Abstract
In cholestatic liver diseases, the ability of hydrophobic bile acids to damage membranes of hepatocytes/ductal cells contributes to their cytotoxicity. However, ursodeoxycholic acid (UDC), a hydrophilic bile acid, is used to treat cholestasis because it protects membranes. It has been well established that bile acids associate with and solubilize free cholesterol (CHOL) contained within the lumen of the gallbladder because of their structural similarities. However, there is a lack of understanding of how membrane CHOL, which is a well-established membrane stabilizing agent, is involved in cytotoxicity of hydrophobic bile acids and the cytoprotective effect of UDC. We utilized phospholipid liposomes to examine the ability of membrane CHOL to influence toxicity of individual bile acids, such as UDC and the highly toxic sodium deoxycholate (SDC), as well as the cytoprotective mechanism of UDC against SDC-induced cytotoxicity by measuring membrane permeation and intramembrane dipole potential. The kinetics of bile acid solubilization of phosphatidylcholine liposomes containing various levels of CHOL was also characterized. It was found that the presence of CHOL in membranes significantly reduced the ability of bile acids to damage synthetic membranes. UDC effectively prevented damaging effects of SDC on synthetic membranes only in the presence of membrane CHOL, while UDC enhances the damaging effects of SDC in the absence of CHOL. This further demonstrates that the cytoprotective effects of UDC depend upon the level of CHOL in the lipid membrane. Thus, changes in cell membrane composition, such as CHOL content, potentially influence the efficacy of UDC as the primary drug used to treat cholestasis.
Keywords: Ursodeoxycholic acid cytoprotection, Deoxycholic acid cytotoxicity, Cholesterol, Membrane permeability, Membrane dipole potential, Turbidity
1. Introduction
Bile acids, derivatives of cholesterol (CHOL), are synthesized in the liver, stored and concentrated in the gallbladder and excreted into the small intestine as a component of the enterohepatic circulation [1]. Because of their amphipathic structures, the major physiological functions of bile acids include solubilization and absorption of CHOL, phospholipids and fat-soluble vitamins [1]. High concentrations of bile acids are toxic to epithelial cells lining the GI tract and the hepatobiliary system [1]. The cytotoxicity of bile acids depends on their hydrophobicity [2] and has been suggested to originate from their ability to damage cell membranes [3–7]. The hydrophilic bile acid, ursodeoxycholic acid (UDC), is unique in that it protects GI and heptic epithelial cells against attack by hydrophobic bile acids, such as deoxycholic acid (SDC) [6–11]. For instance, an in vitro study demonstrates that UDC effectively prevents SDC-induced apoptosis of colon cancer cells [10]. Moreover, UDC modulates mitochondrial transmembrane potential to inhibit SDC-induced apoptosis [6,7]. It is thought that this cytoprotective effect of UDC arises from its ability to protect cell membranes against toxic bile acids [6,7,9–11]. In the gallbladder, bile acids strongly associate with free CHOL and prevent CHOL from precipitating out of solution. As a well known membrane-stabilizing agent and an important component of cell membranes, the possible role of membrane CHOL in protecting cells against the detergent effects of hydrophobic bile acids and its involvement in the cytoprotective effects of UDC has not been explored. In this study, we examine the molecular mechanism underlying the ability of membrane CHOL, to influence bile acid toxicity and the cytoprotective effects of UDC.
To investigate the possible role of CHOL in influencing the cytoprotective effect of UDC against hydrophobic bile acid-membrane interactions, we utilized synthetic liposomes and compared the ability of our test bile acids to affect membrane properties with various levels of CHOL embedded within the membrane. Liposomes were composed of dilinoleoyl phosphatidylcholine (DLPC, or di18:2 PC) because it is one of the most abundant PC lipids found in plasma membranes of epithelial cells, such as hepatocytes [12]. The CHOL composition was chosen 30 and 50 mol% because typical CHOL level found in cell plasma membranes is between 30 and 50% [12,13].
We measured changes in three independent, yet closely related membrane properties: membrane permeability; liposome solubilization; and membrane cohesiveness. In a membrane permeability experiment, a hydrophilic probe calcein, which self-quenches at high concentrations, was loaded into liposomes. A leakage of calcein into the bulk solution caused by a compromise of membrane integrity by bile acid attack will lead to de-quenching of the probe and an increase in the fluorescent intensity. The ability of PC liposomes to resist detergent effect of bile acids was examined by measuring alteration in lipid turbidity. The rate of solubilization was then calculated by fitting a cumulative exponential equation (Eq. (1)) to the experimentally determined turbidity curve. Changes in intramembrane environments upon exposure to bile acids were studied by measuring the membrane dipole potential [14–19], which arises from the sum of molecular dipole moments within the membrane (Fig. 1). Two major contributors to the membrane dipole potential are lipid carbonyl bonds and interfacial water molecules [14–19]. In a stable membrane, lipids are tightly packed, which causes all lipid dipole moments to align in an orderly manner, yielding a large sum of dipole moments and a large dipole potential (Fig. 1 top). Conversely, when the membrane is disrupted and lipid packing within the membrane is loosened, lipids tend to orient in various angles and their electrical dipoles possess high degrees of freedom and cancel each other out, giving a smaller dipole potential (Fig. 1 bottom). In addition, the large positively charged membrane dipole potential (200–400 mV) can also be influenced by partitioning of other charged particles into the interior of the bilayer. Therefore, the membrane dipole potential is an excellent indicator of changes in internal environments in the membrane and association of amphiphiles to the membrane [14–20].
Fig. 1.
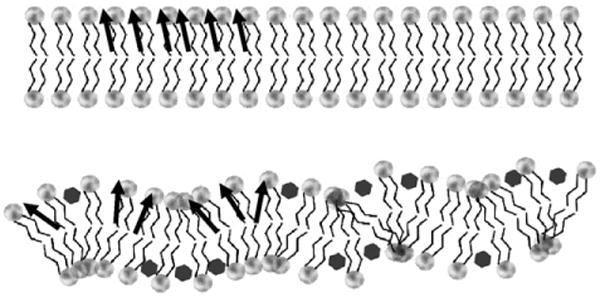
A schematic drawing of the correlation between intramembrane lipid dipole moments and molecular orientation/packing density. When lipids are packed tightly, all membrane internal dipole moments (indicated by the black arrows) are oriented in an orderly fashion, thus giving a large dipole potential (top). When lipid packing is disrupted, lipid dipoles are aligned at various angles, effectively canceling each other out (bottom). This would give a small dipole potential. Note that this intramembrane dipole originates from lipid carbonyl dipole and the molecular dipoles of interfacial water molecules and is different from the headgroup dipole arising from phosphate-choline dipolar interactions.
We found that all three parameters correlate to demonstrate that the membrane CHOL level determines the toxicity of bile acids and cytoprotective ability of UDC. Without CHOL in the membrane, SDC and UDC were found to be toxic (with SDC more toxic than UDC), indicated by all distinct membrane properties examined. The presence of CHOL in the membrane negates some of the bile acid toxicity and is especially effective with UDC. We also found that when combining UDC with SDC, UDC is highly toxic without CHOL in the membrane and only becomes cytoprotective in the presence of membrane CHOL.
2. Materials and methods
2.1. Materials
The phospholipid, dilinoleoyl phosphatidyl-choline (DLPC), was purchased from Avanti Polar Lipids, Inc (Alabaster, AL, USA). Lipids were dissolved in chloroform and stored at −20 °C under nitrogen. Cholesterol and bile acids were purchased from Sigma-Aldrich (St. Louis, MO, USA). The voltage sensitive fluorescent probe di-8 ANEPPS was purchased from Molecular Probes (Carlsbad, CA, USA).
2.2. Methods
2.2.1. Large unilamellar vesicle formation
Approximately 20 mg DLPC in chloroform was dried under nitrogen gas and then exposed to vacuum overnight to completely evaporate chloroform. The lipid film was rehydrated with ∼4 ml of phosphate buffer solution (PBS) at pH 7.4 and incubated at room temperature under nitrogen gas for at least 30 min. Lipid solution was then extruded across a polycarbonate filter with 100 nm pores to form large unilamellar vesicles (LUVs) in a Mini-extruder set-up (Avanti Polar Lipids, Inc). The average size of vesicles was verified to be ∼120 nm by dynamic light scattering (data not shown). All experiments (turbidity, dipole potential and permeability) discussed in this study were performed at room temperature (∼23 °C). In all experiments, liposomes were incubated for 30 min in PBS buffer containing bile acids before measurements.
2.2.2. Turbidity
Liposomes composed of DLPC with 0%, 30% or 50% CHOL were prepared as described above. Turbidity of liposome samples exposed to SDC, UDC, or combinations of the two bile acids was measured as absorbance at 350 nm at room temperature. Measurements were obtained in Genesys 10UV spectrophotometer (Thermo Scientific, Waltham, MA) with a 1 cm path-length cell. Samples were occasionally stirred to ensure uniform mixing. The rate of solubilization, k, by bile acids was calculated by fitting the cumulative exponential equation to experimentally determined time-dependent curve of polydispersity [21,22]:
| (1) |
where Y is the concentration of undissolved lipid in the sample, k is the rate of polydispersity by bile acid, t is time, and μ2w is the second expansion coefficient.
2.2.3. Dipole potential measurements
The membrane dipole potential was obtained by incorporating a low level (∼0.2 mol%) of a voltage sensitive fluorescent probe, di-8 ANEPPS, which shifts its excitation spectrum when exposed to different dipole environments within the membrane [14–19]. Approximately 50 μl of 1 mg/ml di-8 ANEPPS (in 100% ethanol) was added to the LUV solution (4 ml) to achieve a lipid/probe ratio of ∼400:1. The mixture was incubated overnight under nitrogen gas. The small amount of the probe was utilized to ensure that the overall membrane properties of liposomes are not affected by the presence of the probe. Moreover, a large lipid/probe ratio, long incubation time and high affinity of the probe for hydrophobic environment within lipid membranes also guarantee that all probes partition into the membrane. Spectral measurements were performed using a SpectraMax Gemini X spectrophotometer (Molecular Devices, Sunnyvale, CA). The excitation spectra between 400 nm and 550 nm were recorded with the emission wavelength fixed at 680 nm [14,17,23]. The ratio (F420/520) of fluorescence intensities at 420 nm and 520 nm of the excitation spectrum was then directly correlated with the dipole potential, Ψd [14,17]:
| (2) |
2.2.4. Liposome membrane permeability to calcein
The calcein permeability technique has been well-established to determine membrane permeability [24]. Approximately 6 mg of DLPC in chloroform was dried under nitrogen gas and then exposed to vacuum overnight to evaporate chloroform. The lipid film was rehydrated with ∼2 ml of Tris buffer containing ∼4.5 mM calcein. The mixture was vortexed and sonicated for 10 min to form liposomes with high concentrations of calcein encapsulated inside. The liposomal solution was then passed through Sephadex G-50 column to separate liposomes (with encapsulated calcein), which elute in the void volume, from free calcein in the bulk solution. After 30 minute incubation with test solutions, membrane leakage of calcein was indicated by an increase in the fluorescent intensity of the self-quenching calcein (excitation: 472 nm; emission: 516 nm). Total fluorescent intensity of calcein was obtained by adding ∼0.5% Triton X-100 to lyse all liposomes at the end of each experiment. The percent calcein leakage was calculated:
| (3) |
where Ft is the fluorescent intensity of a liposome solution exposed to testing solutions, Fo is the control fluorescent intensity of a liposome solution before being exposed to bile acid solutions and Ftotal is the fluorescent intensity obtained after exposure to Triton X-100.
3. Experimental results
3.1. CHOL alters the effect of deoxycholic acid and ursodeoxycholic acid on membrane permeability
DLPC liposomes were exposed to either SDC or UDC at physiologically relevant concentrations from 0.1 mM to 5 mM [1,25,26]. Both SDC and UDC were found to cause dose-dependent increase in the amount of calcein released from liposomes (Fig. 2). A value of C50 for SDC, the bile acid concentration at which 50% of maximum calcein leakage occurs, was found to be ∼0.56 mM. This C50 value for SDC is in good agreement with Schubert et al. [27] who found that SDC yielded C50 for egg PC membrane encapsulating raffinose (MW 594 g/mol compared to calcein with MW 623 g/mol) was 0.45 mM [27]. When 30 mol% CHOL was incorporated into the liposomal membrane of DLPC, the dose–response curve of calcein leakage in the presence of increasing concentrations of SDC was found to shift to the right (Fig. 2). It can be seen that C50 was shifted to ∼0.9 mM with CHOL, indicating that the liposomal membrane was stabilized by the presence of the sterol although the CHOL protection is not effective since a membrane with a C50 value of 0.9 mM is still considered highly permeable. A higher concentration of UDC was required to increase the calcein permeability across pure DLPC membranes with a C50 of ∼2.2 mM (Fig. 2), also consistent with findings of Schubert et al. [27]. This also agrees with well-established literature that UDC is more hydrophilic and thereby has less interaction with membrane lipids than SDC [1,28]. Incorporation of 30 mol% CHOL into the liposomal membrane of DLPC also shifted the dose curve of calcein leakage to the right, in the presence of increasing concentrations of UDC, with C50 of ∼3.9 mM (Fig. 2). Thus, upon addition of CHOL to lipid membranes, C50 showed a larger shift when the liposomes were exposed to UDC than SDC: ΔC50 was found to be ∼1.7 mM in the presence of UDC; and ∼0.35 mM in the presence of SDC, an approximately 5-fold difference. This data suggests that the stabilizing effect of CHOL was especially effective when liposomes were exposed to UDC, indicating that CHOL is needed for UDC to become nontoxic to membranes concentrations of 3 mM and less, whereas CHOL was only minimally effective in protecting membranes against the destabilizing actions of SDC at concentrations less than 1 mM.
Fig. 2.
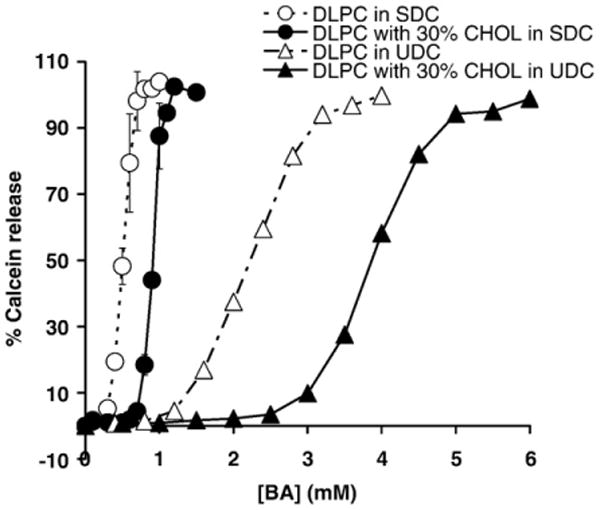
DLPC membrane permeability to calcein was determined as a function of deoxycholic acid (SDC) or ursodeoxycholic acid (UDC) concentration with or without 30% CHOL When exposed SDC, the value of C50 was ∼ 0.56 mM with no CHOL (○) and shifted to ∼0.9 mM with 30 mol% CHOL (●). In UDC, the value of C50 was ∼2.2 mM with no CHOL (Δ) and shifted to ∼3.9 mM with 30 mol% CHOL (▲). The slope of the concentration dependence of increase in calcein permeability in SDC was calculated to be ∼267, while the corresponding slope for UDC is ∼50, indicating more gradual increase in the case of UDC.
Note that calcein permeability data can be interpreted as either a result of a disruption of membrane packing or transformation of the liposomes to micelles. Since the goal of this study is to examine the ability of cholesterol to influence bile acid toxicity and both phenomena (membrane disruption or micelle formation) indicate membrane damage and bile acid toxicity, we believe that we have achieved our goal by directly interpreting the calcein permeability measurements as an indication of membrane damage. Differentiating between the two events would be out of the scope of our current study.
3.2. Effects of bile acids and CHOL on the membrane dipole potential
To better understand the underlying mechanism by which bile acids alter membrane permeability, possible changes in the intramembrane conditions were examined by measuring the membrane dipole potential. The membrane dipole potential of DLPC LUVs was found to be ∼ 180 mV, in agreement with established literature value of the dipole potential for DLPC [14]. As the bile acid concentrations were experimentally increased from 0.5 mM to 8 mM, both SDC and UDC decreased the membrane dipole potential of pure DLPC liposomes in a dose-dependent manner (Fig. 3). This demonstrates that bile acids partition into the interior of the bilayer membrane and possibly alter lipid orientation. Since affinity of bile acids to phospholipids directly correlates with their toxicity [29–31], this potentially explains the loss in integrity and higher permeability of DLPC membranes when exposed to bile acids, which is illustrated in the previous section. Note that bile acids are strong detergents and form mixed micelles with lipids above their critical micelle concentrations (CMCs). Thus it is possible that our dipole potential measurements indicate bilayer-to-micelle transition. We then measured the dipole potential values in pure SDC micelles. We found that the dipole potential of SDC micelles was ∼80 mV, significantly lower than those of bilayers (200–400 mV). We also found that this value is independent of bile acid concentration as long as it is above the CMC of 3 mM. This is expected, since every micelle typically has a fixed number of monomers. An increase in bile acid concentration simply increases the number of micelles and does not have an effect on micelle structure (although simple micelles aggregate to form secondary micelles). In addition, we could not obtain a dipole potential reading (di-8 ANEPPS did not show detectable fluorescence) when SDC concentration was below CMC, further suggesting the validity of the dipole potential in micelles. We thereby have established both ends of the spectrum in terms of how dipole potential changes in different systems: the dipole potential is large in the pure bilayer system; and significantly smaller (by 3–4 folds) in pure bile acid micelle system. Our dipole potential measurements in the presence of both lipids and bile acids lie between these extremes and indicate a transition from bilayer to micelle, a common indicator of membrane damage and bile acid toxicity.
Fig. 3.
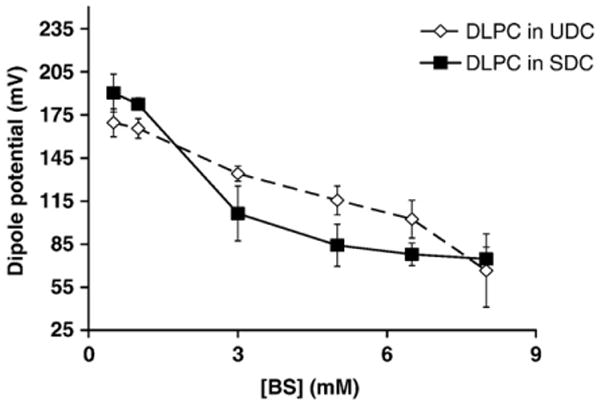
Changes in the DLPC membrane dipole potential as a function of bile acid concentrations. Both bile acids lead to dose-dependent decrease in the dipole potential of DLPC liposomes (p < 0.05). UDC (◊) was found to yield less profound decrease in the dipole potential than SDC (■).
An addition of 30 mol% CHOL to the DLPC membranes significantly increased the dipole potential to ∼220 mV, signifying an alignment of lipids and a stabilization of the overall membrane and is in agreement with findings of Starkes-Peterkovic [19]. Exposure to low concentrations of both bile acids (<1 mM) led to minimal changes in the dipole potential values, indicating significant protection against bile acids (Fig. 4). Interestingly, the larger gap between UDC and SDC treatments shown in Fig. 4 in comparison with Fig. 3 demonstrates that 30 mol% CHOL in the membrane protects liposomes against UDC more effectively than SDC (Fig. 4).
Fig. 4.
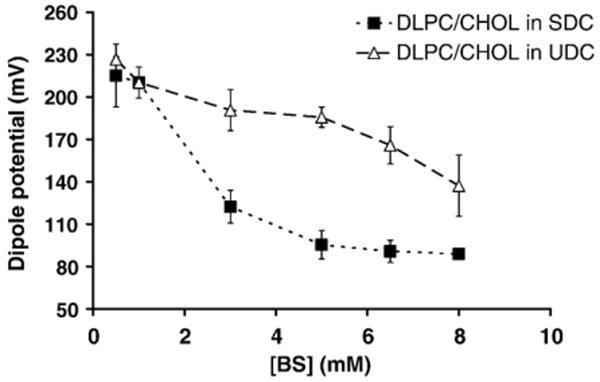
Effects of 30 mol% of CHOL incorporated in DLPC membranes on the dipole potential. When compared with the dipole potential values in liposomes with no CHOL in Fig. 3, CHOL was found to have minimal effect when the liposomes were exposed to SDC (■) (ANOVA two-factor p>0.05). In contrast, the addition of 30% CHOL to DLPC membranes made liposomes more resistant to the effects of UDC on dipole potential (Δ) (ANOVA two-factor p<0.05).
The CHOL-dependence of the cytoprotective actions of UDC on PC membranes was then examined. This is demonstrated in Fig. 5, where addition of CHOL is shown to shift the membrane dipole potential dose-curve significantly upward, illustrating that the presence of CHOL induced alignment of lipids molecules and reduced association of bile acids to the membrane. To further examine UDC cytoprotective effects, the ability of UDC to protect PC membranes with various CHOL levels (30 mol% and 50 mol%) against SDC was then examined by mixing the two bile acids at SDC/UDC molar ratios of 1:1, 1:2, 1:3, 1:4 and 1:5. The combined concentration of the two bile acids was fixed at 5 mM in all cases. As demonstrated in Fig. 6, when exposed to 5 mM SDC, the membrane dipole potential values were markedly reduced in pure DLPC liposomes and increased slightly with the addition of CHOL to the membrane. Increasing concentrations of CHOL in the membrane, however, caused a significant increase in the dipole potential when liposomes were exposed to 5 mM UDC, further suggesting that CHOL interacts with UDC to attenuate its cytotoxicity. When pure DLPC liposomes were exposed to mixture of both bile acids (white bars), UDC was not protective until the level of UDC was 4 to 5 times that of SDC, suggesting that without CHOL in membranes, UDC is not cytoprotective. With 30 mol% or 50 mol% CHOL in the liposomal membrane, a significant recovery of the dipole potential was observed even when exposed to the 1:1 mixture of SDC and UDC, which was not protective at all in pure DLPC membranes, suggesting that the cytoprotective effects of UDC depends on the presence of CHOL within the membrane.
Fig. 5.
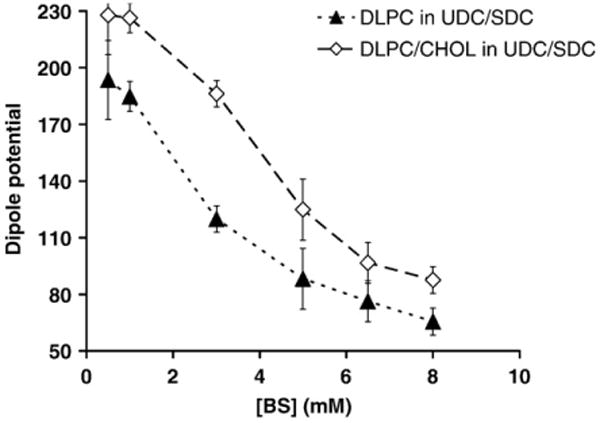
A comparative plot illustrating the effect of DLPC membranes with or without 30 mol% CHOL on the cytoprotective effect of UDC against the membrane-destabilizing action of a combination of UDC and SDC (1:1). The cytoprotective effect of UDC against the membrane disruptive action of SDC is more effective with 30 mol% CHOL in the membrane (◊).
Fig. 6.
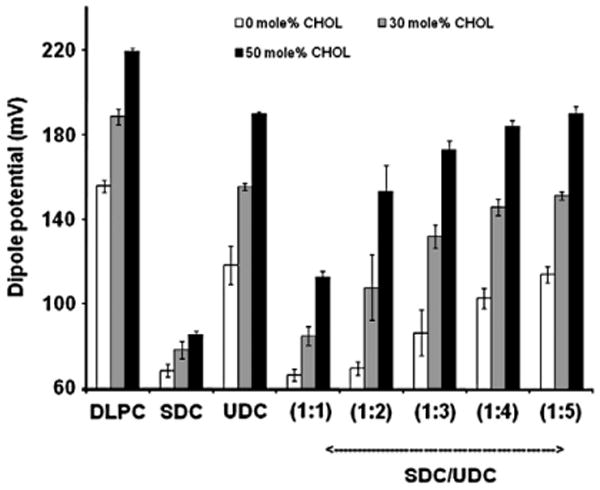
Effects of 30 or 50 mol% CHOL in the membrane on the cytoprotective effect of UDC against SDC in various bile acid combinations. Pure DLPC (white bars), DLPC liposomes containing 30 mol% CHOL in the membrane (gray bars) or DLPC liposomes containing 50 mol% CHOL (black bars) were exposed to PBS (first group), 5 mM SDC (second group), 5 mM UDC (third group), or SDC/UDC mixtures at 1:1, 1:2, 1:3, 1:4 and 1:5. The total concentrations of all SDC/UDC mixtures were fixed at 5 mM. Data is presented as mean+/−SD of 3 individual experiments.
3.3. Turbidity
To further understand the ability of CHOL to influence bile acid toxicity and UDC cytoprotection, we examined the ability of bile acids to solubilize DLPC liposomes containing various levels of CHOL in the membrane by measuring solution turbidity. DLPC liposomes alone showed high turbidity, indicating formation of large water-insoluble vesicles. Addition of 5 mM SDC led to rapid decrease in turbidity, suggesting efficient lipid-solubilizing ability of SDC most likely due to micelle formation (Fig. 7). Fig. 7A illustrates plots of undissolved lipid vs. time, examining the ability of membrane CHOL to influence the ability of SDC alone to solubilize lipids and to alter toxicity of the bile acid. Addition of 30 mol% CHOL did not show any significant protection against SDC, while 50 mol% CHOL in the membrane showed significant protection against SDC (Fig. 7A). These data are in agreement with our dipole potential measurements in that high levels of CHOL are needed to protect the membrane when exposed to SDC alone. Fig. 7B demonstrates plots of undissolved lipid vs. time for SDC treated liposomes and the ability of UDC to protect liposomes containing 0, 30 or 50 mol% CHOL in the membrane. In the presence of UDC, 30 mol% CHOL in the membrane showed observable protection, while the presence of 50 mol% CHOL in the membrane led to minimal solubilization of the lipids and drastically improved protection against bile acids when compared with Fig. 7A (with only SDC). To quantify the bile acid solubilization of lipids, the rate of solubilization, k, was calculated by fitting Eq. (1) to experimentally determined cumulative exponential curves of undissolved lipids vs. time. The data are listed in Table 1. It was found that the presence of either 30 or 50 mol% CHOL in the membrane significantly slowed the solubilization process by SDC, which was more efficient when the membrane contains 50 mol% CHOL. This is in agreement with the dipole potential data, which demonstrate that 30 mol% membrane CHOL was less effective at protecting the integrity of PC liposomes (from being converted to micelles) than 50 mol%.
Fig. 7.
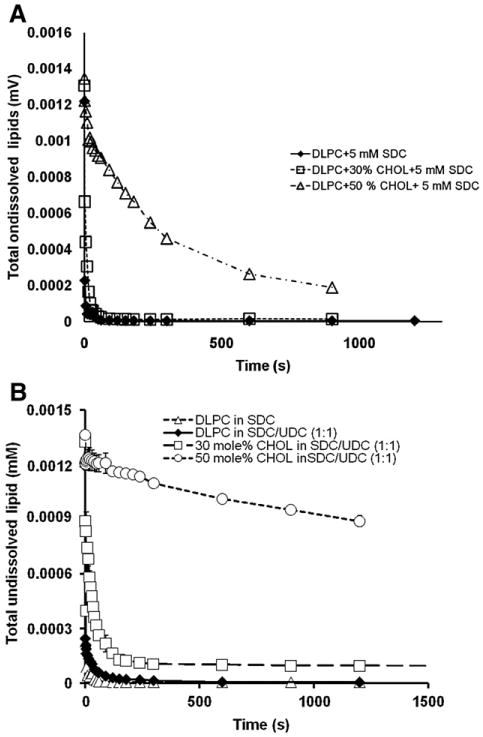
(A) The levels of undissolved lipids indicated by the turbidity measurements at 350 nm were monitored over time. It shows that without CHOL in the membrane, 5 mM SDC alone was highly damaging and completely solubilized DLPC lipids within 10 s. The presence of CHOL in the membrane at 30 mol% was unable to protect the DLPC membrane while 50 mol% CHOL display some level of protection. (B) The levels of undissolved lipids indicated by the turbidity measurements at 350 nm were monitored over time. It shows that without CHOL in the membrane, a SDC/UDC combination was mostly as damaging as SDC alone (both at 5 mM). The presence of CHOL in the membrane at 30 or 50 mol% prevented lipids from being solubilized by bile acids, demonstrating the ability of CHOL in the membrane to influence the cytoprotective effect of UDC against SDC attack.
Table 1.
| Liposome composition | Treatment | k (s−1) | μ2w |
|---|---|---|---|
| DLPC | SDC | 1.67 | 4.57 × 10−9 |
| SDC/UDC | 1.37 | 4.3 × 10−8 | |
| UDC | 0.00015 | 1.52 × 10−14 | |
| DLPC with 30 mol% CHOL | SDC | 0.157 | 9.16 × 10−9 |
| SDC/UDC | 0.0177 | 0.00037 | |
| DLPC with 50 mol% CHOL | SDC | 0.0031 | 9.24 × 10−9 |
| SDC/UDC | 0.00084 | 6.3 × 10−15 |
When both bile acids were mixed at 1:1 molar ratio (total bile acid concentration was fixed at 5 mM as in the dipole potential experiments), k values were found to be highly dependent upon the presence of CHOL in the membrane. It was found that k was ∼1.4 s−1 (compared with ∼1.7 s−1 with SDC alone) with no CHOL in the membrane, in agreement with our dipole potential data demonstrating that UDC is not protective when there is no CHOL in the membrane. The value of k was decreased to ∼0.018 s−1 with 30 mol% CHOL in the membrane and ∼0.00084 s−1 with 50 mol% CHOL in the membrane, demonstrating slowed lipid solubilization process and weaker detergent effect. This further suggests that high CHOL level in the membrane is needed for UDC to be protective.
Table 1 also demonstrates that the second exponential coefficients, μ2w, for all conditions are extremely small. It has been suggested that the parameter μ2w/k is an indication of polydispersity and a value approaching zero suggests a monodisperse system [22]. Thus, the fact that all our μ2w/k values approach zero suggests that all our liposome systems approach monodispersity.
4. Discussion
4.1. The correlation of data obtained using synthetic systems: lipid solubilization, the membrane dipole potential and permeability
Here, we studied the membrane CHOL-dependence on bile acid cytotoxicity and UDC cytoprotection by examining three independent parameters indicative of membrane stability: membrane permeability to calcein, membrane solubilization and the membrane dipole potential. The biophysical parameters tested in this study independently examine different membrane properties that are also closely related: both membrane permeability and solubilization depend on the internal integrity of the membrane, which is indicated by the membrane dipole potential.
We found a strikingly similar trend in changes in the membrane dipole potential data as in the membrane permeability data. Whereas a large dipole potential (or a more cohesive membrane) corresponds to low membrane permeability in the case of low bile acid concentration or in the presence of CHOL, a low dipole potential (or a disrupted membrane) corresponds to an increase in the membrane permeability when exposed to high bile acid concentrations or with no CHOL. To further examine the possible correlation between membrane permeability and the dipole potential, we plotted the values of C50 to calcein permeability against the bile acid concentration at which 50% decrease in the dipole potential was observed, or D50, for corresponding bile acids (Fig. 8A). A linear correlation between these two properties (r2=0.947) strongly suggests that the increase in the membrane permeability and decrease in membrane integrity are caused by binding of bile acids to the membrane and possibly changes in lipid orientation and lipid packing, thus a higher probability of particles permeating across the membrane.
Fig. 8.
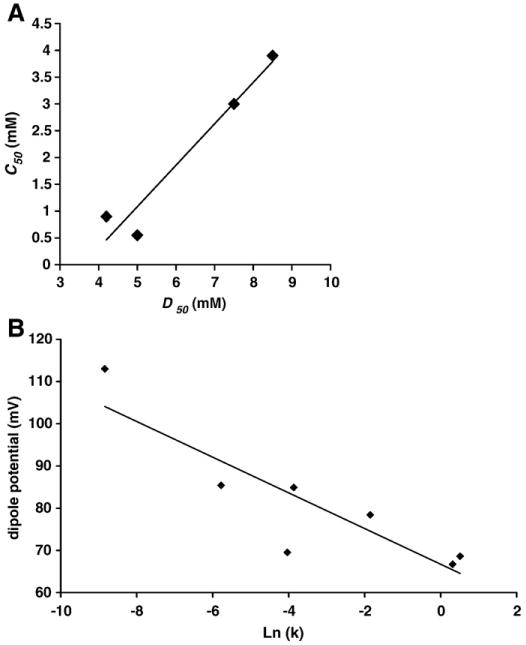
(A) Values of C50, which is the bile acid concentration at which 50% of maximal permeability to calcein was obtained, was plotted against D50, which is the bile acid concentration at which 50% of the maximal dipole potential was obtained. A linear correlation shown in the plot indicates that the change in membrane integrity and permeability is likely caused by the ability of bile acids to influence lipid orient and packing density within the membrane (r2=0.947). (B) The values of the membrane dipole potential of liposomes treated with various bile acid combinations were plotted against Ln (k), the rate of solubilization, for corresponding treatments. A linear correlation (r2=0.78) was found between the two parameters, further suggesting that the ability of bile acids to alter membrane integrity is tied to their ability to change lipid packing density.
One interesting observation is that C50 values (for calcein permeability) were found to be consistently lower than D50 (the 50% change in dipole potential) (see Fig. 8A). This is expected because we used a low molecular weight probe calcein (623 Da) [32] to detect the membrane permeation. The membrane permeability measurements depend on the size of the probe utilized [27,32,33]. Due to its small size, calcein (with a molecular weight of ∼600 Da) can easily cross the membrane, whereas high molecular weight dextrans (>40,000 Da) can only permeate across the membrane when pores in the membrane are sufficiently large [32]. However, the membrane dipole potential measures changes in the molecular dipole orientation of lipids. Since membrane defects or pores can form without significant changes in lipid packing [34,35], a small change in the dipole potential can correspond to a large leakage of a small probe. We also note that this experimental system effectively demonstrates the comparative trend of a possible influence of CHOL on the membrane disruptive action of SDC and UDC, which is the focus of this study.
We also found that our membrane dipole potential data correlate well with the other membrane property we examined: membrane turbidity. For the same bile acid treatment, a higher membrane dipole potential corresponds to a slower lipid solubilization process (smaller k). We plotted the dipole potential vs. Ln (k), which is the rate of solubilization, and found a linear correlation (r2=0.78) between the two parameters (Fig. 8B). This agrees with the established understanding that a stable and cohesive membrane is more resistant to the solubilizing effects of detergents.
Furthermore, the trend of bile acid toxicity closely follows that of the critical micelle concentrations (CMCs) of our test bile acids. Under the ionic strength of 150 mM, which is comparable to the ionic strength of the PBS buffer that we used in all our experiments, the CMC for SDC is approximately 3 mM while the CMC for UDC is ∼7 mM [36]. As expected, because bile acids with low CMCs are more hydrophobic and more likely to associate with phospholipids, this leads to high toxicity. In fact, the octanol-water partition coefficients (log P) for SDC and UDC, indicator of their lipophilicity, also correlate with their CMCs: log P of SDC is ∼446 while the log P of UDC is ∼158 [28], suggesting larger affinity of SDC for the lipid constituents of the bilayer. Our findings agree with this trend in that the concentration of SDC that led to 50% leakage of the DLPC liposomes was much lower than that of UDC. Furthermore, the ability of SDC to decrease the dipole potential and the rate of solubilization also follow that same pattern.
4.2. CHOL-dependent bile acid toxicity and cytoprotection of UDC
Our experimental results suggest that the cytotoxicity of bile acids depends on CHOL levels in the membrane. This is supported in the literature by Langmuir trough experiments studying the influence of CHOL [29]. The Langmuir trough experiments demonstrated that the molecular area of a mixed monolayer composed of PC and bile acids was significantly smaller than the calculated sum of individual areas of the corresponding PC and bile acids under the assumption of ideal mixing [29–31]. This suggests that bile acids interact with lipids to form a tighter complex. When CHOL is added to a lipid monolayer at a molar concentration between 30–50 mol%, the PC-bile acid cross-sectional area is much closer to the theoretically calculated sum of areas of lipid and bile acids under the assumption of ideal mixing [29]. This indicates that the association between bile acids and PC is weakened with CHOL in the membrane. This view is also supported by an adsorption study, demonstrating that increasing amount of CHOL in the membrane decreases the amount of bile acids adsorbed to membranes [37]. This agrees with our finding that the bile acid toxicity is linked to their ability to disrupt phospholipid membranes and that CHOL is the key to the protection of lipid membranes against bile acid attack.
It has been suggested that UDC protects cell membranes possibly by replacing the more toxic hydrophobic bile acids under physiological conditions. Lower levels of toxic bile acids would mean less toxicity. Another possible mechanism for UDC cytoprotection is that UDC, a less membrane damaging bile acid, binds to membranes and causes the bilayer to be negatively charged, which repels the negatively charged SDC. However, here we demonstrate that these influences alone are unlikely to protect cell membranes. Both dipole potential and lipid solubilization data show that a solution containing 5 mM of SDC/UDC (1:1) mix had similar capacity to damage the membrane with no CHOL as 5 mM pure SDC. The dipole potential data in Fig. 3 suggest that 2.5 mM pure SDC should be significantly less damaging than 5 mM SDC. However, 2.5 mM SDC in the 5 mM SDC/UDC 1:1 mixture was found to be as damaging as 5 mM pure SDC (Fig. 6). Simply decreasing SDC concentration to 2.5 mM in the presence of UDC seems unable to attenuate SDC toxicity. This suggests that, in the absence of membrane CHOL, UDC seems to enhance the membrane damaging effect of hydrophobic bile acids. Association of bile acids with lipids to form mixed micelles is the key to solubilize lipids and their cytotoxicity. In the case of pure DLPC liposomes, our observation that the rate of lipid solubilization was hardly changed (Table 1) when comparing 5 mM pure SDC with 5 mM 1:1 mixture of SDC/UDC (k=1.67 s−1 in 5 mM pure SDC vs. k=1.37 s−1 in 5 mM SDC/UDC) suggests that SDC binding to lipids was not weakened in the presence of UDC. However, incorporation of CHOL into the membrane, especially at 50 mol%, the SDC/UDC 1:1 mixture displayed significant protection, which was not observed with pure DLPC. This suggests that UDC cytoprotection depends on presence of CHOL in the membrane. It is possible that UDC associates with CHOL in the membrane to form a complex that could potentially be even more membrane-protective than CHOL alone. Indeed, a fractionation experiment using colon cancer cells exposed to bile acids found that UDC and CHOL were isolated in the same fraction, while SDC was found in a different fraction [38]. This suggests that UDC is capable of forming a complex with membrane CHOL and this complex potentially pushes SDC away from the membrane and decreases toxicity.
4.3. Biological significance
Here we demonstrate that bile acid toxicity and UDC cytoprotection depends on membrane CHOL. Many drugs used to treat cardiovascular diseases, such as lovastatin, aim to inhibit cell CHOL biosynthesis. This would lower CHOL level in cell membranes as well, which may cause the cells to be more susceptible to bile acid toxicity. Indeed, one of the main side effects of lovastatin is liver dysfunction [39,40]. This could also mean that UDC treatment would become less effective and possibly even damaging when CHOL levels in the cell membrane are pharmacologically reduced.
Acknowledgments
This work was in part supported by NIH grant T32 - DK07664 and PHS grant DK56338 from the Digestive Disease Center, Texas Medical Center, Houston, Texas. We thank Dr. Roger G. O'Neil for critiquing a draft of this manuscript and for insightful discussions on bile acid cytotoxicity.
References
- 1.Hofmann AF, Mysels KJ. Bile acid solubility and precipitation in vitro and in vivo: the role of conjugation, pH, and Ca2+ ions. J Lipid Res. 1992;33:617–626. [PubMed] [Google Scholar]
- 2.Shekels LL, Beste JE, Ho SB. Tauroursodeoxycholic acid protects in vitro models of human colonic cancer cells from cytotoxic effects of hydrophobic bile acids. J Lab Clin Med. 1996;127:57–66. doi: 10.1016/s0022-2143(96)90166-3. [DOI] [PubMed] [Google Scholar]
- 3.Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525–531. doi: 10.1053/jhep.2002.36088. [DOI] [PubMed] [Google Scholar]
- 4.Guldutuna S, Zimmer G, Imhof M, Bhatti S, You T, Leuschner U. Molecular aspects of membrane stabilization by ursodeoxycholate [see comment] Gastroenterology. 1993;104:1736–1744. doi: 10.1016/0016-5085(93)90653-t. [DOI] [PubMed] [Google Scholar]
- 5.Akare S, Martinez JD. Bile acid induces hydrophobicity-dependent membrane alterations. Biochim Biophys Acta. 2005;1735:59–67. doi: 10.1016/j.bbalip.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues CM, Fan G, Ma X, Kren BT, Steer CJ. A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest. 1998;101:2790–2799. doi: 10.1172/JCI1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ. Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol Med. 1998;4:165–178. [PMC free article] [PubMed] [Google Scholar]
- 8.Schmassmann A, Hofmann AF, Angellotti MA, Ton-Nu HT, Schteingart CD, Clerici C, Rossi SS, Rothschild MA, Cohen BI, Stenger RJ, et al. Prevention of ursodeoxycholate hepatotoxicity in the rabbit by conjugation with N-methyl amino acids. Hepatology. 1990;11:989–996. doi: 10.1002/hep.1840110613. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann AF. Pharmacology of ursodeoxycholic acid, an enterohepatic drug. Scand J Gastroenterol Suppl. 1994;204:1–15. doi: 10.3109/00365529409103618. [DOI] [PubMed] [Google Scholar]
- 10.Im E, Akare S, Powell A, Martinez JD. Ursodeoxycholic acid can suppress deoxycholic acid-induced apoptosis by stimulating Akt/PKB-dependent survival signaling. Nutr Cancer. 2005;51:110–116. doi: 10.1207/s15327914nc5101_15. [DOI] [PubMed] [Google Scholar]
- 11.Heuman DM, Bajaj R. Ursodeoxycholate conjugates protect against disruption of cholesterol-rich membranes by bile salts. Gastroenterology. 1994;106:1333–1341. doi: 10.1016/0016-5085(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 12.Cazeils JL, Bouillier-Oudot M, Auvergne A, Candau M, Babile R. Lipid composition of hepatocyte plasma membranes from geese overfed with corn. Lipids. 1999;34:937–942. doi: 10.1007/s11745-999-0443-z. [DOI] [PubMed] [Google Scholar]
- 13.Prabhu RB, Perakath, Balasubramanian KA. Isolation of human small intestinal brush border membranes using polyethylene glycol and effect of exposure to various oxidants in vitro. Dig Dis Sci. 2003;48:995–1001. doi: 10.1023/a:1023024301913. [DOI] [PubMed] [Google Scholar]
- 14.Clarke RJ. Effect of lipid structure on the dipole potential of phosphatidylcholine bilayers. Biochim Biophys Acta. 1997;1327:269–278. doi: 10.1016/s0005-2736(97)00075-8. [DOI] [PubMed] [Google Scholar]
- 15.Clarke RJ. The dipole potential of phospholipid membranes and methods for its detection. Adv Colloid Interface Sci. 2001;89–90:263–281. doi: 10.1016/s0001-8686(00)00061-0. [DOI] [PubMed] [Google Scholar]
- 16.Clarke RJ, Kane DJ. Optical detection of membrane dipole potential: avoidance of fluidity and dye-induced effects. Biochim Biophys Acta. 1997;1323:223–239. doi: 10.1016/s0005-2736(96)00188-5. [DOI] [PubMed] [Google Scholar]
- 17.Clarke RJ, Lupfert C. Influence of anions and cations on the dipole potential of phosphatidylcholine vesicles: a basis for the Hofmeister effect. Biophys J. 1999;76:2614–2624. doi: 10.1016/S0006-3495(99)77414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starke-Peterkovic TN, Turner N, Else PL, Clarke RJ. Electric field strength of membrane lipids from vertebrate species: membrane lipid composition and Na+− K+-ATPase molecular activity. Am J Physiol Regul Integr Comp Physiol. 2005;288:R663–R670. doi: 10.1152/ajpregu.00434.2004. [DOI] [PubMed] [Google Scholar]
- 19.Starke-Peterkovic TN, Turner N, Vitha MF, Waller MP, Hibbs DE, Clarke RJ. Cholesterol effect on the dipole potential of lipid membranes. Biophys J. 2006;90:4060–4070. doi: 10.1529/biophysj.105.074666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brockman H. Dipole potential of lipid membranes. Chem Phys Lipids. 1994;73:57–79. doi: 10.1016/0009-3084(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 21.Koppel D. Analysis of macromolecular polydispersity in intensity correlation spectroscopy: the method of cumulants. J Chem Phys. 1972;57:4814–4820. [Google Scholar]
- 22.Rajagopalan N, Lindenbaum S. Kinetics and thermodynamics of the formation of mixed micelles of egg phosphatidylcholine and bile salts. J Lipid Res. 1984;25:135–147. [PubMed] [Google Scholar]
- 23.Zhou Y, Raphael RM. Solution pH alters mechanical and electrical properties of phosphatidylcholine membranes: relation between interfacial electrostatics, intramembrane potential, and bending elasticity. Biophys J. 2007;92:2451–2462. doi: 10.1529/biophysj.106.096362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinstein JN, Yoshikami S, Henkart P, Blumenthal R, Hagins WA. Liposome–cell interaction: transfer and intracellular release of a trapped fluorescent marker. Science. 1977;195:489–492. doi: 10.1126/science.835007. [DOI] [PubMed] [Google Scholar]
- 25.Coleman R, Lowe PJ, Billington D. Membrane lipid composition and susceptibility to bile salt damage. Biochim Biophys Acta. 1980;599:294–300. doi: 10.1016/0005-2736(80)90075-9. [DOI] [PubMed] [Google Scholar]
- 26.Billington D, Evans CE, Godfrey PP, Coleman R. Effects of bile salts on the plasma membranes of isolated rat hepatocytes. Biochem J. 1980;188:321–327. doi: 10.1042/bj1880321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schubert R, Jaroni H, Schoelmerich J, Schmidt KH. Studies on the mechanism of bile salt-induced liposomal membrane damage. Digestion. 1983;28:181–190. doi: 10.1159/000198984. [DOI] [PubMed] [Google Scholar]
- 28.Roda A, Minutello A, Angellotti MA, Fini A. Bile acid structure–activity relationship: evaluation of bile acid lipophilicity using 1-octanol/water partition coefficient and reverse phase HPLC. J Lipid Res. 1990;31:1433–1443. [PubMed] [Google Scholar]
- 29.Fahey DA, Carey MC, Donovan JM. Bile acid/phosphatidylcholine interactions in mixed monomolecular layers: differences in condensation effects but not interfacial orientation between hydrophobic and hydrophilic bile acid species. Biochemistry. 1995;34:10886–10897. doi: 10.1021/bi00034a023. [DOI] [PubMed] [Google Scholar]
- 30.Leonard MR, Bogle MA, Carey MC, Donovan JM. Spread monomolecular films of monohydroxy bile acids and their salts: influence of hydroxyl position, bulk pH, and association with phosphatidylcholine. Biochemistry. 2000;39:16064–16074. doi: 10.1021/bi001316m. [DOI] [PubMed] [Google Scholar]
- 31.Kauffman JM, Pellicciari R, Carey MC. Interfacial properties of most mono-fluorinated bile acids deviate markedly from the natural congeners: studies with the Langmuir–Pockels surface balance. J Lipid Res. 2005;46:571–581. doi: 10.1194/jlr.M400439-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura Y, Lemasters JJ. Glycine blocks opening of a death channel in cultured hepatic sinusoidal endothelial cells during chemical hypoxia. Cell Death Differ. 2001;8:850–858. doi: 10.1038/sj.cdd.4400877. [DOI] [PubMed] [Google Scholar]
- 33.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Raphael RM. Effect of salicylate on the elasticity, bending stiffness, and strength of SOPC membranes. Biophys J. 2005;89:1789–1801. doi: 10.1529/biophysj.104.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans E, Heinrich V, Ludwig F, Rawicz W. Dynamic tension spectroscopy and strength of biomembranes. Biophys J. 2003;85:2342–2350. doi: 10.1016/s0006-3495(03)74658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roda A, Hofmann AF, Mysels KJ. The influence of bile salt structure on self-association in aqueous solutions. J Biol Chem. 1983;258:6362–6370. [PubMed] [Google Scholar]
- 37.Heuman DM, Bajaj RS, Lin Q. Adsorption of mixtures of bile salt taurine conjugates to lecithin-cholesterol membranes: implications for bile salt toxicity and cytoprotection. J Lipid Res. 1996;37:562–573. [PubMed] [Google Scholar]
- 38.Jean-Louis S, Akare S, Ali MA, Mash EA, Jr, Meuillet E, Martinez JD. Deoxycholic acid induces intracellular signaling through membrane perturbations. J Biol Chem. 2006;281:14948–14960. doi: 10.1074/jbc.M506710200. [DOI] [PubMed] [Google Scholar]
- 39.Folkers K, Langsjoen P, Willis R, Richardson P, Xia LJ, Ye CQ, Tamagawa H. Lovastatin decreases coenzyme Q levels in humans. Proc Natl Acad Sci U S A. 1990;87:8931–8934. doi: 10.1073/pnas.87.22.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willis RA, Folkers K, Tucker JL, Ye CQ, Xia LJ, Tamagawa H. Lovastatin decreases coenzyme Q levels in rats. Proc Natl Acad Sci U S A. 1990;87:8928–8930. doi: 10.1073/pnas.87.22.8928. [DOI] [PMC free article] [PubMed] [Google Scholar]


