not simply editing? Trends in Biochemical Sciences. 2007b;32:118–128. doi: 10.1016/j.tibs.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Huthoff H, Malim MH. Identification of amino acid residues in APOBEC3G required for regulation by human immunodeficiency virus type 1 Vif and Virion encapsidation. J Virol. 2007;81:3807–3815. doi: 10.1128/JVI.02795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger S, Kim DY, Hultquist JF, Shindo K, LaRue RS, Kwon E, Li M, Anderson BD, Yen L, Stanley D, et al. Vif hijacks CBF-β to degrade APOBEC3G and promote HIV-1 infection. Nature. 2011:1–5. doi: 10.1038/nature10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S, Khan MA, Miyagi E, Plishka R, Buckler-White A, Strebel K. The human immunodeficiency virus type 1 Vif protein reduces intracellular expression and inhibits packaging of APOBEC3G (CEM15), a cellular inhibitor of virus infectivity. J Virol. 2003;77:11398–11407. doi: 10.1128/JVI.77.21.11398-11407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Ode H, Nakashima M, Imahashi M, Naganawa Y, Kurosawa T, Yokomaku Y, Yamane T, Watanabe N, Suzuki A, et al. The APOBEC3C crystal structure and the interface for HIV-1 Vif binding. Nat Struct Mol Biol. 2012;19:1005–1010. doi: 10.1038/nsmb.2378. [DOI] [PubMed] [Google Scholar]
- Kohli RM, Abrams SR, Gajula KS, Maul RW, Gearhart PJ, Stivers JT. A portable hot spot recognition loop transfers sequence preferences from APOBEC family members to activation-induced cytidine deaminase. J Biol Chem. 2009;284:22898–22904. doi: 10.1074/jbc.M109.025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg JF, Yonemoto W, Greene WC. Endogenous factors enhance HIV infection of tissue naive CD4 T cells by stimulating high molecular mass APOBEC3G complex formation. J Exp Med. 2006;203:865–870. doi: 10.1084/jem.20051856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissinel E, Henrick K. Protein interfaces, surfaces and assemblies service PISA at European Bioinformatics Institute. 2007 [Google Scholar]
- LaRue RS, Andresdottir V, Blanchard Y, Conticello SG, Derse D, Emerman M, Greene WC, Jonsson SR, Landau NR, Lochelt M, et al. Guidelines for naming nonprimate APOBEC3 genes and proteins. Journal of Virology. 2009;83:494–497. doi: 10.1128/JVI.01976-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRue RS, Jonsson SR, Silverstein KA, Lajoie M, Bertrand D, El-Mabrouk N, Hotzel I, Andresdottir V, Smith TP, Harris RS. The artiodactyl APOBEC3 innate immune repertoire shows evidence for a multi-functional domain organization that existed in the ancestor of placental mammals. BMC Mol Biol. 2008;9:104. doi: 10.1186/1471-2199-9-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRue RS, Lengyel J, Jónsson SR, Andrésdóttir V, Harris RS. Lentiviral Vif degrades the APOBEC3Z3/APOBEC3H protein of its mammalian host and is capable of cross-species activity. Journal of virology. 2010;84:8193–8201. doi: 10.1128/JVI.00685-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecossier D, Bouchonnet F, Clavel F, Hance AJ. Hypermutation of HIV-1 DNA in the absence of the Vif protein. Science. 2003;300:1112. doi: 10.1126/science.1083338. [DOI] [PubMed] [Google Scholar]
- Li M, Shandilya SM, Carpenter MA, Rathore A, Brown WL, Perkins AL, Harki DA, Solberg J, Hook DJ, Pandey KK, et al. First-in-class small molecule inhibitors of the single-strand DNA cytosine deaminase APOBEC3G. ACS Chem Biol. 2012;7:506–517. doi: 10.1021/cb200440y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddament MT, Brown WL, Schumacher AJ, Harris RS. APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr Biol. 2004;14:1385–1391. doi: 10.1016/j.cub.2004.06.050. [DOI] [PubMed] [Google Scholar]
- Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- Miyagi E, Opi S, Takeuchi H, Khan M, Goila-Gaur R, Kao S, Strebel K. Enzymatically Active APOBEC3G is Required for Efficient Inhibition of HIV-1. Journal of Virology. 2007:13346–13353. doi: 10.1128/JVI.01361-07. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EN, Holmes RK, Craig HM, Klein KC, Lingappa JR, Malim MH, Sheehy AM. Antiviral function of APOBEC3G can be dissociated from cytidine deaminase activity. Current Biology. 2005;15:166–170. doi: 10.1016/j.cub.2004.12.068. [DOI] [PubMed] [Google Scholar]
- Nik-Zainal S, Alexandrov LB, Wedge DC, Van Loo P, Greenman CD, Raine K, Jones D, Hinton J, Marshall J, Stebbings LA, et al. Mutational processes molding the genomes of 21 breast cancers. Cell. 2012;149:979–993. doi: 10.1016/j.cell.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochnow C, Bransteitter R, Klein MG, Goodman MF, Chen XS. The APOBEC-2 crystal structure and functional implications for the deaminase AID. Nature. 2007;445:447–451. doi: 10.1038/nature05492. [DOI] [PubMed] [Google Scholar]
- Reingewertz TH, Benyamini H, Lebendiker M, Shalev DE, Friedler A. The C-terminal domain of the HIV-1 Vif protein is natively unfolded in its unbound state. Protein engineering design selection PEDS. 2009;22:281–287. doi: 10.1093/protein/gzp004. [DOI] [PubMed] [Google Scholar]
- Reingewertz TH, Shalev DE, Friedler A. Structural disorder in the HIV-1 Vif protein and interaction-dependent gain of structure. Protein and peptide letters. 2010;17:988–998. doi: 10.2174/092986610791498876. [DOI] [PubMed] [Google Scholar]
- Roberts SA, Sterling J, Thompson C, Harris S, Mav D, Shah R, Klimczak LJ, Kryukov GV, Malc E, Mieczkowski PA, et al. Clustered mutations in yeast and in human cancers can arise from damaged long single-strand DNA regions. Mol Cell. 2012;46:424–435. doi: 10.1016/j.molcel.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher AJ, Hache G, Macduff DA, Brown WL, Harris RS. The DNA deaminase activity of human APOBEC3G is required for Ty1, MusD, and human immunodeficiency virus type 1 restriction. Journal of Virology. 2008;82:2652–2660. doi: 10.1128/JVI.02391-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandilya SMD, Nalam MNL, Nalivaika EA, Gross PJ, Valesano JC, Shindo K, Li M, Munson M, Royer WE, Harjes E, et al. Crystal structure of the APOBEC3G catalytic domain reveals potential oligomerization interfaces. Structure. 2010;18:28–38. doi: 10.1016/j.str.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- Simon JHM, Sheehy AM, Carpenter EA, Fouchier RAM, Malim MH. Mutational Analysis of the Human Immunodeficiency Virus Type 1 Vif Protein. Journal of Virology. 1999;73:2675–2681. doi: 10.1128/jvi.73.4.2675-2681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Pathak VK. Identification of specific determinants of human APOBEC3F, APOBEC3C, and APOBEC3DE and African green monkey APOBEC3F that interact with HIV-1 Vif. J Virol. 2010;84:12599–12608. doi: 10.1128/JVI.01437-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Sutton L, Johnson ME, D’Aquila RT, Donahue JP. Signals in APOBEC3F N-terminal and C-terminal Deaminase Domains Each Contribute to Encapsidation in HIV-1 Virions and Are Both Required for HIV-1 Restriction. J Biol Chem. 2012;287:16965–16974. doi: 10.1074/jbc.M111.310839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenglein MD, Burns MB, Li M, Lengyel J, Harris RS. APOBEC3 proteins mediate the clearance of foreign DNA from human cells. Nature structural & molecular biology. 2010;17:222–229. doi: 10.1038/nsmb.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K. APOBEC3G & HTLV-1: Inhibition without deamination. Retrovirology. 2005;2:37. doi: 10.1186/1742-4690-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Rada C, Neuberger MS. Altering the spectrum of immunoglobulin V gene somatic hypermutation by modifying the active site of AID. J Exp Med. 2010;207:141–153. doi: 10.1084/jem.20092238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Dolan PT, Dang Y, Zheng YH. Biochemical differentiation of APOBEC3F and APOBEC3G proteins associated with HIV-1 life cycle. J Biol Chem. 2007;282:1585–1594. doi: 10.1074/jbc.M610150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand HL, Doehle BP, Bogerd HP, Cullen BR. A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. EMBO J. 2004;23:2451–2458. doi: 10.1038/sj.emboj.7600246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Gao L, Li L, Lu Z, Fan X, Patel CA, Pomerantz RJ, DuBois GC, Zhang H. Potent suppression of viral infectivity by the peptides that inhibit multimerization of human immunodeficiency virus type 1 (HIV-1) Vif proteins. J Biol Chem. 2003;278:6596–6602. doi: 10.1074/jbc.M210164200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Yu Y, Liu B, Luo K, Kong W, Mao P, Yu XF. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- Zennou V, Bieniasz PD. Comparative analysis of the antiretroviral activity of APOBEC3G and APOBEC3F from primates. Virology. 2006;349:31–40. doi: 10.1016/j.virol.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, Gao L. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424:94–98. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Du J, Evans SL, Yu Y, Yu X-F. T-cell differentiation factor CBF-β regulates HIV-1 Vif-mediated evasion of host restriction. Nature. 2011;481:376–379. doi: 10.1038/nature10718. [DOI] [PubMed] [Google Scholar]
- Zheng Y-H, Irwin D, Kurosu T, Tokunaga K, Sata T, Peterlin BM. Human APOBEC3F Is Another Host Factor That Blocks Human Immunodeficiency Virus Type 1 Replication. Journal of Virology. 2004;78:6073–6076. doi: 10.1128/JVI.78.11.6073-6076.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.